Abstract
Helicase-like proteins play a crucial role in nucleic acid- and chromatin-mediated reactions. In this study, we identified 134 helicase-like proteins in the nematode Caenorhabditis elegans and classified the proteins into 10 known subfamilies and a group of orphan genes on the basis of sequence similarity. We characterized loss-of-function phenotypes in RNA interference (RNAi)-treated animals for helicase family members, using the RNAi feeding method, and found several previously unreported phenotypes. Fifty-one (39.5%) of 129 genes tested showed development- or growth-defect phenotypes, and many of these genes were putative nematode homologs of essential genes in a unicellular eukaryote, budding yeast, suggesting conservation of these essential proteins in both species. Comparative analyses between these species identified evolutionarily diverged nematode proteins as well as conserved family members. Chromosome mapping of the nematode genes revealed 10 pairs of putative duplicated genes and clusters of C. elegans-specific SNF2-like genes and Helitrons. Analyses of transcriptional profile data revealed a predominantly oogenesis- and germline-enriched expression of many helicase-like genes. Finally, we identified the D2005.5(drh-3) gene in an RNAi-based screen for genes involved in resistance to X-ray irradiation. Analysis of DRH-3 will clarify the potentially novel mechanism by which it protects against X-ray-induced damage in C. elegans.
Key words: C. elegans, comparative genomics, drh-3, helicase family, RNAi-based screen
1. Introduction
The helicase superfamily is made up of three functional classes, DNA helicases, RNA helicases, and chromatin remodeling ATPases, and the members of this family play a crucial role in various nucleic acid- and chromatin-mediated cellular reactions such as DNA replication, repair and recombination, pre-mRNA splicing, ribosome biogenesis, RNA interference, and chromatin remodeling.1–5 Since these reactions are essential for maintenance, expression, and regulation of genetic information in the chromosome, dysfunctions of helicase genes may lead to genetic diseases, including cancers. Indeed, genetic mutations of the human RecQ-like BLM and the WRN DNA helicase result in the early development of various cancers and premature aging, respectively.6 Most helicases share conserved amino acid sequence motifs, and these genes are classified into five families (SF1–SF5) on the basis of the occurrence and characteristics of conserved motifs.7 Because of the biological importance of the helicase family, we conducted a comprehensive analysis of the functions of helicase family members in two model organisms: Saccharomyces cerevisiae and Caenorhabditis elegans. Previously, we examined loss-of-function phenotypes of yeast novel helicase-related genes, using gene knockout strains and characterized gene expression profiles by northern blotting.8 In our previous study, we identified 21 uncharacterized genes including five essential genes YDL031W[DBP10], YDL084W[SUB2], YKL078W[DHR2], YLR276C[DBP9], and YMR128W[ECM16], and YDL070W[BDF2] and YGL150C[INO80] were later shown to be non-essential. Some of these novel genes were subsequently characterized to clarify their molecular functions, for example, SUB2 in pre-mRNA splicing,9 DHR2, ECM16 and several DEAD-box genes such as DBP9 in ribosome biogenesis,10,11 YOL095C(HMI1) in the maintenance of mitochondrial DNA,12 and INO80 and YDR334W(SWR1) in chromatin remodeling and transcription.13,14 Thus, comprehensive analyses focusing on the helicase superfamily can lead to the discovery of novel genes required for basic cellular reactions involved in cell proliferation, development, and aging.
In the current study, we have focused on helicase family members in a multicellular organism, the nematode C. elegans, using an RNA interference (RNAi) technique. Indeed, many novel multicellular specific proteins from other gene families have been discovered by RNAi-mediated comprehensive studies in C. elegans, including SR-related proteins,15,16 proteins for the ubiquitylation system,17 the forkhead proteins,18 and G protein-coupled receptors.19 Comparative analysis of helicase-like proteins in yeast and C. elegans will allow us to identify nematode-specific proteins that likely play an important role in multicellular organism-specific functions such as morphogenesis. Identification and characterization of these higher eukaryote-specific helicases will be useful in understanding the molecular mechanisms of genetic diseases caused by mutations of human helicase-like genes.
Here, we found 134 genes encoding putative helicase-like proteins in C. elegans and systematically prepared RNAi-treated animals for each of these genes to characterize their loss-of-function phenotypes. Fifty-one of 129 genes tested caused embryonic lethality or growth defects by RNAi, and these genes contained many putative homologs of yeast essential helicase-like genes. We identified two divergent gene clusters and 10 pairs of putative gene duplications on chromosomes and found germline- and oogenesis-enriched expression of many helicase-like genes. In addition, an RNAi-based screen was performed to identify genes required for resistance to X-ray irradiation, resulting in successful identification of the novel D2005.5(drh-3) gene.
2. Materials and methods
2.1. Sequence analyses
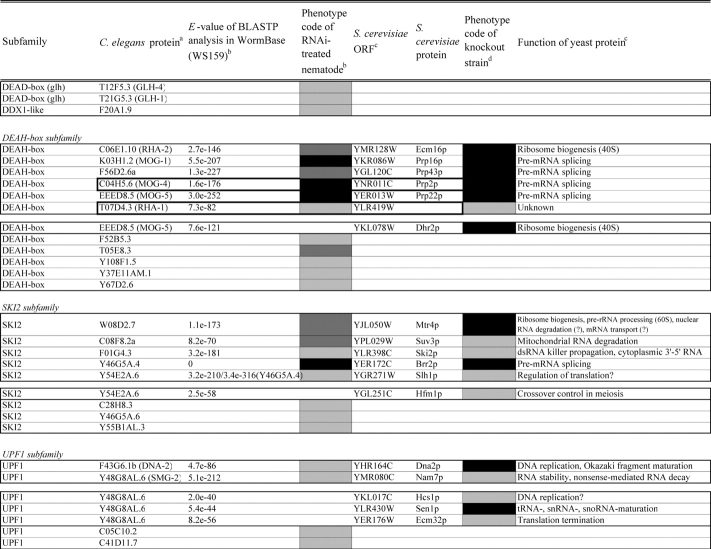
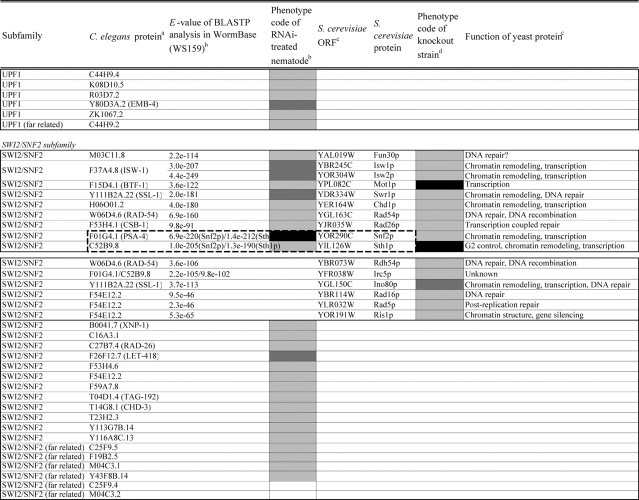
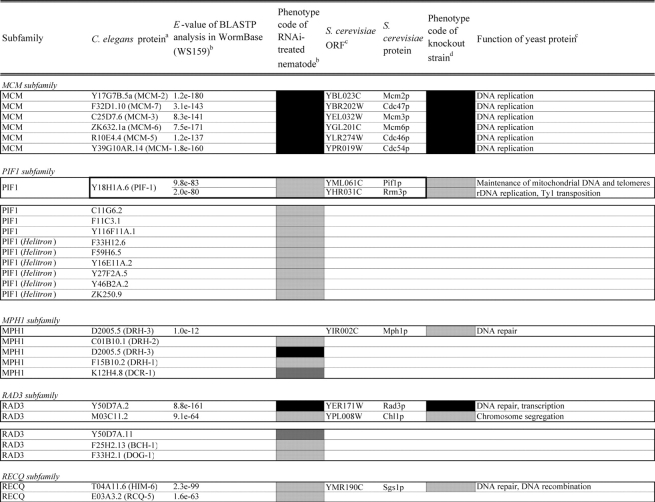
Identification of helicase-like proteins in C. elegans was performed as described in the legend of Table 1. Helicases were classified according to the yeast helicase-like protein subfamilies by Linder (http://www.medecine.unige.ch/~linder/helicases_list.html) with modifications (i.e. addition of the new subfamilies MPH1, PIF1, RAD3, and RECQ). Most orthologous proteins were identified from the InParanoid database20 as described in the legend of Table 2. Homologous members in gene pairs and clusters were identified by homology search in C. elegans nucleotide sequence databases as described in the legend of Supplementary Table S4.
Table 1.
Summary of RNAi analyses of C. elegans helicase-like genes*
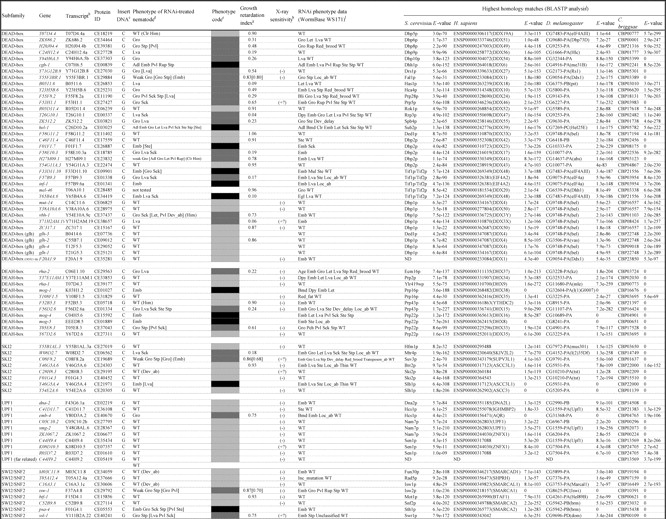
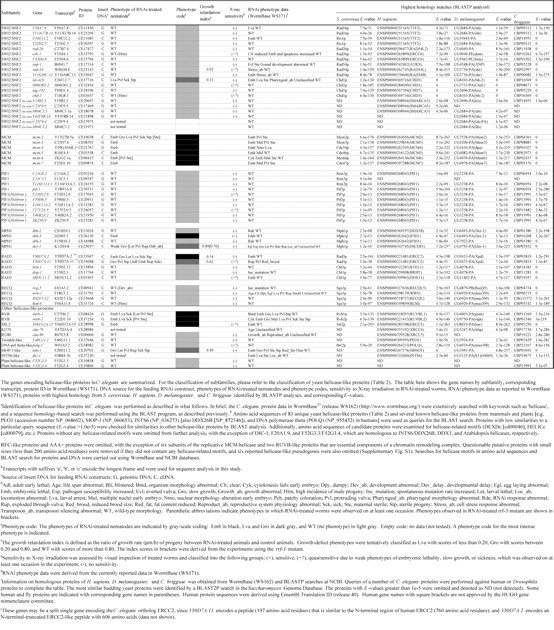
Table 2.
Comparison of loss-of-function phenotypes of helicase-like genes in S. cerevisiae and C. elegans
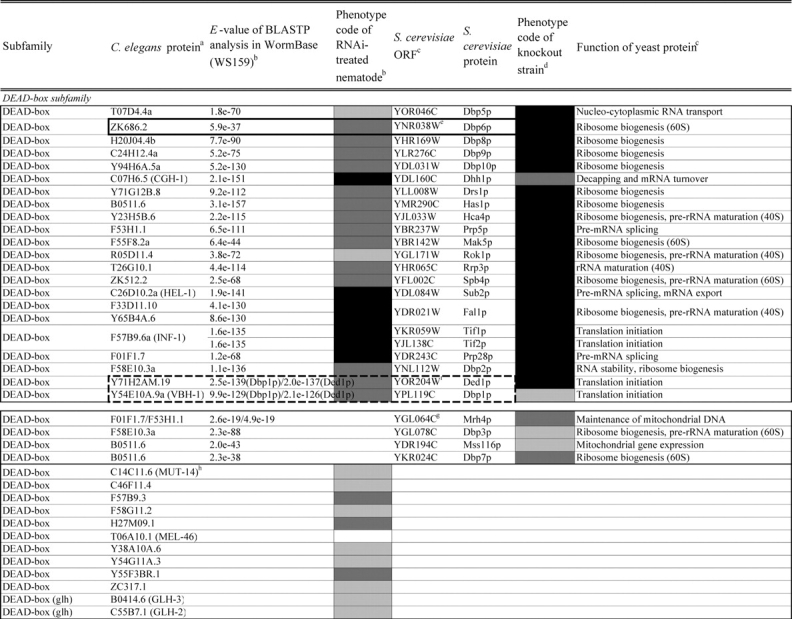
Loss of function phenotypes of helicase like proteins in S. cerevisiae and C. elegans are summarized according to subfamily for comparison between species. aC. elegans proteins putatively orthologous to the yeast helicase like proteins and E values of the BLAST analyses are shown. C. elegans proteins were identified by BLASTP analysis and database search against the InParanoid database (version 4.0 updated April 2005, http://inparanoid.cgb.ki.se/). Several putative orthologs were identified as reciprocal best BLAST hits with an E val ue <1.0e 30 between S. cerevisiae and C. elegans. Suffixes ‘a’ and ‘b’ indicate variants with the highest homology to the yeast protein. bPhenotype code (C. elegans): The phenotypes of RNAi treated nematodes are indicated by gray scale coding: Emb in black, Lva and Gro in dark gray, and WT (no phenotype) in light gray. Empty code: no data (not tested). A phenotype code for the most intense phenotype is indicated. cClassification and functions of yeast helicase like proteins are according to the yeast RNA helicase database by Linder and colleagues. dPhenotypes of the corresponding knockout strains were mainly obtained from the Sacch aromyces Genome Database and our previous report8 and shown by phenotype codes: lethal in black, slow growth in dark gray, and viable in light gray, no data in white. eThe proteins surrounded with bold lines are a putative orthologous pair based on BLASTP scores, but were not in the InParanoid database. The Ku70 and Ku80 homologs in yeast and nematodes are described in the Saccharomyces Genome Database and WormBase. fTwo pairs of yeast proteins (Snf2p and Sth1p, Ded1p and Dbp1p) with two C. elegans orthologs are surrounded by dashed lines. gThe yeast proteins with BLAST scores lower than that of the putative homologs or without any sequence homologies to C. elegans proteins are indicated in separated box for each subfamily. hC. elegans proteins without significant similarities to yeast helicase like proteins are also indicated separately. iND, not detected . Several C. elegans proteins with E values greater than 1e 10 when compared with the Y’ Hel1 proteins were omitted because the similarities were to low complexit y regions in the amino acid sequences. jTwenty five budding yeast specific proteins including subtelomere specific helicase like proteins and four yeast proteins (Hrq1p, Hpr5p, Hmi1p, and Irc3p) and six C. elegans (higher eukaryot e) specific proteins (DIC 1, NSH 1, POLQ 1, F46G11.1, F52G3.3, and F52G3.4) were detected.
2.2. C. elegans strains and culture procedures
C. elegans wild-type strain Bristol N2 and the RNAi-hypersensitive rrf-3 mutant strain NL2099 (rrf-3(pk1426) II)21 (obtained from the Caenorhabditis Genetics Center) were used in this study. Animals were maintained at 20°C on nematode growth medium (NGM) agar plates seeded with the Escherichia coli OP50 strain, as described previously.22
2.3. Construction of recombinant DNA
Genomic DNA or cDNA fragments corresponding to helicase-like genes were cloned into the blunted EcoRI site of the double-stranded RNA (dsRNA) expression vector pPD129.36 (a kind gift of Dr A. Fire, Stanford University School of Medicine, USA). Insert DNA was directly amplified by PCR from a C. elegans embryo cDNA library (No. 937007, Stratagene, La Jolla, CA, USA) or genomic DNA (N2), using a gene-specific primer set for blunt-end cloning. PCR primers were purchased from Sawady (Tokyo) and Proligo LLC (Boulder, USA), and nucleotide sequences of the primers will be provided upon request. The nucleotide sequences of the resultant recombinant clones were determined by dye-terminator cycle sequencing.
2.4. Feeding RNAi
RNAi by feeding bacteria was performed using the N2 and the rrf-3 strains, as described previously,23 with the following modifications. In brief, the HT115(DE3) E. coli strain containing the pDP129.36 with a target gene-specific insert was grown overnight in 2× YT medium containing 100 µg/mL ampicillin (or 50 µg/mL carbenicillin and 12.5 µg/mL tetracycline) with stirring at 37°C. Aliquots of the culture (30 µL) were spread onto NGM agar in a Petri dish (Ø 6 cm) containing 1 mM isopropyl β-D-thiogalactopyranoside (IPTG) and the indicated antibiotics and incubated at 37°C for 18 h for RNAi (RNAi plates). The next day, P0 animals at the fourth larval (L4) to young adult stages were placed onto the RNAi plates and fed the recombinant E. coli strain expressing dsRNA for over 18 h to avoid F1 progeny with leaky phenotypes. Subsequently, P0 animals were transferred onto new RNAi plates with bacteria-expressing dsRNA for 12 h to lay eggs (F1) and then removed. The eggs on this second RNAi plate were used for phenotypic analyses of F1 progeny. HT115(DE3) with vector alone was used as control bacteria for mock RNAi treatments. For double-RNAi treatment, 50 µL of culture suspension equally mixed with growing bacteria for each target gene was seeded on RNAi plates for dsRNA expression.
2.5. Phenotypic analyses of RNAi-treated animals
The hatching rate of the eggs was determined as described previously.24 RNAi-treated animals for 12 h were transferred onto a new RNAi plate to lay eggs for 12 h. F1 eggs laid on the RNAi plate were cultured for 24 h. Subsequently, the numbers of hatched larvae and dead eggs were scored to determine the hatching rate. The experiments were repeated at least twice. Growth of hatched F1 progeny was monitored by body length measurements as described previously.22 The growth-defect phenotypes were tentatively classified as larval arrest (Lva), slow growth (Gro), and normal growth (WT), using the growth retardation index, as described in the legend of Table 1. Brood size of RNAi-treated animals was examined in two ways as described in the legend of Supplementary Table S2. X-ray sensitivity assay of RNAi-treated animals was performed as described in the legend of Tables 3 and 4.
Table 3.
Influence of X-ray irradiation on the viability of F1 progeny from RNAi-treated animals
| X-ray dose (Gy) | Hatching rate (%) |
|||
|---|---|---|---|---|
| Control | D2005.5 (RNAi) | rad-51 (RNAi) | Y66D12A.15 (RNAi) | |
| 0 | 91.8 (n = 622) | 32.5 (n = 382) | 62.4 (n = 86) | 1.1 (n = 451) |
| 40 | 62.8 (n = 756) | 0.6 (n = 313) | 3.2 (n = 313) | 0.0 (n = 574) |
The cDNA fragments corresponding to D2005.5 and rad-51(Y43C5A.6) were amplified from phage cDNA clones yk331a2 and yk401c3 (a kind gift of Dr Y. Kohara, National Institute of Genetics, Japan), respectively, by PCR with the primer set yk5′-F (5′-TGGCGGCCGCTCTAGAACTAGTGGATC-3′) and yk3′-SmaR (5′-TTCCCGGGTGAATTGTAATACGACTCACTATAGGGCG-3′). These cDNAs were used for X-ray-induced embryonic lethality assay. The genomic DNA fragment (∼2.3 kb) corresponding to Y66D12A.15 was amplified from C. elegans genomic DNA (N2 strain) by PCR using the primer set Y66Dex1-3F (5′-AAGCTTGAAAAACCCAGAAAAATGGCA-3′) and Y66Dex1-3R (5′-TTCCACTCCAACCTTGGTCGCATCGGC-3′). These fragments were cloned into the dsRNA expression vector, and the nucleotide sequences were confirmed by sequencing. Four young adult worms were fed bacteria-expressing dsRNA to the target gene on an RNAi plate for 18 h and were subsequently X-ray-irradiated (Radioflex 320CG, RIGAKU, Tokyo) at a rate of 2 Gy/min. Irradiated animals were transferred onto a fresh RNAi plate, cultured for 2 days to lay eggs and then removed. After 24 h, the hatching rate of eggs laid on the plate was determined. The total numbers of eggs counted are indicated in parentheses.
Table 4.
Influence of X-ray irradiation on the growth of F1 progeny from RNAi-treated animals
| Body length (mm) |
|||
|---|---|---|---|
| Control | D2005.5 (RNAi) | gei-17 (RNAi) | |
| Mock irradiated | 0.932 ± 0.062 (n = 40) | 0.923 ± 0.076 (n = 32) | 0.919 ± 0.116 (n = 43) |
| Significance relative to control (P-value) | 0.521 | 0.560 | |
| X-ray irradiated (40 Gy) | 0.992 ± 0.076 (n = 56) | 0.726 ± 0.208 (n = 18) | 0.826 ± 0.211 (n = 31) |
| Significance relative to control (P-value) | <0.0001 | <0.0001 | |
The genomic DNA fragment corresponding to gei-17(W10D5.3) was amplified using the primer set W10D5.3-F (5′-CGCTTCCACTTCCATTCTACGATG-3′) and W10D5.3-R (5′-GGCCATTCCAGATGGAGATGAGCC-3′). The D2005.5 cDNA fragment (∼1.5 kb) was amplified from a C. elegans embryo cDNA library using the primers D1-BF (5′-CCGGGATCCATCGTTGATCTGATGCCTGCGATGG-3′) and ZAP-R (5′-GAATTGTAATACGACTCACTATAGGGC-3′). The D2005.5 cDNA and gei-17 genomic DNA fragment were used for an X-ray-induced growth retardation assay. The growth of larvae from RNAi-treated animals was monitored by determining the mean body length of the animals. The mean ± standard deviation values of body length of animals at 3 days after X-ray or mock irradiation were determined and are indicated . Numbers of animals measured are in parentheses. Statistical significance of the differences in mean body length between control and RNAi-treated animals in each group was analysed by Student's t-test (significance at P < 0.05) using the software package JMP IN5.1.2J (SAS Institute, Cary, USA).
3. Results
3.1. Identification of helicase-like genes in C. elegans
In this study, we have expanded our analyses of helicase family members from unicellular eukaryote S. cerevisiae to a multicellular animal, the nematode C. elegans. A sequence homology search, with known helicase-like proteins as the queries, identified 134 gene products in the recent C. elegans protein data in the public nematode database WormBase25 (release WS162). These proteins were classified into 10 subfamilies (DEAD-box, DEAH-box, SKI2, UPF1, SWI2/SNF2, MCM, PIF1, MPH1, RAD3, and RECQ) on the basis of a modified classification of yeast helicase-like proteins and one group of ‘other helicase-like proteins’ containing 11 orphan proteins (Table 1). Three subfamilies (DEAD-box, DEAH-box, and SKI2) contain many proteins involved in aspects of RNA metabolism, including ribosome biogenesis, pre-mRNA splicing, RNA degradation, and translation.2,3 A number of the proteins in the MCM,26 PIF1,27 RAD3, and RECQ6 subfamilies play roles in DNA-mediated reactions, and the SWI2/SNF2 members act primarily in chromatin remodeling and/or DNA metabolism.4,5 The total number of helicase-like proteins in C. elegans (134 proteins) was greater than the number of yeast helicase-like proteins (103 proteins including 21 subtelomeric helicase-like proteins).28 Among the genes identified were six nematode homologs of mammal- and plant-specific helicase-like genes (polq-1, nsh-1, dic-1, F46G11.1, F52G3.3, and F52G3.4) and five C. elegans-specific SNF2-like genes (C25F9.4, C25F9.5, M04C3.1, M04C3.2, and Y43F8B.14). Six Helitrons, a novel class of mobile genetic elements encoding a ‘rolling circle’ replication protein and a helicase29,30 (F33H12.6, F59H6.5, Y16E11A.2, Y27F2A.5, Y46B2A.2, and ZK250.9), were also identified.
Drosophila melanogaster, Homo sapiens, and Caenorhabditis briggsae proteins homologous to each C. elegans protein are presented in Table 1. Most helicase-like genes were well conserved between C. elegans and C. briggsae, with the exception of the C. elegans-specific SNF2-like genes, several PIF1-like genes including the Helitrons, and plant helicase-like gene homologs (F52G3.3 and F52G3.4). Although homologs of the Helitrons, F52G3.3, and F52G3.4 were detected in plants (data not shown), these three gene groups were not conserved in humans or flies. We detected putative human and fly homologs of many genes from the known subfamilies, as well as the orphan genes, but we were unable to find putative counterparts of several DEAD-box genes including four glh genes, some of the UPF1- and SWI2/SNF2-like genes, and most of the PIF1 members, in addition to the C. elegans-specific SNF2-like genes and two plant helicase-like genes (Table 1).
3.2. Phenotypic analyses of C. elegans RNAi-treated for helicase-like genes
Of the 134 genes identified in this study, 49 corresponded to genes of known function; however, the functions of the remaining genes are unknown. Therefore, we used the feeding RNAi method to identify loss-of-function phenotypes of uncharacterized helicase-like genes to aid in ascertaining the function of the gene products. We prepared 129 dsRNA expression constructs with cDNA or genomic DNA fragments of the target genes, and E. coli transformants expressing dsRNA were fed to P0 animals to examine the RNAi-induced phenotypes of the resultant F1 progeny. Typical culture images of the F1 progeny 3 days after hatching are shown in Fig. 1. The control progeny from mock-treated P0 animals grew to adults and laid F2 eggs (Fig. 1A). In contrast, eggs laid by RNAi-treated animals for mcm-6, encoding a subunit of the replicative MCM helicase,26 exhibited an embryonic lethal phenotype (Fig. 1B). RNAi for the uncharacterized genes W08D2.7 and ZK686.2 encoding a yeast Ski2p-like protein and a DEAD-box protein caused larval growth arrest (Fig. 1C) and growth retardation (Fig. 1D), respectively, suggesting that both gene products are essential for development and/or larval growth. RNAi for Y50D7A.11 which encodes an ERCC2-like protein caused a progeny sterile phenotype (no F2 eggs in the culture) with growth retardation (Fig. 1E). In addition to embryonic lethality and sterility,31 we observed an RNAi-induced developmental abnormality (a protruding vulva phenotype in Fig. 1F) and increased mortality (Fig. 1G) among F1 survivors of cgh-1(RNAi) animals.
Figure 1.
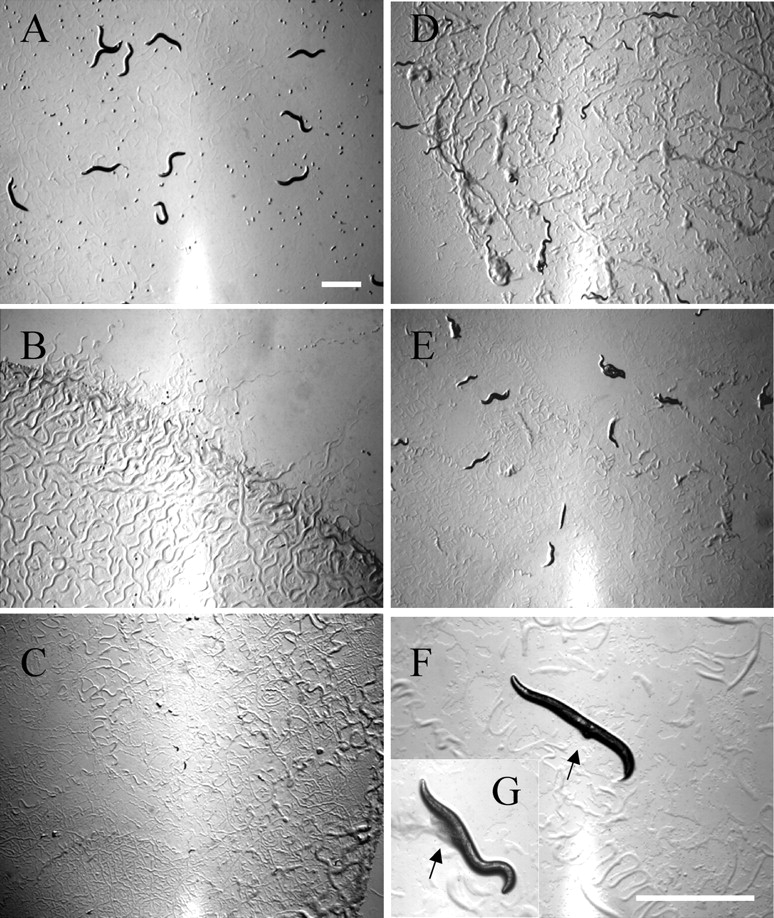
Typical phenotypes of F1 progeny from nematodes RNAi-treated for helicase-like genes. Typical images of the F1 progeny from eggs laid by RNAi-treated P0 animals on RNAi plates for control [vector alone (A)], mcm-6(ZK632.1) RNAi (B), W08D2.7 RNAi (C), ZK686.2 RNAi (D), Y50D7A.11 RNAi (E), and cgh-1 (C07H6.5) RNAi [(F) and (G) in a threefold enlarged image] are shown. The progeny were cultured on RNAi plates supplemented with dsRNA-expressing bacteria for 3 days after laying, and images were then captured. The RNAi phenotypes shown are embryonic lethal (Emb in Table 1) (B), larval arrest (Lva) (C), slow growth (Gro) (D), slow growth and sterile progeny (Gro Stp) (E), and protruding vulva (Pvl) (F and G). Arrows indicate protruded vulva (F) and resultant abdominal burst (G). Bar: 1 mm.
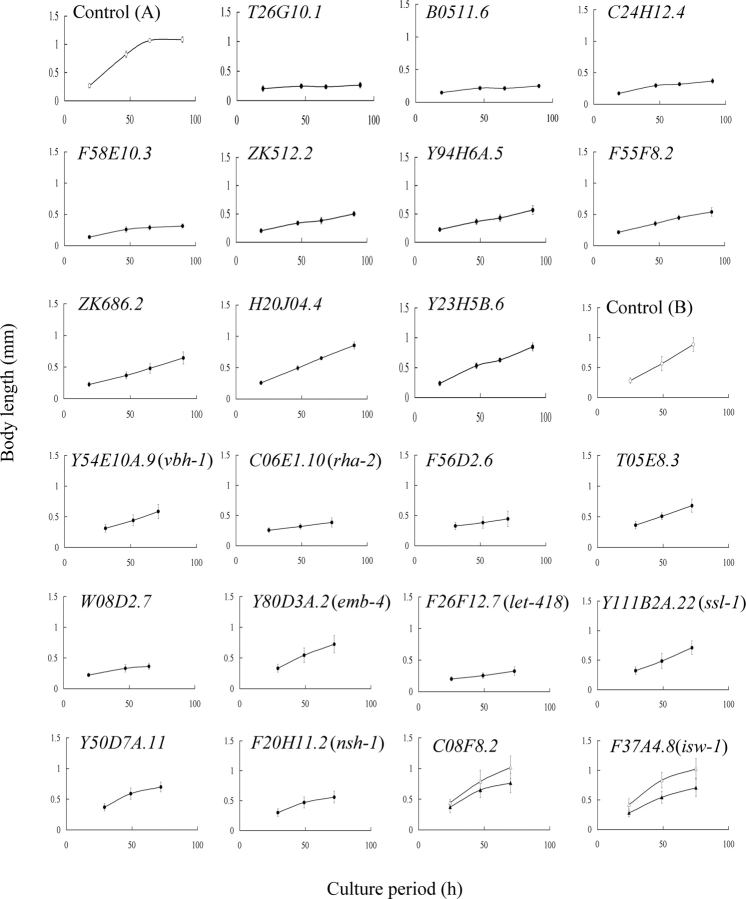
In this study, we examined primarily embryonic lethality and growth-defect phenotypes. Fig. 2 shows growth curves of F1 larvae from animals treated with for 22 helicase members. Growth of progeny from T26G10.1(RNAi) and B0511.6(RNAi) was almost completely arrested (Fig. 2); however, RNAi-induced growth retardation was variable in progeny among the targeted genes. For example, growth rates of progeny from F58E10.3(RNAi), Y23H5B.6(RNAi), and mock-treated animals were calculated to be 3.4, 8.7, and 17.6 µm/h, respectively (Fig. 2). The level of growth defects in the progeny is represented as the growth retardation index in Table 1.
Figure 2.
Influence of RNAi treatment of helicase family genes on larval growth. The growth of F1 larvae from eggs laid by RNAi-treated P0 animals was monitored by measuring the body length of progeny. The resultant growth curves of progeny of animals (N2 strain) that were RNAi-treated for the indicated 10 genes (T26G10.1 to Y23H5B.6) in the DEAD-box subfamily are shown together with the growth curve of progeny without RNAi-treatment [control (A)]. The growth curves obtained from RNAi experiments for the genes in other subfamilies are shown with their control growth curve [control (B)] as follows: Y54E10A.9(vbh-1) from the DEAD-box subfamily; C06E1.10(rha-2), F56D2.6, and T05E8.3 from the DEAH-box subfamily; W08D2.7 from the SKI2 subfamily; Y80D3A.2(emb-4) in the UPF1 subfamily; F26F12.7(let-418) and Y111B2A.22(ssl-1) from the SWI2/SNF2 subfamily; Y50D7A.11 from the RAD3 subfamily; and F20H11.2(nsh-1) as an orphan member, respectively. Experiments for C08F8.2 (SKI2 subfamily) and F37A4.8(isw-1) (SWI2/SNF2 subfamily) indicated in bold letters were carried out using the rrf-3 mutant as a host because of weak slow-growth phenotypes of the RNAi-treated N2 animals, and the resultant growth curves of progeny of control (open triangle) and RNAi-treated (closed triangle) animals are shown. The calculated growth rate for each population was 17.5 µm/h [control (A)], 0.7 (T26G10.1), 1.5 (Y71H2AM.19), 3.3 (B0511.6), 3.4 (C24H12.4), 4.0 (ZK512.2), 4.5 (Y94H6A.5), 5.1 (F55F8.2), 5.5 (ZK686.2), 8.4 (H20J04.4), 8.7 (Y23H5B.6), 12.5 [control (B)], 6.8 (Y54E10A.9(vbh-1)), 2.7 (C06E1.10(rha-2)), 3.0 (F56D2.6), 7.4 (T05E8.3), 3.2 (W08D2.7), 9.1 (Y80D3A.2(emb-4)), 2.6 (F26F12.7(let-418)), 9.1 (Y111B2A.22(ssl-1)), 7.6 (Y50D7A.11), 6.0 (F20H11.2(nsh-1)), 8.5 (C08F8.2) and 12.5 (rrf-3 control), and 8.3 (F37A4.8(isw-1)) and 11.8 (rrf-3 control).
We compared our results with RNAi phenotype data in the public WormBase (WS171) and most phenotypes were in agreement (Table 1). Furthermore, we successfully obtained several new phenotypes; for instance, RNAi for two DEAD-box subfamily members, C24H12.4 and Y71G12B.8, resulted in larval arrest and slow growth, respectively. RNAi for Y66D12A.15, which encodes an ortholog of the human ERCC3-like protein, and psa-4, which is required for embryonic development,32 resulted in embryonic lethality in the current study. [These phenotypes in Y66D12A.15(RNAi) and psa-4(RNAi) were not previously present in the database (WS162), but have been recently confirmed in the updated version (WS171) during revision of the manuscript.] On the other hand, it was reported that RNAi for dna-2, F20A1.9, F52B5.3, F59A7.8, M03C11.2, M03C11.8, Y116A8C.13, and Y37E11AM.1 caused an embryonic lethal phenotype, but no growth defect and/or visible abnormalities were observed in our experiments even using the RNAi-hypersensitive rrf-3 mutant33,34 as a host (data not shown). The discrepancies of RNAi experiments are summarized in Supplementary Table S1.
In this study, we examined effects of suppression of 39 germline- or oocyte-expressed helicase-like genes on brood size by feeding RNAi from L1 stage or from L4 stage (Supplementary Table S2). Reduction in brood size was observed in F55F8.2(RNAi) and T05E8.3(RNAi) animals in the L4 RNAi experiments. In the L1 RNAi experiments, suppression of F56D2.6 and C08F8.2 caused significant reductions of brood size; however, reduction was due to sterility in P0 animals by F56D2.6 RNAi and to embryonic lethality by C08F8.2 RNAi (data not shown). Reduced brood size in C08F8.2(RNAi) and F55F8.2(RNAi) animals has been observed previously by others.35
3.3. An RNAi-mediated screen for helicase-like genes involved in resistance to X-ray irradiation
We tried to identify genes that play important roles in specific conditions—in this case, X-ray irradiation—and applied the feeding RNAi technique to screen for genes involved in protection against X-ray-induced DNA damage. Assuming that dysfunctions in candidate genes would cause hypersensitivity to X-rays, animals RNAi-treated for 87 helicase-like genes that were dispensable for embryonic survival were tested for their X-ray sensitivity. RNAi-treated P0 animals were irradiated with X-rays (40 Gy), and the hatching rate of the resultant F1 progeny was examined. Several candidate genes were detected, but only D2005.5(drh-3) RNAi reproducibly enhanced the sensitivity to X-rays (Table 1). The hatching rate of the F1 progeny from drh-3(RNAi) animals without X-ray irradiation was 32.5% due to embryonic lethality induced by RNAi alone; however, the viability of F1 progeny from irradiated drh-3(RNAi) animals markedly decreased to 0.6% (Table 3). A similar X-ray hypersensitivity was observed in rad-51(RNAi) animals, in which DNA double-strand break repair and meiotic homologous recombination were suppressed.36,37 X-ray-induced growth retardation was also observed in the F1 progeny from drh-3(RNAi) animals. The F1 progeny from drh-3(RNAi) and gei-17(RNAi) animals without irradiation developed normally. However, F1 larvae from irradiated animals exhibited a slow-growth phenotype (Table 4). The gei-17 gene encodes a putative E3 SUMO ligase that participates in embryonic DNA damage responses in C. elegans, and the gei-17(RNAi) embryo is sensitive to other DNA-damaging agents.38,39
4. Discussion
4.1. Identification of helicase family members and RNAi-based phenotypic analyses in C. elegans
This is the first survey of members of helicase-like genes in C. elegans. In this study, several novel members of helicase family were identified by a systematic BLAST-based homology search, including two plant helicase-like genes of F52G3.3 and F52G3.4 and five C. elegans-specific SNF2-like genes. It should be noted that the current total number of helicase-like genes (134 genes) is tentative. For example, both Y50D7A.2 and a neighboring gene, Y50D7A.11, may be a split single gene encoding the C. elegans ortholog ERCC2 (see the legend of Table 1).
In this study, we identified 51 helicase-like genes that are required for viability and/or developmental growth of C. elegans. This percentage (39.5% of 129 genes tested) was significantly higher than the number of phenotype-positive genes from several genome-wide RNAi analyses (∼10%35,40 to 27%41), suggesting the biological importance of helicase-like genes in cellular function. The number of genes required for embryonic development and/or larval growth was variable among the subfamilies. For example, many members of the DEAD-box (63.9% of the members), DEAH-box (54.5%), MCM (100%), and MPH1 (50%) subfamilies exhibited development- and growth-defects by RNAi. In contrast, relatively few of the PIF1 (0%), RECQ (0%), UPF1 (10%), or SWI2/SNF2 (15.4%) subfamily members showed such defects (Supplementary Table S3). These results are consistent with our previous phenotypic analysis using knockout strains of yeast helicase-like genes.8 The difference in the incidence of these phenotypes among the subfamilies could be accounted by the biological roles in the members in each subfamily. It is interesting that the suppressions of half of the genes (dcr-1 and drh-3) in the MPH1 subfamily cause growth-defects or embryonic lethality. This suggests biological importance of RNAi in viability and larval growth in C. elegans because both gene products act in RNAi.42,43 We found some discrepancies in RNAi-induced phenotypes between our experiments and the studies reported in WormBase (Supplementary Table S1). We newly found larval arrest and slow-growth phenotypes caused by C24H12.4 RNAi and Y71G12B.8 RNAi, respectively. C24H12.4 and Y71G12B.8 encode putative homologs of the yeast DEAD-box proteins Dbp9p and Drs1p, respectively. Since both yeast proteins are required for ribosomal RNA biogenesis11,44 and essential for viability in yeast,3,8 our observations on both genes are probably significant (see the legend of Supplementary Table S1 for other discrepancies).
4.2. Loss-of-function phenotypes of helicase family members diverged in C. elegans
The putative C. elegans orthologs of yeast helicase-like proteins were identified and are shown in Table 2 with their loss-of-function phenotypes. The MCM subfamily members26 and two RUVB-like proteins45 were completely conserved and required for viability in both species. The DEAD-box, DEAH-box, SKI2, UPF1, and SWI2/SNF2 subfamilies contained two classes of proteins, those that were conserved in both species and those that were species-specific. For example, 21 putative orthologous pairs of the DEAD-box members were well conserved. In contrast, we could not detect any putative nematode homologs corresponding to the four yeast proteins Mrh4p, Dbp3p, Dbp7p, and Mss116p, or any yeast homologs of 15 nematode proteins. Budding yeast contain one or two members of the PIF1, MPH1, RAD3, and RECQ subfamilies; however, the number of members in each of these subfamilies had increased in C. elegans, and many of these divergent proteins are conserved in humans (Table 1). Twenty-five budding yeast-specific proteins and six higher eukaryote-specific proteins were also detected (Table 2). We found a high degree of conservation of loss-of-function phenotypes for homologs in both organisms. The majority (20 of 22 proteins) of putative C. elegans orthologs of yeast essential DEAD-box members caused embryonic lethality or growth-defect phenotypes by their depletion (Table 2). Similar phenotypic conservation of essential homologs in both species was also found in members of the DEAH-box, SKI2, MCM, and RAD3 subfamilies, as well as in the RUVB-like and SSL2-like proteins, suggesting that these putative conserved orthologs play similar essential cellular roles in C. elegans as in their yeast counterparts (Table 2). Interestingly, depletions of the subfamily members which have diverged in C. elegans were rarely able to induce growth-defect phenotypes (i.e. only nine of 67 genes tested across the subfamilies).
Because of the detection of diverged members in C. elegans, we assigned all helicase-like genes and pseudogenes to the six C. elegans chromosomes to examine the distribution of the extranumerary genes in the genome. The BLAST-based sequence homology searches identified 10 candidate gene pairs, three highly related gene pairs, and two gene clusters in the helicase-like genes (Supplementary Table S4) and mapped to chromosomes (Supplementary Fig. S1). Six putative gene pairs (glh-1 and glh-2, glh-4 and T08D2.3, F33D11.10 and Y65B4A.6, F57B9.3 and inf-1, F53H1.1 and Y73B3B.5, and mut-14 and ZC317.1) belonged to the DEAD-box subfamily (Supplementary Fig. S1A). At least two partners of the paired genes were pseudogenes (T08D2.3 and Y73B3B.5). Four pairs of putative duplicated genes were identified among the SWI2/SNF2 (let-418 and chd-3), SKI2 (Y46G5A.4 and Y46G5A.6), PIF1 (C11G6.2 and Y116F11A.1), and MPH1 (drh-1 and drh-2) subfamilies (Supplementary Figs S1C, D, G and H). In addition, two clusters of C. elegans-specific SNF2-like genes and Helitrons in the PIF1 subfamily were found on the terminal regions of chromosomes V and II, respectively, suggesting that these genes might have been generated by a few rounds of gene duplications (Supplementary Figs S1C and G; see the figure legend).
RNAi for the subfamily members diverged in C. elegans poorly induced growth-defect phenotypes (Table 2). This phenomenon may indicate a functional redundancy with paralogous proteins or diverged members in C. elegans. In fact, two diverged DEAD-box members, GLH-1 and GLH-4, are known to play redundant functions in germline development. Kuznicki et al.46 showed that double RNAi for glh-1/4 was required for significant sterile phenotype. Since some of paired proteins (e.g. LET-418 and CHD-3) have redundant functions,47 we assumed that the products of duplicated genes with unknown function (i.e. Y67D2.6 and Y108F1.5, or C11G6.2 and Y116F11A.1) may be the case. However, none of the detectable phenotypes in animals treated with double RNAi for these paired genes were observed (data not shown).
4.3. Expression profiles of helicase-like genes in C. elegans and influence of RNAi for germline- enriched genes
We examined the expression profiles of the helicase-like genes, using four published genome-wide expression studies of C. elegans genes48–51 (Supplementary Table S2). Reinke et al.51 identified germline-enriched and sex-regulated genes and classified the genes into several expression categories. Assignment of helicase-like genes in each subfamily to each expression category revealed that many helicase-like genes (58 of 134 genes) were categorized as ‘intrinsic’ and ‘oogenesis-enriched’ genes (Supplementary Table S5A). The fraction of helicase-like genes (21.6%) in the oogenesis-enriched category is significantly higher than that of C. elegans genes in general (5.7% of the total genes), suggesting an expression bias for helicase family members in oogenesis in hermaphrodites. Dominant expression of helicase-like genes in the embryonic stages was also detected in another study50 (Supplementary Table S5B). The data reported by Jiang et al.48 show sex-biased expression of the helicase-like genes (12.7% of helicase-like genes versus 27.6% of total C. elegans genes in male; 32.1 versus 24.7% in hermaphrodites in Supplementary Table S5C). This is also consistent with the high proportion of helicase-like genes in the oogenesis-enriched genes. Assignment of the helicase-like genes to the C. elegans gene expression map49 also indicates that helicase-like genes were relatively concentrated (1.9- to 4.2-fold) in six mountains (2, 5, 7, 11, 20, and 25) out of 46 mountains, and mountains 2, 7, and 11 contain predominantly oocyte- and germline-enriched genes49 (Supplementary Table S6). In addition, the dominant expression of PIF1 or SKI2 members in males is interesting, because three of three and three of six genes in the PIF1 and SKI2 subfamilies, respectively, appeared in the ‘male dominant groups’ (Supplementary Table S5C), and this may implicate these genes in male-specific functions such as spermatogenesis. Most Helitrons of the PIF1 members were poorly expressed in the aforementioned studies.
Since oogenesis-enriched expression of many helicase-like genes suggests potential roles of their gene products in the development and proliferation of germ cells, we examined the effect of suppression of 39 germline-enriched genes on brood size by RNAi, and reduced reproductive capacity in F55F8.2(RNAi) and T05E8.3(RNAi) animals was detected (Supplementary Table S2). F55F8.2 encodes a homolog of yeast-splicing factor Prp28p, and the T05E8.3 gene product is similar to yeast Prp22p and a putative homolog of human DHX33 (Table 1). This indicates that both gene products play important roles in the reproduction in C. elegans. In the L1 RNAi analysis, suppression of F56D2.6 and C08F8.2 caused a significant reduction in brood size because of sterility in P0 animals and of embryonic lethality, respectively, suggesting that F56D2.6, encoding a putative homolog of yeast-splicing factor Prp43p, is required for reproduction, and as a putative homolog of yeast mitochondrial RNA helicase Suv3p, C08F8.2 plays an essential role in embryonic viability. In the previous study by Colaiacovo et al.,52 four helicase-like genes have been identified in an RNAi-based screen for genes involved in chromosome morphogenesis and nuclear organization in C. elegans germline. We also found several detectable phenotypes in dcr-1(RNAi) and ruvb-2(RNAi) animals, but did not find weak phenotypes reported in rha-1(RNAi) and C27B7.4(RNAi) animals (Table 1).
4.4. Identification of drh-3 gene involved in resistance to X-ray-induced DNA damage
In this study, we succeeded in identifying D2005.5(drh-3) as a gene for protection against X-ray irradiation. Four Dicer-like proteins in the MPH1 subfamily, including DRH-3, have recently been shown to play an important role in RNAi.43 It remains to be resolved why depletion of the RNAi factor DRH-3 causes X-ray hypersensitivity in C. elegans. Previous RNAi-based studies have shown that some helicase family members are implicated in resistance to X-ray-induced DNA damage. Boulton et al.53 showed that RNAi of rad-54 and Y116A8C.13 caused DNA repair defect phenotypes. Recently, van Haaften et al.54 performed a genome-wide screen for C. elegans genes that protect cells against ionizing radiation to identify three helicase-like genes D2005.5, Y80D3A.2, and isw-1. Although D2005.5 was commonly detected in the screens by us and van Haaften et al., we failed to find marked X-ray-dependent phenotypic defects in the progeny of Y80D3A.2(RNAi) or isw-1(RNAi) animals (Table 1). Several genes including rad-54 or RecQ-like helicase genes are thought to be involved in DNA repair of X-ray-induced DNA damage; however, these genes were not always detected in previous RNAi-based screens. In order to isolate more candidate genes, several technical improvements in the screens may be required, including more sensitive assay systems (e.g. use of reporter animals55), RNAi-based screens using rrf-3 mutants,34 or soaking RNAi-mediated screens.41
In conclusion, we have identified helicase-like genes in C. elegans and characterized loss-of-function phenotypes of these genes. The results obtained from phenotypic analyses, comparative analyses, chromosome mapping, and a study of the expression patterns of helicase family members will be useful for studying helicase-mediated molecular reactions governing dynamic regulation of DNA, RNA, and chromatin. Furthermore, characterization of DRH-3 will elucidate functional interactions between the resistance to X-ray irradiation and RNAi.
Supplementary material
Supplementary Data: Supplementary data are available online at dnaresearch.oxfordjournals.org.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research (C) (No. 17590057), on Priority Areas Genome Biology, and the 21st Century COE Program Ecological Engineering for Homeostatic Human Activities, from the Ministry of Education, Culture, Sports, Science and Technology of Japan (T.E.), the Human Frontier Science Program (HFSP-RGP0012/2004-C), and Solution Oriented Research for Science and Technology from the Japan Science and Technology Agency (J071501010) (F.H.), and the special research program of the Toyohashi University of Technology, the Naito Foundation, and the REIMEI Research Resources of Japan Atomic Energy Research Institute (T.E.).
Supplementary Material
Acknowledgements
We would like to thank Drs A. Fire and Y. Kohara for kindly providing vectors and cDNA clones, and the Caenorhabditis Genetics Center for mutant strains. We are also grateful to M. Nakamura, T. Ohgake, R. Hayashi and other laboratory members for helpful discussions and technical support.
References
- 1.Tuteja N., Tuteja R. Unraveling DNA helicases. Motif, structure, mechanism and function. Eur. J. Biochem. 2004;271:1849–1863. doi: 10.1111/j.1432-1033.2004.04094.x. [DOI] [PubMed] [Google Scholar]
- 2.Tanner N. K., Linder P. DExD/H box RNA helicases: from generic motors to specific dissociation functions. Mol. Cell. 2001;8:251–262. doi: 10.1016/s1097-2765(01)00329-x. [DOI] [PubMed] [Google Scholar]
- 3.Cordin O., Banroques J., Tanner N. K., Linder P. The DEAD-box protein family of RNA helicases. Gene. 2006;367:17–37. doi: 10.1016/j.gene.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Lusser A., Kadonaga J. T. Chromatin remodeling by ATP-dependent molecular machines. BioEssays. 2003;25:1192–1200. doi: 10.1002/bies.10359. [DOI] [PubMed] [Google Scholar]
- 5.Durr H., Flaus A., Owen-Hughes T., Hopfner K. P. Snf2 family ATPases and DExx box helicases: differences and unifying concepts from high-resolution crystal structures. Nucleic Acids Res. 2006;34:4160–4167. doi: 10.1093/nar/gkl540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickson I. D. RecQ helicases: caretakers of the genome. Nat. Rev. Cancer. 2003;3:169–178. doi: 10.1038/nrc1012. [DOI] [PubMed] [Google Scholar]
- 7.Gorbalenya A. E., Koonin E. V. Helicases: amino acid sequence comparisons and structure–function relationships. Curr. Opin. Struct. Biol. 1993;3:419–429. [Google Scholar]
- 8.Shiratori A., Shibata T., Arisawa M., Hanaoka F., Murakami Y., Eki T. Systematic identification, classification, and characterization of the open reading frames which encode novel helicase-related proteins in Saccharomyces cerevisiae by gene disruption and Northern analysis. Yeast. 1999;15:219–253. doi: 10.1002/(SICI)1097-0061(199902)15:3<219::AID-YEA349>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Libri D., Graziani N., Saguez C., Boulay J. Multiple roles for the yeast SUB2/yUAP56 gene in splicing. Genes Dev. 2001;15:36–41. doi: 10.1101/gad.852101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colley A., Beggs J. D., Tollervey D., Lafontaine D. L. Dhr1p, a putative DEAH-box RNA helicase, is associated with the box C + D snoRNP U3. Mol. Cell. Biol. 2000;20:7238–7246. doi: 10.1128/mcb.20.19.7238-7246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daugeron M. C., Kressler D., Linder P. Dbp9p, a putative ATP-dependent RNA helicase involved in 60S-ribosomal-subunit biogenesis, functionally interacts with Dbp6p. RNA. 2001;7:1317–1334. doi: 10.1017/s1355838201010640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuusk S., Sedman T., Joers P., Sedman J. Hmi1p from Saccharomyces cerevisiae mitochondria is a structure-specific DNA helicase. J. Biol. Chem. 2005;280:24322–24329. doi: 10.1074/jbc.M500354200. [DOI] [PubMed] [Google Scholar]
- 13.Shen X., Mizuguchi G., Hamiche A., Wu C. A chromatin remodelling complex involved in transcription and DNA processing. Nature. 2000;406:541–544. doi: 10.1038/35020123. [DOI] [PubMed] [Google Scholar]
- 14.Krogan N. J., Keogh M. C., Datta N., et al. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol. Cell. 2003;12:1565–1576. doi: 10.1016/s1097-2765(03)00497-0. [DOI] [PubMed] [Google Scholar]
- 15.Longman D., Johnstone I. L., Caceres J. F. Functional characterization of SR and SR-related genes in Caenorhabditis elegans. EMBO J. 2000;19:1625–1637. doi: 10.1093/emboj/19.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawano T., Fujita M., Sakamoto H. Unique and redundant functions of SR proteins, a conserved family of splicing factors, in Caenorhabditis elegans development. Mech. Dev. 2000;95:67–76. doi: 10.1016/s0925-4773(00)00339-7. [DOI] [PubMed] [Google Scholar]
- 17.Jones D., Crowe E., Stevens T. A., Candido E. P. Functional and phylogenetic analysis of the ubiquitylation system in Caenorhabditis elegans: ubiquitin-conjugating enzymes, ubiquitin-activating enzymes, and ubiquitin-like proteins. Genome Biol. 2002;3 doi: 10.1186/gb-2001-3-1-research0002. RESEARCH0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hope I. A., Mounsey A., Bauer P., Aslam S. The forkhead gene family of Caenorhabditis elegans. Gene. 2003;304:43–55. doi: 10.1016/s0378-1119(02)01175-7. [DOI] [PubMed] [Google Scholar]
- 19.Keating C. D., Kriek N., Daniels M., et al. Whole-genome analysis of 60 G protein-coupled receptors in Caenorhabditis elegans by gene knockout with RNAi. Curr. Biol. 2003;13:1715–1720. doi: 10.1016/j.cub.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien K. P., Remm M., Sonnhammer E. L. Inparanoid: a comprehensive database of eukaryotic orthologs. Nucleic Acids Res. 2005;33:D476–D480. doi: 10.1093/nar/gki107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sijen T., Fleenor J., Simmer F., et al. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- 22.Harada H., Kurauchi M., Hayashi R., Eki T. Shortened lifespan of nematode Caenorhabditis elegans after prolonged exposure to heavy metals and detergents. Ecotoxicol. Environ. Saf. 2007;66:378–383. doi: 10.1016/j.ecoenv.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Timmons L., Court D. L., Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- 24.Ohkumo T., Masutani C., Eki T., Hanaoka F. Deficiency of the Caenorhabditis elegans DNA polymerase η homologue increases sensitivity to UV radiation during germ-line development. Cell Struct. Funct. 2006;31:29–37. doi: 10.1247/csf.31.29. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz E. M., Antoshechkin I., Bastiani C., et al. WormBase: better software, richer content. Nucleic Acids Res. 2006;34:D475–D478. doi: 10.1093/nar/gkj061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishimi Y. A DNA helicase activity is associated with an MCM4, -6, and -7 protein complex. J. Biol. Chem. 1997;272:24508–24513. doi: 10.1074/jbc.272.39.24508. [DOI] [PubMed] [Google Scholar]
- 27.Boule J. B., Zakian V. A. Roles of Pif1-like helicases in the maintenance of genomic stability. Nucleic Acids Res. 2006;34:4147–4153. doi: 10.1093/nar/gkl561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada M., Hayatsu N., Matsuura A., Ishikawa F. Y'-Help1, a DNA helicase encoded by the yeast subtelomeric Y' element, is induced in survivors defective for telomerase. J. Biol. Chem. 1998;273:33360–33366. doi: 10.1074/jbc.273.50.33360. [DOI] [PubMed] [Google Scholar]
- 29.Kapitonov V. V., Jurka J. Rolling-circle transposons in eukaryotes. Proc. Natl Acad. Sci. USA. 2001;98:8714–8719. doi: 10.1073/pnas.151269298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poulter R. T., Goodwin T. J., Butler M. I. Vertebrate helentrons and other novel Helitrons. Gene. 2003;313:201–212. doi: 10.1016/s0378-1119(03)00679-6. [DOI] [PubMed] [Google Scholar]
- 31.Navarro R. E., Shim E. Y., Kohara Y., Singson A., Blackwell T. K. cgh-1, a conserved predicted RNA helicase required for gametogenesis and protection from physiological germline apoptosis in C. elegans. Development. 2001;128:3221–3232. doi: 10.1242/dev.128.17.3221. [DOI] [PubMed] [Google Scholar]
- 32.Sawa H., Kouike H., Okano H. Components of the SWI/SNF complex are required for asymmetric cell division in C. elegans. Mol. Cell. 2000;6:617–624. doi: 10.1016/s1097-2765(00)00060-5. [DOI] [PubMed] [Google Scholar]
- 33.Simmer F., Tijsterman M., Parrish S., et al. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr. Biol. 2002;12:1317–1319. doi: 10.1016/s0960-9822(02)01041-2. [DOI] [PubMed] [Google Scholar]
- 34.Simmer F., Moorman C., van der Linden A. M, et al. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 2003;1:E12. doi: 10.1371/journal.pbio.0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rual J. F., Ceron J., Koreth J., et al. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 2004;14:2162–2168. doi: 10.1101/gr.2505604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takanami T., Mori A., Takahashi H., Higashitani A. Hyper-resistance of meiotic cells to radiation due to a strong expression of a single recA-like gene in Caenorhabditis elegans. Nucleic Acids Res. 2000;28:4232–4236. doi: 10.1093/nar/28.21.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rinaldo C., Bazzicalupo P., Ederle S., Hilliard M., La Volpe A. Roles for Caenorhabditis elegans rad-51 in meiosis and in resistance to ionizing radiation during development. Genetics. 2002;160:471–479. doi: 10.1093/genetics/160.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holway A. H., Hung C., Michael W. M. Systematic, RNA-interference-mediated identification of mus-101 modifier genes in Caenorhabditis elegans. Genetics. 2005;169:1451–1460. doi: 10.1534/genetics.104.036137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holway A. H., Kim S. H., La Volpe A., Michael W. M. Checkpoint silencing during the DNA damage response in Caenorhabditis elegans embryos. J. Cell. Biol. 2006;172:999–1008. doi: 10.1083/jcb.200512136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamath R. S., Fraser A. G., Dong Y., et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 41.Maeda I., Kohara Y., Yamamoto M., Sugimoto A. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr. Biol. 2001;11:171–176. doi: 10.1016/s0960-9822(01)00052-5. [DOI] [PubMed] [Google Scholar]
- 42.Tabara H., Yigit E., Siomi H., Mello C. C. The dsRNA binding protein RDE-4 interacts with RDE-1, DCR-1, and a DExH-box helicase to direct RNAi in C. elegans. Cell. 2002;109:861–871. doi: 10.1016/s0092-8674(02)00793-6. [DOI] [PubMed] [Google Scholar]
- 43.Duchaine T. F., Wohlschlegel J. A., Kennedy S., et al. Functional proteomics reveals the biochemical niche of C. elegans DCR-1 in multiple small-RNA-mediated pathways. Cell. 2006;124:343–354. doi: 10.1016/j.cell.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 44.Venema J., Tollervey D. Ribosome synthesis in Saccharomyces cerevisiae. Annu. Rev. Genet. 1999;33:261–311. doi: 10.1146/annurev.genet.33.1.261. [DOI] [PubMed] [Google Scholar]
- 45.Jonsson Z. O., Dhar S. K., Narlikar G. J., et al. Rvb1p and Rvb2p are essential components of a chromatin remodeling complex that regulates transcription of over 5% of yeast genes. J. Biol. Chem. 2001;276:16279–16288. doi: 10.1074/jbc.M011523200. [DOI] [PubMed] [Google Scholar]
- 46.Kuznicki K. A., Smith P. A., Leung-Chiu W. M., Estevez A. O., Scott H. C., Bennett K. L. Combinatorial RNA interference indicates GLH-4 can compensate for GLH-1; these two P granule components are critical for fertility in C. elegans. Development. 2000;127:2907–2916. doi: 10.1242/dev.127.13.2907. [DOI] [PubMed] [Google Scholar]
- 47.von Zelewsky T., Palladino F., Brunschwig K., Tobler H., Hajnal A., Muller F. The C. elegans Mi-2 chromatin-remodelling proteins function in vulval cell fate determination. Development. 2000;127:5277–5284. doi: 10.1242/dev.127.24.5277. [DOI] [PubMed] [Google Scholar]
- 48.Jiang M., Ryu J., Kiraly M., Duke K., Reinke V., Kim S. K. Genome-wide analysis of developmental and sex-regulated gene expression profiles in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 2001;98:218–223. doi: 10.1073/pnas.011520898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S. K., Lund J., Kiraly M., et al. A gene expression map for Caenorhabditis elegans. Science. 2001;293:2087–2092. doi: 10.1126/science.1061603. [DOI] [PubMed] [Google Scholar]
- 50.Baugh L. R., Hill A. A., Slonim D. K., Brown E. L., Hunter C. P. Composition and dynamics of the Caenorhabditis elegans early embryonic transcriptome. Development. 2003;130:889–900. doi: 10.1242/dev.00302. [DOI] [PubMed] [Google Scholar]
- 51.Reinke V., Gil I. S., Ward S., Kazmer K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development. 2004;131:311–323. doi: 10.1242/dev.00914. [DOI] [PubMed] [Google Scholar]
- 52.Colaiacovo M. P., Stanfield G. M., Reddy K. C., Reinke V., Kim S. K., Villeneuve A. M. A targeted RNAi screen for genes involved in chromosome morphogenesis and nuclear organization in the Caenorhabditis elegans germline. Genetics. 2002;162:113–128. doi: 10.1093/genetics/162.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boulton S. J., Gartner A., Reboul J., et al. Combined functional genomic maps of the C. elegans DNA damage response. Science. 2002;295:127–131. doi: 10.1126/science.1065986. [DOI] [PubMed] [Google Scholar]
- 54.van Haaften G., Romeijn R., Pothof J., et al. Identification of conserved pathways of DNA-damage response and radiation protection by genome-wide RNAi. Curr. Biol. 2006;16:1344–1350. doi: 10.1016/j.cub.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 55.Pothof J., van Haaften G., Thijssen K., et al. Identification of genes that protect the C. elegans genome against mutations by genome-wide RNAi. Genes Dev. 2003;17:443–448. doi: 10.1101/gad.1060703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.