Abstract
Background
The aim of this paper is to discuss the controversial origins of petals from tepals or stamens and the links between the morphological expression of petals and floral organ identity genes in the core eudicots.
Scope
I challenge the widely held classical view that petals are morphologically derived from stamens in the core eudicots, and sepals from tepals or bracts. Morphological data suggest that tepal-derived petals have evolved independently in the major lineages of the core eudicots (i.e. asterids, Santalales and rosids) from Berberidopsis-like prototypes, and that staminodial petals have arisen only in few isolated cases where petals had been previously lost (Caryophyllales, Rosales). The clear correlation between continuous changes in petal morphology, and a scenario that indicates numerous duplications to have taken place in genes controlling floral organ development, can only be fully understood within a phylogenetic context. B-gene expression plays a fundamental role in the evolution of the petals by controlling petaloidy, but it does not clarify petal homology.
Conclusions
An increased synorganization of the flower in the core eudicots linked with the establishment of floral whorls restricts the petaloid gene expression to the second whorl, reducing the similarities of petals with tepals from which they were originally derived. An increased flower size linked with secondary polyandry or polycarpelly may lead to a breakdown of the restricted gene expression and a reversal to ancestral characteristics of perianth development. An altered ‘sliding boundary’ hypothesis is proposed for the core eudicots to explain shifts in petaloidy of the perianth and the event of staminodial petals. The repetitive changes of function in the perianth of the core eudicots are linked with shifts in petaloidy to the outer perianth whorl, or losses of petal or sepal whorls that can be secondarily compensated for by the inclusion of bracts in the flower. The origin and evolution of petals appears to be as complex on a molecular basis as it is from a morphological point of view.
Key words: Apetala 3, Berberidopsis, bract-derived petals, core eudicots, gene expression, perianth evolution, petaloidy, phylogeny, staminodial petals
MORPHOLOGY AND GENES: TWO CONTRASTING APPROACHES?
The origin and evolution of the perianth has received increased interest in recent years from evolutionary developmental genetic studies (e.g. Jack et al., 1992; Albert et al., 1998; Kramer et al., 1998, 2003; Kramer and Irish, 1999, 2000; Theissen et al., 2002; Lamb and Irish, 2003; Kim et al., 2004b; Zahn et al., 2005). Thanks to a stable molecular phylogeny of the eudicot clade (e.g. Hoot et al., 1999; Magallón et al., 1999; Soltis et al., 2000, 2003; Hilu et al., 2003; Kim et al., 2004a) it has become possible to test hypotheses regarding the evolution of floral structures in the angiosperms within a firm phylogenetic context (e.g. Zanis et al., 2003; Ronse de Craene et al., 2003, unpubl. res.; Endress and Matthews, 2006).
Most angiosperm flowers have an outer envelope enclosing the reproductive organs, called a perianth. The perianth can take a myriad of forms and shapes, ranging from undifferentiated (tepaloid) to differentiated into an outer calyx (sepals) and an inner corolla (petals). Unlike with stamens and carpels it is not possible to homologize the perianth in a satisfactory manner, as the perianth can have different orgins and can take up different forms (Endress, 1994, 2006; Kramer et al., 2003). Although different theories have been proposed in the past, the most commonly accepted classical morphological theories have pointed out that petals in the majority of angiosperms have been derived either from bracts as are the sepals (bracteopetals), or from sterile stamens (andropetals) (e.g. Hiepko, 1965; Takhtajan, 1991). Angiosperm sepals often have characteristics of leaves or bracts (i.e. persistent, protective organs with chlorophyll, a triangular shape with a broad base, three vascular traces and rapid growth), while petals often have characteristics morphologically common to stamens (i.e. deciduous, thin, coloured organs with a single vascular trace, a narrow base and retarded growth) (Hiepko, 1965; see also Endress, 1994). The general consensus is that petals in the core eudicots have a staminodial nature. Because of the strong emphasis on Ranunculaceae petals that show obvious characteristics of staminodes (e.g. Tamura, 1965; Erbar et al., 1998), the temptation is great to use this evidence as the driving force for petal evolution among all eudicots (Fig. 1A).
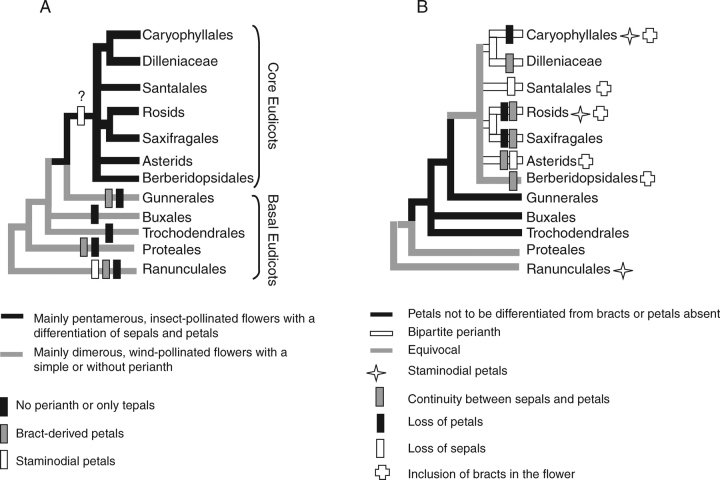
Fig. 1.
Illustration of the phylogenetic relationship of the major clades of eudicots, based on APG (2003). (A) Reconstruction of perianth differentiation in the basal eudicot grade and Gunnerales, in contrast to the supposedly staminodial petals of the core eudicots. (B) Reconstruction of petal homologies in the eudicots. The distribution of characters has been mapped for the major lineages of the core eudicots, indicating cases where petals have a staminodial or a bract-derived origin.
Evolutionary developmental genetics have presented models to understand perianth development and evolution on a molecular genetic basis. The differentiation of the flower rests on the ABC-model, in which identity of organs in each whorl is determined by a combination of three classes of MADS-box organ identity genes: A genes are responsible for sepal formation, A + B function determines petal identity, B + C function specifies stamens, and C-function determines carpel development. This model has been subsequently extended to include D- and E-class genes that also play a role in floral organ development (reviewed in, for example, Ferrario et al., 2004). Most genetic studies of petal identity have focused on the class B genes APETALA3 (AP3) and PISTILLATA (PI) from Arabidopsis or their orthologues DEFICIENS (DEF) and GLOBOSA (GLO) from Antirrhinum (Weigel and Meyerowitz, 1994; Albert et al., 1998; Gustaffson, 2000; Theissen et al., 2002; Kramer et al., 2003; Lamb and Irish, 2003; Litt and Irish, 2003; Kramer and Jaramillo, 2005; Zahn et al., 2005). AP3 or DEF interacts with the PI or GLO function in the formation of obligate heterodimers in conveying petal and stamen identity in both Arabidopsis and Antirrhinum (Jack et al., 1992; Riechmann et al., 1996). The ABC model was initially developed for core eudicot model flowers (i.e. Arabidopsis, Antirrhinum), which have a clearly differentiated bipartite perianth, but is becoming increasingly used to determine the genetic basis for undifferentiated organs (tepals) in all major groups of angiosperms and to understand the ‘true’ nature of the petals (cf. Kramer et al., 2003; Soltis et al., 2003; Di Stilio et al., 2005; Zahn et al., 2005). It is generally assumed that the original perianth of angiosperms was either sepaloid or petaloid and that a homeotic transition from sepaloid to petaloid organ identity or vice versa was responsible for a biseriate perianth (Albert et al., 1998; Kramer and Irish, 2000; Kanno et al., 2003; Hintz et al., 2006). A shift of region in the expression of genes (the ‘sliding-boundary’ model) has been put forward as a mechanism of flower evolution (e.g. Kanno et al., 2003; Kramer et al., 2003, 2006; Ochiai et al., 2004; Kramer and Jaramillo, 2005; Hintz et al., 2006). Shifts of gene expression towards sepals (A genes) would lead to a greater affinity of the petals with sepals or bracts (bracteopetals); ectopic expression of B-function genes AP3 and PI in the first whorl leads to a replacement of sepaloid by petaloid organs. The presence or absence of petaloid tepals in monocots is correlated with DEF-gene expression (equivalent to AP3) in the first whorl or its absence (Kanno et al., 2003; Ochiai et al., 2004). However, other studies show that there is much more variation in petal gene expression than initially thought. For example, in Asparagus with undifferentiated petaloid tepals GLO B-genes are only expressed in the inner tepal whorl (Park et al., 2004). In the orchid Phalaenopsis there is a differential expression of four different DEF-like paralogues in the perianth (Tsai et al., 2004). In addition, petaloid tissue can be produced that does not express AP3 and PI homologues, as was shown for Aristolochia by Jaramillo and Kramer (2004) and for Marcgraviaceae by Geuten et al. (2006). This indicates that a simple model with ectopic expression to provide a global explanation for petal origins and homology (e.g. Albert et al., 1998; Kanno et al., 2003; He et al., 2004) needs to be used with moderation as it cannot be applied to all cases of ectopic petaloidy. Although there is a conserved petal identity programme common for all angiosperms, the explanation of petal derivations based on the biseriate model of Antirrhinum and Arabidopsis does not take any historical or phylogenetic context into account and excludes any other origins of petals from multiple whorls or spirals. For example, the biseriate, tetramerous flower of Arabidopsis has been derived along an evolutionary pathway that is highly different from that of trimerous Tulipa. In between there is scope for much divergent evolution. Although the model explains how organs become petaloid (bear petal features) by a shift of genetic expression, it does not elucidate the homology of the petals. Assuming that Arabidopsis mutants in which sepals are replaced by petals can function as general models for petal evolution is clearly an oversimplification.
EVOLUTIONARY MODELS FOR THE ORIGIN OF PETALS IN THE CORE EUDICOTS
Several studies (e.g. Doyle, 1994; Kramer and Irish, 1999, 2000; Theissen et al., 2000; Kramer et al., 2003; Zahn et al., 2005) have demonstrated that B-class gene expression is found in basal angiosperms as well as gymnosperms, and suggested that there is a single origin of petaloidy in the perianth, followed by many independent derivations of a bipartite perianth. This development rests on a variable but commonly inherited petal identity programme, and the acquisition of different gene lineages leading to different evolutionary pathways for perianth development in angiosperms, which closely follow the phylogeny of the angiosperms (e.g. Kramer and Irish, 1999; Lamb and Irish, 2003; Litt and Irish, 2003; Soltis et al., 2004; Kim et al., 2004b; Kramer and Jaramillo, 2005; Zahn et al., 2005; Kramer et al., 2006). While B-gene function is linked to the specification of male reproductive organs in both gymnosperms and angiosperms, its function became increasingly altered by multiple gene or genome duplications, followed by neofunctionalization events, leading to the core eudicots and derived monocots and this is linked with a higher degree of specialization of organs and the differentiation of a bipartite perianth. Kim et al. (2005a, b) investigated the expression of AP3 and PI genes in several basal angiosperms. They found a broader expression pattern of these genes compared with the eudicots, and this appears to be correlated with the progressive transition from bracts to carpels in flowers, and with a lack of differentiation of the perianth. Kramer and co-workers (Kramer and Irish, 1999, 2000; Kramer et al., 2003; Di Stilio et al., 2005) extensively studied diversification of AP3 and PI lineages in the Ranunculaceae of the lower eudicots. They demonstrated the presence of several duplication events and expression patterns not necessarily associated with petal development. Their results only confirm that petal expression in different whorls results from divergent pathways of AP3 and PI genes, which at least for Ranunculaceae corresponds to the traditional distinction between tepals and nectar leaves (Hiepko, 1965).
Recent molecular phylogenies have explicitly demonstrated that the core eudicots have been derived from a grade comprising the lower eudicots (e.g. Magallón et al., 1999; Hilu et al., 2003; Soltis et al., 2003; Kim et al., 2004a). One outstanding result of the molecular phylogenetic studies is that Gunnerales with two monogeneric families Gunneraceae and Myrothamnaceae is the sister group of the remaining core eudicots, and this has important consequences for our understanding of petal evolution. There is an important floral morphological gap between lower and core eudicots. The lower eudicots immediately sister to the core eudicots (e.g. Tetracentraceae, Myrothamnaceae, Didymelaceae, Buxaceae) and the Gunnerales share small dimerous, often wind-pollinated flowers without perianth or with a perianth that is not clearly differentiated (Fig. 1A; Endress, 1986; von Balthazar and Endress, 2002; von Balthazar et al., 2003; Drinnan et al., 2004; Wanntorp and Ronse De Craene, 2005; Ronse De Craene and Wanntorp, 2006). The majority of core eudicots, by contrast, share well-developed pentamerous, insect-pollinated flowers with a differentiation of sepals and petals (Fig. 1A).
The morphological transition between lower and core eudicots appears to be an abrupt event, coinciding with the rapid radiation of the major lineages of the core eudicots and may be caused by different reasons, including a high extinction rate or an ancient explosive radiation at the base of the core eudicots (Soltis et al., 2003; Ronse De Craene, 2004; Wanntorp and Ronse De Craene, 2005). However, the relationships between the main six lineages of core eudicots (i.e. Berberidopsidales, Santalales, Caryophyllales, rosids, Saxifragales and asterids) remain unresolved (Fig. 1; Magallón et al., 1999; Soltis et al., 2003; Judd and Olmstead, 2004). There is limited fossil evidence to provide transitional forms between lower and core eudicots. Most lower eudicots are not good candidates for understanding core eudicot floral evolution. Gunnerales, the sister group to all other core eudicots (Soltis et al., 2003), are also dimerous and show a strong reductive trend linked with wind-pollination; therefore, they cannot help explain the evolution of core eudicot flowers (Wanntorp and Ronse De Craene, 2005; Ronse De Craene and Wanntorp, 2006).
One of the phylogenetic scenarios of basal core eudicot evolution has placed the Berberidopsidales, a small order with two families Berberidopsidaceae and Aextoxicaceae, as one of the basal core eudicot lineages but with relatively weak support (Soltis et al., 2003; Judd and Olmstead, 2004; Kim et al., 2004a). Berberidopsis has an atypical flower construction for the core eudicots in that the perianth is undifferentiated and spiral with several parts intergrading from outer bracts to inner petals (Ronse De Craene, 2004; Fig. 2A, B). The other genera in Berberidopsidales, such as Streptothamnus and Aextoxicon, have a clearly differentiated perianth. This points to a potential transformation event from the undifferentiated perianth to a biseriate perianth, which can function as a model for petal evolution in the core eudicots.
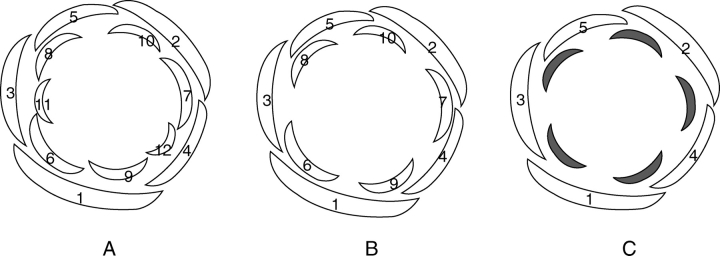
Fig. 2.
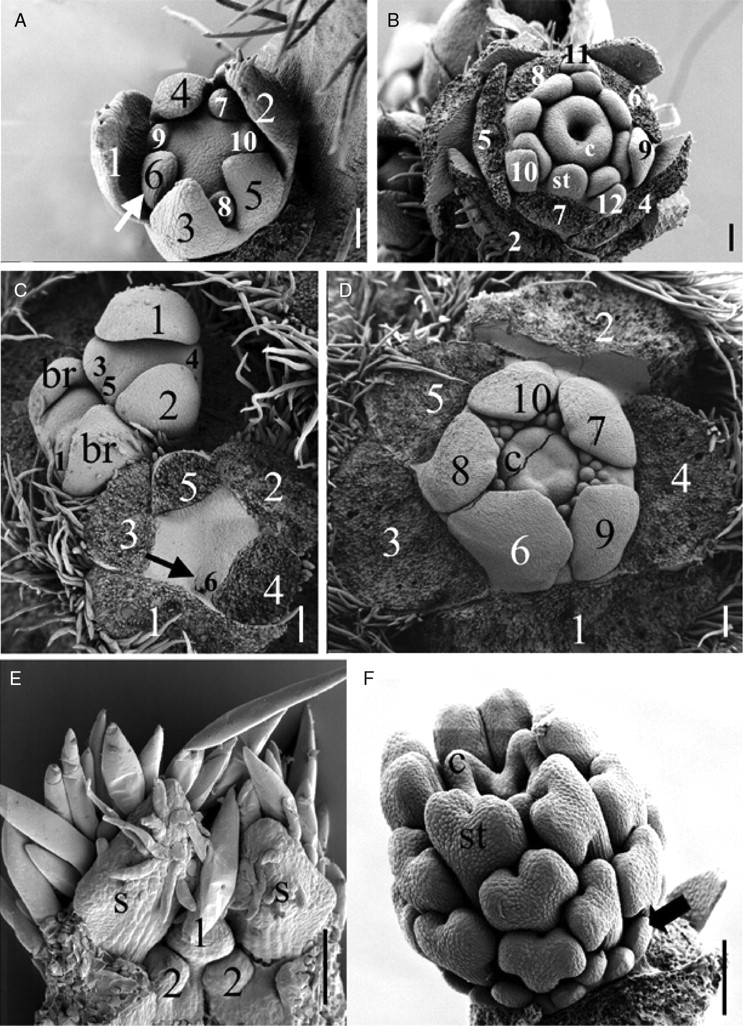
(A,B) Stages of the floral development of Berberidopsis corallina (Berberidopsidaceae). (A) Early stage of perianth initiation showing two pentamerous whorls of tepals arising in a spiral sequence. The arrow points to the sixth tepal initiating a second ‘whorl’. (B) Later stage of development of the flower at gynoecium development. The outer tepals have been removed. Numbers show the sequence of the 12 tepals; c, carpel; st, stamen. (C,D) Stages of the floral development of Cochlospermum vitifolium (Bixaceae – rosids). (C) Young inflorescence with three flowers at different stages of development. Numbers show the sequence of initiation of the sepals on individual flowers and first petal (arrow in front flower); br, bracteole. (D) Older bud at the initiation of the carpels (sepals removed); c, carpel primordium. (E) Section through young flower of Potentilla sp. (Rosaceae). Initiation of three primordia on a common primordium; the uppermost primordium develops as a petal and the lower as stamens. S, sepal. (F) Young flower primordium of Corbichonia decumbens (Lophiocarpaceae). The outer members of a centrifugal androecium develop into petaloid staminodes (arrow). St, stamen; c, carpel. Scale bars = 100 µm.
Because Berberidopsis has many intermediate features, Ronse De Craene (2004) suggested that the genus may help to understand how the transition between a spiral undifferentiated perianth and the common biseriate perianth in the core eudicots came about. Berberidopsis resembles other core eudicots at mid-developmental stage in an incipient pentamerous arrangement following a 2/5 sequence of initiation of outer and inner tepals (Fig. 2A). The pattern of development in Berberidopsis is not much different from the spiral initiation of sepals and petals found in several core eudicots (Fig. 2C, D). It could be suggested that the origin of the biseriate perianth of core eudicots is a matter of progressive readjustement (Fig. 3) by a strict arrangement of tepals in alternating whorls, the reduction of size of the inner tepals, and their nearly simultaneous initiation through shorter plastochrons. Increased synorganization of the petal whorl is responsible for a stronger differentiation of the petals as independent organs with specific attributes. An alternative hypothesis of the extension of petaloidy to the sepals appears improbable owing to the constraints of a spiral arrangement and the absence of similar cases in other core eudicots.
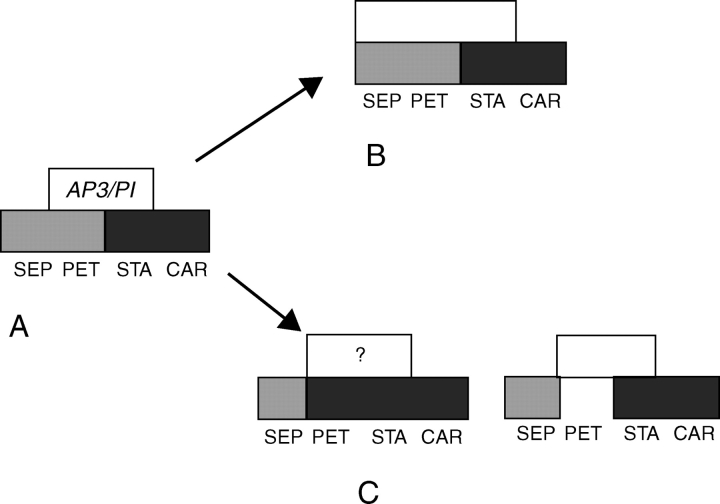
Fig. 3.
Floral diagrams demonstrating the putative sequence for the origin of petals in the core eudicots. (A) Prototype such as Berberidopsis with a spiral perianth of 12 tepals. (B) Core eudicot flower with a bipartite perianth of five broad sepals and five petals arising in a spiral sequence. (C) Core eudicot flower with five sequentially arising sepals and five petals arising simultaneously (in grey).
The node at the transition from basal to core eudicots has been identified with a large-scale gene duplication event at the level of Trochodendraceae–Buxaceae (TM6 and euAP3 arising from a paleoAP3 lineage), and there is a concordant evolution of derived gene lineages with the abrupt evolution of different floral morphologies in the core eudicots, although sampling of genes did not include crucial groups such as Berberidopsidales (Lamb and Irish, 2003; Litt and Irish, 2003; Hernández-Hernández et al., 2007; Kramer et al., 2006). Whereas TM6 and paleoAP3 have a similar motif at the C-terminal, euAP3 has a distinctive motif that has been shown to promote petal formation, unlike that of paleoAP3 (Lamb and Irish, 2003). Although the function of TM6 remains unclear, in Solanaceae it appears to have little or no role in petal development (e.g. Rijpkema et al., 2006). The divergence of euAP3 has therefore been implicated in the evolution of petals in the core eudicots (Lamb and Irish, 2003; Kramer and Jaramillo, 2005; Kramer et al., 2006). A single nucleotide deletion was responsible for the transition of the paleoAP3 motif into the euAP3 motif after duplication (Kramer et al., 2006). Kramer and Jaramillo (2005) questioned whether the petals in the core eudicots evolved by a restriction of the C-gene expression from an outer whorl of stamens (cf. andropetals) or by the expansion of the B-gene expression into previously sterile organs (cf. bracteopetals).
TRENDS IN THE DIFFERENTIATION OF THE PERIANTH IN THE CORE EUDICOTS: LINKS WITH EVO–DEVO
A study of the distribution of selected morphological characters related to petals on a recent morphological phylogeny of the core eudicots (Fig. 1B; L. P. Ronse De Craene, unpubl. res.) revealed that a number of characters traditionally associated with sepals are widely expressed in the petals of the core eudicots; these include a high frequency of broadly built, fast-growing, imbricate petals present in all main core eudicot clades. Thick petals of unequal size as well as the presence of three or more vascular bundles per petal also figure frequently among the major clades of the eudicots. Petals arising as a direct continuation of bracts are widespread in several of the higher lineages (Fig. 1B; Ronse De Craene, 2004). Although aestivation patterns are formed late in the floral development, an imbricate aestivation is usually a reflection of a spiral initiation sequence. These observations contradict the previously presented assumptions of a general staminodial origin of core eudicot petals. Several taxa scattered over the core eudicots possess large flowers with multiples of stamens and carpels and a variable number of sepals and petals arising in a helical order (e.g. Bixaceae, Dilleniaceae, Salicaceae, Clusiaceae, Theaceae: Fig. 2C, D; Ronse De Craene, 1989; Endress, 1997; Tsou, 1998; Bernhard and Endress, 1999; Gustaffson, 2000; Tucker and Bernhardt, 2000). These taxa are nested in clades having more regular flowers with fewer parts, and it remains uncertain whether large, helical flowers represent a truly plesiomorphic condition or a reversal.
It is clear that the general opinion that petals of the majority of angiosperms are derived from stamens is a myth that does not withstand closer scrutiny. The use of floral ontogenetic evidence within a reliable phylogenetic framework supports the derivation of petals by a differentiation of an inner series of tepals, leading to a bipartite perianth (Fig. 3; L. P. Ronse De Craene, unpubl. res.). Contrary to the common assumption of staminodial petals in Arabidopsis or Antirrhinum, distribution patterns based on floral developmental evidence, anatomy and morphology demonstrate that petals can be considered as of staminodial origin on only a few occasions. They tend to be restricted to certain clades where petals have initially been lost and where a visual attraction had to be reinvented (e.g. Fig. 2E, F; Caryophyllales, Rosales: Hofmann, 1994; Ronse De Craene et al., 1998; Leins et al., 2001; Ronse De Craene and Smets, 2001; Ronse De Craene, 2003). Therefore, staminodial petals must be the result of a secondary readjustment of gene expression in an outer whorl of sterile stamens. The observations of a recruitment of a TM6 homologue from the petal into the stamen of Silene (Caryophyllaceae: Zahn et al., 2005) should be interpreted in this context.
The controversy regarding petal homology cannot be solved with gene expression studies alone. Some support for andropetals comes from the fact that AP3 and PI genes in the eudicots are strongly expressed in the stamens as well as the petals (Kramer and Irish, 2000; Zahn et al., 2005). Alternatively, the expression of AP3 and PI homologues appears highly conserved across the angiosperms (e.g. Kramer and Irish, 2000; Stellari et al., 2004; Kramer et al., 2006), having different functions in some basal groups, and being expressed in different degrees across the eudicot phylogeny. All angiosperms are capable of the expression of petaloid features and this expression can run along a gradient (with little morphological distinction between sets of organs and with the expression of petaloid genes in the stamens), or it can be increasingly set as a distinction between strictly defined whorls. Litt and Irish (2003) argue that the molecular mechanisms responsible for floral development may be different outside the core eudicots, and this underlines the important gap that is seen in the morphology. Morphological evidence of petal development in the core eudicots adds more support for a bracteolar derivation of the petals in the majority of core eudicots, including the model systems, such as Arabidopsis, Antirrhinum and Petunia, and only for the possibility of a staminodial nature in some restricted clades (Caryophyllales, Rosales: Fig. 2E, F). This also implies that the independent derivation of petals from either stamens or bracts would either involve the recruitment of different kinds of genes in different lineages of the core eudicots, or the same genes can be recruited independently to the same effect. It will be one of the future challenges to determine how staminodial petals differ genetically from bracteolar petals.
A whorled arrangement of the flower is a fundamental prerequisite for a restricted differentiation of petaloid organs and is fundamental for canalizing the expression of genes towards specific organs. In basal angiosperms B-gene expression extends over all organs and this appears to be correlated with the progressive transition from bracts to carpels in flowers, with a lack of differentiation of the perianth (Kim et al., 2005a). Already in the Ranunculales the expression of the MADS box genes AP3 and PI in the nectar leaves of Ranunculaceae is different from the petaloid tepals, culminating in the strictly whorled genus Aquilegia (Kramer and Irish, 1999; Kramer et al., 2003). The suggestion that new sequences in the C-terminal domain in euAP3 genes in core eudicots is associated with the evolution of a differentiated perianth (e.g. Kramer et al., 1998; Lamb and Irish, 2003) does not clarify the whole picture of petal evolution. I postulate that a stricter synorganization of whorled flowers in the advanced clades of the core eudicots (Endress, 1990, 2006) is linked with a more restricted expression of A-genes in the second whorl and an incursion of C-genes in the developing petals, leading to an increased similarity of petals (and even sepals) with stamens.
However, one often finds massive multistaminate flowers scattered over different clades of the core eudicots which appear less synorganized and have a strong expression of bract features in the petals: spiral initiation sequence, large, unequal petals with rapid growth and imbricate aestivation. Although these flower types may represent a retained plesiomorphic condition (resembling the pattern in Berberidopsis), it is possible that the development of a ring meristem linked with an increase of stamens or carpels has caused a disruption of the regular whorled initiation pattern of floral organs, leading to a reversal to a more diffuse expression of petal-inducing genes.
PETALOIDY AND PETAL EVOLUTION IN THE CORE EUDICOTS
A bipartite perianth is commonly found in all major clades following the divergence of the Berberidopsidales. Although petals appear to have a bract origin in the core eudicots, it is not known whether the transition between tepals and petals occurred once or several times. Petaloidy can easily jump between different whorls, with the possible extension of gene influences in the adjoining whorls. For example, in tetramerous flowers of Stachyurus sinensis (Stachyuraceae), the two outer sepals are brownish as are the bracts, while the two inner sepals bear the same coloration as the four petals (L. P. Ronse De Craene, unpubl. obs.). The coloured staminodes of Jacquinia macrocarpa are morphologically very similar to the petals, although they represent an aborted stamen whorl (Caris and Smets, 2004). Similarly, organ identity can switch at the boundary of petals and stamens, as illustrated in the transition of petals into stamens and vice versa (e.g. Papaveraceae: Ronse De Craene, 2003; Brassicaceae: Nutt et al., 2006). Sudden homeotic transformations may play an important role in the evolution of the perianth (known as hopeful monsters: see Theissen, 2006).
The number of species in the core eudicots with coloured petaloid sepals is very high (L. P. Ronse De Craene, unpubl. res.). Sepals often appear as an outer, pigmented whorl highly similar to the petal whorl. This phenomenon might be used as an indication that sepals and petals are functionally homologous structures but it affects flowers at maturity while sepals and petals maintain their distinct morphologies in earlier developmental stages. Examples of this are widespread, such as Passiflora (Passifloraceae), Impatiens (Balsaminaceae), Rhamnaceae, Fuchsia (Onagraceae), Staphylea (Staphyleaceae), Gomphia (Ochnaceae), Moringa (Moringaceae), Ribes (Grossulariaceae), Clermontia (Campanulaceae) and several Brassicaceae. Petaloidy of the calyx is often correlated with a reduction in size or variability in the presence or absence of the petals (e.g. Cunoniaceae, Oliniaceae, Polygonaceae, Saxifragaceae, Thymelaeaceae). The combination of protection with attraction in the calyx has affected several clades. It would be interesting to know whether this shift has occurred concomitantly with a reduction of the petals (as observed in, for example, Fabaceae: Tucker, 2001; Oliniaceae: Schönenberger and Conti, 2003), or after petals have initially been lost (in wind-pollinated clades: e.g. Begoniaceae, Phytolaccaceae). Endress and Matthews (2006) found an evolutionary connection between reduced petals and lobate petals, as both are frequently associated in the same family. However, they did not observe whether this trend was correlated with petaloidy of the sepals.
Petaloidy in the sepals has arisen independently and on many different occasions, and is clearly a secondary phenomenon linked to a shifting pattern of gene expression. It is clear that this transference of function to the sepals is in no way a replacement of the sepals with the petals (Geuten et al., 2006).
The sliding boundary model proposed by default for all angiosperms can be altered for the core eudicots, as it has been for other groups (Figs 4 and 5). However, this approach needs to be used cautiously as different genetic mechanisms can induce similar morphologies, or vice versa (Theissen, 2005; Zahn et al., 2005; Geuten et al., 2006). Starting with a floral model well established in the core eudicots such as Arabidopsis (Figs 4A and 5A), an ectopic shift of B-genes towards the sepals leads to increased petaloidy of the outer whorl (Figs 4B and 5B). As explained above, this trend is frequently found in several families of rosids, Caryophyllales and asterids. The secondary acquisition of petaloid organs by an extension of the B-gene expression in the stamen whorl, the addition of different B-genes, or the restriction of the C-gene expression in stamens may lead to staminodial petals as found in some taxa of the Caryophyllales and Rosales (Figs 2E, F, 4C and 5F; Ronse De Craene, 2003).
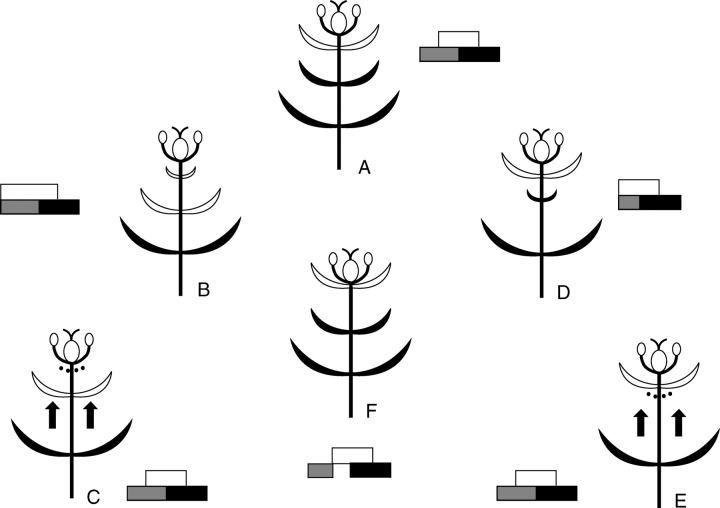
Fig. 4.
Altered ‘sliding-boundary’ hypothesis for the core eudicots. (A) ABC model for flowers with well-differentiated sepals and petals; (B) flowers with petaloid calyx next to a corolla; (C) flowers with staminodial petals, either by an ectopic expression of C genes linked with different B-genes, or by a restriction of the C-gene activity.
Fig. 5.
Floral models showing different levels of perianth evolution in the core eudicots; phyllomes from bottom to top represent bracts, sepals and petals; black colour represents absence of petaloidy; white colour represents petaloidy; broken curves represent lost whorls. For each model the putative ABC-model is shown. (A) Flower with well-differentiated biseriate perianth; (B) reduction or loss of petals and petaloidy of the sepal whorl; (C) secondary insertion of bracts in an apetalous flower; (D) reduction or loss of sepals; (E) secondary insertion of bracts in an asepalous flower; (F) development of staminodial petals after the initial loss of petals.
Once the differentiation of a petal whorl has been firmly established there are certain clear patterns associated with the main clades of the core eudicots (Fig. 1B). In Santalales evidence for a bipartite perianth is only found in some Olacaceae s.l. (e.g. Heisteria, Diogoa: L. Wanntorp and L. P. Ronse De Craene, unpubl. res.). In most Santalales petals represent a well-developed valvate whorl and the calyx is reduced, or replaced by a calyculus of probably bracteolar origin (L. Wanntorp and L. P. Ronse De Craene, unpubl. res.). Asterids and rosids usually have a clear differentiation of sepals and petals, although the development of the petals is highly variable. Rosids (including Saxifragales) have a frequent tendency for petal loss (Endress and Matthews, 2006), linked with petaloidy of the sepals (e.g. Saxifragaceae. Grossulariaceae, Rosaceae, Malvaceae, Fabaceae, Thymelaeaceae). The core Caryophyllales represent a basically apetalous clade with probably wind-pollinated ancestors. Petals have been reinvented several times within the order, either by petaloidy of the sepal whorl (e.g. Nyctaginaceae, Cactaceae, Amaranthaceae), or by the development of petaloid staminodes (e.g. Caryophyllaceae, Stegnospermaceae, Aizoaceae, Molluginaceae p.p.: Fig. 2F; Hofmann, 1994; Ronse De Craene et al., 1998). Asterids have the largest number of taxa with strongly developed, synorganized flowers, and the corolla is often sympetalous. The protective function is occasionally taken over by the corolla, or by the floral bracts, making the calyx redundant. As a result the calyx is repeatedly lost in several larger families (e.g. Rubiaceae, Acanthaceae, Asteraceae, Caprifoliaceae).
CONCLUSIONS
The model of a tepaline origin of the petals through Berberidopsis-like ancestors is supported by the widespread distribution of tepal attributes in the petals of the core eudicots (Fig. 1B) and links up with the assumption that an ancient exclusion of the B function from the outer petaloid whorl in early eudicots may be responsible for the sepal–petal distinction (Albert et al., 1998). The core eudicot flower is a dynamic structure and the evolution of the perianth is reflected in the shifting balance between different gene expressions. The topographical limits of the flower, especially the perianth, are constantly altered during evolution. At this stage only broad lines can be traced regarding changes occurring in the differentiation of a perianth in the core eudicots (Fig. 5), but with better resolved phylogenies it will be possible to reconstruct the steps in perianth evolution more accurately on a morphological as well as a genetic basis. There are various evolutionary patterns possible in the development of the perianth from a prototype such as those present in Antirrhinum or Arabidopsis (Fig. 5A). Loss of petals in certain families has been compensated for by petaloidy of the sepals (Fig. 5B; e.g. Begoniaceae, Rhamnaceae, Saxifragaceae, Thymelaeaceae, Malvaceae); this has occurred before or after total petal loss. In other groups bracts can be included in the flower to produce a secondary calyx (Fig. 5C; e.g. Loranthaceae, Portulacaceae, Didiereaceae). Loss of sepals is also a frequent phenomenon in certain families, especially among asterids (Fig. 5D; e.g. Rubiaceae, Acanthaceae, Asteraceae). An insertion of bracts in the flower (possibly as an epicalyx) leads to a flower with a biseriate perianth with the same or a different gene expression (Fig. 5E). The derivation of petals from staminodes (Fig. 5F) remains a special case with a limited distribution. It will be interesting to discover the details of how different pathways of reduction and increase have evolved in different groups of core eudicots. However, it is important to understand how structural changes fit with changes in gene expression.
There is no strict correlation between the phylogeny of the core eudicots and gene duplications, although there is some concordance (e.g. Hernández-Hernández et al., 2007; Kramer et al., 2006). Genetic changes may reflect changes in morphology, but not necessarily: gene homologies do not necessarily correspond to morphological homologies. Neither is it possible to link gene phylogenies (different orthologues and paralogues arising through duplication events) with their function (Theissen, 2005; Zahn et al., 2005). This makes it extremely difficult to assign a specific role in petal determination, as a reflection of the morphology of the petals. Neither can one unequivocally answer whether there is one origin of petals or several independent developments within the core eudicots. The numerous paralogues of euAP3 and TM6 in the core eudicots seem to indicate the latter scenario, although the duplication event pre-dates the diversification of the core eudicots. The general use of the term ‘petal’ appears unfortunate as it does not convey any information on homology. We could use the terms ‘sepaloid tepal’ and ‘petaloid tepal’, as well as ‘petaloid staminode’ or ‘staminopetal’ (cf. Ronse De Craene and Smets, 2001) to describe petals in the core eudicots, depending on their origin.
The floral phyllotaxis is fundamentally linked to the expression of genes in flowers and this has not been addressed sufficiently in genetic studies (cf. Endress, 2006). An increased arrangement in whorls leads to a restricted petal gene expression. But what genes are responsible for this? It is also a question of whether changes in genetic expression trigger morphological novelties or whether plants have the ability to switch on/off gene activity in response to functional pressures. A breakdown of the strictly whorled arrangement is linked to stamen or carpel increases in the flower and this appears to dilute the strict genetic boundaries between whorls. As a result perianth differentiation becomes less clear with a gradual change between outer and inner organs and unsettled petal boundaries. How is this reflected at the genetic level?
However, sudden homeotic changes can also have an important impact on evolutionary changes. Ectopic petaloidy of the sepal whorl can go hand in hand with a progressive reduction of the petal whorl, involving different genes and processes.
These questions remain largely unanswered at the moment.
ACKNOWLEDGEMENTS
I thank Dr Charlie Scutt for inviting me to give a presentation at the 10th Congress of the FESPB in Lyon in July 2006. I also thank Professor Mike Jackson for accepting my contribution to this collection of papers for Annals of Botany, for an excellent meal, and for financial support. I am grateful to Hélène Citerne and two anonymous referees for critical comments on the manuscript. Financial support of the Tropical group of RBGE for travel is acknowledged.
LITERATURE CITED
- Albert VA, Gustaffson MGH, Di Laurenzio L. Ontogenetic systematics, molecular developmental genetics, and the angiosperm petal. In: Soltis DE, Soltis PS, Doyle JJ, editors. Molecular systematics of plants II. New York: Kluwer Academic; 1998. pp. 349–374. [Google Scholar]
- Angiosperm Phylogeny Group (APG II) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- von Balthazar M, Endress PK. Development of inflorescences and flowers in Buxaceae and the problem of perianth interpretation. International Journal of Plant Sciences. 2002;163:847–876. [Google Scholar]
- von Balthazar M, Schatz GE, Endress PK. Female flowers and inflorescences of Didymelaceae. Plant Systematics and Evolution. 2003;237:199–208. [Google Scholar]
- Bernhard A, Endress PK. Androecial development and systematics in Flacourtiaceae s.l. Plant Systematics and Evolution. 1999;215:141–155. [Google Scholar]
- Caris PL, Smets EF. A floral ontogenetic study on the sister group relationship between the genus Samolus (Primulaceae) and the Theophrastaceae. American Journal of Botany. 2006;91:627–643. doi: 10.3732/ajb.91.5.627. [DOI] [PubMed] [Google Scholar]
- Di Stilio VS, Kramer EM, Baum DA. Floral MADS box genes and homeotic gender dimorphism in Thalictrum dioicum (Ranunculaceae) – a new model for the study of dioecy. The Plant Journal. 2005;41:755–766. doi: 10.1111/j.1365-313X.2005.02336.x. [DOI] [PubMed] [Google Scholar]
- Drinnan AN, Crane PR, Hoot SR. Patterns of floral evolution in the early diversification of non-magnoliid dicotyledons (eudicots) Plant Systematics and Evolution. 1994;8(suppl.):93–122. [Google Scholar]
- Doyle JJ. Evolution of a plant homeotic multigene family: towards connecting molecular systematic and molecular developmental genetics. Systematic Biology. 1994;43:307–328. [Google Scholar]
- Endress PK. Floral structure, systematics, and phylogeny in Trochodendrales. Annals of the Missouri Botanical Garden. 1986;73:297–324. [Google Scholar]
- Endress PK. Patterns of floral construction in ontogeny and phylogeny. Biological Journal of the Linnean Society. 1990;39:153–175. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Endress PK. Relationships between floral organization, architecture, and pollination mode in Dillenia (Dilleniaceae) Plant Systematics and Evolution. 1997;206:99–118. [Google Scholar]
- Endress PK. Angiosperm floral evolution: morphological developmental framework. Advances in Botanical Research. 2006;44:1–61. [Google Scholar]
- Endress PK, Matthews ML. Elaborate petals and staminodes in eudicots: diversity, function, and evolution. Organisms. Diversity and Evolution. 2006;6:257–293. [Google Scholar]
- Erbar C, Kusma S, Leins P. Development and interpretation of nectary organs in Ranunculaceae. Flora. 1998;194:317–332. [Google Scholar]
- Ferrario S, Immink RGH, Angenent GC. Conservation and diversity in flower land. Current Opinions in Plant Biology. 2004;7:84–91. doi: 10.1016/j.pbi.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Geuten K, Becker A, Kaufmann K, Caris P, Janssens S, Viaene T, et al. Petaloidy and petal identity MADS-box genes in the balsaminoid genera Impatiens and Marcgravia. The Plant Journal. 2006;47:501–518. doi: 10.1111/j.1365-313X.2006.02800.x. [DOI] [PubMed] [Google Scholar]
- Gustaffson MHG. Floral morphology and relationships of Clusia gundlachii with a discussion of floral organ identity and diversity in the genus Clusia. International Journal of Plant Sciences. 2000;161:43–53. doi: 10.1086/314229. [DOI] [PubMed] [Google Scholar]
- He H, Münster T, Saedler H. On the origin of floral morphological novelties. FEBS Letters. 2004;567:147–151. doi: 10.1016/j.febslet.2004.02.090. [DOI] [PubMed] [Google Scholar]
- Hernández-Hernández T, Martínez-Castilla, Alvarez-Buylla ER. Functional diversification of B MADS-box homeotic regulators of flower development: adaptive evolution in protein–protein interaction domains after major gene duplication events. Molecular Biology and Evolution. 2007;24:465–481. doi: 10.1093/molbev/msl182. [DOI] [PubMed] [Google Scholar]
- Hiepko P. Vergleichend-morphologische und entwicklungsgeschichtliche Untersuchungen über das Perianth bei den Polycarpicae. Botanische Jarhbücher für Systematik. 1965;84:359–508. [Google Scholar]
- Hintz M, Bartholmes C, Nutt P, Ziermann J, Hameister S, Neuffer B, Theissen G. Catching a ‘hopeful monster’: shepherd's purse (Capsella bursa-pastoris) as a model system to study the evolution of flower development. Journal of Experimental Botany. 2006;57:3531–3542. doi: 10.1093/jxb/erl158. [DOI] [PubMed] [Google Scholar]
- Hilu KW, Borsch T, Müller K, Soltis DE, Soltis PS, Savolainen V, et al. Angiosperm phylogeny based on matK sequence information. American Journal of Botany. 2003;90:1758–1776. doi: 10.3732/ajb.90.12.1758. [DOI] [PubMed] [Google Scholar]
- Hofmann U. Flower morphology and ontogeny. In: Behnke H-D, Mabry TJ, editors. Caryophyllales. Evolution and systematics. Berlin: Springer-Verlag; 1994. pp. 123–166. [Google Scholar]
- Hoot SB, Magallón S, Crane PR. Phylogeny of basal eudicots based on three molecular datasets: atpB, rbcL, and 18S nuclear ribosomal DNA sequences. Annals of the Missouri Botanical Garden. 1999;86:1–32. [Google Scholar]
- Jack T, Brockmann LL, Meyerowitz EM. The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell. 1992;68:683–697. doi: 10.1016/0092-8674(92)90144-2. [DOI] [PubMed] [Google Scholar]
- Jaramillo MA, Kramer EM. APETALA3 and PISTILLATA homologs exhibit novel expression patterns in the unique perianth of Aristolochia (Aristolochiaceae) Evolution and Development. 2004;6:449–458. doi: 10.1111/j.1525-142X.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- Judd WS, Olmstead RG. A survey of tricolpate (eudicot) phylogenetic relationships. American Journal of Botany. 2004;91:1627–1644. doi: 10.3732/ajb.91.10.1627. [DOI] [PubMed] [Google Scholar]
- Kanno A, Saeki H, Kameya T, Saedler H, Theissen G. Heterotopic expression of class B floral homeotic genes supports a modified ABC model for tulip (Tulipa gesneriana) Plant Molecular Biology. 2003;52:831–841. doi: 10.1023/a:1025070827979. [DOI] [PubMed] [Google Scholar]
- Kim S, Soltis DE, Soltis PS, Zanis MJ, Suh Y. Phylogenetic relationships among early-diverging eudicots based on four genes: were the eudicots ancestrally woody? Molecular Phylogeny and Evolution. 2004a;31:16–30. doi: 10.1016/j.ympev.2003.07.017. [DOI] [PubMed] [Google Scholar]
- Kim S, Yoo M-J, Albert VA, Farris JS, Soltis PS, Soltis DE. Phylogeny and diversification of B-Function MADS-box genes in angiosperms: evolutionary and functional implications of a 260-million-year-old duplication. American Journal of Botany. 2004b;91:2102–2118. doi: 10.3732/ajb.91.12.2102. [DOI] [PubMed] [Google Scholar]
- Kim S, Koh J, Yoo M-J, Kong H, Hu Y, Ma H, et al. Expression of floral MADS-box genes in basal angiosperms: implications for the evolution of floral regulators. The Plant Journal. 2005a;43:724–744. doi: 10.1111/j.1365-313X.2005.02487.x. [DOI] [PubMed] [Google Scholar]
- Kim S, Ma H, Hu Y, Endress PK, Hauser BA, Buzgo M, et al. Sequence and expression studies of A-, B-, and E-class MADS-Box homologues in Eupomatia (Eupomatiaceae): support for the bracteate origin of the calyptra. International Journal of Plant Sciences. 2005b;43:724–744. [Google Scholar]
- Kramer EM, Irish VF. Evolution of genetic mechanisms controlling petal development. Nature. 1999;399:144–148. doi: 10.1038/20172. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Irish VF. Evolution of the petal and stamen developmental programs: evidence from comparative studies of the lower eudicots and basal angiosperms. International Journal of Plant Sciences. 2000;161(Suppl. 6):S29–S40. [Google Scholar]
- Kramer EM, Jaramillo MA. Genetic basis for innovations in floral organ identity. Journal of Experimental Zoology. 2005;304B:526–535. doi: 10.1002/jez.b.21046. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Dorit RL, Irish VF. Molecular evolution of genes controlling petal and stamen development: duplication and divergence within the APETALA3 and PISTILLATA lineages of the Ranunculaceae. Genetics. 1998;149:765–783. doi: 10.1093/genetics/149.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer EM, Di Stilio VS, Schlüter PM. Complex patterns of gene duplication in the APETALA3 and PISTILLATA lineages of the Ranunculaceae. International Journal of Plant Sciences. 2003;164:1–11. [Google Scholar]
- Kramer EM, Su H-J, Wu C-C, Hu J-M. A simplified explanation for the frameshift mutation that created a novel C-terminal motif in the APETALA3 gene lineage. BMC Evolutionary Biology. 2006;6:30. doi: 10.1186/1471-2148-6-30. (electronic publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RS, Irish VF. Functional divergence within the APETALA3/PISTILLATA floral homeotic gene lineages. Proceedings of the National Academy of Sciences of the USA. 2003;100:6558–6563. doi: 10.1073/pnas.0631708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leins P, Walter A, Erbar C. Eine morphogenetische Interpretation der Caryophyllaceen-Kronblätter. Botanische Jahrbücher für Systematik. 2001;123:355–367. [Google Scholar]
- Litt A, Irish VF. Duplication and diversification in the APETALA1/FRUITFULL floral homeotic gene lineage: implications for the evolution of floral development. Genetics. 2003;165:821–833. doi: 10.1093/genetics/165.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magallón S, Crane PR, Herendeen PS. Phylogenetic pattern, diversity and diversification of eudicots. Annals of the Missouri Botanical Garden. 1999;86:297–372. [Google Scholar]
- Nutt P, Ziermann J, Hintz M, Neuffer B, Theissen G. Capsella as a model system to study the evolutionary relevance of floral homeotic mutants. Plant Systematics and Evolution. 2006;259:217–235. [Google Scholar]
- Ochiai T, Nakamura T, Mashiko Y, Fukuda T, Yokoyama J, Kanno A, Kameya T. The differentiation of sepal and petal morphologies in Commelinaceae. Gene. 2004;343:253–262. doi: 10.1016/j.gene.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Park J-H, Ishikawa Y, Ochiai T, Kanno A, Kameya T. Two GLOBOSA-like genes are expressed in second and third whorls of homochamydeous flowers in Asparagus officinalis L. Plant Cell Physiology. 2004;45:325–332. doi: 10.1093/pcp/pch040. [DOI] [PubMed] [Google Scholar]
- Rijpkema AS, Royaert S, Zethof J, van der Weerden G, Gerats T, Vandenbussche M. Analysis of the Petunia TM6 MADS box gene reveals functional divergence within the DEF/AP3 lineage. Plant Cell. 2006;18:1819–1832. doi: 10.1105/tpc.106.042937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann JL, Krizek BA, Meyerowitz EM. Dimerization specificity of Arabidopsis MADS domain homeotic proteins APETALA1, APETALA3, PISTILLATA, and AGAMOUS. Proceedings of the National Academy of Sciences of the USA. 1996;93:4793–4798. doi: 10.1073/pnas.93.10.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronse De Craene LP. Floral development of Cochlospermum tinctorium and Bixa orellana with special emphasis on the androecium. American Journal of Botany. 1989;76:1344–1359. [Google Scholar]
- Ronse De Craene LP. The evolutionary significance of homeosis in flowers: a morphological perspective. International Journal of Plant Science. 2003;164(Suppl. 5):S225–S235. [Google Scholar]
- Ronse De Craene LP. Floral development of Berberidopsis corallina: a crucial link in the evolution of flowers in the core eudicots. Annals of Botany. 2004;94:741–751. doi: 10.1093/aob/mch199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronse De Craene LP, Smets E. Staminodes: their morphological and evolutionary significance. Botanical Review. 2001;67:351–402. [Google Scholar]
- Ronse De Craene LP, Wanntorp L. Evolution of floral characters in. Gunnera. Systematic Botany. 2006;31:671–688. [Google Scholar]
- Ronse De Craene LP, Smets E, Vanvinckenroye P. Pseudodiplostemony, and its implications for the evolution of the androecium in the Caryophyllaceae. Journal of Plant Research. 1998;111:25–43. [Google Scholar]
- Ronse De Craene LP, Soltis PS, Soltis DE. Evolution of floral structures in basal angiosperms. International Journal of Plant Sciences. 2003;164(Suppl. 5):S329–S363. [Google Scholar]
- Schönenberger J, Conti E. Molecular phylogeny and floral evolution of Penaeaceae, Oliniaceae, Rhynchocalycaceae, and Alzateaceae (Myrtales) American Journal of Botany. 2003;90:293–309. doi: 10.3732/ajb.90.2.293. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Soltis PS, Chase MW, Mort ME, Albach DC, Zanis M, et al. Angiosperm phylogeny inferred from a combined data set of 18S rDNA, rbcL and atpB sequences. Botanical Journal of the Linnean Society. 2000;133:381–461. [Google Scholar]
- Soltis DE, Senters AE, Zanis MJ, Kim S, Thompson JD, Soltis PS, et al. Gunnerales are sister to other core eudicots: implications for the evolution of pentamery. American Journal of Botany. 2003;90:461–470. doi: 10.3732/ajb.90.3.461. [DOI] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE, Chase MW, Endress PK, Crane PR. The diversification of flowering plants. In: Cracraft J, Donoghue MJ, editors. Assembling the tree of life. Oxford: Oxford University Press; 2003. pp. 154–167. [Google Scholar]
- Stellari GM, Jaramillo MA, Kramer EM. Evolution of the APETALA3 and PISTILLATA lineages of MADS-Box containing genes in the basal angiosperms. Molecular Biology and Evolution. 2004;21:506–519. doi: 10.1093/molbev/msh044. [DOI] [PubMed] [Google Scholar]
- Takhtajan A. Evolutionary trends in flowering plants. New York: Columbia University Press; 1991. [Google Scholar]
- Tamura M. Morphology, ecology and phylogeny of the Ranunculaceae. IV. Scientific Reports of Osaka University. 1965;14:53–71. [Google Scholar]
- Theissen G. Birth, life and death of developmental control genes: new challenges for the homology concept. Theory in Bioscience. 2005;124:199–212. doi: 10.1016/j.thbio.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Theissen G. The proper place of hopeful monsters in evolutionary biology. Theory in Bioscience. 2006;124:349–369. doi: 10.1016/j.thbio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Theissen G, Becker A, Di Rosa A, Kanno A, Kim JT, Münster T, et al. A short history of MADS-box genes in plants. Plant Molecular Biology. 2000;42:115–149. [PubMed] [Google Scholar]
- Theissen G, Becker A, Winter K-U, Münster T, Kirchner C, Saedler H. How the land plants learned their floral ABCs: the role of MADS box genes in the evolutionary origin of flowers. In: Cronk QCB, Bateman RM, Hawkins JA, editors. Developmental genetics and plant evolution. London: Taylor & Francis; 2002. pp. 173–205. [Google Scholar]
- Tsai W-C, Kuoh C-S, Chuang M-H, Chen W-H, Chen H-H. Four DEF-like MADS box genes displayed distinct floral morphogenetic roles in Phalaenopsis orchid. Plant and Cell Physiology. 2004;45:831–844. doi: 10.1093/pcp/pch095. [DOI] [PubMed] [Google Scholar]
- Tsou C-H. Early floral development of Camellioideae (Theaceae) American Journal of Botany. 1998;85:1531–1547. [PubMed] [Google Scholar]
- Tucker SC. The ontogenetic basis for missing petals in Crudia (Leguminosae: Caesalpinoideae: Detarieae) International Journal of Plant Sciences. 2001;162:83–89. [Google Scholar]
- Tucker SC, Bernhardt P. Floral ontogeny, pattern formation, and evolution in Hibbertia and Adrastea (Dilleniaceae) American Journal of Botany. 2000;87:1915–1936. [PubMed] [Google Scholar]
- Wanntorp L, Ronse De Craene LP. Flower morphology of Gunnera – key to eudicot diversification or reductive “dead-end”? International Journal of Plant Sciences. 2005;166:945–953. [Google Scholar]
- Weigel D, Meyerowitz EM. The ABCs of floral homeotic genes. Cell. 1994;78:203–209. doi: 10.1016/0092-8674(94)90291-7. [DOI] [PubMed] [Google Scholar]
- Zahn LM, Leebens-Mack J, DePamphilis CW, Ma H, Theissen G. To B or not to B a flower: the role of DEFICIENS and GLOBOSA orthologs in the evolution of the angiosperms. Journal of Heredity. 2005;96:225–240. doi: 10.1093/jhered/esi033. [DOI] [PubMed] [Google Scholar]
- Zanis MJ, Soltis PS, Qiu YL, Zimmer E, Soltis DE. Phylogenetic analyses and perianth evolution in basal angiosperms. Annals of the Missouri Botanical Garden. 2003;90:129–150. [Google Scholar]