Abstract
Background and Aims
Eschscholzia californica (California poppy) is an emerging model plant for ‘evo–devo’ studies from the basal eudicot clade of Papaveraceae. California poppy has a relatively small genome, a short life cycle and, most importantly, it is amenable for transformation. However, since this transformation protocol is time consuming, virus-induced gene silencing (VIGS) was evaluated as a fast method to obtain functional data for California poppy genes.
Methods
Commercially available California poppy plants were infiltrated with Agrobacterium tumefaciens carrying the tobacco rattle virus plasmids pTRV1 and pTRV2. pTRV2 contained part of the eschscholzia Phytoene Desaturase (EcPDS) gene whose loss of function results in photobleaching of the green parts of the plant and in a lack of floral coloration. The degree and duration of these symptoms was evaluated for vegetative rosettes and plants in flower.
Key Results
It is shown that VIGS is able to effectively down-regulate the EcPDS gene in eschscholzia. Various degrees of silencing were observed starting <2 weeks after infiltration with Agrobacterium tumefaciens in 92 % of the plants. Tissue with silencing symptoms also showed complete or strong reduction of EcPDS transcripts. Strong silencing resulted in almost completely white petals, fruits, shoots and leaves. Plants with a strong degree of silencing will eventually die off; however, others are able to produce EcPDS gene product even after a strong initial silencing and will recover. Silencing was found to be not always systemic, but was often restricted to certain organs or parts of organs.
Conclusions
VIGS is an effective, fast and transient method to down-regulate gene expression in eschscholzia. It serves well to detect prominent phenotypes which may become obvious even if some target gene transcript remains in the plant tissue. However, subtle phenotypes will be more difficult to detect, as extremely strong silencing effects occur in <10 % of all flowers from infected plants.
Key words: Eschscholzia californica, California poppy, Agrobacterium tumefaciens, tobacco rattle virus, Phytoene Desaturase, PDS, virus-induced gene silencing, VIGS, post-transcriptional gene silencing, basal eudicot, EcPDS
INTRODUCTION
Most of our knowledge on the molecular basis of plant development is derived from gene function analyses in a handful of model species, e.g. Arabidopsis thaliana, Petunia × hybrida, Oryza sativa (rice), Zea mays (corn), Nicotiana tabacum (tobacco), Antirrhinum majus (snapdragon), Lycopersicon esculentum (tomato) and Pisum sativum (pea). Functional studies have so far been mainly feasible if large collections of mutants in combination with genetic maps are available (e.g. in arabidopsis, petunia, snapdragon and pea) and/or if stable transformation is easily achieved (e.g. arabidopsis, petunia, tobacco, and rice to some extent). All these species belong to the core eudicots, with the exception of the monocot rice. Thus, the data collected on, for example, gene function, regulation of gene expression or interacting protein partners cover only a part of the angiosperm tree. For a better understanding of the evolution of developmental traits, our knowledge needs to be extended to species which are interesting from a phylogenetic point of view. Functional data for key regulators of development are lacking, for example, for the ANITA grade of basal angiosperms (Qiu et al., 2000), basal eudicots, monocots other than grasses, gymnosperms and non-seed plant lineages.
To overcome the lack of functional data in non-model plants, several studies report on successful overexpression of putative key regulators in heterologous systems, e.g. arabidopsis (Winter et al., 2002; Zhang et al., 2004). But these experiments only address how genes behave in the heterologous systems and may not predict phenotypic effects in the species itself. Therefore, heterologous expression experiments can only provide hints on the function that a gene carries out and need careful interpretation.
Genome and EST sequencing projects are being initiated for many non-model species (e.g. the FGP, Albert et al., 2005; http://www.ncbi.nlm.nih.gov/genomes/PLANTS/PlantList.html) and generate a wealth of sequence information. However, these resources can only be used intensively if functional studies can be undertaken within these same species to elucidate the evolution of gene function.
A promising approach for functional studies among non-model plants, particularly for those where Agrobacterium-mediated transformation is not an option, is virus-induced gene silencing (VIGS). VIGS relies on an intrinsic mechanism against viral invasions that has evolved early in evolution, sharing conserved factors in species such as arabidopsis and Drosophila melanogaster (Benedito et al., 2004).
According to the phylogeny reconstructions utilizing molecular markers the Ranunculales are the earliest branching eudicots (Angiosperm Phylogeny Group, 2003). Information about the role of developmental control genes in this clade is crucial to an understanding of angiosperms as a whole, given its position between core eudicots on the one hand and basal angiosperms and monocots on the other. At the same time, the Ranunculales show unique patterns of diversity, including leaf morphology, flower symmetry and alkaloid metabolism that have attracted researchers to conduct comparative studies in this group. Eschscholzia californica (California poppy), belonging to the family of Papaveraceae, is an emerging model system in the Ranunculales. Unlike its relative Papaver somniferum (opium poppy), eschscholzia is experimentally more accessible as it does not require government research permits. Additionally, its genome is more than three times smaller than that of opium poppy (Carlson et al., 2006) and its generation time can be reduced to <3 months (Becker et al., 2005). Several resources have become available for eschscholzia which include in situ hybridization protocols (Busch and Gleissberg, 2003; Zahn et al., 2006), detailed studies of its morphogenesis (Gleissberg, 2004; Becker et al., 2005) and an EST database for early flower development resulting in >6000 gene sequences (Carlson et al., 2006). These sequences are also represented on microarrays to allow for large-scale expression studies (Claude de Pamphilis, Pennsylvania State University, State College, PA, USA, pers. comm.). Characterization of a couple of developmental control genes has already been started in eschscholzia (Busch and Gleissberg, 2003: Groot et al., 2005: Kölsch and Gleissberg, 2006; Zahn et al., 2006). Additionally, a transformation protocol including somatic embryogenesis from a single cell enables the generation of stable transgenic lines (Park and Facchini, 2000) which can be made available for gene function studies. However, since this transformation protocol proves to be time-consuming, a faster way for high-throughput gene knock-down is therefore desirable.
Reported here is the successful tobacco rattle virus (TRV)-based silencing of the Phytoene Desaturase (EcPDS) gene in the basal eudicot eschscholzia, which proved to be a fast and efficient tool for gene function analysis. The TRV vector system (Liu et al., 2002), which is a bipartite vector system that enables the insertion of a target gene, was used.
The aim was to test if the mechanism of VIGS based on the TRV system (Burch-Smith et al., 2004) is able to suppress the amount of specific transcripts in eschscholzia. It was decided to use the Phytoene Desaturase (PDS) as an easily visible marker gene (Fray and Grierson, 1993). Its corresponding protein is the key enzyme of the carotenoid biosynthesis pathway, it oxidizes and subsequently cyclises phytoene to (α)- and (β)-carotene. The carotenes serve as floral pigments or will be converted into xanthophylls of the antenna pigments of the plant's photosystem. A lack of EcPDS in eschscholzia results in easily visible photobleaching of all green parts of the plant. In the flower, bright orange petals, pollen and stigma will appear white from the lack of carotenes. Phenotype observations can therefore be achieved in all above-ground parts of the plant.
MATERIALS AND METHODS
Plant growth conditions
Virus-induced gene silencing experiments were undertaken at the University of Mainz (MZ) and at the University of Bremen (HB). Eschscholzia seeds were purchased from B & T World Seeds, Aigues-Vive, France and from Larner Seeds (Bolinas, CA, USA). In MZ, seeds were stratified for 2 d in water before sowing in autoclaved standard potting mix (20 % of each peat, Lithuanian peat, cocoa peat, compost, sand). Plants were grown in growth rooms at 23 °C with long days (16 h) at 70 µmol s−1 m−2. Following germination, only one plant was left per pot, and plants were watered twice a week with a 4 g L−1 solution of Hakaphos (Compo) 4 weeks after germination. In HB, the seeds were sown in 70 % soil (Einheitserde, Werkverband, Germany), 20 % sand with 2 g L−1 Osmocote Pro (Scotts, The Netherlands) added, and then covered with approx. 1 cm of peat (Euflor, Germany). After sowing, the seeds were stratified for 5–7 d at 4 °C. Then they were moved to the greenhouse and grown with 16 h light/8 h darkness, normal daylight being intensified with mercury lamps. Day temperature ranged between 18 °C and 23 °C, night temperature between 15 °C and 18 °C. As eschscholzia is strictly self-incompatible, flowers were hand-pollinated. To test wether the observed effects are vertically transmitted into the next generation, plants were grown under sterile conditions according to Park and Facchini (2000) with the exception that for some experiments, 3 % (w/v) sucrose was added to the B5 medium. After stratification at 4 °C they were moved to growth chambers with 18 h light, 6 h darkness in 20 °C.
Isolation of EcPDS
RNA from eschscholzia tissue was isolated using the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) and subsequently reverse transcribed into cDNA using the Omniscript RT kit (Qiagen) according to the manufacturer's instructions. The resulting single-stranded cDNA was used as a template in a PCR reaction with the primers PDS-F1 (5′-CGGTCTAGAGCCACTMAACTTYATAAACC-3′) and PDS-R1(5′-CGGGACTCCTTCAGTTTTCTGTGAAACC-3′) containing XbaI and SacI restriction sites, respectively. The resulting 407-bp PCR fragment was cloned into pGEM-T vector system II (Promega, Madison, WI, USA) and verified by sequencing. The restriction sites present in the primers were utilized to excise the EcPDS fragment from pGEM-T and to insert it into the XbaI and SacI restriction sites of the pTRV2 multiple cloning site (Liu et al., 2002), resulting in the vector pTRV2-EcPDS. After verification of the sequence, the pTRV2-EcPDS vector was then transformed into Agrobacterium tumefaciens strain GV3101 and used for inoculation.
Preparation of agrobacteria and infiltration of agrobacteria carrying TRV1 and TRV2 constructs into California poppy
Agrobacteria for the VIGS inoculation of eschscholzia plants were prepared essentially according to the protocol IV in Lu et al. (2003), with the following exceptions: transformed agrobacterium colonies containing TRV1 and TRV2 plasmids were selected using gentamycin and kanamycin (50 µL mL−1 each). The cells were harvested at an OD550 of 0·7–0·85.
For agrobacterium infiltration, one agrobacterium strain containing TRV1 and the other strain containing the TRV2 vectors were mixed in a 1 : 1 ratio.
Eschscholzia plants were infiltrated in three different ways: (1) seedlings with mature cotyledons but without a visible rosette leaf (7 d after germination) were infiltrated by applying small droplet of agrobacterium suspension to the shoot apical meristem; (2) plants with 5–12 rosette leaves were infiltrated by applying approx. 1 mL of agrobacterium suspension to the lower surface of two to three leaves; (3) plants with 5–22 rosette leaves (up to 31 d after germination) were inoculated by adding 3–4 mL of agrobacterium suspension to the shoot apical meristem and the petioles of the surrounding leaves. The plants were pinched gently to allow the dispersal of the agrobacteria.
Expression analysis of EcPDS by RT-PCR
Several days following observed leaf photobleaching, emerging leaves from node 6, which arose from the silenced part of the shoots above the infection site, were collected from 19 plants, six from mock-treated plants and 13 from poppy plants inoculated with pTRV1 and pTRV2-EcPDS exhibiting photobleaching. RNA was extracted using the RNeasy Kit and cDNA was generated using Omniscript RT. Approximately 360 ng of RNA from eschscholzia leaf (tissue) were reverse transcribed (see paragraph on the isolation of EcPDS). To distinguish between amplification of endogenous EcPDS transcripts from the virus-derived sequence, a larger portion of the gene not included in the virus was amplified using primers PDS-F2 (5′-TGGATGARAAAGCAGGGYGTWCC-3′) and PDS-R3 (5′-CCTTRCAAGTTACWGACATGTCWGCA-3′). To avoid amplification of genomic DNA contaminations, primers used for RT-PCR amplification were chosen to span introns. Expression levels of EcPDS (after 40 PCR cycles) were determined relative to rRNA transcript abundance (after 26 cycles; Cho and Cosgrove, 2000). Each semi-quantitative RT-PCR was replicated three times and to minimize pipetting errors, puRE Taq Ready-To-Go PCR Beads (Amersham Bioscience) were used. The semi-quantitative RT-PCR gels were scanned and saved in jpeg format.
RESULTS
Tobacco rattle virus has no detected effects on leaf and flower morphology
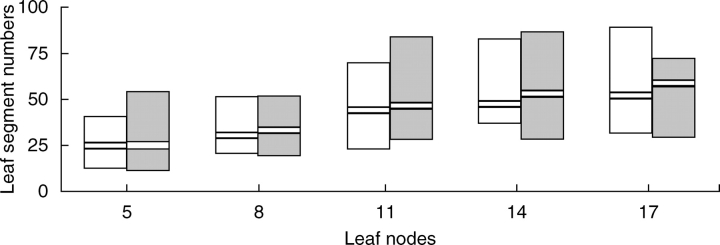
Leaf and flower morphological traits of plants inoculated with TRV1 and empty TRV2 (TRV2-E) vectors were compared with untreated plants to infer any perturbation on leaf or floral morphology. Plants infected with the virus showed no obvious differences in overall shoot and leaf morphology compared with the control. To see if the degree of leaf dissection was specifically affected, leaflet numbers at nodes five, eight, 11, 14 and 17 were compared (Fig. 1, and Table S1 in Supplementary material available online). While segment numbers varied considerably between individual plants, particularly at higher nodes, virus infected plants did not show any consistent deviation of this trait from untreated plants. Also it was not possible to detect any deviation of the floral architecture, size of floral organs or time to flowering in plants infected with the TRV1 and TRV2-E vector (data not shown). All plants inoculated with the TRV1 and TRV2-E following the three infection strategies a, b and c, and all non-treated plants survived for a period of several weeks before being discarded (eschscholzia plants are potentially perennial). Therefore, the tobacco rattle virus appears to have little or no effect on leaf and shoot morphology in its host eschscholzia.
Fig. 1.
Comparison of leaf segment numbers between TRV-treated and untreated plants. The columns show the range of segment numbers in 30 plants at nodes 5, 8, 11, 14 and 17. Black bars show the median for each node. Open columns are untreated, grey are TRV-treated plants.
VIGS of EcPDS in eschscholzia
First the PDS gene was isolated from an eschscholzia cDNA pool with degenerate primers targeting the conserved regions of known PDS genes (Genbank accession no. EF208023). Then a 407-bp fragment of the eschscholzia PDS gene (EcPDS) was generated and this was cloned into the pTRV2 vector (Liu et al., 2002). The TRV vectors were delivered to the cells by a simple agrobacterium leaf infiltration of young plants. Three different strategies of application of the agrobacterium strain GV3101 carrying TRV1 and either TRV2-EcPDS or TRV2-E to the plants were examined.
Interest was in the efficiency of three different inoculation methods and therefore their success in conferring photobleaching to poppy plants was tested systematically. For strategy (a), the aim was to infect the shoot apical meristem of seedlings. From the 17 plants that were infected with the TRV vectors, only three (17·5 %) showed signs of photobleaching. However, the degree of silencing for these three plants was so strong that two of the plants died shortly after the photobleaching phenotype was observed, most likely as a result of excessive photobleaching caused by VIGS of EcPDS. One plant managed to survive by overcoming the infection and generated green leaves after several leaves that showed strong symptoms of VIGS.
In strategy (b), when the lower surface of a few leaves were infected with agrobacteria carrying the TRV plasmids, 94 % of 17 plants infected with pTRV2-EcPDS showed photobleaching. However, the silencing effect was restricted to the infected leaves for several days before it spread through the plant. In the majority of the cases observed, the silencing did not spread evenly through the plants but rather appeared in a striped fashion (Fig. 2E, F): Many shoots showing a striped pattern resulted in leaves that were partly green and partly white from the petiole to the blade along the lateral axis (Fig. 2E). In some cases, a green leaf was observed forming on a fully photobleached shoot (Fig. 2C).
Fig. 2.
Effects of EcPDS VIGS on the vegetative parts and inflorescences of eschscholzia plants. (A) non-treated plant, (B) treated plant showing strong silencing leading to necrosis in several leaves (red arrows). However, this plant escapes silencing by producing newly expanding, non silenced leaves. (C) Unbleached leaf (red arrow) emerging from a fully silenced shoot. (D) Untreated leaf. (E) Partially silenced leaf blade emerging from a partially photobleached petiole. Silenced and unsilenced tissues are separated by a sharp boundary along the middle of the petiole and blade (red arrow) running parallel to the vascular bundles. (F) Partially silenced leaf blade showing a pattern of photobleaching that follows the vascular system. (G) Completely white (photobleached) leaf showing only small patches of pink anthocyanin pigments. (H) Eschscholzia inflorescence exhibiting complete photobleaching in the shoot, leaves, axillary buds and sepals of the terminal flower. Patches of anthocyanin pigmentation as in (H) are also visible in this picture. Ab, Axillary bud; L, leaf; Tb, terminal bud.
Several additional millilitres of agrobacterium suspension were used to inoculate the shoot apical meristem and the surrounding leaves of older plants in infection strategy (c). Ninety-two per cent of 100 plants infected using this strategy showed photobleaching after 12 ± 3 d. The photobleaching phenotype spread rapidly through the plant, starting with newly formed leaves (Fig. 2B). The photobleaching effect reached the shoot tip after several days. The speed by which the silencing spread throughout the plant was strongly enhanced once the shoot appeared to be affected. In some cases, full photobleaching of leaves and sepals, leaving anthocyanins in a few apical patches visible, could be observed (Fig. 2G, H).
The data presented in the following paragraphs are based on the experimental set-up of strategies (b) and (c).
Reduced eschscholzia petal coloration as a result of EcPDS VIGS
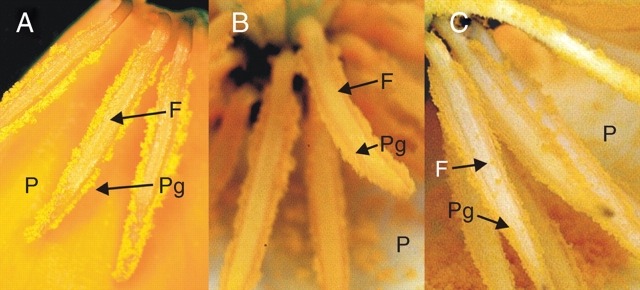
One aim of this study was to test the applicability of VIGS for functional studies of flower development genes. Therefore the EcPDS silencing effect on flowers was recorded in detail. First, the occurring variations in phenotype were classified according to its strength as visible in petal decoloration on the adaxial side: (a) strong lack of carotenoids (loc): all petals completely white or with a narrow orange rim at the posterior end, or three white petals and one petal with <50 % striped area (Fig. 3B and C); (b) weak loc: three fully coloured petals, or petals with yellow stripes, or orange petals with only patchy decoloration; (c) intermediate loc: phenotypes observed intermediate to strong and weak, e.g. two-coloured petals and two showing white stripes or four striped petals (Fig. 3D–F). Remarkably, the abaxial side of the petals always showed a stronger silencing effect than the adaxial side. The sepals, gynoecia and, subsequently, the fruits (Fig. 3H) showed a photobleaching phenotype that was comparable to the classification based on petal discoloration. However, fully white pollen grains were never observed, even though California poppy pollen pigments are derivatives of the carotenoid biosynthesis pathway (Wakelin et al., 2003). Pollen grains sometimes showed weakly reduced pigment amount (Fig. 4B) but the amount of pollen pigments appeared not to be correlated to the loc in the petals. Even when the petals showed strong loc, pollen grains mostly looked like wild type (Fig. 4C).
Fig. 3.
Effect of virus-induced gene silencing of EcPDS on flower and fruit coloration. (A) top view of a flower of an untreated plant at anthesis. (B) and (C) show two flowers with strong silencing phenotype characterized by the absence of petal coloration (B, top view; C, side view); (C) also shows a partially photobleached sepal. In (D–F) three different variations of medium silencing phenotypes are documented: while (F) shows a homogenous distribution of white stripes throughout the petals, the petals in (D) and (E) are heavily segmented showing different degrees of silencing in one flower. (G) and (H) show mature fruits of eschscholzia before desiccation (length approx. 9 cm). The silenced fruit (H) differs from the fruit of an untreated plant (G) only in its photobleached appearance.
Fig. 4.
View of the androecium of untreated and TRV1 plus TRV2-EcPDS-treated plants. (A) Part of an untreated eschscholzia flower at anthesis showing petals with several stamens attached. Anthers and pollen grains are bright orange. (B) Close-up of the anthers and gynoeceum of an EcPDS-silenced flower whose petals show a strong discoloration indicating strong EcPDS silencing; however, pollen grains and anther filaments are fully coloured. (C) Close-up of anthers and gynoecium of a flower showing medium EcPDS silencing effects in the petals: the usual orange coloration of the pollen grains has been reduced to a pale yellow and the anthers lack any coloration. F, Filament; P, adaxial side of the petal; Pg, pollen grains
The visible effects of EcPDS VIGS were recorded in 95 flowering plants from the day of infection until the plants were removed from the greenhouse, approx. 2 months after their infection. These poppy plants produced a total of 550 flowers. 60 % of all flowers showing a loc phenotype. Of the total number of flowers, 8 % showed strong loc, 38 % intermediate loc and 14 % weak loc. All flowers were hand-pollinated so that the VIGS effects could be followed in the next generation. Flowers showing strong loc produced completely white gynoecia which continued to grow into mature fruits, and produced a seed set comparable to non-infected plants. These photobleached fruits (Fig. 3H) produced seeds which were undistinguishable from seeds of non-treated plants in shape and coloration.
Sixty seeds collected from EcPDS-silenced white fruits were grown under sterile conditions on B5 medium and 50 % of these seeds germinated. For seeds from untreated fruits a germination rate of 61·7 % was obtained.
Additionally, 60 seeds were grown in sterile conditions on B5 medium supplemented with sucrose. Using this approach, it was possible to grow strongly photobleached seedlings unable to actively maintain photosynthesis. However, none of the germinating seedlings (germination rate of 55 %) from EcPDS-silenced fruits showed any signs of photobleaching suggesting that VIGS does not affect the next generation. Of the seeds from untreated plants, 56·7 % (n = 60) germinated on B5 medium supplemented with sucrose. These results suggest that VIGS does not have a significant effect on the seed germination rate and that eschscholzia does not transfer VIGS to the next generation.
Virus-induced gene silencing reduces the transcript level of EcPDS
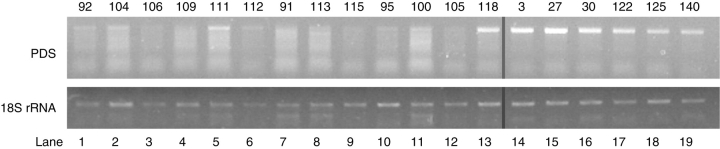
An investigation was carried out to see if individual plants showing leaf photobleaching also had reduced levels of the EcPDS transcript. Figure 5 shows amplification of an endogenous EcPDS product from 13 plants that showed symptoms after inoculation with pTRV2-EcPDS, in comparison to six control plants that were infected (inoculated with pTRV2-E and untreated). In 11 of the 13 treated plants, expression levels were severely reduced or undetectable while the untreated control plants show high EcPDS transcript levels, confirming that photobleaching is due to down-regulation of EcPDS transcript. Leaves from individuals with full photobleaching of emerging leaves (lanes 1–6 in Fig. 5) and with partial photobleaching (lanes 7–13 in Fig. 5) did not correlate with consistent differences in the degree of silencing.
Fig. 5.
Effects of virus-induced gene silencing of EcPDS on EcPDS transcript abundance. Total RNA was isolated from leaves of vegetative plants showing symptoms of silencing (lanes 1–13), from plants infected only with the pTRV2-E vector (lanes 14–16), and from untreated plants (lanes 17–19), and reverse transcribed as described in the experimental procedures. The abundance of endogenous EcPDS transcripts was visualized after 40 cycles of RT-PCR with EcPDS-specific primers (top). As a control, RT-PCR samples were carried out with primers specific to 18S ribosomal RNA (26 cycles, bottom). Numbers above the gel photos denote individual plants. Lanes 1–6 are from plants with complete photobleaching of emerging leaves, whereas lanes 7–13 are from plants with partial photobleaching.
In two plants (111 and 118, in lanes 5 and 13 of Fig. 5), expression of EcPDS was similar to untreated plants.
Time course of the observed silencing effects in vegetative plant parts and flowers
The first effects of the silencing became visible around 7 d after agrobacterium inoculation as discoloration of lamina tissue of newly emerging leaves (Fig. 6A). A few days later, some leaves showed complete discoloration (Fig. 6B). In most plants, EcPDS silencing remained easily detectable throughout subsequent developmental stages. Colourless, white tissue occurred to variable degrees in both vegetative and flowering stages, and could comprise entire leaves or parts of them, entire shoots above a certain node, or only certain leaves, and flower pedicels and flowers or sectors of these (Figs 2 and 3). While the EcPDS silencing response did not seem to move below the node of leaf infection, many plants showed complete discoloration of all newly emerging leaves. In some cases, the silencing response disappeared so that normal green leaves developed again above colourless leaves (Fig. 6C). Figure 6 shows an individual in which silencing symptoms disappeared completely during rosette development, and reappeared at flowering stage.
Fig. 6.

Example of progression of EcPDS silencing symptoms over time in an individual plant. One week after infection (A), first decoloration is perceptible in unexpanded leaves (arrows). After two weeks (B), strong decoloration affects leaves at 3 to 4 nodes, while older and younger leaves are green. Four weeks (C) and 6 weeks (D) post-infection, newly expanding leaves remain green, while symptoms progressively disappear from the affected leaves (arrows). After 16 weeks (E), symptoms have disappeared. Upon flowering after 20 weeks (F), photobleaching resumes again in leaves and elongated stems.
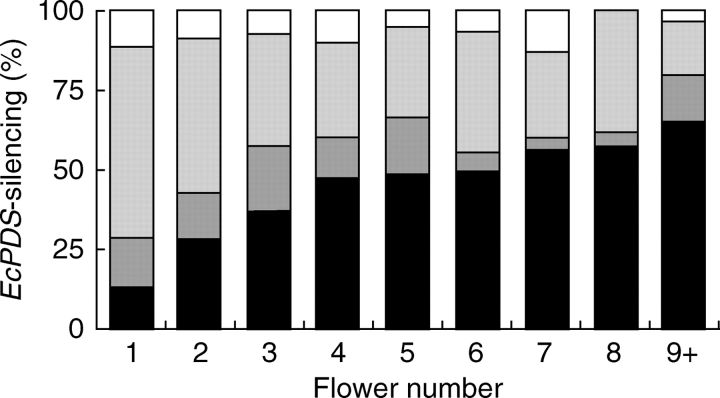
Interest then moved to the effect flower position has on VIGS and when the silencing effect starts to fade. Thus the degree of loc in petals of all flowers from infected plants was recorded (Fig. 7, and Table S2 in Supplementary material available online). The highest rate of gene silencing success was found in the petals of the first flower (n = 95) of all infected plants. Only about 14 % of all flowers did not show any loc, around 15 % showed weak loc, 60 % showed intermediate loc and almost 12 % showed very strong loc. The percentage of flowers not showing any signs of VIGS increases from about 14 % in first emerging flowers to almost 58 % in the latest scored flowers, indicating a strong decrease of VIGS in later emerging flowers. A weak silencing effect was also noted to diminish in later emerging flowers. This effect was apparent in 15 % of the first flower to emerge on inoculated plants, but in only approx. 4 % of the eight flowers to emerge on the same plant. Approximately double the number of first flowers showed intermediate loc in comparison to the seventh or eighth flowers. Only the very strong silencing appears to remain between 5 % (fifth flowers) and about 13 % (seventh flowers) without a tendency to decrease in later emerging flowers, except for flower number 8 and later, for example, in none of the eighth flowers (n = 26) could strong silencing of the petals be detected. Taken together it can be concluded from these observations that the chances of successful silencing is highest in the first flower and the probablility of flowers not showing any silencing effects increases with flower number.
Fig. 7.
Percentage of the different EcPDS-silencing degrees in petals according to flower number of TRV1 and TRV2-EcPDS-treated eschscholzia plants. White, Strong lack of carotenoids (loc) in petals; light grey, intermediate loc in petals; dark grey, weak loc in petals; black, no observed silencing.
DISCUSSION
It was demonstrated in this study that VIGS via the TRV system is a fast and highly effective tool for gene down-regulation in eschscholzia. Silencing effects were visible <2 weeks post-infiltration, and a significant proportion of plants exhibited strong silencing of entire shoots and inflorescences.
Advantages and limitations of VIGS
The PDS gene was chosen to test the ability of the system to induce gene silencing because it allows the detection of symptoms both in green organs such as stems, leaves, pedicels, sepals and gynoecia, and in petals and stamens that accumulate carotenoids in this species.
Hileman et al. (2005) demonstrated that a TRV-based VIGS system is also able to induce gene silencing in another member of Papaveraceae, Papaver somniferum. Possibly, other Papaveraceae and Ranunculales will be susceptible as well, allowing comparative studies of effects of gene silencing in this family/order in the future. VIGS in Papaver, however, requires vacuum infiltration to elicit a response. This involves temporary removal of seedlings from the substrate, which is likely to result in perturbance of shoot development that can hinder developmental studies. It might also lower the survival rate and appears to delay the onset of photobleaching compared with eschscholzia.
Another advantage is, as compared with VIGS in tomato and tobacco, that TRV infection in eschscholzia appears to perturb morphological development much less. Therefore, the study of morphological effects of silenced genes, particularly in leaves and flowers, will be more feasible in eschscholzia (Burch-Smith et al., 2004).
Phenotypes for different genes besides PDS in several species have been observed already, e.g. for Prohibitins in petunia (Chen et al., 2005), rbcS in chilli pepper (Chung et al., 2004), defence genes in Nicotiana attenuata (Saedler and Baldwin, 2004), resistance genes in arabidopsis (Cai et al., 2006) and used for functional gene characterization. Interestingly, developmental phenotypes have not been described in the literature so far. However, the severity and penetrance of the present phenotypes suggests that functional developmental studies involving leaf, inflorescence and shoot development are possible when VIGS is employed in eschscholzia.
The photobleaching phenotype conferred by post-transcriptional silencing of the EcPDS gene in eschscholzia is capable of validating silencing only in rather mature tissues in which chloroplasts or chromoplasts have developed. At this point it remains uncertain whether the silencing response extends to meristematic tissues of the shoot apical meristem and of leaf primordia or floral primordia, where many important developmental regulators are expressed. In tobacco, TRV has been shown to be effective in silencing meristem-specific genes such as NFL, a tobacco homologue of FLORICAULA, and DEFICIENS, a floral MADS-box gene (Ratcliff et al., 2001; Liu et al., 2004). In this study a significant proportion of eschscholzia plants was seen developing, at some point, photobleaching of the entire shoot above a certain node, including all emerging leaves and flowers. This indicates a full systemic response that possibly includes the shoot apical meristem. Subsequent experiments using meristem-expressed genes will be necessary to demonstrate silencing in meristems of eschscholzia.
As in other species, partial silencing in which photobleaching was constricted to specific parts of the shoot system was frequently observed (Fig. 1). Interestingly, white (photobleached) and green tissue were sometimes separated by a sharp boundary that ran parallel to vascular bundles in elongated stems and petioles. This indicates that virus propagation and/or systemic silencing response occurs mainly along the vascular system and may be blocked where lateral vein anastomoses are rare or lacking, such as in intercalary elongating organs (leaf petioles, flower pedicels).
Partial silencing, because it occurs in an unpredictable fashion, can impair the use of VIGS in the analysis of genes with less obvious knockout phenotypes or in genes whose products act non-cell-autonomously. This problem can be addressed by increasing the sample size to include enough individuals with whole-shoot silencing. Another possibility is to use TRV2 constructs that include an easily detectable silencing marker, such as PDS, along with the gene of interest. However, additional strong silencing of, for example, PDS might decrease the overall fitness of the plant up to a level where the plant eventually dies off from the lack of carotenoids. Nevertheless, in some cases the observed sharp boundaries between silenced and wild-type tissues may provide an advantageous system to study knock-out phenotypes next to the wild-type condition, comparable with a chimeric condition.
By designing the TRV2 insert to conserved gene regions of paralogous genes of the host plant, it should be possible to simultaneously down-regulate redundantly acting genes, but this requires enough genomic information or previous hybridization experiments. The lack of genomic information can also lead to unwanted silencing of unknown targets, for example, while working with members of a gene family. A control experiment using a different insert from the same target gene would exclude artefacts produced by simultaneous silencing genes different from the target gene. Another interesting possibility would be the simultaneous knockout of two genes unrelated in sequences by cloning two corresponding gene fragments into one TRV2 vector. For intensive and continuous silencing, 150–500 nucleotide large inserts are routinely used and these are genetically stable in the virus vector (Lu et al., 2003). However, up to 690 nucleotides have been used successfully with the TRV vector system (Liu et al., 2002).
In eschscholzia, VIGS can provide a rapid approach to the assessment of gene function, as it circumvents the need to establish transgenic lines that requires tissue culture, somatic embryogenesis, and a time-consuming whole-plant regeneration step. Another advantage above a transformation approach is that specific developmental stages can be targeted by appropriate timing of infection, and that embryo lethal knock outs can be studied postembryonically.
Since no isogenic lines are available, variability is substantial in eschscholzia, for example in leaf morphology (A. Scholz and S. Gleissberg, unpubl. res.), growth habit, floral organ number, inflorescence formation, diameter of buds during flower development, etc. (Becker et al., 2005). Moreover, incomplete reduction of target gene transcript levels may, for example, in the case of transcription factors, still allow gene function to be maintained, resulting in a lack of phenotypes. As a result, subtle phenotypes may remain undetected.
As some genes require an almost complete knock-down of expression to show a phenotype, the percentage of infected plants showing a silencing phenotype might be lower than 10 %, e.g. only 8 % of all flowers showed a strong lack of carotenoids in the present experiments. These numbers correspond to the percentage of floral phenotypes if a transcription factor regulating floral development is silenced by VIGS (S. Wege and A. Becker, unpubl. res.). However, infecting larger numbers of plants will circumvent the problem of low phenotypic penetrance.
Even though the wild-type TRV is transmitted into subsequent generations in up to 40 % of the cases in, for example, spinach, beet, potato and tobacco, the TRV vectors are not (Robertson, 2004). In this work, it is shown that the effects of VIGS are also not heritable in eschscholzia. Further studies using the TRV vector system are therefore confined to individual plants which may display widely varying degrees of silencing even in different parts of a single plant. This complicates the correlation of symptoms with expression levels. Particularly for developmental regulators, interrelation of expression level and phenotype is challenging due to late occurrence of phenotypes and the destructive nature of expression studies. However, the failure of TRV vector transmission into subsequent generations reduces bio safety-related risks.
Viral infection might also modify the miRNA production of the plant. The DICER-LIKE1 (DCL1) protein in arabidopsis is the only one that processes microRNAs. However, upon viral infection, DCL1 switches to cleave viral dsRNAs instead of producing miRNA. This might lead to dramatic changes in post-transcriptional processing of miRNA-regulated genes, as temporal and spatial misexpression will occur (Bouché et al., 2006). However, as TRV infection of eschscholzia does not result in morphological changes, this problem does not seem to be relevant in this species. Possibly, the eschscholzia genome encodes for several DCL1-like proteins without overlapping functions as for DCL1 of arabidopsis. For plant species exhibiting morphological differences between untreated plants and individuals treated with pTRV1 and pTRV2-E, the overall decrease in miRNAs might help to explain at least some of the differences. Phenotypes attributed to VIGS might be more difficult to interpret if the target genes are indirectly regulated by VIGS and some general morphological differences unrelated to the target gene between wild-type and VIGS-infected plants could be attributed to an overall decrease of miRNAs.
Despite these limitations, TRV-mediated gene silencing is a very promising approach to functional analyses in eschscholzia. As the California poppy has already become the focus of evolutionary developmental studies in the basal eudicots, the present findings open new ways for functional analyses of developmental control genes, enhancing the significance of this emerging model system in the Ranunculales.
SUPPLEMENTARY MATERIAL
Supplementary material is available online at http://aob.oxfordjournals.org/ and provides leaf segment numbers in TRV-treated and untreated plants, and observed EcPDS silencing effects in eschscholzia petals according to flower number of TRV1- and TRV2-EcPDS-treated plants.
ACKNOWLEDGEMENTS
We are indebted to Dinesh Kumar, Yale University, New Haven, CT, USA, for providing the base set of TRV vectors used in this study. This study was supported in parts by DFG grants (GL 213/3-1 to S.G. and BE 2547/6-1 to A.B.). We thank Sandra S. Meyer, Mainz, and Sabrina Lange, Bremen, for experimental help and Gisela Krüger for professional photography.
LITERATURE CITED
- Albert VA, Soltis DE, Carlson JE, Germerie WG, Wall PK, Ilut DC, et al. Floral gene resources from basal angiosperms for comparative genomics research. BMC Plant Biology. 2005;5:5. doi: 10.1186/1471-2229-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APGII. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- Becker A, Gleissberg S, Smyth DR. Floral and vegetative morphogenesis in California poppy (Eschscholzia californica Cham.) International Journal of Plant Sciences. 2005;166:537–555. [Google Scholar]
- Benedito VA, Visser PB, Angenent GC, Kerns FA. The potential of virus-induced gene silencing for speeding up functional characterization of plant genes. Genetics and Molecular Research. 2004;3:323–341. [PubMed] [Google Scholar]
- Bouché N, Lauressergues D, Gasciolli V, Vaucheret H. An antagonistic function for Arabidopsis DCL2 in development and a new function for DCL4 in generating viral siRNAs. EMBO Journal. 2006;25:3347–3356. doi: 10.1038/sj.emboj.7601217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Anderson JC, Martin GB, Dinesh-Kumar SP. Applications and advantages of virus-induced gene silencing for gene function studies in plants. The Plant Journal. 2004;39:734–746. doi: 10.1111/j.1365-313X.2004.02158.x. [DOI] [PubMed] [Google Scholar]
- Busch A, Gleissberg S. EcFLO, a FLORICAULA-like gene from Eschscholzia californica is expressed during organogenesis at the vegetative shoot apex. Planta. 2003;217:841–848. doi: 10.1007/s00425-003-1046-z. [DOI] [PubMed] [Google Scholar]
- Cai X-Z, Xu Q-F, Wang C-C, Zheng Z. Development of a virus-induced gene-silencing system for functional analysis of the RPS2-dependent resistance signalling pathways in Arabidopsis. Plant Molecular Biology. 2006;62:223–232. doi: 10.1007/s11103-006-9016-z. [DOI] [PubMed] [Google Scholar]
- Carlson JE, Leebens-Mack JH, Wall PK, Zahn LM, Mueller LA, Landherr LL, et al. EST database for early flower development in California poppy (Eschscholzia californica Cham., Papaveraceae) tags over 6000 genes from a basal eudicot. Plant Molecular Biology. 2006;62:351–369. doi: 10.1007/s11103-006-9025-y. [DOI] [PubMed] [Google Scholar]
- Chen J-C, Jiang C-Z, Reid MS. Silencing a prohibitin alters plant development and senescence. The Plant Journal. 2005;44:16–24. doi: 10.1111/j.1365-313X.2005.02505.x. [DOI] [PubMed] [Google Scholar]
- Cho HT, Cosgrove DJ. Altered expression of expansin modulates leaf growth and pedicel abscission in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the USA. 2000;97:9783–9788. doi: 10.1073/pnas.160276997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Seong E, Kim Y-C, Chung EJ, Oh S-K, Lee S, et al. A method of high frequency virus-induced gene silencing in chili pepper (Capsicum annuum L. cv. Bukang) Molecules and Cells. 2004;17:377–380. [PubMed] [Google Scholar]
- Fray RG, Grierson D. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Molecular Biology. 1993;22:589–602. doi: 10.1007/BF00047400. [DOI] [PubMed] [Google Scholar]
- Gleissberg S. Comparative analysis of leaf shape development in Eschscholzia californica and other Papaveraceae–Eschscholzioideae. American Journal of Botany. 2004;91:306–312. doi: 10.3732/ajb.91.3.306. [DOI] [PubMed] [Google Scholar]
- Groot EP, Sinha N, Gleissberg S. Expression patterns of STM-like KNOX and histone H4 genes in shoot development of the dissected-leaved basal eudicot plants Chelidonium majus and Eschscholzia californica (Papaveraceae) Plant Molecular Biology. 2005;58:317–331. doi: 10.1007/s11103-005-4548-1. [DOI] [PubMed] [Google Scholar]
- Hileman LC, Drea S, de Martino G, Litt A, Irish VF. Virus-induced gene silencing is an effective tool for assaying gene function in the basal eudicot species Papaver somniferum (opium poppy) The Plant Journal. 2005;44:334–341. doi: 10.1111/j.1365-313X.2005.02520.x. [DOI] [PubMed] [Google Scholar]
- Kölsch A, Gleissberg S. Diversification of CYCLOIDEA-like TCP genes in the basal eudicot families Fumariaceae and Papaveraceae s.str. Plant Biology. 2006;8:680–687. doi: 10.1055/s-2006-924286. [DOI] [PubMed] [Google Scholar]
- Liu YL, Schiff M, Dinesh-Kumar SP. Virus-induced gene silencing in tomato. The Plant Journal. 2002;31:777–786. doi: 10.1046/j.1365-313x.2002.01394.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nakayama N, Schiff M, Litt A, Irish VF, Dinesh-Kumar SP. Virus induced gene silencing of a DEFICIENS ortholog in Nicotiana benthamiana. Plant Molecular Biology. 2004;54:701–711. doi: 10.1023/B:PLAN.0000040899.53378.83. [DOI] [PubMed] [Google Scholar]
- Lu R, Martin-Hernandez AM, Peart JM, Malcuit I, Baulcombe DC. Virus-induced gene silencing in plants. Methods. 2003;30:296–303. doi: 10.1016/s1046-2023(03)00037-9. [DOI] [PubMed] [Google Scholar]
- Park S-U, Facchini PJ. Agrobacterium-mediated genetic transformation of California poppy, Eschscholzia californica Cham., via somatic embryogenesis. Plant Cell Reports. 2000;19:1006–1012. doi: 10.1007/s002990000213. [DOI] [PubMed] [Google Scholar]
- Qiu YL, Lee Y, Bernasconi-Quadroni F, Soltis DE, Soltis PS, Zanis M, et al. Phylogeny of basal angiosperms: analyses of five genes from three genomes. International Journal of Plant Sciences. 2000;161:S3–S27. [Google Scholar]
- Ratcliff F, Martin-Hernandez AM, Baulcombe DC. Technical advance: tobacco rattle virus as a vector for analysis of gene function by silencing. The Plant Journal. 2001;25:237–245. doi: 10.1046/j.0960-7412.2000.00942.x. [DOI] [PubMed] [Google Scholar]
- Robertson D. VIGS vectors for gene silencing: many targets, many tools. Annual Review of Plant Biology. 2004;55:495–519. doi: 10.1146/annurev.arplant.55.031903.141803. [DOI] [PubMed] [Google Scholar]
- Saedler R, Baldwin IT. Virus-induced gene silencing of jasmonate-induced direct defences, nicotine and trypsin proteinase-inhibitors in Nicotiana attenuata. Journal of Experimental Botany. 2004;55:151–157. doi: 10.1093/jxb/erh004. [DOI] [PubMed] [Google Scholar]
- Wakelin AM, Lister CE, Conner AJ. Inheritance and biochemistry of pollen pigmentation in California poppy (Eschscholzia californica Cham.) International Journal of Plant Sciences. 2003;164:867–875. [Google Scholar]
- Winter KU, Saedler H, Theissen G. On the origin of class B floral homeotic genes: functional substitution dominant inhibition in Arabidopsis by expression of an orthologue from the gymnosperm Gnetum. The Plant Journal. 2002;31:457–475. doi: 10.1046/j.1365-313x.2002.01375.x. [DOI] [PubMed] [Google Scholar]
- Zahn LM, Leebens-Mack JH, Arrington JM, Hu Y, Landherr LL, dePamphilis CW, et al. Conservation and divergence in the AGAMOUS subfamily of MADS-box genes: evidence of independent sub- and neofunctionalization events. Evolution and Development. 2006;8:30–45. doi: 10.1111/j.1525-142X.2006.05073.x. [DOI] [PubMed] [Google Scholar]
- Zhang P, Tan HT, Pwee KH, Kumar PP. Conservation of class C function of floral organ development during 300 million years of evolution from gymnosperms to angiosperms. The Plant Journal. 2004;37:566–577. doi: 10.1046/j.1365-313x.2003.01983.x. [DOI] [PubMed] [Google Scholar]