Abstract
Background and Aims
Root growth and development are closely dependent upon nitrate supply in the growth medium. To unravel the mechanism underlying dependence of root growth on nitrate, an examination was made of whether endogenous nitric oxide (NO) is involved in nitrate-dependent growth of primary roots in maize.
Methods
Maize seedlings grown in varying concentrations of nitrate for 7 d were used to evaluate the effects on root elongation of a nitric oxide (NO) donor (sodium nitroprusside, SNP), a NO scavenger (methylene blue, MB), a nitric oxide synthase inhibitor (Nω-nitro-L-arginine, L-NNA), H2O2, indole-3-acetic acid (IAA) and a nitric reducatse inhibitor (tungstate). The effects of these treatments on endogenous NO levels in maize root apical cells were investigated using a NO-specific fluorescent probe, 4, 5-diaminofluorescein diacetate (DAF-2DA) in association with a confocal microscopy.
Key Results
Elongation of primary roots was negatively dependent on external concentrations of nitrate, and inhibition by high external nitrate was diminished when roots were treated with SNP and IAA. MB and L-NNA inhibited root elongation of plants grown in low-nitrate solution, but they had no effect on elongation of roots grown in high-nitrate solution. Tungstate inhibited root elongation grown in both low- and high-nitrate solutions. Endogenous NO levels in root apices grown in high-nitrate solution were lower than those grown in low-nitrate solution. IAA and SNP markedly enhanced endogenous NO levels in root apices grown in high nitrate, but they had no effect on endogenous NO levels in root apical cells grown in low-nitrate solution. Tungstate induced a greater increase in the endogenous NO levels in root apical cells grown in low-nitrate solution than those grown in high-nitrate solution.
Conclusions
Inhibition of root elongation in maize by high external nitrate is likely to result from a reduction of nitric oxide synthase-dependent endogenous NO levels in maize root apical cells.
Key words: Nitrate supply, nitric oxide (NO), IAA, root elongation, maize, Zea mays
INTRODUCTION
Nitrogen (N) is an essential mineral nutrient and frequently limits plant growth and development. Nitrate is a major N source available in aerobic soils, and plays a critical role in root growth (Forde, 2002). Root growth and development, particularly for later roots, are closely dependent upon nitrate availability in soils (Zhang et al., 1999; Zhang and Ford, 2000; Linkohr et al., 2002; Tian et al., 2005). In Arabidopsis, a dual effect of external nitrate on root elongation has been recognized; low and high external concentrations of nitrate stimulate and inhibit lateral root growth, respectively (Zhang and Ford, 2000). The involvement of several phytohormones in nitrate-dependent root growth has been implicated (Signora et al., 2001; Guo et al., 2005; Tian et al., 2005). For example, ABA is involved in mediating nitrate-dependent root branching in Arabidopsis (Signaora et al., 2001). Transport of auxin from shoot to root is inhibited by localized supply of nitrate in Arabidopsis (Zhang et al., 2000) and maize (Guo et al., 2005). In addition to ABA and auxin, possible involvement of cytokinin in nitrate-mediated root growth in maize has also been implicated (Tian et al., 2005).
Nitric oxide (NO) is emerging as an important messenger molecule associated with many biochemical and physiological processes in plants (Pagnussat et al., 2002; Lamattina et al., 2003; Stohr and Stremlau, 2006). Two potential enzymatic sources of NO production in plants are NO synthase (NOS) and nitrate reductase (NR). In mammals, NO production is mediated by NOS, which catalyzes the conversion of L-arginine to L-citrulline and NO (Furchgott, 1995). Mammalian NOS inhibitors inhibit NO production in response to various stimuli in plants (Lamattina et al., 2003; Neill et al., 2003), implying that an arginine-dependent NOS activity may also exist in plants. However, the molecular identity of plant NOS remains controversial and elusive (Crawford et al., 2006; Zemojtel et al., 2006). NR is a central enzyme of nitrogen assimilation in plants, catalysing the transfer of two electrons from NAD(P)H to nitrite and further catalysing the NAD(P)H-dependent reduction of nitrite to NO (Kaiser et al., 2002). The plasma-membrane-bound form of NR (PM-NR) that is located on the outer surface of the plasma-membrane has been suggested to act as a nitrate sensor (Forde and Clarkson, 1999). A recent study has revealed that a plasma-membrane-bound enzyme is capable of catalysing the reduction of nitrite to NO, and that this nitrite–NO reductase may act in concert with the PM-NR to produce NO by converting nitrate to NO (Stohr et al., 2001). However, experimental evidence on the involvement of NO in sensing and signalling for nitrate is lacking. Furthermore, NR-mediated generation of NO is involved in ABA-dependent stomatal closure in Arabidopsis (Desikan et al., 2002). There are compelling examples that NO plays an important role in mediating root elongation and development (Gouvea et al., 1997; Pagnussat et al., 2002; Correa-Aragunde et al., 2004).
Cross-talk between NO and other molecular signals, such as H2O2 (Neill et al., 2002) and phytohormones (Tun et al., 2001; Desikan et al., 2002), exists in the response of plants to environmental stress. Recent studies have revealed that reactive oxygen species (ROS), particularly H2O2 production, are sensitive to nitrogen supply (Shin et al., 2005; Schachtman and Shin, 2007). However, whether H2O2 is involved in nitrate-dependent root growth remains to be determined. Auxin is an essential phytohormone in regulation of cell division and elongation (Blilou et al., 2005). There is evidence to show that auxin is associated with nitrate-dependent root growth and development in maize (Gouvea et al., 1997) and Arabidopsis (Zhang et al., 1999). The involvement of NO in IAA-induced adventitious root development has also been reported (Pagnussat et al., 2003). Given that nitrate is a substrate for NR-catalysed NO production, and root development and growth are closely related to NO, it is expected that NO may play a role in nitrate-dependent root growth. A previous study has demonstrated that growth of primary, seminal and crown roots of maize was significantly reduced with increasing external nitrate concentrations up to 5 mm (Tian et al., 2007a). To elucidate the mechanisms underlying nitrate-mediated root growth, we investigated the roles of NO, IAA and H2O2 in nitrate-dependent growth of primary roots in maize.
MATERIALS AND METHODS
Plant material and growth conditions
Seeds of maize (Zea mays L., inbred line 478) were surface-sterilized for 20 min in 10 % H2O2 and then rinsed in distilled water several times. The sterilized seeds were soaked in an aerated CaSO4 solution for 6 h in the dark and then placed on filter paper moistened with CaSO4 in Petri dishes for 2 d. The germinated seeds were incubated in sand quartz for 2 d and the seedlings were transferred to grow hydroponically in nutrient solutions containing varying nitrate concentrations (0·01, 1 and 10 mm) for another 7 d. Nitrogen was supplied in nutrient solution as Ca(NO3)2. To exclude the possibility that the Ca2+ may play a role in the treatments, the concentration of Ca2+ in low-nitrate treatments was supplemented to the same level as that of the high-nitrate concentration by using CaCl2. The other nutrients in the bulk solutions consisted of (in mm): 0·75 K2SO4, 10 KCl, 0·25 KH2PO4, 0·65 MgSO4, 0·13 FeSO4; and (in μm): 1 MnSO4, 1 ZnSO4, 0·1 CuSO4, 0·035 MoO3. The pH of the nutrient solutions was adjusted to 6·0. The maize seedlings were grown in a controlled environment with a temperature of 28/22 °C, 14/10 h light cycle, and photosynthetic photon flux density of 250–300 µm m−2 s−1 at canopy height.
Measurement of root elongation
After being pre-treated in nutrient solutions with varying concentrations of nitrate for 7 d, the maize seedlings were incubated for 48 h in treatment solutions of low (0·01 mm) and high (10 mm) nitrate containing the following chemicals (μm): 100 H2O2, 1 sodium nitroprusside (SNP), 1 methylene blue (MB), 100 Nω-nitro-L-arginine (L-NNA), 100 tungstate and 0·1 indole-3-acetic acid (IAA). Root length was measured before and after the treatments and the difference between the two sets of data was used to compute root elongation. At least eight independent replicates were used for each treatment.
Determination of H2O2
H2O2 contents were determined by the peroxidase-coupled assay protocols described by Veljovic-Jovanovic et al. (2002). About 0·5 g of maize roots were ground in liquid N2 and the powder was extracted in 2 mL of 1 m HClO4 in the presence of insoluble PVP (5%). The homogenate was centrifuged at 12 000 g for 10 min and the supernatant was neutralized with 5 m K2CO3 to pH 5·6 in the presence of 100 mL of 0·3 m phosphate buffer (pH 5·6). The solution was centrifuged at 12 000 g for 1 min and the sample was incubated for 10 min with 1 U ascorbate oxidase in order to oxidize ascorbate prior to assay. The reaction mixture was composed of 0·1 m phosphate buffer (pH 6·5), 3·3 mm DMAB, 0·07 mm MBTH and 0·3 U POX. The reaction was initiated by addition of 200 mL of sample. The absorbance change at 590 nm was monitored at 25 °C.
Determination of endogenous NO levels in roots
NO content in root apices was determined by the method described by Tian et al. (2007b). Briefly, after the 7-d cultivation in varying nitrate concentrations, the primary root apices (about 2 cm from the root apices) were excised, rinsed in 20 mm HEPES–NaOH buffer (pH 7·5) for several minutes and then incubated in 15 µm 4,5-diaminofluorescein diacetate (DAF-2DA) in HEPES–NaOH in a rotary shaker for 1 h at 23 °C. The DAF-2DA-dependent fluorescence was detected by a laser confocal scanning microscope (LSM 510; Zeiss, Oberkochen, Germany) with excitation and emission wavelengths of 488 nm and 525 nm, respectively. Three-dimensional scanning was done with a 2-μm Z-series project step, and the three-dimensional reconstructed images of individual root apices were used to calculate the relative fluorescence. The fluorescence intensity of the individual root apices (approx. 4 mm in length) was determined using Zeiss LSM 510 software and was expressed in pixel numbers on a scale ranging from 0 to 255.
RESULTS
Effect of nitrate on root elongation
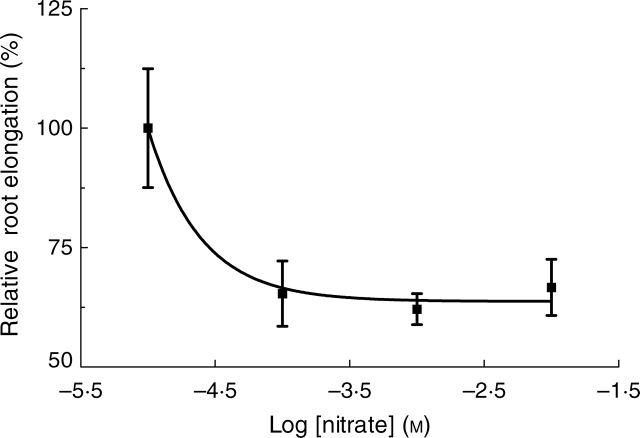
As shown in Fig. 1, there was an exponential reduction in growth for primary roots when nitrate concentrations in the external medium were increased from 0·01 to 10 mm. For instance, elongation of primary roots was inhibited by 30 and 36 % when exposed to 0·1 and 1 mm nitrate, respectively, compared with maize plants grown in solution containing 0·01 mm nitrate.
Fig. 1.
Effect of external nitrate supply on elongation of primary roots of maize seedlings. Root elongation of maize seedlings pre-grown in varying concentrations of nitrate (0·01 mm, 0·1 mm, 1 mm or 10 mm) for 7 d were used to study relative elongation during a 24-h period. The curve was fitted by an exponential decay with the equation y = (1·03 × 10−4)e−2·56x + 63·72. Data were normalized for elongation in 0·01 mm nitrate solution, and represent the mean ± s.e of at least eight roots for each treatment.
Effect of nitrate supply on H2O2 concentration in roots
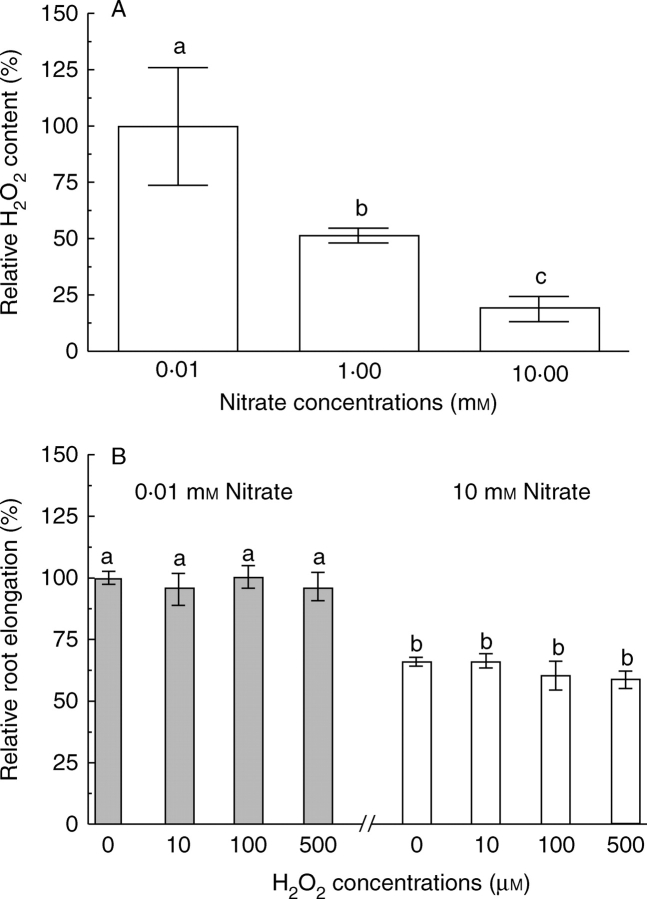
Maize roots grown in 0·01 mm nitrate solution accumulated higher levels of H2O2 than maize roots grown in 1 and 10 mm nitrate (Fig. 2A). For example, H2O2 concentrations were decreased by 50 and 82% with an increase in the external nitrate concentration from 0·01 to 1, and 10 mm, respectively.
Fig. 2.
(A) Effect of external nitrate supply on H2O2 in maize seedlings, and (B) responses of root elongation of maize seedlings incubated in 0·01 mm and 10 mm nitrate solutions to exposure to exogenous H2O2 for 48 h. In (A), seedlings were cultivated for 7 d in nutrient solutions varying in nitrate concentration as indicated, and the H2O2 content in the root apices is expressed as relative to that observed in the 0·01 mm nitrate treatment. Data are means ± s.e. of three independent measurements. In (B), relative root elongation was determined in the presence of varying concentrations of H2O2 for seedlings grown in 0·01 or 10 mm nitrate solution, and the data are normalized to the value at 0·01 mm nitrate solution without H2O2. Data are mean ± s.e. of at least eight roots for each treatment. Different letters indicate significantly different values (P = 0·05).
In order to test whether the external nitrate-induced reduction in H2O2 concentration underlies the arrest of root elongation, the effect of exogenous H2O2 on root elongation growth in low (0·01 mm) and high (10 mm) nitrate solutions was investigated. Figure 2B shows that treatment of maize roots with varying concentrations of H2O2 (up to 500 mm) had no effect on root elongation regardless of the level of nitrate supply. This result suggests that the reduction of H2O2 levels by higher concentrations of external nitrate are unlikely to account for the nitrate-induced inhibition of root elongation.
Effect of IAA and NO on root growth under varying nitrate concentrations
No significant change in root elongation was found when maize seedlings grown in 0·01 mm nitrate were treated with 100 nm IAA (Fig. 3A). By contrast, the same treatment significantly enhanced elongation of roots incubated in 10 mm nitrate (Fig. 3A). Thus these results suggest that the inhibition of root growth caused by high nitrate may result from a reduction of auxin in the roots.
Fig. 3.
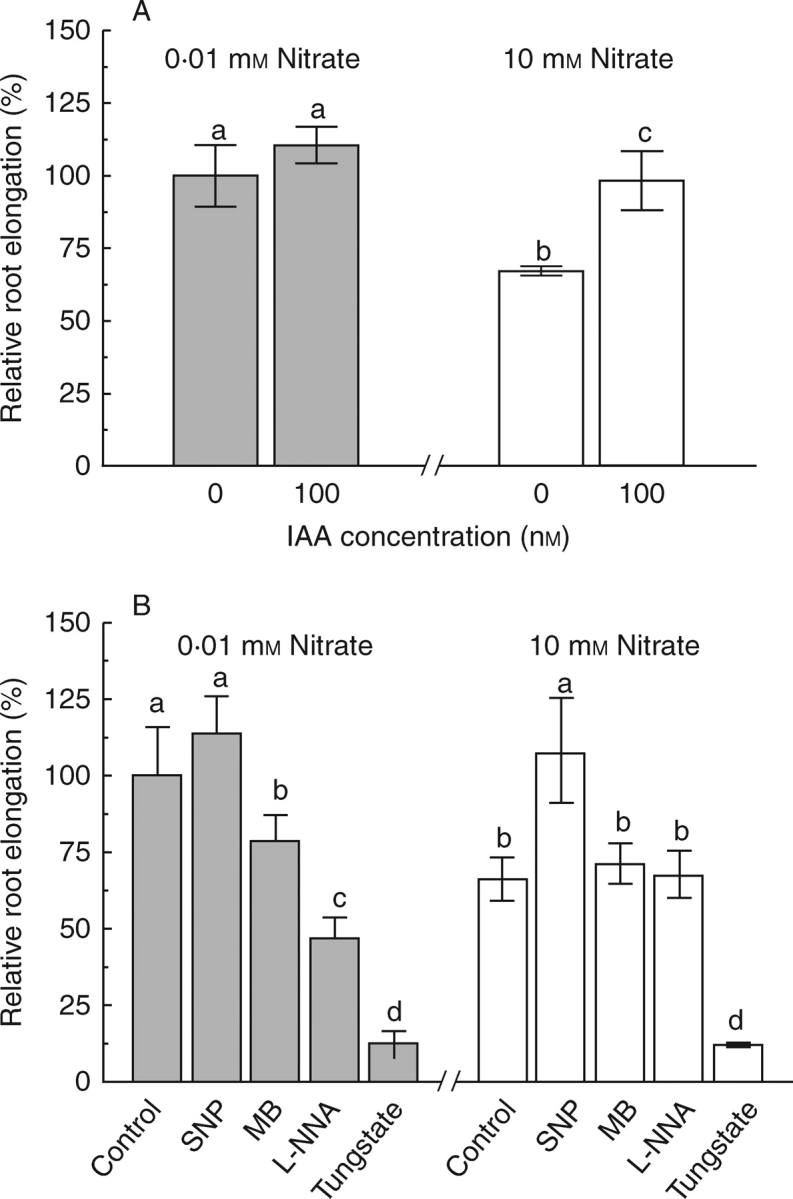
Responses of maize root elongation for seedlings grown in low- or high-nitrate solutions to (A) IAA, and (B) SNP, methylene blue (MB), L-NNA or tungstate. Seedlings were grown for 7 d in either 0·01 mm or 10 mm nitrate and then treated for 48 h with either 0·1 µm IAA, 1 µm SNP, 1 µm methylene blue, 100 µm L-NNA or 100 µm tungstate. Root elongation was expressed relative to the value obtained for seedlings grown in 0·01 mm nitrate without any other chemicals. Data are means ± s.e. of at least eight roots for each treatment. Different letters indicate significantly different values (P = 0·05).
The NO donor SNP, applied at 1 µm, reversed the root inhibition induced by 10 mm nitrate, but had no effect on root elongation of maize grown in 0·01 mm nitrate (Fig. 3B). Treatment with a NO scavenger (MB) and an antagonist of nitric oxide synthase (L-NNA) significantly inhibited root growth of maize seedlings grown in low-nitrate solution (0·01 mm), but these treatments had no effect on root growth of plants grown in high-nitrate solution (10 mm, Fig. 3B). To test whether the nitrate reductase (NR)-dependent NO production is involved in the high-nitrate-induced inhibition of root elongation, the effect of a NR inhibitor, tungstate, on root elongation of maize plants grown in high- and low- nitrate solution was investigated. Tungstate at 100 µm equally inhibited root elongation of maize seedlings grown in both high- and low-nitrate solutions (Fig. 3B). These findings indicate that the endogenous NO levels may have been reduced as a result of down-regulation of NOS activity in roots grown under high nitrate.
Effect of high external nitrate concentrations on endogenous NO levels in roots
Endogenous levels of NO in the root apices of maize seedlings grown in 10 mm nitrate solution were much lower than those in apices grown in 0·01 mm nitrate, as demonstrated by the observation of less DAF-2DA-dependent fluorescence intensity (Fig. 4A, E). When maize roots grown in 10 mm nitrate were treated with IAA and a NO donor, SNP, the endogenous NO levels in the root apices were significantly increased (Fig. 4E–G). The IAA-induced increase in the NO levels in root apical cells grown in high-nitrate solution was greater than that induced by SNP. By contrast, the same treatment had no effect on the endogenous NO levels in root apical cells grown in low-nitrate solution (Fig. 4A–C, G). Thus these findings indicate that high nitrate supply induces a reduction of endogenous NO levels in root apical cells of maize seedlings, and that the high-nitrate-induced reduction in the NO levels can be reversed by a supplement of external IAA and SNP. The NR inhibitor tungstate markedly increased the DAF-2DA-dependent fluorescence intensity of root apices grown in both low- and high-nitrate solutions, with the effect being greater in roots grown in low-nitrate solution than in high nitrate solution (Fig. 4G). No fluorescence signals were detected from the maize roots that were not loaded with DAF-2DA (data not shown), suggesting that the observations were not due to autofluorescence.
Fig. 4.
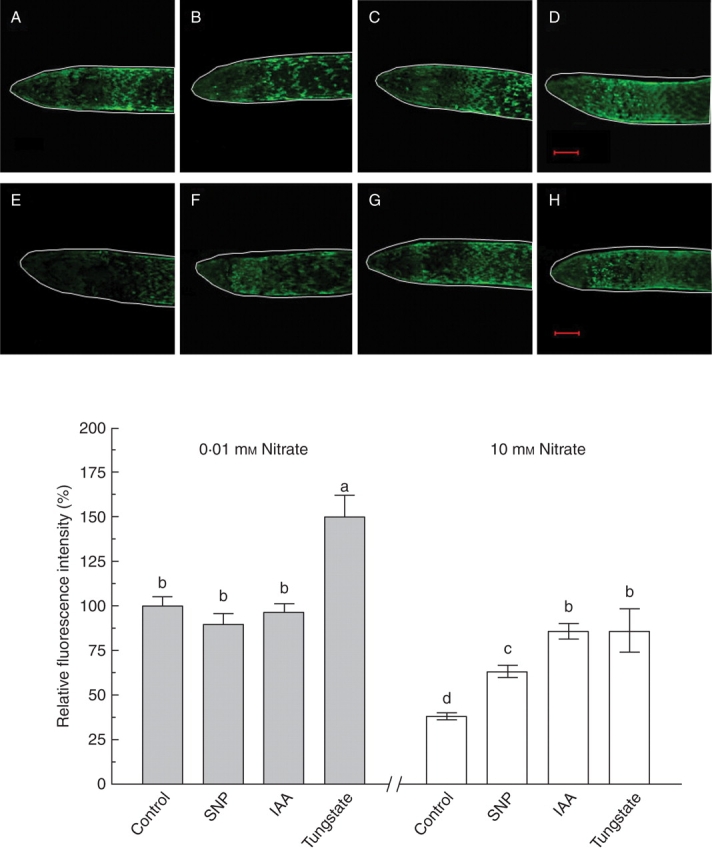
Effects of incubate with SNP, IAA and tungstate for 48 h on endogenous NO levels in primary root apices of maize plants grown in low- (0·01 mm) or high- (10 mm) nitrate solution for 7 d. The NO-specific fluorescent dye DAF-2DA was loaded into roots and fluorescence was detected by a confocal microscopy. Representative images are shown, and scale bars = 500 µm. (A–D) are roots grown in low nitrate (0.01 mM) without any other treatment (A), (B–D) are roots grown in low nitrate and treated with 1 µM SNP (B), 100 nM IAA (C) and 100 µM tungstate (D); (E–F) are roots grown in high nitrate (10 mM) without other treatment (E) and treated with 1 µM SNP (F), 100 nM IAA (G) and 100 µM tungstate (H), respectively. The graph below shows mean fluorescence intensities in the root apices expressed as pixel numbers relative to those measured in 0·01 mm nitrate (G). Data are means ± s.e. from measurements of at least six roots for each treatment. Different letters indicate significantly different values (P = 0·05).
DISCUSSION
It is well known that root growth and development are closely related to nitrate concentrations in the external medium, such that low and high concentrations of nitrate respectively stimulate and inhibit growth (Granatoand Raper, 1989; Zhang et al., 1999, 2000; Forde, 2002). The responses of root growth and development to external nitrate concentrations have frequently been studied on lateral roots (e.g. Forde, 2002). In the present study, we examined the effects of external nitrate supply on growth of primary roots of maize and found that elongation of these roots was markedly inhibited when they were grown in solutions with nitrate concentrations greater than 0·1 mm (Fig. 1). This result is in contrast to a previous study in which growth of primary roots in Arabidopsis was found to be independent of external nitrate concentrations ranging from 0·01 to 100 mm (Zhang and Forde, 1998). However, our results are in agreement with those of Linkohr et al. (2002), who reported that elongation of primary roots of Arabidopsis is reduced with increasing concentrations of nitrate in the growing medium.
One important finding in the present study is that the nitrate-induced inhibition of root elongation in maize was markedly reversed by treatments of the roots with a NO donor (SNP) and IAA (see Fig. 3). These findings prompt us to propose that the arrest of root elongation by high levels of external nitrate concentrations may result from an alteration of endogenous NO levels in root apical cells. Consistent with this hypothesis is that the endogenous NO levels in root apices of maize seedlings incubated in 10 mm nitrate were only 30 % of those grown in 0·01 mm nitrate (Fig. 4). When roots were treated with IAA, the endogenous NO levels in apices grown in high-nitrate solutions were increased to levels comparable with those grown in low-nitrate solutions (Fig. 4). Therefore, these findings provide experimental evidence to support that NO is likely to play a role in mediation of nitrate-dependent root growth in maize.
Endogenous NO production in plant cells is mainly catalysed by NR and NOS (Neill et al., 2003). A NR inhibitor (tungstate) induced a greater increase in the endogenous NO levels in maize root apical cells grown in low-nitrate solution than those grown in high-nitrate solution (Fig. 4G), but it had similar inhibitory effect on root elongation of plants grown in low- and high-nitrate solutions (Fig. 3). Activity of NR in the same maize genotype is enhanced with increasing external nitrate concentrations (Tian et al., 2005). It is expected that NR-mediated NO production would be greater in root cells grown in high-nitrate solution than in those grown in low-nitrate solution. In contrast, our results demonstrated that root cells incubated in high-nitrate solution had reduced NO production compared with those incubated in low-nitrate solution (Fig. 4A, E). Taken together, these observations suggest that NR-mediated NO production is unlikely to be involved in the nitrate-dependent NO production and root elongation. A NO scavenger and NOS inhibitor reduced root elongation in maize plants grown in the low-nitrate medium, while the same treatment had no effect on elongation of roots grown in the high-nitrate solution (Fig. 3). These findings suggest that NOS activity may be inhibited in plants grown in high-nitrate solution, thus leading to a reduction of the endogenous NO levels. To the best of our knowledge, the results presented in this study show for the first time that NOS-dependent NO production in plants is modulated by external nitrate supply.
NO levels in root apices of maize plants grown in the high-nitrate solutions were enhanced more by treatment with IAA than SNP (Fig. 4), but SNP and IAA were equalling effective in reversing the nitrate-induced inhibition of root elongation (Fig. 3). We are not able to provide an explanation for this difference, but it may be speculated that SNP concentration in the solution might have been reduced substantially after 48 h, thus resulting in the observed low endogenous NO levels. Furthermore, the findings that IAA reversed high-nitrate-induced inhibition of root elongation (Fig. 3) and induced elevation of endogenous NO levels in roots grown in high nitrate (Fig. 4) suggest that the effect of IAA on root elongation may occur through modulation of endogenous NO. This explanation is also in line with a previous study in which NO was shown to operate downstream of IAA in promoting adventitious root development (Pagnussat et al., 2003). Although our data indicate that both IAA and NO may be involved in nitrate-induced inhibition of root elongation, the mechanism underlying sensing and signalling of the external nitrate and how NO and IAA interact to affect root elongation remains to be unravelled. Future studies using Arabidopsis mutants that are defective in NO synthesis, auxin transport and sensing may potentially shed some light on this issue. Moreover, the present study is also consistent with observations that high concentrations of nitrate induced a decrease in auxin concentration in roots – specifically in root apices – of the same maize genotype (Tian et al., 2007a). In Arabidopsis, stimulation and inhibition of root growth by low and high nitrate supply have been suggested to result from a localized and systemic effect, respectively (Zhang and Forde, 1998). Our finding that high nitrate supply suppresses the endogenous NO levels in maize root apical cells suggests that NO may function as an important molecule in mediating the proposed systemic effect of nitrate on root growth.
An increase in accumulation of H2O2 in roots has been reported upon exposure of plants to nutrient-deficient solutions, including N-deficiency (Shin and Schachtman, 2005; Schachtman and Shin, 2007). The H2O2 plays an important role in mediating physiological changes for adaptation to the nutrient deficiency (Schachtman and Shin, 2007). In the present study, a similar increase in H2O2 concentration was observed with a decrease in the nitrate concentration in the external solution (Fig. 2A). There are reports showing that both cytochrome c-dependent respiration (Millar and Day, 1996) and respiration through the alternative pathway (Huang et al., 2002) are sensitive to NO. It is conceivable that alterations of the endogenous NO by the elevated external nitrate supply could affect respiration activities, resulting in reductions in H2O2 content. The reductions in H2O2 contents under high nitrate conditions appear to be a consequence rather than a cause, as treatment with exogenous H2O2 of maize roots grown under high nitrate did not alleviate the nitrate-induced inhibition of root elongation.
In conclusion, the present study reveals that endogenous NO levels in maize root apical cells were closely dependent upon nitrate supply and IAA, such that high nitrate supply reduced the endogenous NO levels and reductions in NO levels were markedly reversed by exogenous IAA. Thus, these findings indicate that high nitrate supply may reduce IAA levels and subsequently inhibits NOS activity, leading to a decrease in the endogenous NO level, which serves as a trigger to elicit nitrate-dependent root growth.
ACKNOWLEDGEMENTS
This research was supported by an innovative group research grant No. 30521002 from the National Natural Science Foundation of China and the Chinese Academy of Sciences through its Hundred Talent Program. We thank Dr Fan-Jun Chen, China Agricultural University, for kindly supplying the maize seeds.
LITERATURE CITED
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta. 2004;218:900–905. doi: 10.1007/s00425-003-1172-7. [DOI] [PubMed] [Google Scholar]
- Crawford NM, Galli M, Tischner R, Heimer YM, Okamoto M, Mack A. Response to Zemojtel et al. 2006. Plant nitric oxide synthase: back to square one. Trends in Plant Science. 2006;11:526–527. [Google Scholar]
- Desikan R, Griffiths R, Hancock J, Neill S. A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proceedings of the National Academy of Sciences USA. 2002;99:16314–16318. doi: 10.1073/pnas.252461999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde BG. Local and long-range signaling pathways regulating plant responses to nitrate. Annual Review of Plant Biology. 2002;53:203–224. doi: 10.1146/annurev.arplant.53.100301.135256. [DOI] [PubMed] [Google Scholar]
- Forde BG, Clarkson DT. Nitrate and ammonium nutrition of plants: physiological and molecular perspective. Advances in Botanical Research. 1999;30:1–90. [Google Scholar]
- Furchgott RF. Special topic: nitric oxide. Annual Review of Physiology. 1995;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- Gouvea CMCP, Souza JF, Magalhaes CAN, Martins IS. NO-releasing substances that induce growth elongation in maize root segments. Plant Growth Regulation. 1997;21:183–187. [Google Scholar]
- Granato TC, Raper CD. Proliferation of maize (Zea mays L) roots in response to localized supply of nitrate. Journal of Experimental Botany. 1989;40:263–275. doi: 10.1093/jxb/40.2.263. [DOI] [PubMed] [Google Scholar]
- Guo YF, Chen FJ, Zhang FS, Mi GH. Auxin transport form shoot to root is involved in the response of lateral root growth to localized supply of nitrate in maize. Plant Science. 2005;169:894–900. [Google Scholar]
- Huang X, von Rad U, Durner J. Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cells. Planta. 2002;215:914–923. doi: 10.1007/s00425-002-0828-z. [DOI] [PubMed] [Google Scholar]
- Kaiser WM, Weiner H, Kandlbinder A, Tsai CB, Rockel P, Sonoda M, Planchet E. Modulation of nitrate reductase: some new insights, an unusual case and a potentially important side reaction. Journal of Experimental Botany. 2002;53:875–882. doi: 10.1093/jexbot/53.370.875. [DOI] [PubMed] [Google Scholar]
- Lamattina L, Garcìa-Mata C, Graziano M, Pagnussat G. Nitrate oxide: the versatility of an extensive signal molecule. Annual Review of Plant Biology. 2003;54:109–36. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- Linkohr BI, Williamson LC, Fitter HA, Leyser HMO. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. The Plant Journal. 2002;29:751–760. doi: 10.1046/j.1365-313x.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- Millar AH, Day DA. Nitric oxide inhibits the cytochrome oxidase but not the alternative oxidase of plant mitochondria. FEBS Letters. 1996;398:155–158. doi: 10.1016/s0014-5793(96)01230-6. [DOI] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Hancock J. Hydrogen peroxide signaling. Current Opinion in Plant Biology. 2002;5:388–395. doi: 10.1016/s1369-5266(02)00282-0. [DOI] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Hancock JT. Nitric oxide signaling in plants. New Phytologist. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L. Nitric oxide is required for root organogenesis. Plant Physiology. 2002;129:954–956. doi: 10.1104/pp.004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Lanteri ML, Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiology. 2003;132:1241–1248. doi: 10.1104/pp.103.022228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Shin R. Nutrient sensing and signaling: NPKS. Annual Review of Plant Biology. 2007;58:47–69. doi: 10.1146/annurev.arplant.58.032806.103750. [DOI] [PubMed] [Google Scholar]
- Shin R, Berg RH, Schachtman DP. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiology. 2005;46:1350–1357. doi: 10.1093/pcp/pci145. [DOI] [PubMed] [Google Scholar]
- Signora L, Smet ID, Foyer CH, Zhang H. ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. The Plant Journal. 2001;28:655–662. doi: 10.1046/j.1365-313x.2001.01185.x. [DOI] [PubMed] [Google Scholar]
- Stohr C, Stremlau S. Formation and possible role of nitric oxide in plant roots. Journal of Experimental Botany. 2006;57:463–470. doi: 10.1093/jxb/erj058. [DOI] [PubMed] [Google Scholar]
- Stohr C, Strube F, Marx G, Ullrich WR, Rockel P. A plasma membrane-bound enzyme of tobacco roots catalyses the formation of nitric oxide from nitrite. Planta. 2001;212:835–841. doi: 10.1007/s004250000447. [DOI] [PubMed] [Google Scholar]
- Tian QY, Chen FJ, Zhang FS, Mi GH. Possible involvement of cytokinin in nitrate-mediated root growth in maize. Plant and Soil. 2005;100:1–12. [Google Scholar]
- Tian QY, Chen FJ, Liu JX, Zhang FS, Mi GH. Journal of Plant Physiology. 2007a. Inhibition of maize root growth by high nitrate supply is correlated to reduced IAA levels in roots. (in press) [DOI] [PubMed] [Google Scholar]
- Tian QY, Sun DH, Zhao MG, Zhang WH. Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos L. New Phytologist. 2007b;174:322–331. doi: 10.1111/j.1469-8137.2007.02005.x. [DOI] [PubMed] [Google Scholar]
- Tun NN, Holk A, Scherer GF. Rapid increase of NO release in plant cell cultures induced by cytokinin. FEBS Letters. 2001;509:174–176. doi: 10.1016/s0014-5793(01)03164-7. [DOI] [PubMed] [Google Scholar]
- Veljovic-Jovanovic S, Noctor BG, Foyer CH. Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiology and Biochemistry. 2002;40:501–507. [Google Scholar]
- Zhang H, Forde BG. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]
- Zhang H, Forde BG. Regulation of Arabidopsis root development by nitrate availability. Journal of Experimental Botany. 2000;51:51–59. [PubMed] [Google Scholar]
- Zhang H, Jennings A, Barlow PW, Forde BG. Dual pathways for regulation of root branching by nitrate. Proceedings of National Academy of Sciences USA. 1999;96:6529–6534. doi: 10.1073/pnas.96.11.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemojtel T, Frohlich A, Palmieri MC, Kolanczyk M, Mikula I, Wyrwicz LS, et al. Plant nitric oxide synthase: a never-ending story? Trends in Plant Science. 2006;11:524–525. doi: 10.1016/j.tplants.2006.09.008. [DOI] [PubMed] [Google Scholar]