Abstract
Background and Aims
Legume nitrogen is derived from two different sources, symbiotically fixed atmospheric N2 and soil N. The effect of genetic variability of root and nodule establishment on N acquisition and seed protein yield was investigated under field conditions in pea (Pisum sativum). In addition, these parameters were related to the variability in preference for rhizobial genotypes.
Methods
Five different spring pea lines (two hypernodulating mutants and three cultivars), previously identified in artificial conditions as contrasted for both root and nodule development, were characterized under field conditions. Root and nodule establishment was examined from the four-leaf stage up to the beginning of seed filling and was related to the patterns of shoot dry matter and nitrogen accumulation. The genetic structure of rhizobial populations associated with the pea lines was obtained by analysis of nodule samples. The fraction of nitrogen derived from symbiotic fixation was estimated at the beginning of seed filling and at physiological maturity, when seed protein content and yield were determined.
Key Results
The hypernodulating mutants established nodules earlier and maintained them longer than was the case for the three cultivars, whereas their root development and nitrogen accumulation were lower. The seed protein yield was higher in ‘Athos’ and ‘Austin’, the two cultivars with increased root development, consistent with their higher N absorption during seed filling.
Conclusion
The hypernodulating mutants did not accumulate more nitrogen, probably due to the C cost for nodulation being higher than for root development. Enhancing exogenous nitrogen supply at the end of the growth cycle, by increasing the potential for root N uptake from soil, seems a good option for improving pea seed filling.
Key words: Pisum sativum, hypernodulating mutants, nodules, roots, nitrogen fixation, mineral nitrogen absorption, seed protein content, Rhizobium leguminosarum biovar viciae
INTRODUCTION
In Western Europe, Pea (Pisum sativum) seeds constitute an alternative to soybean as a protein-rich feedstock. However, seed yields and protein contents are highly variable, largely because of biotic and environmental stresses. Nitrogen nutrition is one of the key processes involved in determining yield (Sagan et al., 1993). As for other legumes, in pea nitrogen nutrition relies in part on mineral nitrogen absorption by roots and mostly on symbiotic fixation of atmospheric nitrogen occurring in nodules. Nitrogen-fixing activity varies during the pea growth cycle, increasing during the vegetative phase up to flowering, concomitant with the development of nodules, which represent the largest carbon sink for the plant (Voisin et al., 2003b). Conversely, at the end of the growth cycle, filling seeds become the largest carbon sink (Jeuffroy and Warembourg, 1991) at the expense of roots and, in particular, nodules. Thus, both symbiotic fixation and mineral N root absorption decline during seed filling (Jensen, 1987; Voisin et al., 2003a). Seed growth is consequently dependent on N remobilization from vegetative tissues (Pate and Flinn, 1973; Jensen, 1987; Schiltz et al., 2005). This affects photosynthesis and eventually curtails the seed-filling period because it induces senescence of the aerial parts (Sinclair and de Wit, 1975). Therefore, it would be desirable to increase the exogenous nitrogen supply at the end of the growth cycle, to reduce or delay N remobilization and improve pea seed filling (Salon et al., 2001).
One strategy to enhance exogenous nitrogen supply late during the growth cycle could be to select pea lines with a prolonged period of nodule development, and thus symbiotic N2 fixation, during seed filling. In grain legumes, the presence of existing nodules inhibits nodulation on younger root tissues, a phenomenon termed autoregulation of nodulation (Caetano-Anolles and Gresshoff, 1991). Mutagenesis experiments have confirmed the host's control of nodulation (Engvild, 1987; Duc and Messager, 1989; Caetano-Anolles and Gresshoff, 1991). Hypernodulating mutants are potential candidates for enhancing N2 fixation through an increase in nodule number: they are defective in the autoregulation of nodulation (Sagan and Gresshoff, 1996). Three different genes have been shown to control nodule number in pea: NOD3 (Postma et al., 1988), SYM28 and SYM29 (Sagan and Duc, 1996). Roots are the principal source of the systemic signal contributing to hypernodulation phenotype in nod3 mutant lines (Postma et al., 1988), whereas the hypernodulation phenotype of sym28 and sym29 mutants is under shoot control (Sagan and Duc, 1996). However, various sym28 and sym29 hypernodulating mutants did not accumulate more N than the wild-type, and accumulated less dry matter in the aerial parts (Sagan et al., 1993; Salon et al., 2001). New nod3 or sym29 mutant pea lines have been recently selected (Krusell et al., 2002), in which the high-level consumption of assimilates triggered by symbiosis is less detrimental to shoot biomass accumulation than in the previous mutants studied.
Studying the plant genotype × rhizobial strain combination represents another way of improving nitrogen fixation. Significant effects of rhizobial strain and of strain × pea cultivar interactions on nodule and shoot biomass or N acquisition have been reported (Skot, 1983; Fezenko et al., 1995; Martensson and Rydberg, 1996). Moreover, a Rhizobium strain variant was found to extend the meristematic activity of nodules, increasing nodule dry matter and branching in pea (Sagan and Gresshoff, 1996).
Another strategy to improve late exogenous nitrogen acquisition could be selection of pea lines with increased root development, thus enhancing soil mineral N uptake during seed filling. More developed root systems should also improve water uptake under drought conditions, which are frequently encountered in late spring and affect N fixation in particular. Despite the vital role of roots for both water and nutrient uptake and tolerance to root diseases, very few experiments have been performed to investigate the genetic variation in root establishment of pea. Most of the available data result from experiments conducted under controlled conditions on young seedlings (Ali-Khan and Snoad, 1977; McPhee, 2005). Although Veitenheimer and Gritton (1984) observed similar relative differences in root dry weight at flowering, for four pea lines studied either in the field or in the greenhouse, they pointed out that both growth stage and culture medium had significant effects on ranking for root traits. Because root number can increase during the reproductive phase (Thorup-Kristensen, 1998; Voisin et al., 2002), additional studies at different stages of plant growth would be advisable for selecting pea lines with improved root development.
The aims of the present study were: (1) to investigate under field conditions how root and nodule genetic variability previously identified in artificial conditions may influence N acquisition throughout the growth cycle; (2) to understand whether differences in seed protein yield among pea genotypes were related to differences in nitrogen and carbon acquisition; and (3) to investigate whether variability for developmental responses and N acquisition was associated with variability in preference for rhizobial genotypes.
MATERIALS AND METHODS
Plant material
Five lines of pea Pisum sativum L. were selected from previous greenhouse experiments on the basis of either contrasting nodulation ability or variation in root development. P118 and P121 are mutants of the leafy cultivar ‘Frisson’ (registered in 1979 in the French variety catalogue) derived from ethyl methane sulfonate mutagenesis. They were both selected for their hypernodulation character and the maintenance of shoot dry matter production, as compared with their isogenic parent line. P118 and P121 are mutants of the SYM29 and NOD3 genes, respectively. Because there is no mutant for root development available in the ‘Frisson’ genetic background, two pea cultivars were chosen, ‘Athos’ and ‘Austin’, having higher root biomass than ‘Frisson’ but similar aerial development rate and date of beginning of flowering in greenhouse tests. ‘Athos’ and ‘Austin’ are recent afila spring pea cultivars widely cultivated in France.
Site and soil characteristics
The field study was carried out at Labruyère (Côte d'Or, France). The soil is a loamy sand with a pH (in H2O) of 7·3. At the sowing date, the soil contained about 2·85 mg kg−1 of mineral N (NO3− and NH4+) and 6 g kg−1 of organic C in the ploughed layer (0–30 cm). One week after sowing, 0·1 g N m−2 (i.e. 0·28 mg kg−1) of 15N-labelled ammonium nitrate (1 % 15N) was applied. The soil P and K contents (P = 70 mg kg−1; K = 150 mg kg−1) were non-limiting for the crops. The size of indigenous Rhizobium leguminosarum bv. viciae populations was estimated by plant tests following the method of the most probable numbers (MPN) as previously described (Louvrier et al., 1995). The MPN of rhizobia were in the range 103–105 g−1 soil in the 0–30-cm layer (three replicate soil samples analysed), which was considered to be non-limiting for nodulation (Ballard et al., 2004). Weeds, pests and diseases were well controlled by adequate pesticides used in conventional agricultural practices. Irrigation was provided at the beginning and end of flowering to avoid any drought stress.
Field experimental design
The field was subdivided into two neighbouring blocks. Within each block, the five pea genotypes were sown on 12 March, 2002, in a randomized design with three replicates. One block was used for successive plant samplings during plant growth cycle, the other for mechanized seed yield determination at harvest. The 15N isotope dilution technique was used to estimate the contribution of symbiotic N fixation to overall N acquisition by the pea genotypes, with spring barley (Hordeum vulgare L.) as the non-fixing reference crop (Voisin et al., 2002). The pea and barley plants were grown in six-row plots of 7-m length with 20 cm between rows and 50 cm between plots, at a density of, respectively, 80 and 350 plants m−2.
Sampling in the field
At five successive dates from the four-leaf stage to physiological maturity, roots and aerial parts of pea genotypes were harvested in the three replicate plots of the block reserved for plant sampling. In each plot and for each sampling date, a new sampling area 80 cm in length was delimited in the four central rows. Within this area, two different root samples of 4–7 plants each were extracted by the excavation of two different soil blocks whose dimensions were 40 cm depth, 25 cm length and 15 cm breadth. Each excavated block was centred within one of the four central rows of the delimited area. The aerial parts of the plants from each soil block were cut before soil excavation. These plants were used for developmental observation: total number of nodes, node of first flower and number of podded nodes. Vegetative parts were oven-dried for shoot dry matter measurement and further nitrogen content determination. The root systems of the plants from each soil block were placed on a sieve and carefully extracted by hand-crumbling the soil around them. Root systems were then rinsed with water. Successive sieves of 1·5-cm and 2-mm mesh size were used to prevent the loss of any fine roots.
All of the remaining plants in the selected area were cut above ground and counted in order to determine plant density. After oven drying at 80 °C for 48 h, shoot dry matter was measured. Nitrogen content measurement and isotopic analyses were conducted later with these dried samples.
Plant measurement procedures
Root system analyses
For the first four sampling dates, i.e. from the four-leaf stage to the beginning of seed filling (BSF), water-washed root samples were placed in plastic bags and stored at 4 °C. Within the following week, two different root samples per genotype and replicate were separately used. The first root sample was used for measuring separately nodules and root characteristics. A sub-sample of 20 nodules was randomly collected from this root sample of 4–7 plants and weighed. All the nodules remaining on the root systems were then carefully separated from the roots, and the fresh and dry matter weights were determined, as well as the corresponding root dry matter weight after oven drying at 80 °C for 48 h. Two to three representative intact root systems were selected from the second root sample. Each root system was carefully spread out in a transparent tray with a shallow layer of water, adjusted to help untangle the roots and minimize root overlapping. Eight-bit grey-level images of spread root systems were then recorded from above with a camera driven by the software Samba (TITN-Alcatel, Grenoble, France). Total root length and surface area were determined from analysis of the images, using a program developed locally on the software Visilog 5·4 (Noesis, France). After image analysis, the intact root systems selected were pooled together with the remaining root systems from the same second root sample, then oven-dried and weighed.
At the last sampling date, i.e. physiological maturity, both root and nodule compartments had begun to decay and the two different root samples were directly used for nodulated root dry matter determination.
Characterization of R. leguminosarum bv. viciae by PCR fingerprinting
At the beginning of flowering, the randomly collected sub-sample of 20 nodules per plot was used for isolation and characterization of the rhizobial populations. The procedure of isolation of R. leguminosarum bv. viciae from nodules was previously described by Vincent (1970). The isolates were maintained on MGY agar medium (Louvrier et al., 1995) at 4 °C. The isolates were characterized by restriction fragment length polymorphism analysis of PCR-amplified DNA fragments (PCR-RFLP) with restriction enzyme HaeIII as previously described (Laguerre et al., 2003). The target DNAs were the intergenic spacer regions between 16S and 23S rDNA (IGS) and the nodulation gene region nodD–F.
Shoot nitrogen content and estimation of N remobilized in seeds
Nitrogen content of ground samples was determined by the Kjeldahl procedure. From the four-leaf stage to BSF, ground samples came from whole aerial parts. At physiological maturity, they constituted vegetative parts. At harvest, ground samples of seeds were analysed.
The amount of N remobilized in seeds during seed filling was estimated as the decline in amount of nitrogen accumulated in vegetative aerial parts between BSF and physiological maturity.
Measurement of symbiotic N fixation
The percentage of nitrogen derived from symbiotic fixation (%Ndfa) was determined at BSF and at physiological maturity, using the isotope dilution technique (Mariotti et al., 1983; Duc et al., 1988):
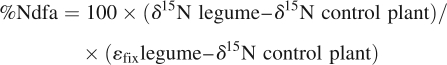 |
where δ15N legume and δ15N control plant are the isotopic compositions of the legume plant and of the non-fixing control plant, respectively. Comparing an N2-fixing legume cultivar with an isogenic non-nodulating line is the best strategy to estimate the percentage of symbiotic fixation, and data obtained using a non-isogenic mutant cannot be used for this estimation (Biedermannova et al., 2002). As the non-nodulating mutant specific for all the pea cultivars experimented was not available, the spring barley cultivar ‘Scarlett’ was used, which gave good estimations of %Ndfa in previous field experiments (Voisin et al., 2002). εfix legume is the isotopic enrichment factor of the legume plant for which N2 fixation is the only N source. According to Mariotti et al. (1980), the value of εfix legume is –1 ‰ for pea. However, considering the large variability between the five pea genotypes studied for their nodulation ability and according to previous studies (Mariotti et al., 1980; Ledgard, 1989), it was suspected that the value of εfix depends both upon the pea genotype and the stage of development. Therefore, a specific greenhouse experiment was conducted in order to determine the εfix of each of the five pea genotypes at BSF and at physiological maturity.
Seed yield at harvest
In the reserved block with three replicate plots per pea plant genotype, seeds were mechanically harvested then weighed for seed yield determination, when their water contents were about 14 %, i.e. on 5 July, 2002 for the three earliest genotypes, ‘Frisson’, P118 and P121 and on 12 July, 2002 for the two remaining cultivars ‘Athos’ and ‘Austin’. The thousand-seed weight of each plot was estimated by weighing two different samples of 250 seeds.
Calculations and statistical analyses
Plant development throughout the growing season in the field experiment was expressed as a function of cumulative degree-days (°Cd) from sowing, using a 0 °C-base temperature (Ney and Turc, 1993).
Analyses of variance for all the plant characters and for the proportions of rhizobial genotypes (after arcsine transformation) were performed using XLSTAT software (version 7·5·2, http://www.xlstat.com), testing both genotype and replicate effects. These analyses did not reveal any significant replicate effect (data not shown). Means were classified using the least significant difference (LSD) range test at the 0·05 probability level.
RESULTS
Shoot biomass accumulation
There was a general increase in shoot dry matter pattern throughout the growth cycle (Fig. 1A). After a slow increase from the four-leaf until the ten-leaf stage, a five-fold increase in shoot dry matter was observed for all the five pea genotypes before seed filling followed by a slower increase up to physiological maturity. However, two different groups of genotypes could be distinguished according to their shoot dry matter accumulation during seed filling. For ‘Frisson’ and the two mutants, the shoot dry matter increased by less than 40 % during seed filling, and the lowest shoot dry matter was recorded for P118. In the second group constituted by ‘Athos’ and ‘Austin’, the increase during seed filling was higher than 50 % and ‘Athos’ clearly had the highest shoot biomass. At physiological maturity, the total shoot dry matter was higher than 1 kg m−2 for ‘Athos’ and ‘Austin’, which was significantly different from the values of about 800 g observed for the two mutants and ‘Frisson’ (Fig. 1A). The differences in shoot dry biomass between genotypes were not correlated to differences in rate of leaf appearance, as the total number of nodes at physiological maturity was about 19 for ‘Frisson’ and P118, 17 for P121 and ‘Austin’, and 16 for ‘Athos’ (data not shown). Conversely, the total shoot dry matter accumulation at physiological maturity was correlated positively to the root dry matter (r = 0·82) and negatively to the nodule dry matter at BSF (r = −0·73).
Fig. 1.
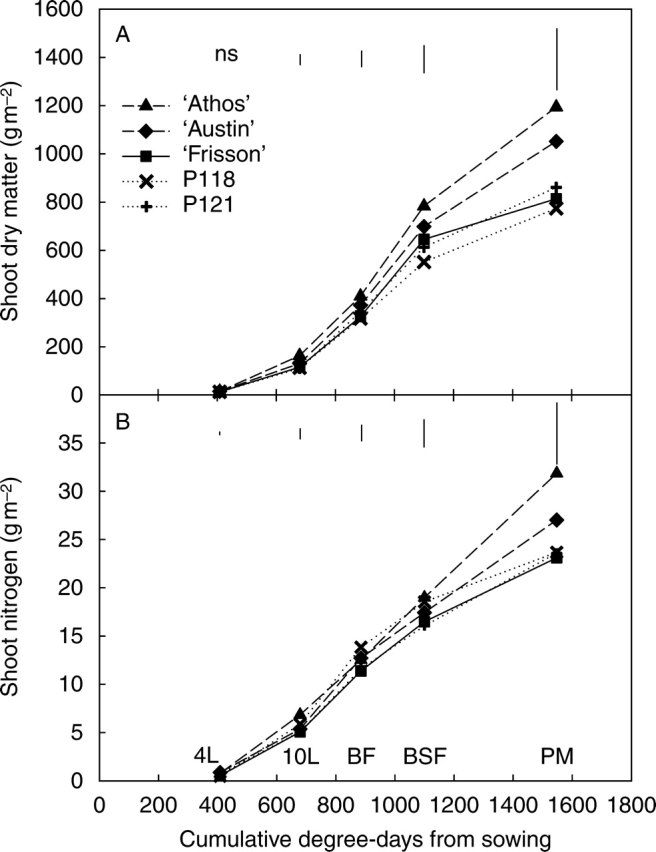
(A) Shoot dry matter and (B) shoot nitrogen of five pea genotypes, from the four-leaf stage until physiological maturity. 4L, 10L, BF, BSF and PM indicate the developmental stages, and correspond to four-leaf stage, ten-leaf stage, beginning of flowering, beginning of seed filling and physiological maturity, respectively. Each point is the mean value of three replicates. Vertical bars represent LSD (P < 0·05).
Root growth pattern
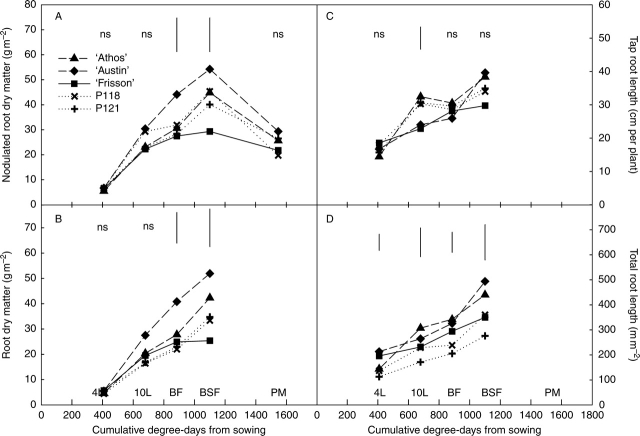
The nodulated root dry biomass per plant, which includes both nodule and root biomass, was about 6 g m−2 and similar for all of the five genotypes at the four-leaf stage (Fig. 2A). Thereafter, there was a general trend in root biomass pattern across time: root biomass increased from the four-leaf stage up to BSF, and then decreased until physiological maturity when most of the roots were decaying. However, differences in root biomass patterns were observed among the genotypes. ‘Austin’ displayed the highest increase up to BSF, whereas the nodulated root biomass of the four other lines increased less between the ten-leaf stage and the beginning of flowering (BF). After BF, a major increase in biomass occurred for ‘Athos’ and the hypernodulating mutants, but not for ‘Frisson’. At BSF, ‘Austin’ displayed the highest biomass incorporation in nodulated roots followed by ‘Athos’ and the two hypernodulating mutants. ‘Frisson’ had the lowest value at about 29 g m−2.
Fig. 2.
(A) Nodulated root dry matter, (B) root dry matter, (C) tap root length and (D) total root length of five pea genotypes, from the four-leaf stage until physiological maturity or the beginning of seed filling. 4L, 10L, BF, BSF and PM indicate the developmental stage and correspond to four-leaf stage, ten-leaf stage, beginning of flowering, beginning of seed filling and physiological maturity, respectively. Each point is the mean value of three replicates. Vertical bars represent LSD (P < 0·05).
At the four-leaf stage, the root biomass was similar at about 5 g m−2 for all five genotypes (Fig. 2B). As already observed for the nodulated root biomass, root biomass continued to rise until BSF for all genotypes except ‘Frisson’, for which the increase stopped at the beginning of flowering. However, for P121 and especially for P118, root biomass accumulation was different from that of nodulated roots: the increase was less pronounced before the ten-leaf stage. At BSF, the root biomasses of ‘Austin’ and ‘Athos’ were 34 and 20 % higher, respectively, than that of the mutants.
The tap and total (tap + primary laterals) root length of ‘Athos’ greatly increased up to the ten-leaf stage and then rose slowly or stabilized (Fig. 2C, D), whereas for ‘Austin’, both parameters increased rapidly up to BSF after a slow increase up to the time of flowering. However, at BSF, both cultivars attained a similar highest value of about 500 m m−2 of total length with 40 cm of tap root length. As seen for ‘Athos’, the tap root length of the two hypernodulating mutants increased mainly before the ten-leaf stage, and at this stage was significantly longer than for ‘Frisson’ and ‘Austin’. For all other stages, the tap root length was very similar between all genotypes. Regardless of stage, the total root length per plant was significantly reduced for P121 compared with its parental line ‘Frisson’, while the value was intermediate for P118. Grey-level camera images of spread root systems at the ten-leaf stage showed that ‘Athos’, ‘Austin’ and ‘Frisson’ had longer primary lateral roots than the two mutants P118 and P121 (data not shown). Most of the nodules of ‘Athos’ and ‘Austin’ were on the tap root, but there were more secondary lateral roots on ‘Athos’ than on ‘Austin’. ‘Frisson’ and its mutant P118 had very large numbers of nodules along their primary lateral roots. Conversely, mutant P121 had many secondary lateral roots and most of the nodules were located on the tap root and at the base of the primary lateral roots.
Variation in nodulation and rhizobial population
At the four-leaf stage, ‘Frisson’, ‘Athos’ and ‘Austin’ had about 2000 nodules m−2, whereas mutant P118 had a significantly higher number of 8000 (Fig. 3A), and mutant P121 had an intermediate value of 5000 nodules m−2. Whereas for the two hypernodulating mutants, nodule number continued to rise thereafter exponentially, a linear progression in nodule number was observed for ‘Frisson’, ‘Athos’ and ‘Austin’. At BSF, P118 and P121 had more than 70 000 nodules m−2, whereas there were about 20 000 for ‘Frisson’ and 12 000 for ‘Athos’ and ‘Austin’.
Fig. 3.
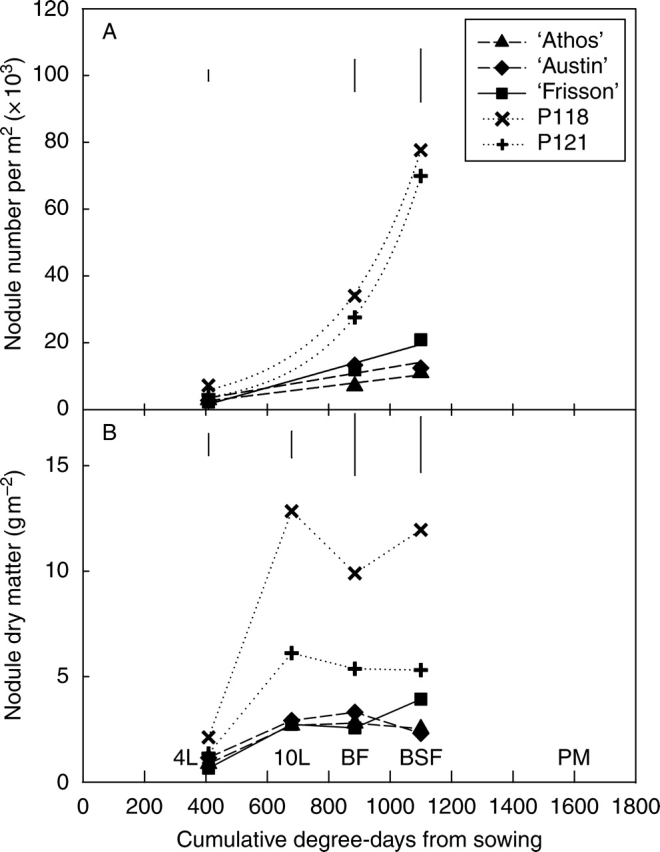
(A) Nodule number and (B) nodule dry matter of five pea genotypes, from the four-leaf stage until physiological maturity or the beginning of seed filling. 4L, 10L, BF, BSF and PM indicate the developmental stage, and correspond to four-leaf stage, ten-leaf stage, beginning of flowering, beginning of seed filling and physiological maturity, respectively. Each point is the mean value of three replicates. Vertical bars represent LSD (P < 0·05).
The nodule dry biomass per plant was significantly higher for P118 than for ‘Frisson’ as early as the four-leaf stage (Fig. 3B). All the other lines had intermediate values. Nodule dry biomass increased thereafter, and peaked for all five genotypes at around the ten-leaf stage, with significantly higher values for the hypernodulating mutants than for the other genotypes. From this stage up to BSF, total nodule biomass either stabilized (P118, P121, ‘Athos’ and ‘Austin’) or slightly increased (‘Frisson’).
The genetic structure of the nodulating populations associated with the five different pea genotypes was investigated at the beginning of flowering. An average of 52 nodule isolates was characterized for each pea genotype by PCR-RFLP with two molecular markers, the rDNA IGS and the nodD gene regions. Six IGS and six distinct nod haplotypes were identified among a total of 259 isolates with a total of 13 composite genotypes defined by the IGS/nod type associations. Seven of these 13 genotypes were predominant, comprising more than 5 % of at least one of the five populations associated with the five pea genotypes. The rhizobial genotype frequency varied according to the pea genotype (Table 1). The three populations related to ‘Frisson’ and its mutants could be differentiated from the two populations associated with ‘Athos’ and ‘Austin’ by their significantly higher proportion of genotype 6/b, and their smaller proportion of genotype 6/a. However, some differences were observed between the population from P118 and those from ‘Frisson’ and P121, as the former showed a lower frequency of the genotype 16/c and a preponderance of the genotypes 17/a and 17/b. Similarly, the ‘Athos’ population differed from the ‘Austin’ one by a lower proportion of genotype 6/b.
Table 1.
Distribution (%) of rhizobial isolates from the Labruyère field in 13 genotypes defined by IGS/nod type associations*
| Rhizobial population sampled from | 6/a | 6/b | 16/c | 17/a | 17/b | 18/a | 18/b | Others† |
|---|---|---|---|---|---|---|---|---|
| ‘Athos’ | 18a | 9c | 2b | 18a | 27a | 9a | 7a | 10 |
| ‘Austin’ | 18a | 16b | 10ab | 16a | 25a | 14a | 0a | 1 |
| ‘Frisson’ | 9a | 31a | 17a | 7a | 17a | 11a | 4a | 4 |
| P118 | 6a | 25a | 8ab | 24a | 22a | 10a | 4a | 1 |
| P121 | 9a | 30a | 15a | 9a | 17a | 11a | 2a | 7 |
* The IGS haplotypes are identified by numbers and the nod haplotypes by letters following the classification given in Depret et al. (2004). Means followed by different letters are significantly different based on multiple comparisons (LSD test) at the 5 % probability level.
† The distribution is given for the seven predominant rhizobial genotypes (>5 % in at least one population); the isolates distributed in six other genotypes were pooled.
Nitrogen fixation and accumulation
Significant differences for nitrogen accumulation were observed among the five genotypes (Fig. 1B). However, it was not possible to distinguish the same two groups as for dry matter accumulation. P118 shared with ‘Athos’ the highest shoot N accumulation from the ten-leaf stage until BSF stage, whereas ‘Frisson’ and P121 both had the lowest N accumulation and ‘Austin’ was intermediate. During seed filling, shoot N accumulation in ‘Athos’ was at the same rate as before seed filling (29 mg m−2 °Cd−1). This rate was reduced for ‘Austin’, ‘Frisson’ and P121 from about 27 to 17 mg m−2 °Cd−1 and fell even further for P118, from 30 to 11 mg m−2 °Cd−1. Finally, at physiological maturity, shoot nitrogen accumulation was significantly correlated to shoot dry matter accumulation (r = 0·96). At the end of its growth cycle, P118 had accumulated no more nitrogen than ‘Frisson’ or P121 (23 g m−2), which was lower than the amount reached by ‘Athos’ and ‘Austin’ (32 and 27 g m−2, respectively).
The respective contributions of symbiotic fixation and root N absorption to shoot N accumulation varied during the pea growth cycle and depended upon the pea genotype. The percentage of N derived from symbiosis was higher at BSF than at maturity for all the genotypes except for ‘Frisson’ (Table 2). When compared with the other genotypes, ‘Frisson’ had the highest contribution of symbiotic nitrogen fixation at BSF and especially at maturity, whereas P121 had the lowest values. P118 was intermediate with values slightly higher than for ‘Athos’ and ‘Austin’. However, ‘Frisson’ accumulated less nitrogen from fixation than did P118 before seed filling, and less than ‘Athos’ throughout the crop cycle. P121 was always the genotype with the least N accumulation deriving from fixation, throughout the growth cycle. By contrast, P121 accumulated large amounts of N derived from absorption, although not higher than ‘Athos’. At BSF, the shoot N accumulation was significantly correlated with the amount of N derived from fixation, but not with the amount of N derived from absorption (Table 3). During seed filling, exogenous N supply still depended on N2 fixation but more on mineral N absorption than at earlier stages (Table 3). Of the five pea genotypes studied, ‘Athos’ and P118 were those with the highest N accumulation before seed filling, and also the highest estimated amount of N remobilized in seeds during seed filling, significantly higher than for P121 (Table 2). ‘Austin’ and ‘Frisson’ had remobilized intermediate quantities. There was a significant positive correlation between the estimated amount of apparent N remobilized in seeds during seed filling and shoot N accumulation, especially from N2 fixation, before seed filling (Table 3).
Table 2.
Nitrogen accumulation and remobilization for the five pea genotypes studied
| Genotype | Shoot N accumulation from fixation (%) |
Shoot N accumulation from fixation (g m−2) |
Shoot N accumulation from absorption (g m−2) |
N remobilization in seeds during seed filling from vegetative aerial parts (g m−2)* | |||
|---|---|---|---|---|---|---|---|
| At beginning of seed filling | At maturity | At beginning of seed filling | At maturity | At beginning of seed filling | At maturity | ||
| ‘Athos’ | 86·9ab | 80·3b | 16·5a | 25·6a | 2·5ab | 6·3a | 13·7a |
| ‘Austin’ | 86·5ab | 82·6b | 15·1ab | 22·4ab | 2·3ab | 4·6b | 12·3ab |
| ‘Frisson’ | 88·6a | 91·3a | 14·6ab | 21·1ab | 1·9b | 2·0c | 12·6ab |
| P118 | 88·2a | 83·3b | 16·3a | 19·8b | 2·2ab | 3·9b | 13·7a |
| P121 | 81·3b | 75·0c | 13·1b | 17·7b | 3·0a | 5·9a | 10·0b |
Results of LSD-range test are shown where means followed by different letters are significantly different at the 5 % probability level.
* N remobilized in seeds = N accumulated in vegetative aerial parts at beginning of seed filling minus N accumulated in vegetative aerial parts at maturity.
Table 3.
Matrix of correlation coefficients between N accumulation parameters at the beginning and during seed filling
| At the beginning of seed filling |
During seed filling |
||||||
|---|---|---|---|---|---|---|---|
| Shoot N accumulation (1) | Shoot N accumulation from fixation (2) | Shoot N accumulation from absorption (3) | Shoot N accumulation (4) | Shoot N accumulation from fixation (5) | Shoot N accumulation from absorption (6) | N remobilization in seeds (7) | |
| (1) | |||||||
| (2) | 0·96* | ||||||
| (3) | –0·20 ns | –0·46 ns | |||||
| (4) | 0·41 ns | 0·30 ns | 0·25 ns | ||||
| (5) | 0·29 ns | 0·29 ns | –0·12 ns | 0·90* | |||
| (6) | 0·43 ns | 0·17 ns | 0·75 ns | 0·73 ns | 0·36 ns | ||
| (7) | 0·85* | 0·96* | –0·67 ns | 0·18 ns | 0·29 ns | –0·07 ns | |
ns, non-significant; *, significant at the 0·05 level.
The protein content in mature seeds was significantly higher for the two mutants than for their parental line ‘Frisson’ and the two other cultivars ‘Athos’ and ‘Austin’ (Table 4). Overall, it was observed that the mature seed protein content was highly correlated with the nodule biomass at BSF (Table 5). Conversely, the seed yield was slightly depressed in P118 and P121 compared with ‘Frisson’ and significantly lower than for ‘Austin’ and ‘Athos’ (Table 4). Interestingly, seed yield was similar for the two mutants but with different seed components: P118 had a lower seed weight but a similar number of seeds as ‘Frisson’, whereas the seed number of P121 was lower than for ‘Frisson’ but its thousand-seed weight was the same (Table 4). ‘Frisson’ and its mutants had a significantly lower thousand-seed weight and a significantly higher seed number than the two more recent cultivars ‘Athos’ and ‘Austin’.
Table 4.
Seed yield and protein content; yield components of the five pea genotypes studied
| Genotype | Seed protein content (mg g−1) | Seed yield (g m−2) | Seed protein yield (g m−2) | Thousand-seed weight (g) | Seed number m−2 |
|---|---|---|---|---|---|
| ‘Athos’ | 243b | 545a | 133a | 238·4a | 2285c |
| ‘Austin’ | 236b | 467b | 110b | 238·4a | 1959c |
| ‘Frisson’ | 244b | 430bc | 105b | 122·5b | 3511a |
| P118 | 277a | 379c | 105b | 110·6c | 3425a |
| P121 | 267a | 379c | 101b | 127·8b | 2963b |
Results of LSD-range test are shown where means followed by different letters are significantly different at the 5 % probability level.
Table 5.
Correlation coefficients of seed protein content and seed protein yield at maturity with dry matter and N accumulation parameters at the beginning of seed filling, during seed filling and at maturity
| At the beginning of seed filling |
During seed filling |
At maturity |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Nodule dry matter | Root dry matter | Shoot dry matter | Shoot N accumulation | Shoot dry matter | Shoot N accumulation | N remobilization in seeds | Shoot dry matter | Shoot N accumulation | |
| Seed protein content | 0·91* | −0·53 ns | −0·80 ns | 0·01 ns | −0·48 ns | −0·65 ns | −0·16 ns | −0·66 ns | −0·52 ns |
| Seed protein yield | −0·48 ns | 0·52 ns | 0·87 ns | 0·72 ns | 0·82 ns | 0·89* | 0·56 ns | 0·88* | 0·97* |
ns, non-significant; *, significant at the 0·05 level.
Finally, seed protein yields were similar for all genotypes except ‘Athos’, for which it was higher (Table 4), without any correlation to the nodule biomass at BSF (Table 5). By contrast, the mature seed protein yield was highly correlated with the total shoot dry matter accumulation and with the total shoot N accumulation (Table 5). More precisely, the final seed protein yield was significantly correlated to the exogenous N accumulation during seed filling but not to the N remobilization from vegetative tissues during the same period or to the N accumulation before seed filling (Table 5).
DISCUSSION
Genetic variability of pea root and nodule establishment was observed under field conditions
The hypernodulating mutants P118 and P121 had significantly higher nodule dry matter and number than their isogenic parent line ‘Frisson’, as early as at the four- or ten-leaf stage of development. Thereafter, their exponential increase of nodule number up to BSF suggests a major defect in the autoregulation of nodulation. Moreover, shoot biomass production of these two mutants was not reduced as compared with ‘Frisson’, although in other hypernodulating mutants shoot dry matter accumulation was reduced more than two-fold (Sagan et al., 1993; Salon et al., 2001).
The maximum root biomass of ‘Austin’ was high compared with that previously observed for other cultivars in the literature. At BSF, the peak root biomass of ‘Austin’ was about 52 g m−2, and only 38 g m−2 for cultivar ‘Baccara’ (Voisin et al., 2002), whereas both cultivars produced similar quantities of shoot dry matter (800 g m−2). The higher dry weight but mid-range root length of ‘Austin’, in comparison with ‘Athos’, could be explained by its thicker roots. The root biomass greatly increased up to BSF for all genotypes except ‘Frisson’. ‘Frisson’ showed intermediate values of root biomass up to flowering, but it had the lowest biomass at BSF. This change of ranking between genotypes depending on the growth stage is in agreement with Veitenheimer and Gritton (1984) and emphasized the importance of dynamics studies of nodulated root establishment.
For all five genotypes, nodule biomass peaked at the ten-leaf stage, in agreement with Pate (1958) who found the maximum around the eight-leaf stage in two different varieties. The subsequent decrease in nodule biomass and accompanying increase in root biomass observed for all the genotypes except ‘Frisson’ might indicate that roots are less affected than nodules by the decreasing C availability during the reproductive stages, in agreement with Tricot et al. (1994, 1997). For ‘Athos’ and ‘Austin’, the nodule biomass proportion of the nodulated root biomass decreased linearly from about 16 % at the four-leaf stage to 5 % at BSF. ‘Frisson’ contrasted with the other genotypes by its stability in the proportion of nodule biomass across the growth cycle (10–13 %). The mutants had the highest proportion of nodule biomass out of the entire root system: at least 28 % for P118 and 13 % for P121 throughout the growth cycle with a maximum at the ten-leaf stage (44 % for P118 and 27 % for P121). Thus, for the hypernodulating mutants, especially during the vegetative period, the nodules made the highest C demand, in line with Voisin et al. (2003b) who showed that the C sink strength of nodules increased with their biomass. For these genotypes, nodule growth probably occurred at the expense of root growth.
The hypernodulating mutants accumulated less nitrogen in seeds than genotypes with higher root development
The mature seed protein yield was highly correlated with the total shoot N accumulation, which was not increased in the hypernodulating mutants compared with ‘Frisson’. At physiological maturity, the mutants and ‘Frisson’ have both accumulated the same amount of nitrogen, which was lower than the amount reached by ‘Athos’ and ‘Austin’. Before seed filling, the contribution of symbiotic fixation to N accumulation was predominant for all five genotypes, and hypernodulating mutants, especially P121, had not incorporated more N by fixation than ‘Frisson’. These genotypes with numerous nodules finally acquired less shoot dry matter and nitrogen than the genotypes with higher root development. This could be explained by the increased C cost of symbiotic fixation compared with mineral N acquisition by roots, as demonstrated by Voisin et al. (2003c), or by a huge proportion of ineffective nodules. Finally, the genotypes with a higher root development at BSF had a higher mature seed protein yield than the hypernodulating mutants. Moreover, the final seed protein yield depended more upon exogenous N accumulation during seed filling than upon N remobilization from vegetative tissues during the same period. Such results demonstrate that enhancing exogenous nitrogen supply at the end of the growth cycle, by increasing the potential for root N absorption, is a promising way of improving pea seed filling. Therefore, root studies should not be limited to early vegetative stages but be more focused on the late stages of growth. Among the two genotypes with high root biomass, ‘Athos’ seems to offer the most potential because of its higher shoot N absorption, which may be explained by a greater ability to develop numerous secondary lateral roots.
Host preference for particular rhizobial genotypes may contribute to variability in N acquisition
The genetic background of the host plant influenced the composition of the R. leguminosarum bv. viciae populations in nodules. ‘Athos’ and ‘Austin’ formed nodules with quite similar populations, except for the proportion of genotype 6/b. Both cultivars have been obtained recently by the same breeder and share a common ancestor (C. Duchêne, pers. comm.), which could explain the similarities between them and the differences with the old and no longer used cultivar ‘Frisson’. However, the sym29 mutation (P118) influenced the selection of rhizobial genotypes by comparison with its isogenic parent line, while the nod3 mutation did not (P121). Although no significant differences in nodule number between the two hypernodulating mutants were observed from the ten-leaf stage up to BSF, nodule dry matter in P118 was two-fold higher than in P121, which may reflect a more efficient symbiotic nitrogen fixation in the sym29 mutant than in the nod3 one. Moreover, P121 developed more secondary thin lateral roots than P118, which might permit more N absorption. This contrasted biomass repartition between roots and nodules for the two mutants could indicate different regulation and nodule by root interactions between SYM29 and NOD3 genes. Indeed, in nod3 mutant lines, roots have been identified as the main source of the systemic signal involved in regulation of nodule number (Postma et al., 1988), whereas hypernodulation phenotype of sym29 mutant lines is under shoot control (Sagan and Duc, 1996). SYM29 is the pea orthologue of the HAR1 gene, which is a putative receptor kinase required in Lotus japonicus for shoot-controlled regulation of both nodule organogenesis and root development (Krusell et al., 2002).
Differences or variations in plant physiology, and in root and nodule morphogenesis between pea genotypes, may result in variation in root exudation, or more specifically, in a non-nutritional way, in variation of signal exchange between the bacterial and plant partners. It has in fact been reported that rhizosphere microbial community structure varies in relation to root location, and that such variation is related to quantitative and qualitative changes in root exudation (Baudoin et al., 2001; Yang and Crowley, 2000). Such changes in plant metabolism may lead to differential efficiency of interaction with rhizobial genotypes, and the rhizobial genotype may consequently influence root and nodule physiology, and N2 fixation efficiency. In alfalfa lines selected for high levels of nitrogen fixation, Hardarson et al. (1982) reported a preference for efficient strains of indigenous rhizobia. In the present study, ‘Athos’ accumulated more N derived from fixation than the other genotypes. It was also observed that ‘Athos’ did not show affinity for rhizobial genotype 6/b, whereas it was among the most frequent in the nodules formed with the other pea genotypes. Interestingly, strains with genotype 6/b were found to be among the least effective in nitrogen fixation when compared with a diversity of rhizobial genotypes tested with ‘Frisson’ and ‘Austin’ (G. Laguerre and G. Depret, unpubl. data).
CONCLUSIONS
The present study confirms nitrogen accumulation as a key determinant of seed protein yield, and underlines its dependency on shoot dry matter acquisition. The two hypernodulating mutants did not accumulate more nitrogen than their near-isogenic parental line, probably because the C cost for nodule over-production was higher than for root development and functioning, and occurred at the expense of shoot proliferation. However, P118 has more potential than P121 as its N2 fixation was higher before seed filling. ‘Athos’, with a higher root development than P118, acquired more shoot dry matter and nitrogen during seed filling. An improvement in nitrogen nutrition could be obtained by combining the ‘beneficial effects’ of both the sym29 line and ‘Athos’. The marker-assisted construction of a sym29 isogenic line of ‘Athos’ now in progress will be of great interest to analyse more precisely the relationships between hypernodulation and prolific root development, and their synergistic effects on nitrogen nutrition. Moreover, quantitative trait locus analyses are ongoing in different populations of recombinant inbred line populations to obtain a better understanding of genetic relationships between nodulated root establishment and nitrogen acquisition. The consequence of the rhizobial genetic diversity observed among the cultivars on the efficiency of nitrogen-fixing symbiosis will be evaluated. Investigations on the ability of pea lines to select the most effective rhizobial strains are in progress.
ACKNOWLEDGEMENTS
For help in the field, we thank Henri de Larembergue, Hervé Houtin, Patrick Mathey and Vincent Durey of the UMRLEG, and Didier Contour, Norbert Blanc, Bernard Monier, Stéphanie Sarrasin and Pierre Mangin of the Experimental Unit. We also thank Myriam Huart, Bernadette Roy and Olivier Delfosse for the laboratory analyses, Michèle Bours, Amandine Gauthier, Stéphanie Jaxel and Guillaume Legay for the Rhizobium population analyses, Charles Schneider, who developed the root image analysis program, and Delphine Meunier-Beillard for root image and data analysis. Christiane Duchêne is acknowledged for her guidance in the choice of cultivars. The experiment was carried out in a field of Jean-Luc Calabre at Labruyère (Côte-d'Or, France). This work was funded by the Regional Council of Burgundy. We are grateful to Sergio Ochatt and Richard Thompson for their critical reading of the manuscript and to the anonymous referees for their constructive criticisms.
LITERATURE CITED
- Ali-Khan ST, Snoad B. Root and shoot development in peas. I. Variability in seven root and shoot characters of seedlings. Annals of Applied Biology. 1977;85:131–136. [Google Scholar]
- Ballard RA, Charman N, McInnes A, Davidson JA. Size, symbiotic effectiveness and genetic diversity of field pea rhizobia (Rhizobium leguminosarum bv. viciae) populations in South Australian soils. Soil Biology and Biochemistry. 2004;36:1347–1355. [Google Scholar]
- Baudoin E, Benizri E, Guckert A. Metabolic fingerprint of microbial communities from distinct maize rhizosphere compartments. European Journal of Soil Biology. 2001;37:85–93. [Google Scholar]
- Biedermannova E, Novak K, Vondrys J. Pea mutant Risnod27 as reference line for field assessment of impact of symbiotic nitrogen fixation. Journal of Plant Nutrition. 2002;25:2051–2066. [Google Scholar]
- Caetano-Anolles G, Gresshoff PM. Plant genetic control of nodulation. Annual Review of Microbiology. 1991;45:345–382. doi: 10.1146/annurev.mi.45.100191.002021. [DOI] [PubMed] [Google Scholar]
- Depret G, Houot S, Allard MR, Breuil MC, Nouaïm R, Laguerre G. Long-term effects of crop management on Rhizobium leguminosarum biovar viciae populations. FEMS Microbiology Ecology. 2004;51:87–97. doi: 10.1016/j.femsec.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Duc G, Messager A. Mutagenesis of pea (Pisum sativum L.) and the isolation of mutants for nodulation and nitrogen fixation. Plant Science. 1989;60:207–213. [Google Scholar]
- Duc G, Mariotti A, Amarger N. Measurements of genetic variability for symbiotic dinitrogen fixation in field-grown faba bean (Vicia faba L.) using a low level 15 N-tracer technique. Plant and Soil. 1988;106:269–276. [Google Scholar]
- Engvild KC. Nodulation and nitrogen fixation mutants of pea, Pisum sativum. Theoretical and Applied Genetics. 1987;74:711–713. doi: 10.1007/BF00247546. [DOI] [PubMed] [Google Scholar]
- Fezenko AN, Provorov NA, Orlova IF, Orlov VP, Simarov BV. Selection of Rhizobium leguminosarum strains for inoculation of Pisum sativum L. cultivars: analysis of symbiotic efficiency and nodulation competitiveness. Plant and Soil. 1995;172:189–198. [Google Scholar]
- Hardarson G, Heichel G, Barnes DK, Vance CP. Rhizobium strain preference of alfalfa populations selected for characteristics associated with N2 fixation. Crop Science. 1982;22:55–58. [Google Scholar]
- Jensen ES. Seasonal patterns of growth and nitrogen fixation in field-grown pea. Plant and Soil. 1987;101:29–37. [Google Scholar]
- Jeuffroy MH, Warembourg FR. Carbon transfer and partitioning between vegetative and reproductive organs in Pisum sativum L. Plant Physiology. 1991;97:440–448. doi: 10.1104/pp.97.1.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusell L, Madsen LH, Sato S, Aubert G, Genua A, Szczyglowski K, et al. Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature. 2002;420:422–425. doi: 10.1038/nature01207. [DOI] [PubMed] [Google Scholar]
- Laguerre G, Louvrier P, Allard MR, Amarger N. Compatibility of rhizobial genotypes within natural populations of Rhizobium leguminosarum biovar viciae for nodulation of host legumes. Applied and Environmental Microbiology. 2003;69:2276–2283. doi: 10.1128/AEM.69.4.2276-2283.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgard SF. Nutrition, moisture and rhizobial strain influence isotopic fractionation during N2 fixation in pasture legumes. Soil Biology and Biochemistry. 1989;21:65–68. [Google Scholar]
- Louvrier P, Laguerre G, Amarger N. Semiselective medium for isolation of Rhizobium leguminosarum from soils. Soil Biology and Biochemistry. 1995;27:919–924. [Google Scholar]
- Mariotti A, Mariotti F, Amarger N, Pizelle G, Ngambi J-M, Champigny M-L, Moyse A. Fractionnements isotopiques de l'azote lors des processus d'absorption des nitrates et de la fixation de l'azote atmosphérique par les plantes. Physiologie Végétale. 1980;18:163–181. [Google Scholar]
- Mariotti A, Mariotti F, Amarger N. Utilisation du traçage isotopique naturel par 15N pour la mesure du taux d'azote fixé symbiotiquement par les légumineuses. Physiologie Végétale. 1983;21:279–291. [Google Scholar]
- Martensson AM, Rydberg I. Cultivar × rhizobial strain interactions in peas with respect to early symbiosis, nodule initiation and N uptake. Plant Breeding. 1996;115:402–406. [Google Scholar]
- McPhee K. Variation for seedling root architecture in the core collection of pea germplasm. Crop Science. 2005;45:1758–1763. [Google Scholar]
- Ney B, Turc O. Heat-Unit Based description of the reproductive development of pea. Crop Science. 1993;33:510–514. [Google Scholar]
- Pate JS. Nodulation studies in legumes. I. The synchronisation of host and symbiotic development in the field pea, Pisum arvense L. Australian Journal of Biological Science. 1958;11:361–381. [Google Scholar]
- Pate JS, Flinn AM. Carbon and nitrogen transfer from vegetative organs to ripening seed of field pea (Pisum arvense L.) Journal of Experimental Botany. 1973;24:1090–1099. [Google Scholar]
- Postma JG, Jacobsen E, Feenstra WJ. Three pea mutants with an altered nodulation studied by genetic analysis and grafting. Journal of Plant Physiology. 1988;132:424–430. [Google Scholar]
- Sagan M, Duc G. Sym28 and Sym29, two new genes involved in regulation of nodulation in pea (Pisum sativum L.) Symbiosis. 1996;20:229–245. [Google Scholar]
- Sagan M, Gresshoff PM. Developmental mapping of nodulation events in pea (Pisum sativum L.) using supernodulating plant genotypes and bacterial variability reveals both plant and Rhizobium control of nodulation regulation. Plant Science. 1996;117:167–179. [Google Scholar]
- Sagan M, Ney B, Duc G. Plant symbiotic mutants as a tool to analyse nitrogen and yield relationship in field-grown peas (Pisum sativum L.) Plant and Soil. 1993;153:33–45. [Google Scholar]
- Salon C, Munier-Jolain NG, Duc G, Voisin A-S, Grandgirard D, Larmure A, et al. Grain legume seed filling in relation to nitrogen acquisition: a review and prospects with particular reference to pea. Agronomie. 2001;21:539–552. [Google Scholar]
- Schiltz S, Munier-Jolain N, Jeudy C, Burstin J, Salon C. Dynamics of exogenous nitrogen remobilization from vegetative organs in pea revealed by 15N in vivo labeling throughout seed filling. Plant Physiology. 2005;137:1463–1473. doi: 10.1104/pp.104.056713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, de Witt CT. Photosynthate and nitrogen requirements for seed production by various crops. Science. 1975;189:565–567. doi: 10.1126/science.189.4202.565. [DOI] [PubMed] [Google Scholar]
- Skot L. Cultivar and Rhizobium strain effects on the symbiotic performance of pea (Pisum sativum). Physiologia Plantarum. 1983;59:585–589. [Google Scholar]
- Thorup-Kristensen K. Root growth of green pea (Pisum sativum L.) genotypes. Crop Science. 1998;38:1445–1451. [Google Scholar]
- Tricot F, Pellerin S, Angevin F, Crozat Y. Elaboration de la biomasse des nodosités: Influence de la nutrition carbonée. In: Ney B, Duchêne E, Carrouée B, Angevin F, editors. Agrophysiologie du pois protéagineux. Paris: UNIP-ITCF-INRA; 1994. pp. 75–91. [Google Scholar]
- Tricot F, Crozat Y, Pellerin S. Root system growth and nodule establishment on pea (Pisum sativum L.) Journal of Experimental Botany. 1997;48:1935–1941. [Google Scholar]
- Veitenheimer EE, Gritton ET. Preliminary root studies of Pisum sativum. Pisum Newsletter. 1984;16:73–74. [Google Scholar]
- Vincent JM. A manual for the practical study of root-nodule bacteria. Oxford: Blackwell Scientific Publications; 1970. IBP handbook no. 15. [Google Scholar]
- Voisin A-S, Salon C, Munier-Jolain NG, Ney B. Effect of mineral nitrogen on nitrogen nutrition and biomass partitioning between the shoot and roots of pea (Pisum sativum L.) Plant and Soil. 2002;242:251–262. [Google Scholar]
- Voisin A-S, Salon C, Jeudy C, Warembourg FR. Seasonal patterns of 13C partitioning between shoots and nodulated roots of N2- or nitrate-fed Pisum sativum L. Annals of Botany. 2003a;91:539–546. doi: 10.1093/aob/mcg055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin A-S, Salon C, Jeudy C, Warembourg FR. Root and nodule growth in Pisum sativum L. in relation with photosynthesis: analysis using 13C-labelling. Annals of Botany. 2003b;92:557–563. doi: 10.1093/aob/mcg174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin A-S, Salon C, Jeudy C, Warembourg FR. Symbiotic N2 fixation activity in relation to C economy of Pisum sativum L. as a function of plant phenology. Journal of Experimental Botany. 2003c;54:2733–2744. doi: 10.1093/jxb/erg290. [DOI] [PubMed] [Google Scholar]
- Yang C-H, Crowley DE. Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Applied and Environmental Microbiology. 2000;66:345–351. doi: 10.1128/aem.66.1.345-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]