Abstract
Background and Aims
In kiwifruit (Actinidia), the number of nodes per shoot is highly variable and is influenced by genotype and environmental conditions. To understand this developmental plasticity, three key processes were studied: organogenesis by the shoot apical meristem during shoot growth; expansion of phytomers; and shoot tip abortion.
Methods
Studies were made of organogenesis and shoot tip abortion using light and scanning electron microscopy. The effect of temperature on shoot growth cessation was investigated using temperature indices over the budbreak period, and patterns of shoot tip abortion were quantified using stochastic modelling.
Key Results
All growing buds began organogenesis before budbreak. During shoot development, the number of phytomers initiated by the shoot apical meristem is correlated with the number of expanding phytomers and the mean internode length. Shoot tip abortion is preceded by growth cessation and is not brought about by the death of the shoot apical meristem, but occurs by tissue necrosis in the sub-apical zone. For most genotypes studied, the probability of shoot tip abortion is higher during expansion of the preformed part of the shoot. Lower temperatures during early growth result in a higher probability of shoot tip abortion.
Conclusions
Organogenesis and shoot tip abortion are controlled independently. All buds have the potential to become long shoots. Conditions that increase early growth rate postpone shoot tip abortion.
Key words: Actinidia, kiwifruit, shoot fate, neoformation, organogenesis, shoot tip abortion, developmental plasticity, temperature
INTRODUCTION
The shoots of vascular plants are comprosed of phytomers – repeating units of leaf, axillary meristem, node and internode – that are initiated by the shoot apical meristem (SAM). The enormous diversity of plant form originates from various combinations of a limited number of developmental events that occur within each of these components (Steeves and Sussex, 1989). Architectural models of plant growth predict how a specific set of growth parameters give rise to a characteristic form (Hallé et al., 1978). While most plant form is genetically determined, environmental factors also influence plant architecture. Developmental plasticity is the ability of an organisim of a given genotype to change phenotype and has adaptive significance to plants, which are immobile by nature (Bradshaw, 1965). A high degree of developmental plasticity enables plants to colonize disturbed areas or new environmental niches or rapidly adapt to climatic change or abiotic stress (Sultan, 2000).
One factor that influences developmental plasticity is the ability to exhibit variable growth patterns from season to season. Most woody perennials undergo a rhythmic periodicity of shoot growth, in which phytomers are initiated but do not fully expand and mature until after a dormancy period (Hallé et al., 1978; Puntieri et al., 1998; Sabatier and Barthélémy, 1999). This is termed preformation and allows for a rapid flush of growth, generally in spring (Puntieri et al., 1998, 2000; Sabatier et al., 1998). In some species, additional phytomers are also initiated that expand without a dormancy period (Hallé et al., 1978; Barthélémy and Caraglio, 2007). This is called neoformation, a process which enables plants to respond to favourable growing conditions, and can permit essentially indeterminate seasonal growth (Remphrey and Steeves, 1984). Both the occurrence and extent of neoformation can vary even within the same shoot system (Puntieri et al., 2000; Souza et al., 2000; Gordon et al., 2006). The variability in neoformation may be due to plastic responses to local conditions at the time of shoot extension (Remphrey and Powell, 1984; Gordon et al., 2006; Guédon et al., 2006).
Kiwifruit (Actinidia) is a perennial vine of horticultural importance and a subject of architectural interest (Ferguson, 1984; Warrington and Weston, 1990; Snowball, 1995; Snowball, 1997a; Seleznyova et al., 2002). In kiwifruit, growth and flowering occur in a 2-year cycle, with the current season's shoots originating from axillary meristems on the previous season's growth (Snowball, 1997b) (Fig. 1). In the summer of the first year, axillary meristems initiate a set number of ‘preformed’ phytomers prior to winter dormancy. During spring budbreak of the second season, the active bud begins to swell and develops into an open cluster containing a few leaves (Brundell, 1975). In many shoots, growth cessation occurs soon after this time, followed by the abortion of the shoot tip. Other shoots continue to grow and do not terminate until late in the season, resulting in a final number of phytomers that exceeds the number of preformed phytomers present during budbreak (Snowball, 1997a, b). This indicates that initiation of new phytomers during the current growing season or ‘neoformation’ must occur in some shoots.
Fig. 1.
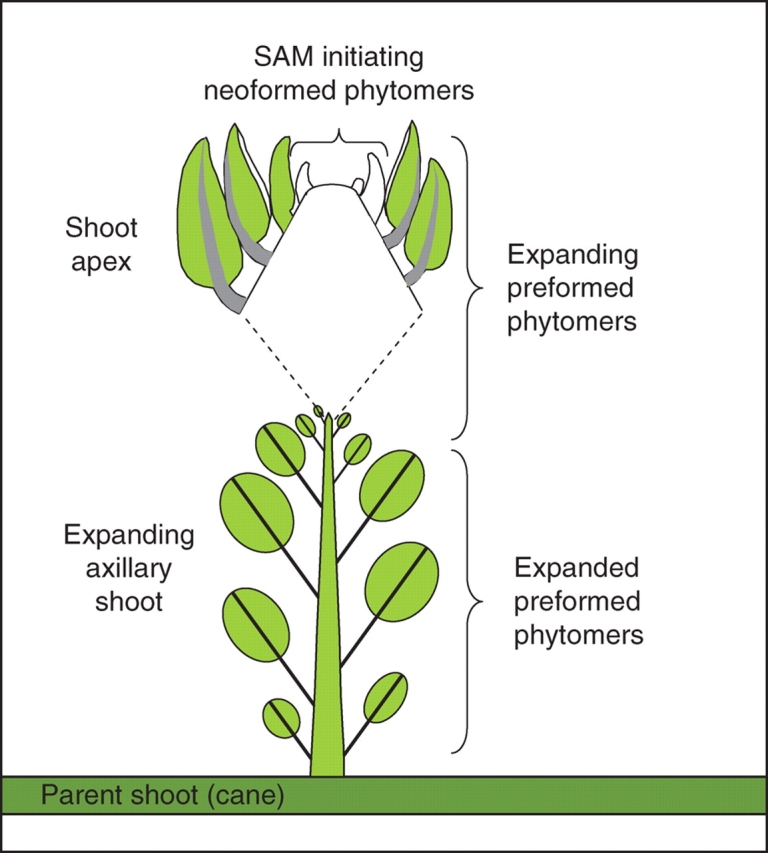
Schematic illustrating shoot organization in Actinidia. In kiwifruit, growth and flowering occur in a 2-year cycle, with the current season's shoots originating from axillary meristems on the previous season's growth (Snowball, 1997b). In the first year, axillary meristems on the parent shoot (cane) initiate a set number of ‘preformed’ phytomers prior to winter dormancy. During the second season, these preformed phytomers expand, and the shoot apical meristem (SAM) initiates additional ‘neoformed’ phytomers that may or may not expand within the current season.
Actinidia species differ significantly in the length and number of nodes per shoot, suggesting that there is a considerable genotype effect on shoot growth (Snowball, 1997b). Controlled environment data have demonstrated a strong effect of temperature on shoot growth (Seleznyova and Halligan, 2006). Actinidia species differ in timing of budbreak (Snowball, 1997b) and are therefore subject to different temperatures during early shoot development. Hence the differences in shoot growth between species could be partly a result of temperature effects. Manipulations such as removal of neighbouring shoots result in a higher proportion of long shoots (Clearwater et al., 2006). Together, these data indicate that genotype, environmental conditions, and competition between shoots affect patterns of shoot growth cessation in kiwifruit. At an individual level, shoot growth appears to be affected by counteracting processes. Initiation and expansion of neoformed phytomers set a potential for indeterminate growth, while growth cessation and shoot tip abortion might occur even before the expansion of all preformed phytomers present in the bud (Snowball, 1997b; Seleznyova et al., 2002). The interplay between these processes dictates final shoot fate; shoots can be short, stopping growth soon after budbreak, or long, continuing growth until the end of the season.
The objective of this study was to understand better the nature of this developmental plasticity and, specifically, to answer the following questions. Do all shoots begin organogenesis in spring? What are the first signs of shoot tip abortion and where do they occur? Does the temperature during early budbreak affect the determination of shoot fate? Light and scanning electron microscopy was used to study organogenesis and shoot tip abortion, temperature indices to investigate the possible effects of temperature on shoot fate, and stochastic modelling to quantify patterns of shoot growth cessation.
MATERIALS AND METHODS
Examination of bud and shoot development in Actinidia deliciosa ‘Hayward’ and Actinidia chinensis ‘Hort16A’
Buds and shoots were collected from ‘Hayward’ and ‘Hort16A’ cultivars grown at Massey University Research Orchard at Palmerston North, New Zealand (40°21·3′S, 175°36·7′E). Mature vines were managed by standard orchard practices and trained on T-bars (Warrington and Weston, 1990). In 2004, between 10 and 15 buds/shoots were collected weekly from late winter, 3 August, until late spring, 1 November (‘Hort16A’) or 9 November (‘Hayward’). Based on data from 2004, it was decided to repeat this analysis over a longer duration and with larger weekly sample sizes. In 2005, between 20 and 25 buds/shoots were collected weekly from midwinter, 9 June (‘Hort16A’) or 13 June (‘Hayward’), until early summer, 23 December. Buds and/or shoots were sampled at random from the middle sections of long parent shoots. Dormant buds were removed from the parent shoot (cane) with a razor blade to ensure that all the bud scales and leaf primordia were included. Dormant buds were fixed in 3 % glutaraldehyde and 2 % paraformaldehyde in 0·1 m phosphate buffer, dehydrated in acetone, critical-point dried in liquid CO2, and then dissected under a stereomicroscope (Leica, Wetzlar, Germany) to count leaf primordia number. Once shoots had begun to expand, fresh material was dissected under a stereomicroscope to ensure an accurate count of leaf primordia. For each sample, the number of bud scales, expanded leaves (>5 mm), leaf primordia (≤5 mm) and the total shoot length were recorded. Bud scales were differentiated from true leaves based on overall morphology and density of hairs (Brundell, 1975). Stage of budbreak (Brundell, 1975) was recorded for each bud sample.
Scanning electron and light microscopy
In some shoots, leaf appearance and expansion ceases shortly after expansion of the initial cluster (Fig. 2A). These shoots are macroscopically identifiable by the large differential in leaf size between the youngest fully expanded leaf and the next youngest leaf, which does not expand (Fig. 2B). In the non-expanding shoots, the entire apex will eventually wither and detach from the shoot. In contrast, in expanding shoots, leaves and internodes appear and expand at a constant rate, creating a gradient of leaf and internode sizes along the shoot axis (Fig. 2C, D). The SAMs of expanding and non-expanding ‘Hayward’ shoots were compared at three time points in 2004 (20 October, 1 November and 11 November) to determine if shoot tip abortion was associated with inactivity or death of the SAM.
Fig. 2.
Non-expanding (A) and expanding (D) ‘Hayward’ shoots become morphologically distinct shortly after budbreak. In non-expanding shoots, there is a large size differential between the youngest expanded leaf and the next youngest leaf (B), which will not expand. In expanding shoots, there is a gradient of leaf sizes at the shoot apex (C).
Shoot apices were dissected as described above, then examined by scanning electron microscopy using a modified replica technique (Sylvester et al., 1990). Specimens were sputter-coated with 25 nm gold (Bal-Tec, Blazers, Liechtenstein) and examined on a Cambridge 250 Mark III scanning electron microscope (Cambridge Instruments, Cambridge, UK). For light microscopy, apices were fixed in FAA (50 % EtOH, 3·7 % formaldehyde, 5 % glacial acetic acid) overnight, dehydrated, and embedded in paraffin. Samples were sectioned at 10 µm, stained with 0·05 % Toluidine Blue, and photographed with a 35-mm camera attached to an Axioplan microscope (Zeiss, Jena, Germany).
Data analysis
Distributions of total leaf number (expanding leaves and primordia) per shoot were calculated for each collection date. Because budbreak did not occur simultaneously across a population, total leaf number was examined as a function of bud developmental stage (Brundell, 1975). Bud stages, sample size and collection dates for this analysis were as follows: dormant (D), n = 11, 3 August 2004; advanced budbreak (ABB), n = 28, pooled from 10, 28 and 29 August 2004; advanced budbreak plus (ABB+), n = 27, pooled from 10, 28 and 29 August 2004; open cluster (OC), n = 14, 14 September 2004; advanced open cluster (AOC), n = 7, 14 September 2004; advanced open cluster plus (AOC+), n = 1, 14 September 2004.
To establish the relationship between internode elongation and leaf production, shoot length and mean internode length of shoots were regressed against the number of expanded leaves, the number of leaf primordia and the total number of leaves (expanding leaves plus primordia). On sampling dates when expanding and non-expanding shoots could clearly be distinguished (Fig. 2), total shoot length and mean internode length were compared for these two shoot types.
Genotype studies
Patterns of shoot tip abortion were compared in different genotypes to see if the differences in the proportion of terminated shoots in different genotypes were correlated with the differences in temperature during budbreak and early shoot development. Two vines from each of the nine Actinidia species studied (A. purpurea, A. arguta, A. deliciosa, A. chinensis, A. latifolia, A. eriantha, A. guilinensis, A. polygama and A. indochinensis) were randomly selected in July 2004 (winter) at the HortResearch Te Puke Research Centre, New Zealand (37°49·3′S, 176°19·1′E). All vines were situated in close proximity to each other. Mature vines were managed by standard orchard practices and trained on T-bars (Warrington and Weston, 1990). After winter pruning, four typical long parent shoots on each of the two vines (a total of eight shoots per species) were selected to record timing of budbreak and shoot development. Mean hourly air temperature was recorded by a permanent weather station located at the Te Puke Research Centre.
Timing of budbreak
Budbreak was recorded on each of the selected canes by counting the number of buds at the green tip stage (tips of bud scales visible) on each cane. Canes were observed twice weekly at the start of budbreak, when budbreak was occurring rapidly, and then once a week until budbreak was completed (no new buds opening). Budbreak was only recorded on the primary cane. Shoot growth on all vines was left unpruned over the growing season so that axillary shoot types could be measured.
Measurements of axillary production
In early January 2005 (summer) axillary shoot type and nodal position from the base of the cane were recorded on selected parent canes. By this time of year, many shoots terminate growth during the expansion of preformed phytomers; these shoots are referred to as terminated or self-terminated shoots (Seleznyova et al., 2002). In the current study, the terminated shoots were further categorized into short shoots (nine expanded phytomers or less) and medium shoots (more than nine expanded phytomers). Shoots that expand neoformed phytomers are long shoots. In total, five axillary shoot types were distinguished: latent bud, and short, medium, long shoot, and damaged/broken shoot. After leaf fall, node number and number of secondary shoots from each shoot type were recorded for all species studied. The node number on shoots of A. arguta, A. polygama and A. indochinensis was recorded at the end of the growing season.
Data analysis and modelling
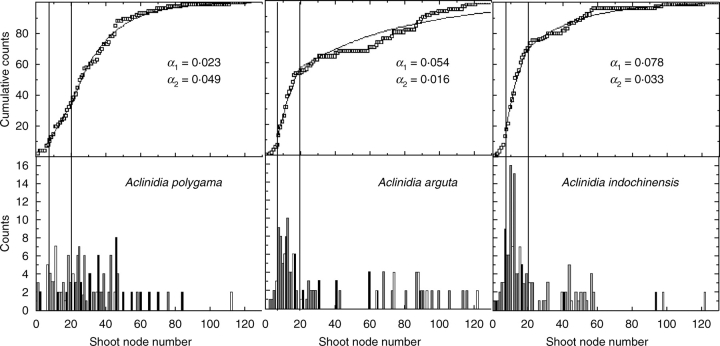
Stochastic modelling was used to explore hypotheses about patterns of shoot growth cessation. It was assumed that, at each developmental step after the opening of the initial cluster of leaves, shoot growth can stop with a probability α or continue with another phytomer beginning expansion. The hypothesis was that the probabilities for growth cessation of the preformed and neoformed parts of the shoot differ. Two probabilities, α1 for 7 ≤ n ≤ 20 and α2 for 20 < n when n is the node number, were considered. For simplicity, the value of 20 was chosen because it approximates the number of preformed nodes in many Actinidia species (Snowball, 1997a). From this hypothesis, the model value of the proportion of terminated shoots that did not exceed a given number of nodes, n, was calculated. The parameter values of α1 and α2 for A. arguta, A. polygama and A. indochinensis were estimated by fitting this model to the corresponding distributions for the data using the list squares method.
The time-course of budbreak and the hourly temperature T(h) at the Te Puke Research Orchard were used to calculate the following temperature indices for each of the species:
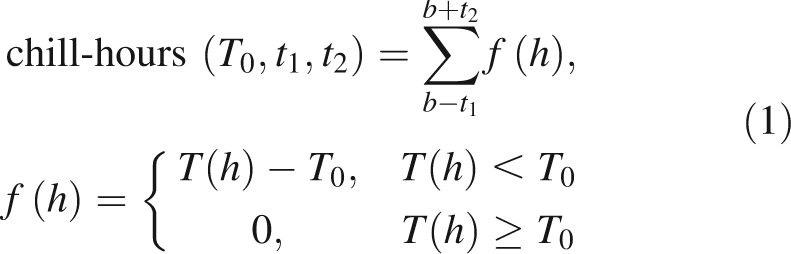 |
1 |
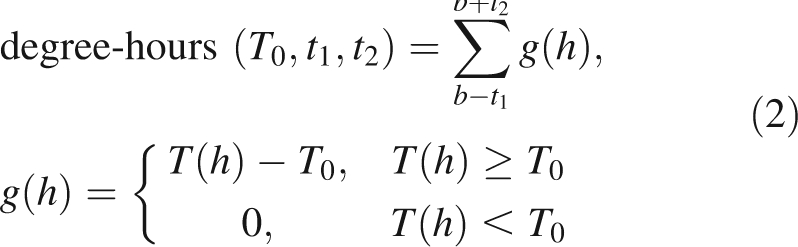 |
2 |
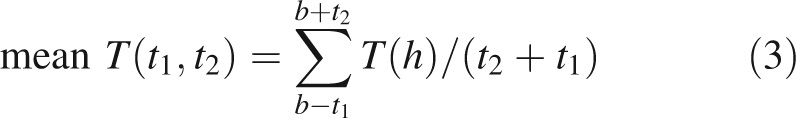 |
3 |
where h = time measured in hours, b = timing of 50 % budbreak, t1 and t2 = durations of time periods before and after 50 % budbreak, respectively; and T0 = base temperature.
The above indices were calculated for several combinations of t1 and t2 (each taking values from 0 to 4 weeks) and for two values of T0: 8 °C and 12 °C. The former value was used in by Buwalda (1991) for modelling shoot growth in kiwifruit. However, according to Morgan et al. (1985), kiwifruit plants grown at a constant temperature of 10 °C showed symptoms of chilling injury including shoot tip abortion. Also controlled environment studies (Seleznyova and Halligan, 2006) showed that 12 °C applied during budbreak and early shoot growth resulted in shoot tip abortion after the opening of the initial cluster of leaves, hence 12 °C was considered as a possible base temperature. A linear function:
| 4 |
where A is a parameter, was fitted to the natural logarithm of proportions of terminated shoots (short and medium) for each genotype, as a function of the temperature index.
RESULTS
Bud and shoot development
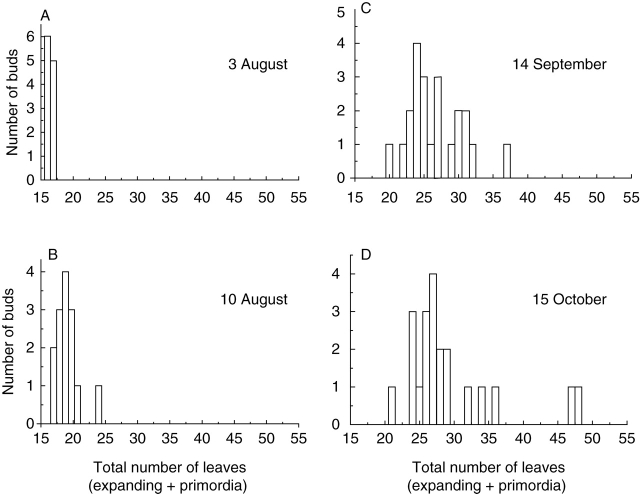
Both ‘Hort16A’ and ‘Hayward’ buds resumed organogenesis several weeks before budbreak (Fig. 3). From budbreak onwards, mean leaf number increased in both genotypes. Data were similar over both 2004 and 2005 growing seasons. Data from 2005 are shown in Fig. 3 because these data represent a larger sample size and longer sampling period than data from 2004. Figure 4 shows the distributions of total leaf number (expanding and primordia) within ‘Hort16A’ buds/shoots at four time points in 2004. Before budbreak, all buds had 16 or 17 leaf primordia (Fig. 4A). One week later, most buds had an increased number of primordia, indicating that organogenesis had begun (Fig. 4B). As the season progressed, the distribution of total leaf number became wider across the population (Fig. 4C). Figure 4D illustrates that all shoots had more leaves than existed in the dormant bud, indicating that all shoots began organogenesis in the spring. Shoot development results were similar for ‘Hayward’ and ‘Hort16A’ apart from the time course of bud activity (Fig. 4). Data from 2005 replicated trends seen in 2004 for both genotypes.
Fig. 3.
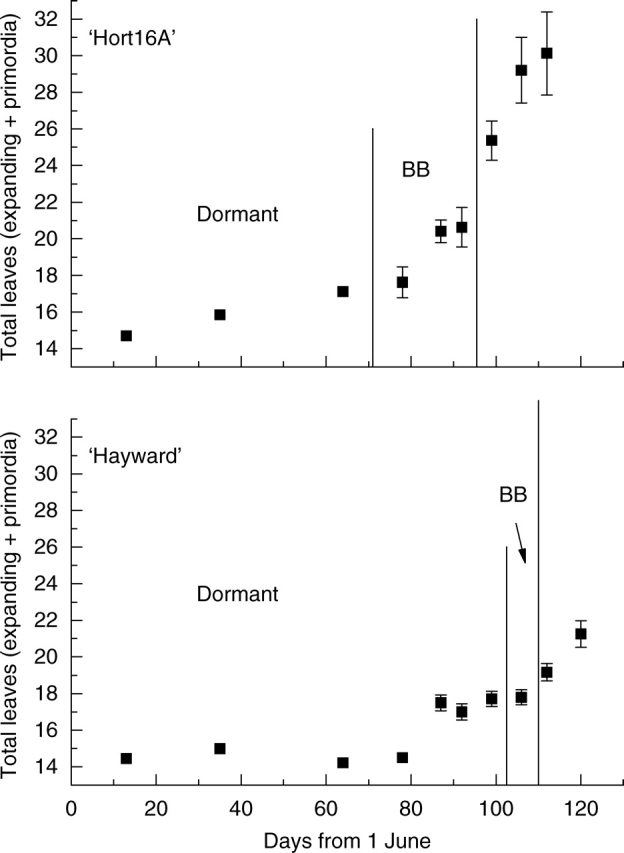
Changes with time in mean number of leaves and primordia in kiwifruit buds and shoots in 2005. BB = budbreak. Vertical bars represent s.e.
Fig. 4.
Histograms showing the number of ‘Hort16A’ buds with a given total leaf number at four time points in 2004: (A) 3 August, (B) 10 August, (C) 14 September, (D) 15 October.
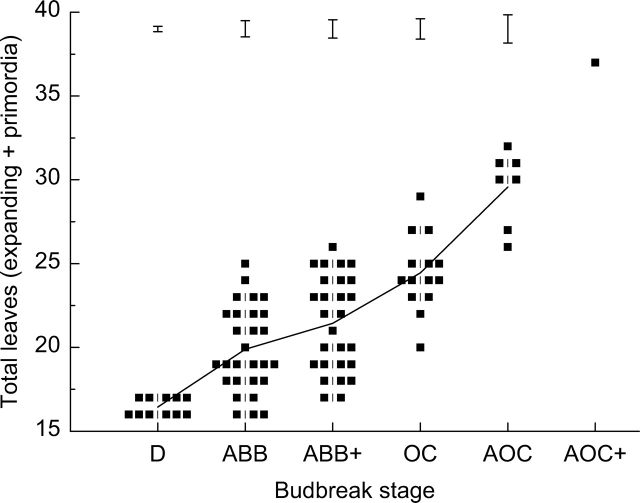
Because budbreak does not occur simultaneously across a population, total leaf number was examined in buds at the same developmental stage (as defined by Brundell, 1975). Total leaf number data from four time points were pooled according to bud stage (Fig. 5). There was very little variation in total leaf number in dormant buds (D). By advanced budbreak (ABB) stage, most buds had begun organogenesis. At each bud stage, there was some variation in total leaf number but, overall, leaf number was related to stage of bud development.
Fig. 5.
Total leaf number within ‘Hort16A’ buds of equivalent developmental stage (as defined by Brundell, 1975). Bud stages are: dormant, D, advanced budbreak, ABB; advanced budbreak plus, ABB+; open cluster, OC; advanced open cluster, AOC; advanced open cluster plus, AOC+. Offset printing is used for overlapping data points. The line shows mean values and standard errors are represented as vertical bars above. Only one sample was at the AOC+ stage, so there is no error bar.
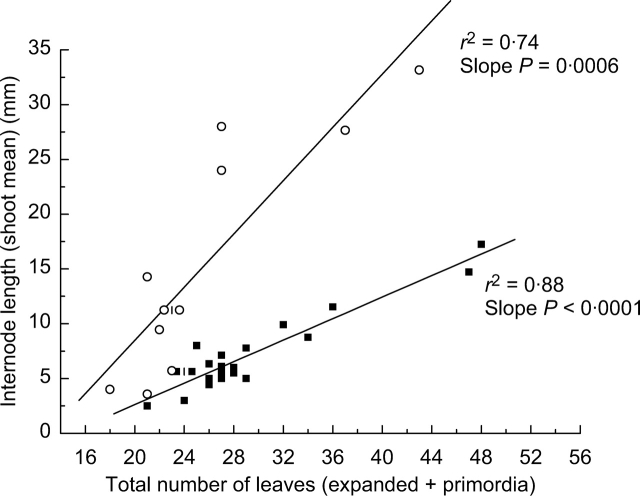
For each sampling date there was a strong positive correlation between mean internode length and total leaf number in both ‘Hayward’ and ‘Hort16A’. Note that the variation in the total leaf number is determined mostly by the variation in organogenesis, because the number of preformed leaves is very consistent (Fig. 4A). Figure 6 shows a typical relationship between the total leaf number and internode length in ‘Hayward’ and ‘Hort16A’ shoots on a given sampling date. For individual shoots, a similar relationship was observed between the number of phytomers on shoots that were expanding or had reached their final size (shoot node number) and mean internode length (data not shown). The notion of shoot vigour is often used as a qualitative measure of vegetative growth (Garrison and Wetmore, 1961; Remphrey and Powell, 1984; Snowball, 1997b; Clearwater et al., 2006). From the current study, the numbers of newly initiated phytomers, expanded phytomers and mean internode length are highly correlated and can be defined as quantitative components of shoot vigour.
Fig. 6.
Typical relationship between mean internode length and total leaf number on a single collection date in 2004. Collection dates shown for ‘Hort16A’ (5 October, closed squares) and ‘Hayward’ (2 November, open circles) were different because of differences in timing of budbreak. Offset printing is used for overlapping data points.
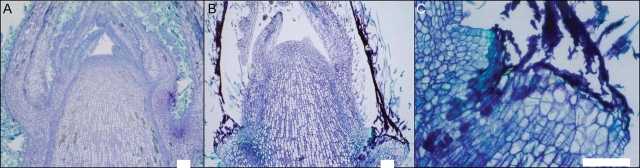
The SAMs of expanding and non-expanding ‘Hayward’ shoots were examined to determine if shoot tip abortion was associated with inactivity or death of the SAM (Fig. 2). Scanning electron micrographs illustrate that the SAM morphology of both shoot types is similar and that they do not show any signs of meristem deterioration (Fig. 7A, B). Longitudinal sections through the SAM of expanding and non-expanding shoots sampled in early November did not reveal any differences in histological organization, cell size or density, or overall morphology between the SAMs of the two shoot types (Fig. 7C, F). Based on the frequency of mitotic figures observed, the SAMs of non-expanding shoots were clearly alive and undergoing cell divisions at the time of sampling.
Fig. 7.
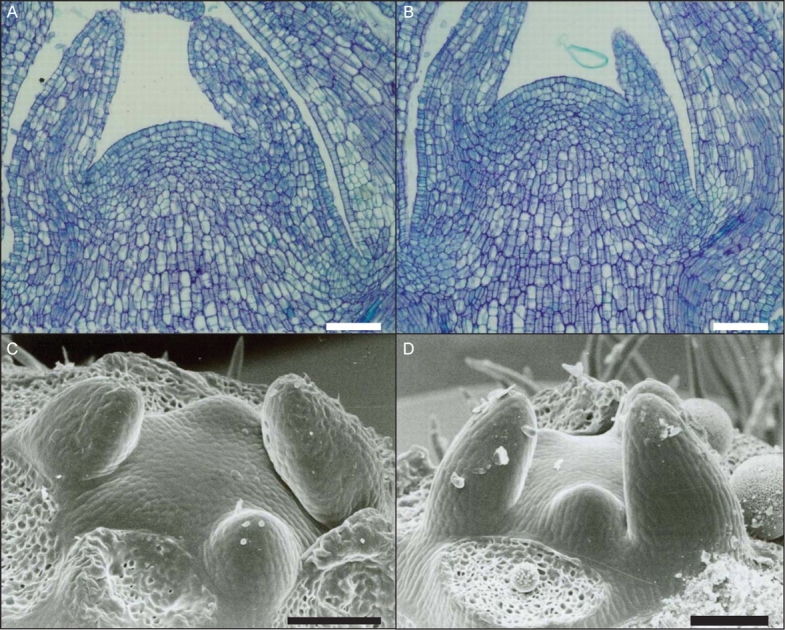
Comparison of shoot apical meristems from expanding (A, C) and non-expanding (B, D) ‘Hayward’ shoots indicates no morphological differences. (A, B) Longitudinal sections stained with Toluidine Blue (samples collected 1 November 2004); (C, D) scanning electron micrographs (samples collected 20 October 2004). Scale bars = 100 µm.
Longitudinal sections through ‘Hayward’ apices sampled several weeks later indicated that leaves five to seven nodes below the SAM had abscised in non-expanding shoots, but not in expanding shoots (Fig. 8A, B). A close-up of the abscission zone illustrates a clear line of cell death distal to the axillary meristem (Fig. 8C). Densely staining cells at the base of younger leaves suggests the formation of an abscission zone. Half of the non-expanding shoots sampled (10 out of 20) exhibited a similar pattern of leaf abscission, whereas none (0 out of 10) of the expanding shoots did. Eventually, cells throughout the sub-apical stem began to decay, and the entire apex detached from the shoot.
Fig. 8.
Comparison of sub-apical zones in expanding (A) and non-expanding (B) ‘Hayward’ shoots reveals that leaf abscission occurs five to seven nodes below the shoot apical meristem in non-expanding shoots (samples collected 23 November 2004). (C) Close up of leaf abscission zone in (B). Scale bars = 100 µm.
Patterns of shoot termination
The sample sizes in this study varied between 146 and 150 shoots per genotype. Final shoot node number distributions for A. polygama, A. arguta and A. indochinensis (Fig. 9, bottom) showed considerable variability in the number of phytomers that expanded before termination. The cumulative node number distribution functions were better represented by the fitted models for leaves between 7 and 20 (Fig. 9, top). This corresponds approximately to expansion of the preformed part of the shoot (Snowball, 1997a). The shapes of the node number distribution and the cumulative distribution function for A. polygama were different from those for A. arguta and A. indochinensis. Based on previous work, the distributions for Polygama were also different from the corresponding distributions for A. deliciosa and A. chinensis (Snowball, 1995; Seleznyova et al., 2002). For A. arguta and A. indochinensis, the estimated probabilities of termination during development of the preformed leaves (α1) were higher than for neoformed leaves (α2), while this relationship was reversed for A. polygama. Also α1 for A. polygama was much smaller than α1 for A. arguta and A. indochinensis.
Fig. 9.
Patterns of shoot growth cessation in Actinidia. Final shoot node number distributions (bottom) and cumulative node number distributions (top). Bars and symbols represent data, continuous lines represent the stochastic models. The model parameters α1 and α2 correspond to the probabilities of shoot growth cessation at each developmental step between expansion of phytomers 7–20 and over 20, respectively.
Effect of temperature on shoot fate
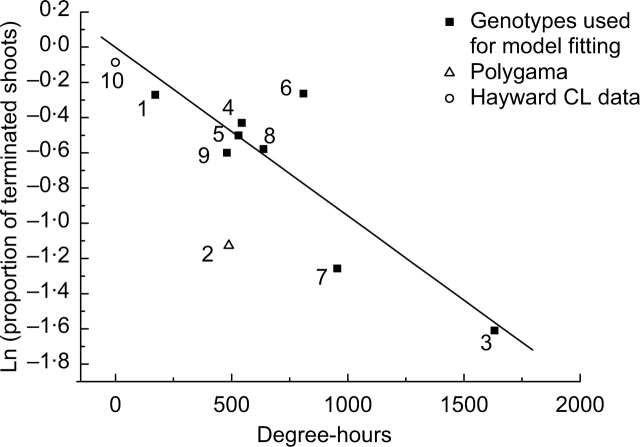
Based on the results of the previous section, patterns of shoot termination were qualitatively similar in all species apart from A. polygama. Hence, the latter was excluded from model fitting in this section. The species studied had a wide range of budbreak dates from August to October. Of all considered combinations of T0, t1 and t2, the values T0 = 12 °C, t1 = 1 week and t2 = 2 weeks were the most suitable parameters [i.e. gave the best fit of eqn (4) to the data] for modelling the effects of temperature on shoot fate during and after budbreak. Temperature indices (1–3) calculated for the same time interval (t1, t2) were highly correlated, as demonstrated by the relationships between degree-hours and chill-hours (r2 = 0·99), and between mean temperature and degree-hours (r2 = 0·98) (not shown). Because of these relationships, correlation coefficients between each of the indices (1–3) and the proportion of terminated (short and medium) shoots were of comparable value. However, the degree-hours (2) was more consistent with the controlled environment data, when extended into a wider range of values (Fig. 10). The relationship between the degree-hours accumulated over 3 weeks and the natural logarithm of the proportion of terminated shoots was significant with P = 0·0027 and r2 = 0·74. The fitted parameters for the linear model (eqn 4) were
| 5 |
Fig. 10.
Effect of accumulated degree-hours on shoot fate: field studies for Actinidia species. Genotypes are numbered as follows: A. indochinensis (1), A. polygama (2), A. purpurea (3), A. chinensis (4), A. arguta (5), A. deliciosa (6), A. latifolia (7), A. eriantha (8), A. guilinensis (9). Controlled environment datum for “Hayward” (10) is from Seleznyova and Halligan (2006). Each symbol represents the natural logarithm of the total proportion of terminated (short and medium) axillary shoots from eight parent canes. Line represents a linear model, y = Ax, r2 = 0·74, slope P = 0·0027 (A. polygama and “Hayward” not included for model fitting).
In this study, each genotype was represented by one data point, thus variability within each vine with respect to the timing of budbreak and shoot fate was not considered.
DISCUSSION
All kiwifruit buds have an equivalent developmental potential for neoformation
In many temperate woody species, neoformation is variable and multiple factors influence the extent of neoformation. The position of a bud on the parent shoot (Davidson and Remphrey, 1994; Sabatier and Barthélémy, 1999; Puntieri et al., 2002; Lebon et al., 2004) or the relative age of the tree (Gunckel et al., 1949; Fontaine et al., 1999) can both influence patterns of neoformation within a shoot system. Favourable environmental conditions such as higher temperatures or water availability during the growing season can promote neoformation, which is advantageous in variable climatic regions (Critchfield, 1960; Remphrey and Steeves, 1984; Powell, 1991).
In contrast, the results of this study indicate that all breaking kiwifruit buds resume organogenesis, adding to the phytomers preformed during the previous season. With the exception of the extreme basal and distal tips, the position of a bud on the parent shoot has no effect on its ability to initiate organogenesis. Hence, all shoots have the potential to develop into long, vigorous shoots containing a large number of neoformed phytomers. One interpretation of the present results is that the onset of organogenesis is the default state in kiwifruit. Alternatively, the lack of a terminal meristem on the parent shoot may reduce apical dominance effects observed in shoots with an apical bud.
The rate of organogenesis is correlated with the appearance of phytomers
Although kiwifruit buds initially have equivalent developmental potential, early shoot vigour is related to the developmental stage of the bud and is not synchronous across the population. As shoots expand, it becomes apparent that there is a relationship between the expansion of the preformed phytomers that are already in the bud and the initiation of new phytomers. The rate of organogenesis is variable and is positively correlated with the rates of leaf appearance and shoot elongation.
Previous studies have shown that there is a relationship between the expansion of leaves and internodes. Removal of expanding leaves caused an immediate cessation of stem elongation in apple shoots (Abbott, 1970). Wetmore and Garrison (1966) demonstrated that the removal of a pair of young leaves decreased both the rate of elongation and the final internode length in Helianthus annus (Wetmore and Garrison, 1966). This effect was limited to the internode below the detached leaf pair and was related to the age of the leaves at the time of removal. The application of gibberellic acid following leaf removal could restore internode elongation in Helianthus (Jones and Phillips, 1966). In other species, auxin application was essential to restore internode elongation following leaf removal (Jacobs and Bullwinkel, 1953). Auxin and gibberellic acid are produced in expanding leaves and have been implicated in the causal relationship between the expansion of leaves and internodes (Kato and Ito, 1962; Jones and Phillips, 1966). Studies of the hormonal control of internode elongation indicate that the response is likely to involve the complex interplay of several hormones and may vary between woody and non-woody species.
Shoot fate is associated with the rate of shoot expansion
During the early stages of development, shoot growth rate and shoot fate can be affected by temperature (Seleznyova and Halligan, 2006), rootstock, and manipulations such as girdling and/or removal of neighbouring shoots (Piller et al., 1998; Clearwater et al., 2006). Slowly expanding shoots have short internodes and stop growth early, whereas rapidly expanding shoots have longer internodes, and grow for longer periods. This relationship between the rate of shoot growth and growth cessation is consistent with a previous architectural study of an A. chinensis genotype that demonstrated a strong relationship between final shoot node number and average shoot internode length (Seleznyova et al., 2002). Similarly, shoot growth cessation in apple is induced by low growth rate (Abbott, 1984). In apple, cessation of growth results in the formation of a terminal bud, whereas in kiwifruit the shoot tip aborts rather than forming a terminal bud.
Organogenesis and shoot tip abortion are controlled independently
This study demonstrates that shoot tip abortion in non-expanding kiwifruit shoots is not initiated by the termination of the SAM. The present results indicate that the kiwifruit SAM is active and continues cell division until the entire shoot tip aborts. The first sign of shoot tip abortion is leaf abscission five to seven nodes below the apical meristem. It is noteworthy that leaf abscission occurs such that the associated axillary meristems remain intact. The preservation of the SAM and axillary meristems provides a developmental reserve for the non-expanding shoot. There is anecdotal evidence that non-expanding kiwifruit shoots are capable of responding to changes in growth conditions and resuming growth up until the entire shoot tip aborts. In certain controlled environment conditions, long fruiting shoots show a pause in leaf appearance followed by resumption of growth (A. N. Seleznyova and D. Greer, unpubl. res.). In Syringa vulgaris, the shoot apex aborts in a similar manner, although organogenesis clearly ceases prior to shoot tip abortion (Garrison and Wetmore, 1961). These authors demonstrated that aborting Syringa shoot apices could be stimulated to resume neoformation by destruction of the axillary meristems. The present results suggest that kiwifruit meristems do not cease organogenesis and there is no fixed time for shoot tip abortion. Both of these factors permit a high degree of developmental plasticity.
Intrinsic variability of bud and shoot development
The variation in the extent of organogenesis during the period between dormancy and advanced budbreak (Fig. 4) demonstrates an intrinsic variability in the expression of vigour during early shoot development. This early difference sets up a potential for great variability in final shoot node number and length (Snowball, 1997b; Seleznyova et al., 2002). A simple stochastic model of shoot termination had a better fit for early stages of shoot development suggesting that, at these stages, the pattern of shoot termination resulted from variability present in the shoot populations. The conclusion from this study and previous work (Snowball, 1997b) is that, for most species, the probability of growth cessation is considerably higher during the expansion of the preformed part of the shoot. In many tree species, most or all of the preformed phytomers expand during shoot development (Guédon et al., 2006), whereas in Actinidia shoot tip abortion can occur at any time during extension of the preformed phymoters, resulting in short or medium shoots. However, once neoformation begins, growth becomes largely indeterminate, resulting in long shoots. Only A. polygama showed a markedly different pattern of growth cessation with a higher probability of termination during neoformation. The less-regular patterns observed for termination during neoformation in A. polygama could be due to the small sample size, but they could also result from effects of local conditions such as nutrient supply on shoot growth (Guédon et al., 2006).
Effect of temperature and genotype on shoot fate
The Actinidia species examined here (A. purpurea, A. arguta, A. deliciosa, A. chinensis, A. latifolia, A. eriantha, A. guilinensis and A. indochinensis) had a wide spread of budbreak dates, which occurred over a broad range of temperatures and enabled the study of temperature effects on shoot fate. The results suggest that, on average, shoot fate (probability of growth cessation) is related to the temperature during a 3-week period starting 1 week before 50 % of budbreak. Species breaking buds earlier and experiencing lower accumulated degree-hours (e.g. A. indochinensis) had more terminated shoots, whereas, species breaking buds later and experiencing higher accumulated degree-hours (e.g. A. latifolia and A. purpurea) had lower proportions of terminated shoots. This relationship reflects the effect of temperature on the total number of terminated shoots for different genotypes. At the individual shoot level, there are other factors such as variability in initial vigour or favourable location within the canopy that can override the temperature effect on shoot fate.
CONCLUSIONS
The focus of this study was to increase our understanding of vigour and developmental plasticity of kiwifruit shoots. It was concluded that kiwifruit shoot fate is an outcome of three processes: initiation of new phytomers by the apical meristem, expansion of phytomers, and abortion of the shoot tip. Organogenesis creates a reserve of phytomers for shoot development, while shoot tip abortion sets the limit for this development. One of the key findings of this study is that these two counter processes are controlled independently. Organogenesis occurs in the SAM, which remains mitotically active until the entire apex has aborted. Shoot tip abortion occurs via necrosis well below the SAM and is preceded by a slowing of phytomer expansion and leaf abscission in the sub-apical zone. Another conclusion from this work is that the macroscopic processes of phytomer appearance and internode elongation are strongly correlated with the microscopic process of organogenesis. The present genotype studies demonstrated that lower temperatures during early shoot development result in higher proportions of terminated shoots.
Based on these results and previous analyses of shoot growth (Clearwater et al, 2006), we propose that shoot tip abortion may be triggered when shoot expansion rate falls below a certain threshold. We suggest that, apart from intrinsic variability expressed as early as budbreak, shoot expansion rate is influenced by multiple factors such as temperature, availability of resources, and competition with neighbouring shoots. Thus, the developmental fate of each shoot is plastic and responds to both environmental and intrinsic factors.
ACKNOWLEDGEMENTS
We thank M. Clearwater and R. Johnston for helpful comments on the manuscript. This research was supported as a HortResearch Kiwifruit Royalty Investment Project.
LITERATURE CITED
- Abbott DL. The role of budscales in the morphogenesis and dormancy of the apple fruit bud. In: Luckwill LC, Cutting CV, editors. Physiology of tree crops. New York, NY: Academic Press; 1970. pp. 65–82. [Google Scholar]
- Abbott DL. The apple tree: physiology and management. London: Grower Books; 1984. [Google Scholar]
- Barthélémy D, Caraglio Y. Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Annals of Botany. 2007;99:375–407. doi: 10.1093/aob/mcl260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics. 1965;13:115–155. [Google Scholar]
- Brundell DJ. Flower development in the Chinese gooseberry (Actinidia chinensis Planch). I. Development of the flowering shoot. New Zealand Journal of Botany. 1975;13:473–483. [Google Scholar]
- Buwalda J. A mathematical model of carbon acquisition and utilisation by kiwifruit vines. Ecological Modelling. 1991;57:43–64. [Google Scholar]
- Clearwater MJ, Seleznyova AN, Thorp TG, Blattmann P, Barnett AM, Lowe RG, et al. Rootstock effects on shoot growth and leaf area development of kiwifruit. Tree Physiology. 2006;26:505–515. doi: 10.1093/treephys/26.4.505. [DOI] [PubMed] [Google Scholar]
- Critchfield WB. Leaf dimorphism in Populus trichocarpa. American Journal of Botany. 1960;47:699. [Google Scholar]
- Davidson CG, Remphrey WR. Shoot neoformation in clones of Fraxinus pennsylvanica in relation to genotype, site and pruning treatments. Trees – Structure and Function. 1994;8:205. [Google Scholar]
- Ferguson AR. Kiwifruit: a botanical review. Horticultural Reviews. 1984;6:1–64. [Google Scholar]
- Fontaine F, Chaar H, Colin F, Clément C, Burrus M, Druelle JL. Preformation and neoformation of growth units on 3-year-old seedlings of Quercus petraea. Canadian Journal of Botany – Revue Canadienne de Botanique. 1999;77:1623–1631. [Google Scholar]
- Garrison R, Wetmore RH. Studies in shoot-tip abortion – Syringa vulgaris. American Journal of Botany. 1961;48:789–795. [Google Scholar]
- Gordon D, Damiano C, DeJong TM. Preformation in vegetative buds of Prunus persica: factors influencing number of leaf primordia in overwintering buds. Tree Physiology. 2006;26:537–544. doi: 10.1093/treephys/26.4.537. [DOI] [PubMed] [Google Scholar]
- Guédon Y, Puntieri JG, Sabatier S, Barthélémy D. Relative extents of preformation and neoformation in tree shoots: analysis by a deconvolution method. Annals of Botany. 2006;98:835–844. doi: 10.1093/aob/mcl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunckel JE, Thimann KV, Wetmore RH. Studies of development in long shoots and short shoots of Ginkgo biloba L. 4. Growth habit, shoot expression and the mechanism of its control. American Journal of Botany. 1949;36:309–316. [PubMed] [Google Scholar]
- Hallé F, Oldeman RAA, Tomlinson PB. Tropical trees and forests: an architectural analysis. New York, NY: Springer Verlag; 1978. [Google Scholar]
- Jacobs WP, Bullwinkel B. Compensatory growth in Coleus shoots. American Journal of Botany. 1953;40:385–392. [Google Scholar]
- Jones RL, Phillips IDJ. Organs of gibberellin synthesis in light-grown sunflower plants. Plant Physiology. 1966;41:1381–1386. doi: 10.1104/pp.41.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Ito H. Physiological factors associated with the shoot growth of apple trees. Tohoku Journal of Agricultural Research. 1962;13:1–21. [Google Scholar]
- Lebon E, Pellegrino A, Tardieu F, Lecoeur J. Shoot development in grapevine (Vitis vinifera) is affected by the modular branching pattern of the stem and intra- and inter-shoot trophic competition. Annals of Botany. 2004;93:263–274. doi: 10.1093/aob/mch038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DC, Warrington IJ, Halligan EA. Effect of temperature and photosynthetic photon flux density on vegetative growth of kiwifruit (Actinidia chinensis) New Zealand Journal of Agricultural Research. 1985;28:109–116. [Google Scholar]
- Piller GJ, Greaves AJ, Meekings JS. Sensitivity of floral shoot growth, fruit set and early fruit size in Actinidia deliciosa to local carbon supply. Annals of Botany. 1998;81:723–728. [Google Scholar]
- Powell GR. Preformed and neoformed extension of shoots and sylleptic branching in relation to shoot length in Tsuga canadensis. Trees – Structure and Function. 1991;5:107–116. [Google Scholar]
- Puntieri J, Barthelemy D, Martinez P, Raffaele E, Brion C. Annual-shoot growth and branching patterns in Nothofagus dombeyi (Fagaceae) Canadian Journal of Botany. 1998;76:673–685. [Google Scholar]
- Puntieri JG, Souza MS, Barthelemy D, Brion C, Nunez MA, Mazzini C. Preformation, neoformation, and shoot structure in Nothofagus dombeyi (Nothofagaceae) Canadian Journal of Botany – Revue Canadienne de Botanique. 2000;78:1044–1054. [Google Scholar]
- Puntieri JG, Stecconi M, Barthelemy D. Preformation and neoformation in shoots of Nothofagus antarctica (G. Forster) Oerst. (Nothofagaceae) shrubs from northern Patagonia. Annals of Botany. 2002;89:665–673. doi: 10.1093/aob/mcf108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remphrey WR, Powell GR. Crown architecture of Larix laricina saplings – shoot preformation and neoformation and their relationships to shoot vigor. Canadian Journal of Botany – Revue Canadienne de Botanique. 1984;62:2181–2192. [Google Scholar]
- Remphrey WR, Steeves TA. Shoot ontogeny in Arctostaphylos uva-ursi (Bearberry) – the annual cycle of apical activity. Canadian Journal of Botany – Revue Canadienne de Botanique. 1984;62:1925–1932. [Google Scholar]
- Sabatier S, Barthélémy D. Growth dynamics and morphology of annual shoots, according to their architectural position, in young Cedrus atlantica (Endl.) Manetti ex Carriere (Pinaceae) Annals of Botany. 1999;84:387–392. [Google Scholar]
- Sabatier S, Barthelemy D, Ducousso I, Germain E. Elongation modes and morphology of annual growth in the walnut tree, Juglans regia L. ‘Lara’ (Juglandaceae) Canadian Journal of Botany. 1998;76:1253–1264. [Google Scholar]
- Seleznyova A, Halligan L. Modelling effect of temperature on area expansion at the leaf the shoot and the whole plant level. Acta Horticulturae. 2006;707:167–174. [Google Scholar]
- Seleznyova AN, Thorp TG, Barnett AM, Costes E. Quantitative analysis of shoot development and branching patterns in Actinidia. Annals of Botany. 2002;89:471–482. doi: 10.1093/aob/mcf069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowball AM. The seasonal cycle of leaf, shoot and bud development in kiwifruit. Journal of Horticultural Science. 1995;70:787–797. [Google Scholar]
- Snowball AM. Axillary shoot bud development in selected Actinidia species. New Zealand Journal of Crop and Horticultural Science. 1997a;25:233–242. [Google Scholar]
- Snowball AM. Seasonal cycle of shoot development in selected Actinidia species. New Zealand Journal of Crop and Horticultural Science. 1997b;25:221–231. [Google Scholar]
- Souza MS, Puntieri JG, Barthelemy D, Brion C. Bud content and its relation to shoot size and structure in Nothofagus pumilio (Poepp. et Endl.) Krasser (Nothofagaceae) Annals of Botany. 2000;85:547–555. [Google Scholar]
- Steeves TA, Sussex IM. Patterns in plant development. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Sultan SE. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science. 2000;5:537–542. doi: 10.1016/s1360-1385(00)01797-0. [DOI] [PubMed] [Google Scholar]
- Sylvester AW, Cande WZ, Freeling M. Division and differentiation during normal and Liguleless-1 maize leaf development. Development. 1990;110:985–1000. doi: 10.1242/dev.110.3.985. [DOI] [PubMed] [Google Scholar]
- Warrington IJ, Weston GC. Kiwifruit science and management. Auckland: New Zealand Society for Horticultural Science; 1990. [Google Scholar]
- Wetmore RH, Garrison R. The morphological ontogeny of the leafy shoot. In: Cutter EG, editor. Trends in plant morphogenesis. London: Longmans; 1966. pp. 187–199. [Google Scholar]