Abstract
Background and Aims
Pitcher plants Nepenthes alata and N. mirabilis are carnivorous species with leaves composed of a photosynthetic part (lamina) and a pitcher trap. This characteristic permitted direct physiological and anatomical comparison between these two distinct parts of the leaves to determine those features involved in the ‘carnivorous syndrome’, which include low net photosynthetic assimilation rate (AN) and low photosynthetic nitrogen use efficiency (PNUE).
Methods
Photosynthetic rate (AN) and respiration rate (Rd) were measured gasometrically, chlorophyll concentration was determined spectrophotometrically and nitrogen concentration was determined using a CHN elemental analyser in lamina and trap separately. Anatomy of N. alata was observed using light, fluorescence and transmission electron microscopy. AN, foliar nitrogen and chlorophyll concentration were also compared with values for other carnivorous plant species (genera Sarracenia, Drosera) that combine both autotrophic and carnivorous functions into the same physical organ.
Key Results
It was found that the AN in Nepenthes lamina was low and PNUE was only slightly higher or similar in comparison with other carnivorous plants. It was not observed that the pitcher had a higher Rd than the lamina, but AN in the pitcher was significantly lower than in the lamina. Nepenthes possesses a cluster of characters that could result in reduced photosynthesis in the pitcher and be responsible for carnivorous function of the leaf: replacement of chlorophyll-containing cells with digestive glands, low chlorophyll and nitrogen concentration, compact mesophyll with a small portion of intercellular spaces, absence of palisade parenchyma and low stomatal density.
Conclusion
Low photosynthetic capacity, nitrogen efficiency, chlorophyll and nitrogen concentration of Nepenthes pitchers was found, together with a set of features that characterized the carnivorous syndrome. Dual use of leaves for photosynthesis and nutrient gain can decrease photosynthetic efficiency in carnivorous plants in general.
Key words: chlorophyll concentration, cost/benefit analysis, Drosera capensis, leaf anatomy, leaf nitrogen concentration, Nepenthes alata, Nepenthes mirabilis, pitcher plant, photosynthetic rate, respiration rate, Sarracenia psittacina, stomatal density
INTRODUCTION
Carnivorous plants have evolved independently in several families. Approximately 600 carnivorous species are known, belonging to six angiosperm subclasses and including monocotyledons and eudicotyledons (Ellison and Gotelli, 2001). This has motivated several authors to suggest that similar ecological factors have resulted in evolutionary convergence (Givnish et al., 1984; Benzing, 1987). According to Givnish's cost/benefit model, carnivorous plants are restricted to environments with an abundant supply of water and light, but are poor in nutrients (Givnish et al., 1984). In other environments, the cost of producing traps would apparently exceed the benefits gained by trapping prey. The costs of carnivory include investment in non-photosynthetic structures such as glands, hairs, glue and digestive enzymes. With regard to photosynthesis, costs represent extra energy requirements for the respiration of carnivorous organs. On the other hand, benefits of carnivory can include an increased rate of photosynthesis, although results are often conflicting (e.g. Ellison and Gotelli, 2002 vs. Wakefield et al., 2005), growth (Karlsson et al., 1991; Moran and Moran, 1998), and higher flowering frequency and seed mass (Thorén and Karlsson, 1998), through increased supply of nitrogen and phosphorus from captured prey. Carnivorous plants possess a cluster of characters (i.e. a ‘syndrome’) that enables them to attract, catch and digest prey, at the cost of photosynthetic capacity (Givnish et al., 1984; Juniper et al., 1989). Carnivorous plants generally have a poorly developed root system (Adamec, 1997b) and may partially trade root biomass for highly specialized traps (Schnell, 2002), the benefit of which was not considered by Givnish when he proposed the cost/benefit model. Thus, Givnish's model is still a matter of debate (Wakefield et al., 2005; Ellison, 2006).
From a morphological point of view, two types of carnivorous plants can be distinguished. Butterwort (Pinguicula), sundew (Drosera) and North American pitcher plants (Sarracenia, Heliamphora and Darlingtonia) have leaves that can both photosynthesize and capture prey. The Australian pitcher plant (Cephalotus), venus fly trap (Dionaea), bladderworts (Utricularia) or Nepenthes pitcher plants produce leaves that can photosynthesize but do not capture prey, and/or produce traps that capture prey and photosynthesize little or not at all (Ellison and Gotelli, 2001). This trait allows them to vary their investment in carnivory as a function of habitat (sensu Givnish et al., 1984). For instance, Sarracenia produces phyllidoform leaves, with higher portion of photosynthetically efficient tissue (keel) than prey trapping tissue (tube), and Nepenthes fails to produce pitchers if light or humidity is too low, or nutrient availability is too high (Clarke, 1997).
The term ‘pitcher plants’ is used for a number of different carnivorous plants with pitcher-like leaves. Many of them have evolved independently in several genera belonging to different families (Sarraceniaceae, Cephalotaceae, Nepenthaceae; Albert et al., 1992). Nepenthes are tropical pitcher plants, growing mainly in Indonesia and belonging to the Nepenthaceae family. Although approx. 90 species of the genus Nepenthes have been identified, their leaf morphology is very similar and consists of a photosynthetic part of the leaf or lamina (extremely enlarged leaf base) and a tendril that carries the pitcher (trap, ascidium). The pitchers are divided into zones that are visibly discernible (Clarke, 1997). These include: a lid, a peristome (ribbed upper rim of the pitcher) involved in attracting and trapping prey, a waxy zone involved in trapping and preventing escape of prey (Gaume et al., 2002), and a digestive zone with a pool of digestive fluid to retain and digest prey (Gorb et al., 2004). Insects are attracted to the peristome by a combination of visual UV reflection patterns and a sweet fragrance produced by the fluid (Moran et al., 1999). When visiting the pitchers, insect can fall into the traps, from which they are mostly unable to escape (Gaume et al., 2002; Bohn and Federle, 2004). This is a most effective way to obtain nutrients that are not presented in the soil (Adamec, 1997b).
There is little knowledge about photosynthetic assimilation (AN) and respiration (Rd) rates in carnivorous plants, restricted to about nine species (Aldrovanda vesiculosa, Darlingtonia californica, Drosera rotundifolia, Pinguicula alpina, P. villosa, P. vulgaris, Sarracenia purpurea, Utricularia macrorhiza). Only five photosynthetic studies in six terrestrial carnivorous plants have been published to date (Small, 1972; Méndez and Karlsson, 1999; Ellison and Gotelli, 2002; Ellison and Farnsworth, 2005; Wakefield et al., 2005). The studies have found low AN and photosynthetic nitrogen use efficiency (PNUE) in carnivorous plants (Ellison, 2006). Reworking leaf morphology and physiology to lure, trap, digest and absorb nutrients apparently reduces the efficiency of AN, mainly because of changes in leaf shape or the replacement of chlorophyll-containing cells with digestive glands (Schnell, 2002). With regard to anatomy, most studies have been confined to digestive and trapping mechanisms (Owen and Lennon, 1999; Owen et al., 1999; Gaume et al., 2002; Gorb et al., 2004) and not to the leaf mesophyll responsible for photosynthetic assimilation. Therefore, the principal aims of the present study were as follows. (1) To compare AN and Rd between pitcher and lamina in two Nepenthes species in relation to leaf chemistry (chlorophyll, nitrogen and carbon concentration) and anatomy (mesophyll and stomata density) to determine those features involved in the carnivorous syndrome. This was not possible in former studies of Drosera, Pinguicula (Méndez and Karlsson, 1999), Darlingtonia (Ellison and Farnsworth, 2005) or Sarracenia (Small, 1972; Wakefield et al., 2005) because the entire leaf is involved in both photosynthesis and capture of prey (whole leaf blade is covered with glands), which makes it difficult to determine gas exchange of photosynthetic and trapping parts of the leaf separately. Although Nepenthes lamina and pitcher are attached physiologically by the tendril, they are distinct morphologically and the pitcher can be dissected easily from the lamina, allowing separate assessment of their AN and Rd. The red tints of pitchers suggest that they may not have much chlorophyll, resulting in diminished AN compared with lamina. Because AN is correlated positively with N concentration and stomatal conductance, it is also hypothesized that there is lower N concentration and lower stomata density in pitchers than in the lamina. Increased Rd in the pitcher of Nepenthes is expected as a cost of carnivory sensu stricto (Givnish et al., 1984). Carbon metabolism in the genus Nepenthes has not been studied as far as we are aware. (2) To compare AN, PNUE, stomata density, chlorophyll and leaf nitrogen concentration with other carnivorous plants (Sarracenia psittacina, Drosera capensis) combining both autotrophic and trap functions in the same physical organ. AN, PNUE, chlorophyll and N concentration, and stomatal density in the pitcher of Nepenthes are hypothesized to be lower than in the leaves of another carnivorous species, because it can rely on assimilates from the photosynthetically active lamina. Therefore, the Nepenthes pitcher should be more specialized to carnivorous function than other carnivorous plants.
MATERIALS AND METHODS
Plant material and culture conditions
Two species of the carnivorous genus Nepenthes were investigated. The endemic carnivorous plant Nepenthes alata Blanco grows as an epiphyte in the mossy mountain forests of the Philippines (Fig. 1A). Nepenthes mirabilis Lour. (Fig. 1B) can climb up the trees and can be found in numerous South East Asian countries and in Australia (Clarke, 2001). Experimental plants were grown from cuttings in the botanical garden of Comenius University in Bratislava under greenhouse conditions in moss without fertilizer at a maximum daily irradiance of 200 µmol m−2 s−1 photosynthetically active radiation (PAR). Each leaf was carrying 10- to 15-cm-long upper pitchers, N. alata red- and N. mirabilis green-coloured, which indicates that cultivated plants have sufficient amount of light and water and low nutrient availability in the soil. Five-year-old plants were used for measurements in June, 2004.
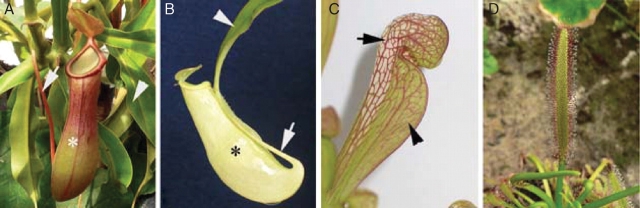
Fig. 1.
Species of carnivorous plants studied. (A) Nepenthes alata: lamina (arrowhead), tendril (arrow) and pitcher (asterisk). (B) Nepenthes mirabilis: lamina (arrowhead), tendril (arrow) and pitcher (asterisk); the tendril was considered as a part of the pitcher in gas exchange measurements and chemical analysis. (C) Sarracenia psittacina: keel (arrowhead) and tube (arrow). (D) Drosera capensis.
For comparison of AN, leaf N and chlorophyll concentration, and PNUE among carnivorous plants the following species were used: Nepenthes alata (Fig. 1A), Sarracenia psittacina (Fig. 1C) and Drosera capensis (Fig. 1D). These plants were grown in moss under greenhouse conditions at the Department of Plant Physiology at a maximum daily irradiance of 400 µmol m−2 s−1 PAR, except for N. alata, which was grown in a Sphagnum/perlite/bark/moss mixture (1 : 1 : 1 : 1). Whereas pitcher plants of the genus Sarracenia are confined to North America (Schnell, 2002), species of the genus Drosera catch their prey on sticky leaves and are distributed worldwide. All plants were measured from May to July, 2006 and were non-reproductive at the time of the study. The experimental plants had access to prey during the experiment.
Gas exchange and leaf chemistry
Measurements and equipment were as described in detail by Masarovičová and Král'ová (2005). In the first experiment, Nepenthes leaf samples were collected from upper parts (1st to 5th adult leaf) of five plants from each species with fully developed pitchers. As Schulze et al. (1997) found that N concentration for N. mirabilis increased with leaf position (the youngest mature leaves in the upper part of the stem had higher N concentrations than older leaves), it was decided to include two young fully expanded leaves with undeveloped pitchers in the second experiment, to achieve higher N concentration comparable with other carnivorous plants. At first, the pitcher was cut near the apex of the lamina, the contents were emptied and the whole pitcher with tendril was enclosed in a thermostabilized chamber. After 10 min of stabilization, CO2 exchange rate (An,pitcher) was measured via an infrared gas analyser (CIRAS-2, PP-Systems, Hitchin, UK) at an air temperature in the chamber of 25 ± 1 °C, a saturating irradiance of 350 µmol m−2 s−1 PAR and a concentration of 360 ppm CO2. Thereafter, the light was switched off, the assimilation chamber was covered with aluminium foil and allowed to stabilize for 10 min, and the pitcher dark respiration (Rd,pitcher) was measured at an air temperature of 25 °C in the dark. Finally, the rate of photosynthesis (An,lamina) and respiration of lamina without tendril (Rd,lamina) were measured under the same conditions (25 °C, 350 µmol m−2 s−1 PAR, 360 ppm CO2). Due to the smaller leaf size of Drosera capensis, about ten leaves per measurement were used. For Sarracenia psittacina, one or two leaves (pitcher) from four plants were used per measurement (approx. 1 month after pitcher opening).
Chlorophyll from five leaves of each species was extracted with 80 % (v/v) chilled acetone and amounts of chlorophyll a and chlorophyll b were determined spectrophotometrically (Jenway 6400, London, UK): chlorophyll a at 663·2 nm and chlorophyll b at 646·8 nm. Chlorophyll concentration was calculated according to Lichtenthaler (1987).
Leaf tissue from photosynthetic measurements was dried at 70 °C for 5 d and its N and C concentration determined using an EA 1108 CHN analyser (Fisons Instruments, Milan, Italy).
Nepenthes alata anatomy
Samples for anatomical and ultrastructural observation were taken from the lamina, lid, waxy zone and digestive zone of N. alata, fixed in 5 % glutaraldehyde for 4 h and post-fixed in 2 % OsO4 for 2 h. After dehydration in a graded acetone series they were embedded in Durcupan ACM resin (Fluka, Switzerland). Semithin sections (1·5 µm thick) for light microscopy were cut on an ultramicrotome (Ultracut E, Reichert – Jung, Vienna, Austria) with a diamond knife, double stained with toluidine blue and basic fuchsin (Lux, 1981), and observed via an Axioskop 2 plus microscope (Zeiss, Jena, Germany). The volume of air spaces in semithin sections was estimated using Laboratory Universal Computer Image Analysis software (LUCIA G, ver. 4·80, Laboratory Imaging, Prague, Czech Republic) in ten randomly chosen views from four leaves. Ultrathin sections (60–100 nm thick) for transmission electron microscopy (TEM) were cut using the ultramicrotome mentioned above, stained with uranyl acetate and lead citrate and observed using a JOEL FX 2000 electron microscope (Tokyo, Japan). Unstained free-hand sections were checked for fluorescence using the Axioskop 2 plus microscope (Zeiss) with filter set 25 (excitation 400, 495, 750 nm; emission 460, 530, 610 nm) and filter set 16 (excitation 485 nm; emission 515 nm; Zeiss).
The density, length and width of stomata were determined in three leaves from each species using a microrelief method. Epidermal impressions were prepared by coating the leaf surface with nail varnish, peeling off the dried layer of nail varnish using sticky tape and adhering onto a microscope slide. Ten randomly chosen views of each slide were evaluated and averaged using the same microscope and software, as described above.
Statistical analyses
Prior to statistical tests, data were analysed for normality and homogeneity of variance. When non-homogeneity was present, the t-test was employed with the appropriate corrected degrees of freedom. To evaluate the significance of the data (AN, Rd, PNUE, chlorophyll and nitrogen content, C/N ratio, stomata density, stomata width and length) the t-test was used (comparison between Nepenthes alata and N. mirabilis). All paired data were statistically evaluated by a two-tailed paired t-test (comparison between lamina and pitcher in the same leaf). A one-way ANOVA followed by least significant difference (LSD) test (Statgraphics) was used to test for significant differences among carnivorous species in AN, PNUE, chlorophyll and N content, and stomata density. ANCOVA (StatistiXL ver. 1·6 for Microsoft Excel) was used to test the homogeneity of slopes and y-intercepts of the relationship between AN and N content for different carnivorous plants. The results are expressed as the mean of five replicated measurements except for the stomatal investigation, where n = 3.
RESULTS
Gas exchange and leaf chemistry
Intraspecific comparison between pitcher and lamina revealed a significantly lower photosynthetic rate of the pitcher (Table 1). An,lamina ranged from 34·8 to 53·0 nmol CO2 g−1 d. wt s−1 and An,pitcher from −6·2 to 0·1 nmol CO2 g−1 d. wt s−1 in N. alata (P = 0·001). An,lamina ranged from 16·3 to 40·6 nmol CO2 g−1 d. wt s−1 and An,pitcher from –3·2 to 4·4 nmol CO2 g−1 d. wt s−1 in N. mirabilis (P = 0·009, Table 1). Only three pitchers out of 15 had low positive AN (max. 4·4 nmol CO2 g−1 d. wt s−1 at 350 µmol m−2 s−1 PAR). No significant differences (P > 0·05) in An,pitcher between species were observed, although N. alata had higher An,lamina than N. mirabilis (P = 0·046).
Table 1.
Physiological, biochemical and anatomical characteristics of two Nepenthes species
| Characteristics |
N. alata |
N. mirabilis |
||
|---|---|---|---|---|
| Pitcher | Lamina | Pitcher | Lamina | |
| AN (nmol CO2 g−1 d. wt s−1) | −2·4 ± 1·4** | 42·3 ± 4·5† | −0·0 ± 1·6** | 24·4 ± 5·5 |
| Rd (nmol CO2 g−1 d. wt s−1) | 6·3 ± 1·1**† | 9·7 ± 0·9 | 11·6 ± 0·9 | 8·0 ± 0·6 |
| Chl a + b (mg g−1 d. wt) | 1·7 ± 0·2** | 8·0 ± 0·8 | 1·6 ± 0·3** | 9·2 ± 1·1 |
| N (%) | 0·28 ± 0·08** | 1·08 ± 0·12 | 0·29 ± 0·06** | 0·70 ± 0·11 |
| C (%) | 43·71 ± 0·06**†† | 46·50 ± 0·14†† | 46·26 ± 0·09** | 47·64 ± 0·05 |
| C/N ratio | 156 ± 11** | 43 ± 8 | 159 ± 14 | 68 ± 9** |
| PNUE (µmol CO2 mol N−1 s−1) | −0·14 ± 6·0** | 56·5 ± 6·7 | −0·17 ± 7·9** | 52·2 ± 9·8 |
| Stomata density (mm−2) | 10·8 ± 1·0**†† | 99·5 ± 4·5†† | 0·8 ± 0·4** | 178·9 ± 16·6 |
| Stomata length (µm) | 41·5 ± 0·5*†† | 39·8 ± 0·6†† | 38·7 ± 0·7** | 34·0 ± 0·8 |
| Stomata width (µm) | 34·0 ± 0·5 * | 32·5 ± 0·5 †† | 33·3 ± 0·7 ** | 25·0 ± 0·4 |
Values shown are means ± s.e., n = 5. Comparisons were made between pitcher and lamina (paired t-test) at P < 0·05 (*), P < 0·01 (**) and between species (t-test) at P < 0·05 (†), P < 0·01 (††).
We found significantly lower Rd,pitcher for N. alata than N. mirabilis (P = 0·011, Table 1). The Rd,pitcher of N. alata was significantly lower than Rd,lamina (P = 0·002). N. mirabilis had slightly, but not significantly, higher Rd,pitcher than Rd,lamina (P = 0·087).
The chlorophyll content was higher in the lamina than in the pitcher of N. alata and N. mirabilis (P < 0·001, Table 1). In addition, significantly lower N and C content was found in the pitcher compared with the lamina for both species (P < 0·001). The values of PNUE did not differ between species, but were significantly lower in the pitchers than in the lamina (P < 0·001). Leaf carbon content was comparable with that in non-carnivorous plants and the combination of low N and high C yielded high C/N ratios especially in the pitcher (Table 1).
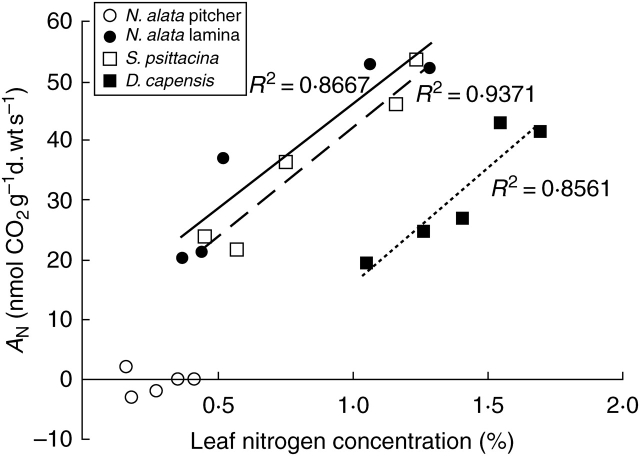
The interspecific comparison revealed significant differences (P < 0·05) between species in AN, N, PNUE and chlorophyll concentration (Table 2). The photosynthetic rate of N. alata lamina was similar to those of Drosera and Sarracenia; however, the PNUE of Nepenthes lamina was significantly higher than in Drosera and similar to that of Sarracenia. Nitrogen and chlorophyll concentration, AN, PNUE and stomatal density in the pitcher of Nepenthes were significantly lower than for all others. Chlorophyll concentration was highest in Nepenthes lamina (Table 2). In all species (except Nepenthes pitcher, P = 0·937) leaf nitrogen concentration was positively correlated with AN (P < 0·05, Fig. 2). The rate of photosynthesis and leaf nitrogen concentration in young lamina without pitchers in the upper part of the stem were twice as high as in the older lamina carrying functional pitchers (AN = 51·3 ± 4·1 vs. 26·3 ± 3·0 nmol CO2 g−1 d. wt s−1, P < 0·01; N = 1·1 ± 0·1 vs. 0·4 ± 0·1 %, P < 0·01); therefore, young lamina without pitcher is the most efficient photosynthetic tissue in Nepenthes. Spring phyllodiform leaves of S. psittacina with a higher portion of tissue invested into the photosynthetic efficient keel than into the tube had significantly higher photosynthetic rates and nitrogen concentration than leaves from the end of the growing season with a higher portion of tissue invested into tube than into the keel (AN = 48 ± 3·2 vs. 23·4 ± 2·9 nmol CO2 g−1 d. wt s−1, P < 0·01; N = 1·0 ± 0·1 vs. 0·5 ± 0·1 %, P < 0·01).
Table 2.
Mass-based photosynthetic rates (AN, nmol CO2 g−1 d. wt s−1), leaf nitrogen concentration (N, %), photosynthetic nitrogen use efficiency (PNUE, µmol CO2 mol N−1 s−1), chlorophyll content (Chl a + b, mg g−1 d. wt) and stomata density (mm−2) in three carnivorous species
| Species | AN | N | PNUE | Chl a + b | Stomata density |
|---|---|---|---|---|---|
| N. alata pitcher | −0·6 ± 0·9a | 0·27 ± 0·05a | −2·9 ± 7·0a | 1·60 ± 0·11a | 12·3 ± 0·8a |
| N. alata lamina | 36·8 ± 7·1b | 0·73 ± 0·18b | 74·5 ± 7·1b | 3·20 ± 0·26b | 110·8 ± 2·4b |
| D. capensis | 31·2 ± 4·7b | 1·39 ± 0·11c | 31·0 ± 3·2c | 2·19 ± 0·25ac | 44·4 ± 1·9c † |
| S. psittacina | 36·4 ± 6·1b | 0·83 ± 0·16b | 62·6 ± 4·0b | 2·30 ± 0·21c | 50·0 ± 3·4c |
| ANOVA | F3,23 = 11·49 | F3,23 = 11·29 | F3,23 = 38·70 | F3,23 = 9·34 | F4,07 = 189·8 |
| P = 0·0003 | P = 0·0003 | P < 0·0001 | P = 0·0008 | P < 0·0001 |
Values shown are mean ± s.e. Different letters in the columns indicate significant differences at P = 0·05 (ANOVA, LSD test, n = 5, for stomata density n = 3).
† Stomata density in Drosera is the sum from both sides of leaves (amfistomatous type of leaf).
Fig. 2.
Net photosynthetic rate (AN) in three carnivorous species in relation to leaf nitrogen content. Regression lines (solid line, N. alata lamina; dashed line, S. psittacina; dotted line, D. capensis) are shown only for significant relationships. The lines have similar slopes (P = 0·083) but D. capensis has different y-intercept (P < 0·001) according to ANCOVA at P = 0·05.
Nepenthes alata anatomy
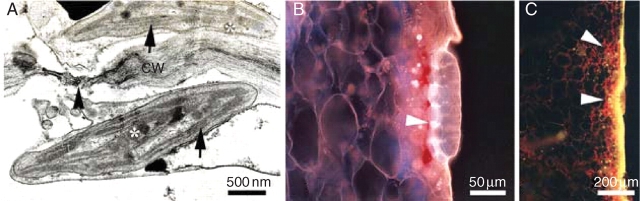
Anatomical observations revealed considerable differences between the pitcher and the lamina (Fig. 3). Under the epidermis of the lamina there were two or three layers of vacuolated hypodermal cells. The mesophyll of tissue dedicated to C-assimilation was composed of a scattered palisade layer with numerous chloroplasts and spongy parenchyma with large intercellular spaces (Fig. 3E, G). The leaf mesophyll of the Nepenthes pitcher was not differentiated into palisade and spongy parenchyma. Pitchers [digestive zone (Fig. 3B), waxy zone (Fig. 3C) and lid (Fig. 3D)] had very small intercellular spaces and the tissue was very compact (Fig. 3F). The volume of air space inside the pitcher was small (0–4·8 %), but in the lamina it was much higher (13–21 %). The digestive zone (Fig. 3B) and the abaxial side of the lid (Fig. 3D) had many glands that are presumably responsible for attracting (lid) and digesting (digestive zone) prey. Stomata occurred on the abaxial side of the lamina (hypostomatous leaf, Fig. 4A, B) and the outer side of the pitcher (Fig. 4C, D), with significantly lower stomatal density on the pitcher surface (Tables 1 and 2). The external side of the pitcher and the abaxial side of the lamina were covered with nectar glands (Fig. 4A, C, D). The mesophyll cells of lamina and pitcher had well-developed chloroplasts with grana and stroma thylakoid lamellae (Fig. 5A). A greater number of chloroplasts were found in cells in the pitcher beneath the digestive glands (Fig. 5C). Cell walls beneath the glands reflect UV light, indicating the presence of large deposits of suberin and lignin (Fig. 5B, C).
Fig. 3.
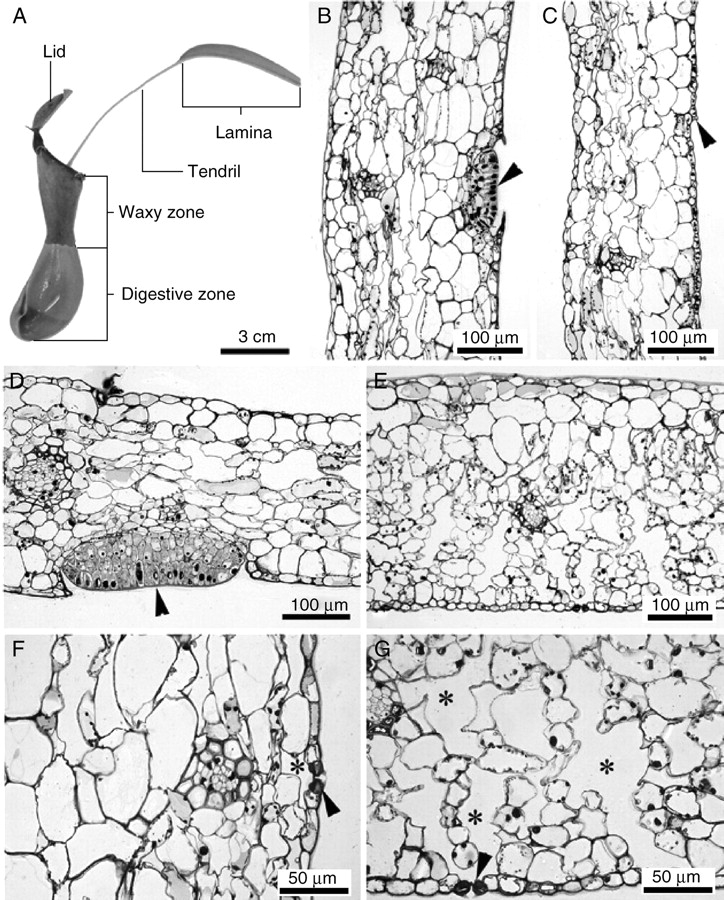
Leaf anatomy of Nepenthes alata. (A) Longitudinal section of the N. alata leaf. (B) Anatomy of digestive zone with multicellular digestive gland (arrowhead); general view. (C) Waxy zone with typical lunate cell (arrowhead) without digestive glands that is responsible for preventing prey escape; general view. (D) Lid with prominent nectar gland (arrowhead). (E) Lamina of Nepenthes alata with palisade and spongious parenchyma; general view. (F) Detail of stomata (arrowhead) and small intercellular spaces (asterisk) with compact mesophyll in the pitcher. (G) Detail of stomata (arrowhead) and large intercellular spaces (asterisk) in lamina mesophyll.
Fig. 4.
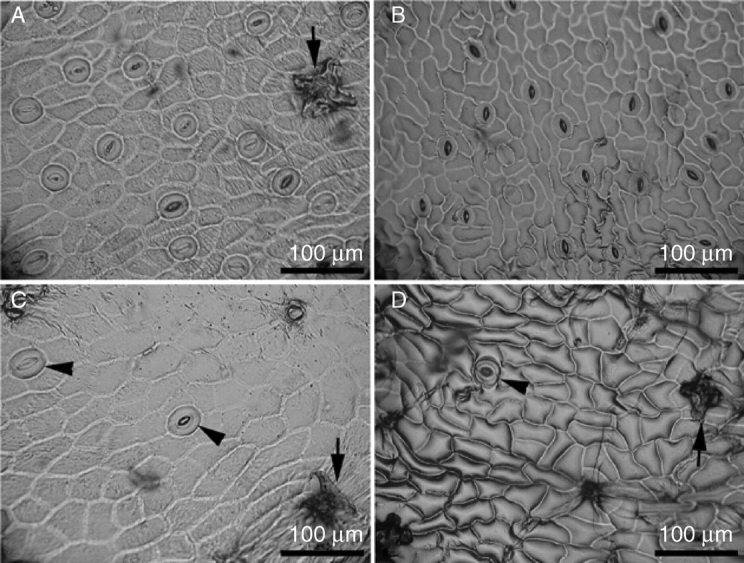
Stomata on the abaxial side of the lamina in Nepenthes alata (A) and in N. mirabilis (B). Stomata on external surface of N. alata (C) and N. mirabilis (D) pitcher. Note only one or two stomata per figure (arrowheads) and extrafloral nectaries on both types of surfaces (arrows).
Fig. 5.
Fluorescence and transmission electron microscopy of N. alata pitcher. (A) Chloroplast with granal membrane (arrow) and plastoglobuli (asterisk) from pitcher mesophyll. Note the plasmodesmata (arrowhead) in cell wall (CW), which promote symplastic transport. (B) Apoplastic barriers (arrowhead) in N. alata beneath digestive glands. (C) Autofluorescence of chloroplasts and apoplastic barriers. Note the absence of photosynthetically active chloroplasts in digestive glands and their increased number beneath apoplastic barriers (arrowheads).
For comparison, the stomatal density, width and length in S. psittacina and D. capensis were also measured. The lowest stomatal density was on the surface of Nepenthes pitchers and the highest was on Nepenthes lamina (P < 0·001, Table 2). D. capensis had stomata on both sides of the leaf, but they were significantly smaller than in Nepenthes (data not shown). S. psittacina had stomata on the external surface of the pitcher, similar to Nepenthes, with a higher stomatal density in the keel (tube = 50 ± 3·4, keel = 72 ± 3·7 mm−2, P < 0·001).
DISCUSSION
Gas exchange and leaf chemistry
Nepenthes is a good experimental genus for studying the cost/benefit model of carnivory. The leaves are divided into photosynthetically active lamina and a pitcher trap, resulting in a functional specialization of different tissues in the same leaf. We supported the belief that photosynthetic rate of carnivorous plants was low in comparison with the Glopnet data set for non-carnivorous plants (Wright et al., 2004; Ellison and Farnsworth, 2005; Ellison, 2006). Méndez and Karlsson (1999) compared AN in subarctic carnivorous and non-carnivorous plants. Photosynthetic performance was also lower in carnivorous plants. Values of AN in Nepenthes lamina agreed with those found by other authors for various carnivorous plants: values for Pinguicula alpina, P. villosa, P. vulgaris, Drosera rotundifolia ranged between 41·8 and 69·3 nmol CO2 g−1 d. wt s−1 (Méndez and Karlsson, 1999). Small (1972) found AN = 29·7 nmol CO2 g−1 d. wt s−1 for the terrestrial carnivorous species Sarracenia purpurea. Darlingtonia californica had from 10 to 50 nmol CO2 g−1 d.wt s−1 depending on leaf mass area (negative correlation), N (positive correlation) and P (positive correlation) concentration (Ellison and Farnsworth, 2005). Although our values of AN agreed with those for other terrestrial carnivorous plants, we expected higher An,lamina, because it does not incur the investment in carnivory (cost). This may be due to low leaf N content in our pitcher plants. Nitrogen concentration in the lamina was low and was even lower in the pitcher (Tables 1 and 2) in comparison with data published by Aerts and Chapin (2000) and may be considered as limiting primary productivity. Moran et al. (2001) found average lamina values of 1·06 % N in Nepenthes rafflesiana and 1·45 % in Nepenthes albomarginata, and Moran and Moran (1998) found lamina N concentrations of 0·57 % in N. rafflesiana from its natural habitat.
Values of PNUE in lamina (Tables 1 and 2) were similar or only slightly higher than in other carnivorous plants. PNUE in Nepenthes lamina was higher than in Drosera rotundifolia (33·6 µmol CO2 mol N−1 s−1) and Pinguicula spp. (29·4–45·4 µmol CO2 mol N−1 s−1; Méndez and Karlsson, 1999) or Darlingtonia (26·4 µmol CO2 mol N−1 s−1, A. M. Ellison, Harvard University, MA, USA, pers. comm.). However, the PNUEs of Sarracenia and Nepenthes were not significantly different in the present study (Table 2). There are two possible explanations for this. The first is that the Nepenthes lamina may not exclude the cost of carnivory completely. The lower surface of the Nepenthes lamina was covered with extrafloral nectaries (Fig. 4A). The second explanation is that the lamina of N. alata was thick and leathery and therefore had higher leaf mass area (LMA = 87 g m−2) than S. psittacina (LMA = 30 g m−2). At least three factors could contribute to species with higher LMA having lower PNUE: CO2 diffusion limitation, light limitation and a higher fraction of N in non-photosynthetic components (Wright et al., 2005). Lower PNUE in D. capensis is perhaps a consequence of the higher cost on carnivory in comparison with Sarracenia (stalked vs. sessile glands). This is consistent with the findings of L. Adamec (Institute of Botany, Academy of Science of Czech Republic, Třeboň, Czech Republic, pers. comm.) who observed seven times higher leaf respiration rates in D. capensis than in S. psittacina. PNUE of Nepenthes pitchers was negative (Table 2), due to negative AN, and it was lower than in Drosera or Sarracenia. Despite the morphological similarity between Nepenthes and Sarracenia (both are pitcher plants), there are wide differences in their physiology. The pitcher of Nepenthes had considerably lower mass-based foliar N and chlorophyll content, AN, PNUE and stomata density than Sarracenia (Fig. 2, Table 2). No negative AN was found in Sarracenia leaf. Sarracenia does not have an additional organ for C-assimilation, and therefore the trap must function in both photosynthesis and prey capture to achieve a positive carbon gain, unlike the pitcher of Nepenthes, which can rely on assimilates from the photosynthetically active lamina. Under certain ecological conditions (dry, shade, increased nitrogen deposition), the plants of the genus Sarracenia produce flattened phyllodia (enlarged keel and reduced tube) that are more efficient in photosynthesis (Ellison and Gotelli, 2002). The plants studied here did not have this type of leaves, but in spring they produced phyllodiform leaves with a higher proportion of tissue invested in photosynthetic (keel) than in prey trapping tissue (tube) with higher AN. The proportion of keel gradually decreased during the growing season as did AN. The pitcher in Fig. 1C represents an intermediate type between these two extremes. The studied species (except the Nepenthes pitcher) had a positive correlation between nitrogen content and AN (Fig. 2A), which is considered typical for plants (Wright et al.,2005) and it was confirmed also for Darlingtonia californica (Ellison and Farnsworth, 2005).
Low An,pitcher is related to both a low tissue nitrogen content and a low chlorophyll content (Tables 1 and 2). Lower chlorophyll content in traps than in the leaves of aquatic Utricularia macrorhiza was observed by Knight (1992). Carnivorous plants tend to have lower chlorophyll content than non-carnivorous plants and pitchers of Nepenthes had the lowest chlorophyll content. Masarovičová and Eliáš (1980) found in 19 herbaceous species chlorophyll a + b = 14·3–27·3 mg g−1 and Eliáš and Masarovičová (1980) found in four tree species chlorophyll a + b = 4·9–14·2 mg g−1 in oak–hornbeam forest. Lower chlorophyll content is probably due to lower nitrogen content in carnivorous plants than in non-carnivorous plants, because a positive correlation between chlorophyll and nitrogen content is generally found for plants (Masarovičová et al., 2000).
The costs to Nepenthes in term of nitrogen invested in its pitcher were low (high C/N ratio), but the benefit is probably much higher. Moran et al. (2001) estimated that prey contributed from 53·8 to 68·1 % of the total foliar N in Nepenthes. From the viewpoint of the cost/benefit model of Givnish et al. (1984), the cost of carnivory can include a reduced photosynthetic and heightened respiration rate in traps. Consistent with this, in the present study pitchers do not have as high a photosynthetic rate as the lamina. Therefore, the pitcher can be considered a cost to the pitcher plant in that the carbon invested in pitchers does not return as much carbon as assimilatory tissue. Although lower positive AN of traps was found by Knight (1992) in the aquatic Utricularia macrorhiza and by Adamec (1997a) in Aldrovanda vesiculosa, mostly negative AN was found here in pitchers. A negative rate of net photosynthesis was also found in plant reproductive structures (Obeso, 2002). Contrary to expectation, the respiration rate of the pitcher was not significantly higher than that of the leaves (Table 1). Respiration of bladders of six aquatic species was 75–200 % greater than in the leaves (Adamec, 2006). These differences are consistent with the specific amino acid changes in Utricularia cytochrome c-oxidase (Leu113, Ser114 replaced by Cys113, Cys114), which accelerates the rate of respiration. These changes were not confirmed for Nepenthes (Jobson et al., 2004). It seems that the reduced AN of trap organs represents the cost of carnivory in general, but the higher Rd of traps is dependent on plant species and type of trap. Nevertheless, it can be concluded that the respiration of pitchers may represent extra energy requirements for pitcher plants. Méndez and Karlsson (1999) found Rd values of 9·8 and 9·0 nmol CO2 g−1 d. wt s−1 in P. alpina and P. vulgaris leaves, but they could not determine which part of the respiration was involved in the carnivorous and assimilatory function.
Nepenthes anatomy in relation to AN and PNUE
The values of PNUE and AN found for carnivorous plants are comparable with values for conifers (Méndez and Karlsson, 1999). The often-published interpretation of the low PNUE and AN values, namely replacement of chlorophyll-containing cells with nectar and digestive glands (Schnell, 2002), must be augmented by another interpretation based on other differences in the anatomy of carnivorous and non-carnivorous leaves. It was found that low stomatal (Lusk et al., 2003) and mesophyll conductance (De Lucia et al., 2003) restricted photosynthesis in some conifer species. Méndez and Karlsson (1999) suggested that lower AN and PNUE of carnivorous plants might be a result of their carnivorous habit. Drosera and Sarracenia leaves do not have a palisade layer, the mesophyll being made up of more or less rounded cells containing chloroplasts (Juniper et al., 1989). A more compact leaf anatomy results in lower mesophyll conductance for CO2 (gm) and thus lower AN. A compact anatomy was observed here, with a small proportion of intercellular spaces, in the pitcher (Fig. 3F), but the lamina had developed palisade and spongy parenchyma with large intercellular spaces (Fig. 3G) such as in leaves of non-carnivorous plants. Despite negative AN in the trap, TEM observation revealed that mesophyll cells contained well-developed chloroplasts with numerous thylakoid membranes and plastoglobuli (Fig. 5A). An increased number of chloroplasts was found in the cells beneath the digestive glands (Fig 5C), although the digestive glands themselves probably do not have chloroplasts. Rudimentary plastids without granal membranes in digestive glands of N. alata were found by Owen et al. (1999). Chia et al. (2004) showed that the generation of oxygen free radicals by pitcher fluids is the first step of the digestion process. We assume that increased numbers of chloroplasts beneath digestive glands, which are efficient for the conduction of solutes into the pitcher, are involved in this process.
Stomata density in the lamina is comparable with non-carnivorous plants; the pitcher surface had a very low stomata density (Tables 1 and 2). Therefore, besides low mesophyll conductance (gm) there is also a low stomatal conductance (gs). In Drosera the stomata occur on both sides of the leaf (amphistomatous leaf; Juniper et al., 1989), but their density is also low. We predict that the above-mentioned characteristics, which make photosynthesis in traps inefficient, may be responsible for the carnivorous function in nutrient-poor habitats. As in roots, a compact anatomy might serve the symplastic transport of nutrients gained from prey through plasmodesmata (Fig. 5A). An endodermal layer beneath the digestive glands, which restricts apoplastic flows in a manner similar to the Casparian strips in the root endodermis, has been found by Owen and Lennon (1999) and Gorb et al. (2004). Owen et al. (1999) observed that the glandular uptake in Nepenthes occurred through the apoplast, but was blocked from entering the underlying mesophyll cell walls by thick endodermal-like regions. The most important role of stomata is in optimizing carbon fixation per unit water loss (Raven, 2002). Whether the low stomata density in Nepenthes pitchers is a consequence of, or reason for, the low assimilation capacity in traps is a matter of debate. Because respiration supplies sufficient amounts of CO2 for photosynthesis, the pitcher does not rely on atmospheric CO2. Carbon dioxide refixation is well known in woody stems without stomata (Wahid and Rasul, 2005). Furthermore, transpiration in traps may be undesirable, when the water is secreted into the pitcher as a digestive fluid (in some cases more than 1 L of digestive fluid per pitcher) and low stomata density may provide a selective advantage. Carnivorous plants, in which the entire shoot is both a photosynthetic and a capture organ (e.g. Drosera and Sarracenia), have intermediate physiology and leaf anatomy (compact mesophyll without palisade parenchyma with higher stomata density than in the pitcher and lower stomata density than in the lamina of Nepenthes) because it is not possible to maximize for these two contrasting functions – there must be trade-offs.
It is not known to what degree the stomatal and mesophyll conductance result in low AN and PNUE in carnivorous plants. Is the low stomatal density and compact mesophyll a consequence of, or a reason for, the low assimilation capacity in carnivorous plants? Future work will require measurements of stomatal limitation (cf. Jones, 1998) and simultaneous measurements of gas exchange and chlorophyll fluorescence by the constant J method (Harley et al., 1992).
CONCLUSIONS
The present study underlines the main features involved in a syndrome of terrestrial carnivorous plants, especially in Nepenthes. Nepenthes are carnivorous plants with leaves divided into a structure for photosynthetic assimilation (lamina) and a pitcher trap. This allowed us to study gas exchange and anatomy in assimilation and trapping parts separately. It is clear that pitchers are less productive than lamina and represent a sizable cost to the plant. There was a prediction that costs of carnivory (carbon metabolism) result in a reduced photosynthetic and increased respiration activity in traps. Reduced photosynthesis was confirmed, but increased respiration was not. Nepenthes possess clusters of characters that result in reduced photosynthesis in traps: replacement of chlorophyll-containing cells with digestive glands, low chlorophyll and nitrogen content, low stomatal density, absence of palisade parenchyma, small intercellular spaces and thus lower stomatal and mesophyll conductance. By contrast, digestive glands, compact mesophyll and low stomatal density are responsible for a digestive function that may provide a selective advantage and the evolution of carnivorous plants growing in nutrient-poor, sunny and wet habitats. In general, the present results confirmed low photosynthetic capacity, nitrogen efficiency, and chlorophyll and nitrogen content in carnivorous plants.
ACKNOWLEDGEMENTS
This work was supported by grant VEGA1/3288/06 and UK/176/2007 from the Comenius University in Bratislava. We thank Mrs J. Šramková for excellent technical assistance, Mr K. Paule (Institute of Chemistry, Slovak Academy of Science in Bratislava) for elemental analyses, Dr Milan Baláž for photographic work, Dr L. Adamec and Prof. L. Nátr for critical reading of the manuscript and Prof. P. White for language corrections.
LITERATURE CITED
- Adamec L. Photosynthetic characteristics of the aquatic carnivorous plant. Aldrovanda vesiculosa. Aquatic Botany. 1997a;59:297–306. [Google Scholar]
- Adamec L. Mineral nutrition of carnivorous plants: a review. Botanical Review. 1997b;63:273–299. [Google Scholar]
- Adamec L. Respiration and photosynthesis of bladders and leaves of aquatic Utricularia species. Plant Biology. 2006;8:765–769. doi: 10.1055/s-2006-924540. [DOI] [PubMed] [Google Scholar]
- Aerts R, Chapin III FS. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Advances in Ecological Research. 2000;30:1–67. [Google Scholar]
- Albert VA, Williams E, Chase MW. Carnivorous plants: phylogeny and structural evolution. Science. 1992;257:1491–1495. doi: 10.1126/science.1523408. [DOI] [PubMed] [Google Scholar]
- Benzing DH. The origin and rarity of botanical carnivory. Trends in Ecology and Evolution. 1987;2:364–369. doi: 10.1016/0169-5347(87)90137-6. [DOI] [PubMed] [Google Scholar]
- Bohn HF, Federle W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proceedings of the National Academy of Sciences of USA. 2004;101:14138–14143. doi: 10.1073/pnas.0405885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia TF, Aung HH, Osipov AN, Goh NK, Chia LS. Carnivorous pitcher plant uses free radicals in the digestion of prey. Redox Report. 2004;9:255–261. doi: 10.1179/135100004225006029. [DOI] [PubMed] [Google Scholar]
- Clarke C. Nepenthes of Borneo. Kota Kinabalu, Malaysia: Natural History Publication; 1997. [Google Scholar]
- Clarke C. Nepenthes of Sumatra and Peninsular Malaysia. Kota Kinabalu, Malaysia: Natural History Publication; 2001. [Google Scholar]
- De Lucia EH, Whitehead D, Clearwater MJ. The relative limitation of photosynthesis by mesophyll conductance in co-occuring species in a temperate rainforest dominated by the conifer. Dacrydium cupresssium. Functional Plant Biology. 2003;30:1197–1204. doi: 10.1071/FP03141. [DOI] [PubMed] [Google Scholar]
- Eliáš P, Masarovičová E. Chlorophyll content in leaves of plants in an oak-hornbeam forest 3. Three species. Photosynthetica. 1980;14:604–610. [Google Scholar]
- Ellison AM. Nutrient limitation and stoichiometry of carnivorous plants. Plant Biology. 2006;8:740–747. doi: 10.1055/s-2006-923956. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Farnsworth EJ. The cost of carnivory for Darlingtonia californica (Sarraceniaceae): evidence from relationships among leaf traits. American Journal of Botany. 2005;92:1085–1093. doi: 10.3732/ajb.92.7.1085. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ. Evolutionary ecology of carnivorous plants. Trends in Ecology and Evolution. 2001;16:623–629. [Google Scholar]
- Ellison AM, Gotelli NJ. Nitrogen availability alters the expression of carnivory in the northern pitcher plant. Sarracenia purpurea. Proceedings of the National Academy of Sciences of USA. 2002;99:4409–4412. doi: 10.1073/pnas.022057199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaume L, Gorb S, Rowe N. Function of epidermal surfaces in the trapping efficiency of Nepenthes alata pitchers. New Phytologist. 2002;156:479–489. doi: 10.1046/j.1469-8137.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD. Carnivory in the bromeliad Brocchinia reducta with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient poor habitats. American Naturalist. 1984;124:479–497. [Google Scholar]
- Gorb E, Kastner V, Peressadko A, Arzt E, Gaume L, Rowe N, Gorb S. Structure and properties of the glandular surfaces in the digestive zone of the pitcher in the carnivorous plant Nepenthes ventrata and its role in insect trapping and retention. Journal of Experimental Biology. 2004;207:2947–2963. doi: 10.1242/jeb.01128. [DOI] [PubMed] [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiology. 1992;98:1429–1443. doi: 10.1104/pp.98.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobson RW, Nielsen R, Laakkonen L, Wikström M, Albert VA. Adaptive evolution of cytochrome c oxidase: infrastructure for a carnivorous plant radiation. Proceedings of the National Academy of Sciences of USA. 2004;101:18064–18068. doi: 10.1073/pnas.0408092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HG. Stomatal control of photosynthesis and transpiration. Journal of Experimental Botany. 1998;49:387–398. [Google Scholar]
- Juniper BE, Robins RJ, Joel DH. The carnivorous plants. London: Academic Press; 1989. [Google Scholar]
- Karlsson PS, Nordell KO, Carlsson BÅ, Svensson BM. The effect of soil nutrient status on prey utilization in four carnivorous plants. Oecologia. 1991;86:1–7. doi: 10.1007/BF00317381. [DOI] [PubMed] [Google Scholar]
- Knight SE. Costs of carnivory in the common bladderwort. Utricularia macrorhiza. Oecologia. 1992;89:348–355. doi: 10.1007/BF00317412. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology. 1987;148:350–382. [Google Scholar]
- Lusk CH, Wright I, Reich PB. Photosynthetic differences contribute to competitive advantage of evergreen angiosperm trees over evergreen conifers in productive habitats. New Phytologist. 2003;160:329–336. doi: 10.1046/j.1469-8137.2003.00879.x. [DOI] [PubMed] [Google Scholar]
- Lux A. [Rapid method for staining of semithin sections from plant material] Biológia (Bratislava) 1981;36:753–757. [in Slovak] [Google Scholar]
- Masarovičová E, Eliáš P. Chlorophyll content in leaves of plants in an oak-hornbeam forest 1. Herbaceous species. Photosynthetica. 1980;14:580–588. [Google Scholar]
- Masarovičová E, Král'ová K. Approaches to measuring plant photosynthetic activity. In: Pessarakli M, editor. Handbook of photosynthesis. Boca Raton, FL: LLC CRC Press, Taylor and Francis Group; 2005. pp. 617–656. [Google Scholar]
- Masarovičová E, Welschen R, Lux A, Lambers H, Argalášová K, Brandšteterová E, Čaniová A. Photosynthesis, biomass partitioning and peroxisomicine A1 production of Karwinskia species in response to nitrogen supply. Physiologia Plantarum. 2000;108:300–306. [Google Scholar]
- Méndez M, Karlsson PS. Costs and benefits of carnivory in plants: insights from photosynthetic performance of four carnivorous plants in a subarctic environment. Oikos. 1999;86:105–112. [Google Scholar]
- Moran JA, Moran AJ. Foliar reflectance and vector analysis reveal nutrient stress in prey-deprived pitcher (Nepenthes rafflesiana) International Journal of Plant Science. 1998;159:996–1001. [Google Scholar]
- Moran JA, Booth WE, Charles JK. Aspects of pitcher morphology and spectral characteristics of six Bornean Nepenthes pitcher plants species: implications for prey capture. Annals of Botany. 1999;83:521–528. [Google Scholar]
- Moran JA, Merbach MA, Livingstone NJ, Clarke CM, Booth WE. Termite prey specialization in the pitcher plant Nepenthes albomarginata – Evidence from stable isotope analysis. Annals of Botany. 2001;88:307–311. [Google Scholar]
- Obeso JR. The cost of reproduction in plants. New Phytologist. 2002;155:321–348. doi: 10.1046/j.1469-8137.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- Owen TP, Lennon KA. Structure and development of the pitcher from the carnivorous plant Nepenthes alata (Nepenthaceae) American Journal of Botany. 1999;86:1382–1390. [PubMed] [Google Scholar]
- Owen TP, Lennon KA, Santo MJ, Anderson AN. Pathways for nutrient transport in the pitchers of the carnivorous plant. Nepenthes alata. Annals of Botany. 1999;84:459–466. [Google Scholar]
- Raven JA. Selection pressures on stomatal evolution. New Phytologist. 2002;153:371–386. doi: 10.1046/j.0028-646X.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Schulze W, Schulze ED, Pate JS, Gillinson AN. The nitrogen supply from soils and insects during growth of the pitcher plants Nepenthes mirabilis, Cephalotus follicularis and Darlingtonia californica. Oecologia. 1997;112:464–471. doi: 10.1007/s004420050333. [DOI] [PubMed] [Google Scholar]
- Schnell DE. Carnivorous plants of the United States and Canada. Portland, OR: Timber Press, Inc; 2002. [Google Scholar]
- Small E. Photosynthetic rates in relation to nitrogen recycling as an adaptation to nutrient deficiency in peat bog plants. Canadian Journal of Botany. 1972;50:2227–2233. [Google Scholar]
- Thorén ML, Karlsson PS. Effects of supplementary feeding on growth and reproduction of three carnivorous plant species in a subarctic environment. Journal of Ecology. 1998;86:501–510. [Google Scholar]
- Wahid A, Rasul E. Photosynthesis in leaf, stem, flower, and fruit. In: Pessarakli M, editor. Handbook of photosynthesis. Boca Raton, FL: LLC CRC Press, Taylor and Francis Group; 2005. pp. 4794–4797. [Google Scholar]
- Wakefield AE, Gotelli NJ, Wittman SE, Ellison AM. Prey addition alters nutrient stoichiometry of the carnivorous plant. Sarracenia purpurea. Ecology. 2005;86:1737–1743. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Garnier E, Hikosaka K, et al. Assessing the generality of global leaf trait relationships. New Phytologist. 2005;166:485–496. doi: 10.1111/j.1469-8137.2005.01349.x. [DOI] [PubMed] [Google Scholar]