Abstract
This study investigated the physiologic and sedative parameters associated with a low-dose infusion of dexmedetomidine (Dex). Thirteen healthy volunteers were sedated with Dex at a loading dose of 6 mcg/kg/h for 5 minutes and a continuous infusion dose of 0.2 mcg/kg/h for 25 minutes. The recovery process was observed for 60 minutes post infusion. The tidal volume decreased significantly despite nonsignificant changes in respiratory rate, minute ventilation, oxygen saturation, and end-tidal carbon dioxide. The mean arterial pressure and heart rate also decreased significantly but within clinically acceptable levels. Amnesia to pin prick was present in 69% of subjects. A Trieger dot test plot error ratio did not show a significant change at 30 minutes post infusion despite a continued significant decrease in bispectral index. We conclude that sedation with a low dose of Dex appears to be safe and potentially efficacious for young healthy patients undergoing dental procedures.
Keywords: Dexmedetomidine, Sedation, α2-Agonist, Amnesia, Dental procedure
Dexmedetomidine (Dex) is a sedative and analgesic agent that acts through an α2-agonist effect.1 In Japan and the United States, it is licensed as a sedative agent for intensive care unit (ICU) sedation after surgery. The effects of α2-agonists have been associated with reduced anesthetic requirements and attenuated blood pressure and heart rate in response to stressful events.2–5 The α2-receptors within the spinal cord modulate pain pathways, thereby providing some degree of analgesia.6–8 In addition, Dex induces a sedative response that exhibits properties similar to natural sleep, unlike other anesthetics. Patients who are given Dex experience a clinically effective sedation yet are still easily and uniquely arousable—an effect that has not been observed with any other clinically available sedative.9,10 Sedation with Dex may be optimal for dental procedures because it possesses many of the properties of an ideal sedative agent, such as minimal influence on respiration and circulation, easy and rapid control of sedative and conscious levels, amnesia, and rapid recovery after sedation.
The present study investigated the effects on respiration, circulation, sedative level, recovery parameters, and amnesia during and after intravenous infusion of low-dose Dex.
Methods
Subjects
Subjects consisted of 13 healthy volunteers who ranged in age from 24 to 37 years. Informed consent was obtained for this institutional review board–approved study.
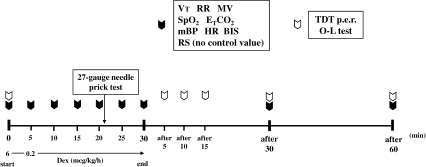
Dex Infusion (Figure 1)
Figure 1.
Time course of the investigation. Subjects were sedated with dexmedetomidine (Dex) at a loading dose of 6 mcg/kg/h for 5 minutes and a continuous infusion dose of 0.2 mcg/kg/h for 25 minutes. The recovery process was observed for 60 minutes after cessation of the Dex infusion. (Control value was not measured in Ramsay score.)
An intravenous catheter (Insyte 20-gauge, Becton Dickinson, Franklin Lakes, NJ) was inserted into a medial cubital vein and an infusion of lactated Ringer's solution was started at 2 mL/kg/h. Subjects were kept in a supine position for 20 minutes. After cardiovascular parameters had achieved steady state (change in vital signs <10%), subjects were sedated with Dex at a loading dose of 6 mcg/kg/h (1 mcg/kg per 10 minutes) for 5 minutes followed by a continuous infusion dose of 0.2 mcg/kg/h for 25 minutes; the recovery process then was observed for 60 minutes after the Dex infusion was stopped. A pin prick test with a 27-gauge needle was performed in the gingival labial mucosa to assess amnesia at 21 minutes after the loading infusion was started (16 minutes after maintenance infusion). The following parameters were measured; tidal volume (TV), respiratory rate (RR), minute volume (MV), oxygen saturation (SpO2), end-tidal carbon dioxide (ETCO2), mean arterial pressure (MAP), heart rate (HR), bispectral index (BIS), and Ramsay score (RS).11 Recovery parameters included the Trieger dot test12 plot error ratio (TDT p.e.r.), which was used to determine the level of psychomotor function, and a 1-leg standing with eyes closed test (O-L test), which was performed to assess the level of recovery of equilibrium. Respiratory parameters were measured with a MAGTRAK (IMI, Saitama, Japan) with a tight-fitting mask; SpO2 and ETCO2 were assessed with a Capnomac Ultima (Datex, Milwaukee, Wis); MAP and HR were evaluated with a Dinamap 8100 (Criticon, Tampa, Fla); and sedative level was determined with a BIS A-2000 (Aspect Medical System, Norwood, Mass).
Statistical Analysis
Friedman's test was applied for the statistical analysis, followed by the Wilcoxon t test with Bonferroni's correction. P values of <.05 were considered statistically significant. However, a statistical analysis was not performed for RS and O-L tests.
Results
Subjects (Table 1)
Table 1.
Background of Subjects. We studied healthy adult volunteers (we obtained informed consent from them). Age, weight, and ASA physical status are following.
Table 2.
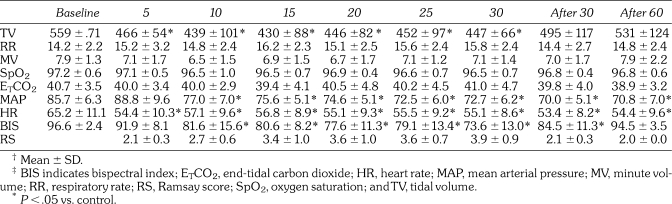
Summary of the Results in Respiration, Circulation, and Sedative Level (Minutes).†‡
Table 3.
The Results of TDT p.e.r. and O-L Test (Minutes).†
Subjects were on average 28.8 ± 3.3 years old and weighed 69.3 ± 8.1 kg.
Respiration (Figures 2 And 3; Table 2)
Figure 2.
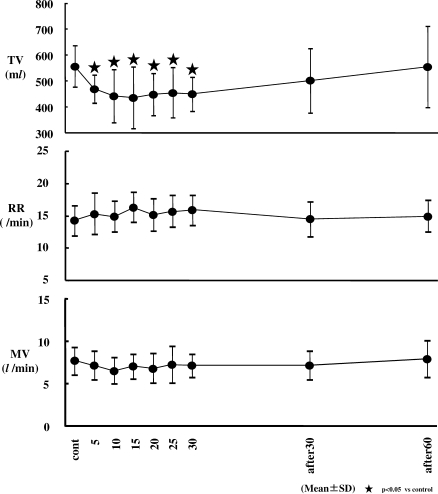
Changes in tidal volume (TV), respiratory rate (RR), and minute volume (MV). TV decreased significantly from 5 minutes to 30 minutes after the start of dexmedetomidine (Dex) infusion. However, RR and MV did not show significant changes. TV decreased significantly from an average of 580 mL (control value) to approximately 430 to 470 mL (P < .05).
Figure 3.
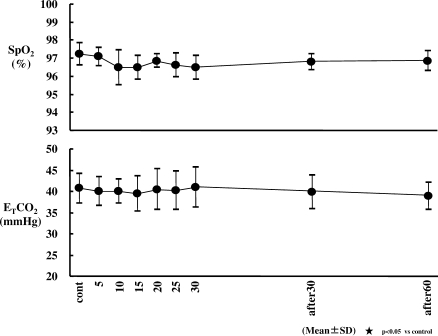
Changes in end-tidal carbon dioxide (ETCO2) and oxygen saturation (SpO2). Respiratory rate (RR) and SpO2 did not show significant changes. Sedation with dexmedetomidine (Dex) had no effect on ETCO2 and SpO2.
No significant changes in RR, MV, SpO2, or ETCO2 were observed. TV decreased significantly at 5, 10, 15, 20, 25, and 30 minutes after the start of the Dex infusion (from an average of 560 mL to 430 to 466 mL) (P < .05).
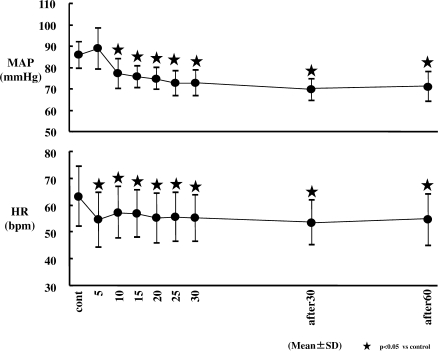
Circulation (Figure 4; Table 2)
Figure 4.
Changes in mean arterial pressure (MAP) and heart rate (HR). The transient increase in MAP was observed at 5 minutes after the start of dexmedetomidine (Dex) infusion (not significant); MAP decreased significantly from an average of 86 mm Hg (control value) to approximately 70 to 77 mm Hg over 80 minutes since 10 minutes after the start of Dex infusion (P < .05). HR decreased significantly since 5 minutes after the start of Dex infusion, and HR decreased significantly from an average of 65 beats per minute (bpm) (control value) to approximately 53 to 57 bpm (P < .05).
After a brief, statistically insignificant increase in MAP following infusion initiation, the MAP decreased significantly from 10 minutes after infusion throughout the 60 minute postinfusion period. The MAP decreased from an average of 86 mm Hg to 70 to 77 mm Hg over this 80 minute period. HR decreased significantly from 5 minutes after infusion throughout the 60 minute postinfusion period (beats per minute [bpm] range, low 60s to mid 50s).
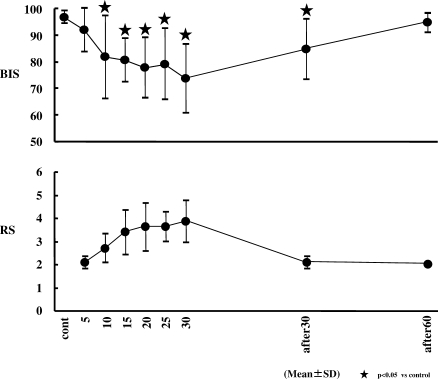
BIS (Figure 5)
Figure 5.
Changes in bispectral index (BIS) and Ramsay score (RS). BIS decreased significantly from 10 minutes after the start of dexmedetomidine (Dex) infusion to 30 minutes after the end of Dex infusion (P < .05). RS showed the optimal sedation level from 10 minutes to 30 minutes after the start of Dex infusion.
BIS decreased significantly from 10 minutes after the start of the Dex infusion to 30 minutes after the end of the Dex infusion. The lowest average BIS was 73.6 at 30 minutes after the start of the Dex infusion.
RS (Figure 5)
Statistical analysis was not performed in RS, which was within the optimal sedative score (∼3 to 4) at 15, 20, 25, and 30 minutes after the start of the Dex infusion.
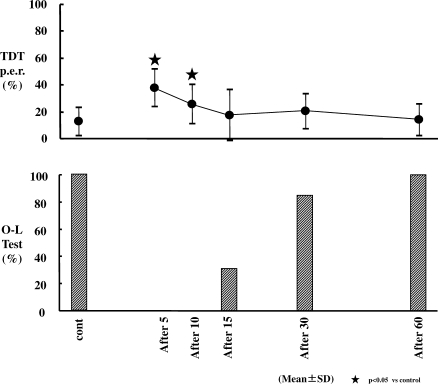
TDT P.e.r. (Figure 6; Table 3)
Figure 6.
Changes in Trieger dot test plus error ratio test (TDT p.e.r.) and 1-leg standing with eyes closed test (O-L test). TDT p.e.r. increased significantly at 5 and 10 minutes from the end of dexmedetomidine (Dex) infusion (P < .05). All subjects passed the O-L test at 60 minutes after cessation of Dex infusion.
TDT p.e.r. increased significantly at 5 and 10 minutes from the end of the Dex infusion. Values were 37.5% and 25.4%, respectively (P < .05), in comparison with the control value of 12.4%.
O-L Test (Figure 6; Table 3)
At 15 minutes post infusion, only 31% of subjects had successfully completed this test. At 30 minutes post infusion, 85% of subjects were successful, and all subjects were successful 60 minutes after cessation of the Dex infusion.
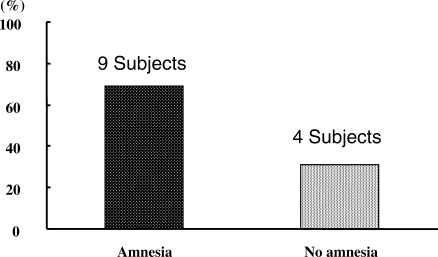
Amnesia (Figure 7)
Figure 7.
Amnesia. Amnesia was recognized with a 27-gauge needle prick test performed in 69% of subjects at 21 minutes after the start of dexmedetomidine (Dex) infusion.
Amnesia was demonstrated with the 27-gauge needle prick test in 69% of subjects at 21 minutes after the start of the Dex infusion.
Discussion
Dex was originally developed for sedation of the intubated ICU patient for short periods. To maximize safety prior to study of the use of Dex for sedation during actual dental procedures, this initial pilot study used a low initial loading dose and the lowest continuous infusion dose within the typical recommended range of 0.2 to 0.7 mcg/kg/h.
Respiration
Results presented here show that TV decreased significantly despite the absence of significant changes in RR, MV, SpO2, and ETCO2. This respiratory change is similar to that seen in studies in which other α2-agonists are used.13,14 Belleville15 reported a small but statistically significant decrease in MV caused by Dex, thus reflecting a reduction in TV. The significant decrease in TV was consistent with Belleville's report, although MV did not show a significant change in the current study. Presumably, in individual subjects with a greater decrease in TV, a compensatory increase in RR reflects minimal changes in MV. The decrease in TV suggests inhibition of the central respiratory drive. α2-Adrenoceptors are ubiquitous throughout the central nervous system, including the brainstem regions, which are instrumental in control of breathing.16 However, the mechanism of α2-adrenoceptors in the control of respiration has not yet been elucidated.
In clinical situations, the decrease in TV may be affected not only by inhibition of the central respiratory drive but also by an upper airway obstruction. However, the current results indicate that a significant decrease in TV has no influence on MV; therefore, low-dose Dex infusion can be safely used for a healthy patient without causing hypoxemia and hypercapnia.
Circulation
Although MAP increased in a statistically and clinically insignificant manner at 5 minutes following the loading dose of Dex, it decreased significantly after 10 minutes. This transient increase is thought to be due to activation of the peripheral α2B-adrenoceptors that mediate vasoconstriction, which appeared earlier than that of α2-adrenoceptors in the central nervous system that mediate decreased sympathetic outflow.17 At the same time as the early transient increase in MAP, HR showed a significant decrease from 65.2 ± 11.1 (control value) to 54.4 ± 10.3. This bradycardic effect may occur as a reflexive response to the MAP increase caused by activation of α2B-adrenoceptors on the peripheral vasculature.18 With regard to the early effect, Hall et al17 suggested that this effect might be unavoidable when α2-agonists are infused because of the time differential between direct binding to the peripheral vascular receptors and diffusion into the central nervous system with resultant sympatholytic effects. In addition, MacDonald et al18 reported that the bradycardiac effect induced by α2-adrenoceptor agonists is mediated in part by α2-adrenoceptors and in part by a baroreflex-mediated response.
MAP showed significant decreases from the 10 minute time point following infusion throughout the 60 minute post-infusion period. It decreased from an average of 85 mm Hg to 70 to 77 mm Hg over an 80 minute period. The decrease in MAP from 10 minutes until 60 minutes post infusion (80 minutes total time) likely reflects inhibition of sympathetic outflow, which overrode the direct effects of Dex on the vasculature.17 Generally, local anesthesia such as epinephrine is used in the clinical setting of dental procedures, and this can lead to an increase in blood pressure.19,20 It may be advisable to delay the administration of local anesthesia with epinephrine until an appropriate time is reached after the start of the Dex maintenance infusion following a loading dose of 6 mcg/kg/h.
Both MAP and HR still showed a significant decrease at 60 minutes after the end of the Dex infusion. Because the elimination half-life of Dex is 2 hours, this suggests that at least 120 minutes must pass post Dex infusion before cardiovascular parameters have fully recovered.21 From the viewpoint of the cardiovascular system, the decrease in MAP within the normal range combined with the decrease in HR should produce some advantages that may be helpful for patients with ischemic heart disease due to decreasing myocardial oxygen demand.
Sedative Level
A sedative condition was demonstrated on the BIS monitor from 10 minutes after the start of the Dex infusion to 30 minutes after the end of the Dex infusion and was observed with the RS from 10 minutes to 30 minutes after the start of Dex infusion. Results of the present study indicate that dental procedures should be started 10 minutes after the start of the Dex infusion. However, if the optimal sedative score is between 3 and 4 in RS, then dental procedures should be started at least 13 minutes after the start of Dex infusion at these doses. This result suggests that combining Dex with other sedatives such as a benzodiazepine and/or increasing the loading dose or the continuous infusion dose of Dex to improve onset time may be clinically necessary.
Subjectively, subjects were in a sedative state based on RS even if they seemed to be clearly conscious. It is interesting to note that a sedative state based on RS was not recognized despite demonstration of a sedative condition on the BIS at 30 minutes post infusion. Discontinuing Dex infusion prior to the time of completion of the dental procedure may be advisable; this may result in acceptable sedation at the end of the procedure.
Recovery Process
Subjects regained their orientation from 15 minutes after the end of the Dex infusion based on TDT p.e.r., which was not significantly different from baseline. However, the sedative condition on the BIS monitor was seen at 30 minutes after cessation of the Dex infusion. This shows that Dex has a unique property that allows subjects to arouse easily with cognition from a sedative state.
Although the elimination half-life of Dex is 2 hours,21 all subjects could perform on an OL-test at 60 minutes after the end of the infusion. It should be appreciated that subjects in this study were young healthy volunteers who might have been able to recover their sense of equilibrium more quickly than older or more medically compromised patients. In addition, we used the lowest recommended dose of Dex at 0.2 mcg/kg/h. Higher doses may delay recovery.
Amnesia
A 27-gauge needle prick test was chosen for use in the present study because no significant difference in the perception of pain has been described with penetration of 25-, 27-, and 30-gauge needles.22 It has been reported that 50% of subjects described amnesia during propofol sedation when a loading dose of propofol of 6 mg/kg/h (100 mcg/kg/min) was provided for 10 minutes, followed by infusion at 4 mg/kg/h (66.7 mcg/kg/min) for 20 minutes.23 However, it is not possible to directly compare these findings with those of the current study because the infusion method used in this study was different from the one used in the earlier report.23 Dex may have a slightly stronger amnesic effect than propofol when given at sedative doses. To improve the incidence of amnesia with a Dex infusion, a sedative such as a benzodiazepine may have to be added during sedation.
Conclusion
The use of a Dex infusion in the present study was observed to have minimal influence on respiration or circulation in young healthy subjects. For this patient population, it should be possible to use this infusion safely for dental procedures. Increasing the maintenance infusion dose of Dex may allow the dental procedure to start earlier in the sedation process and/or may achieve an improved amnesic effect during sedation. In addition, discontinuing the infusion at least 15 minutes prior to procedure completion may prove valuable because it takes at least 60 minutes for such patients to recover sufficiently for discharge, even though the patient may recover his or her orientation within 15 minutes of cessation of the Dex infusion.
References
- Venn R.M, Grounds R.M. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: patient and clinician perceptions. Br J Anaesth. 2001;87:684–690. doi: 10.1093/bja/87.5.684. [DOI] [PubMed] [Google Scholar]
- McSPI-EUROPE Research Group. Perioperative sympatholysis: beneficial effects of the α2-adrenoceptor agonist mivazerol on hemodynamic stability and myocardial ischemia. Anesthesiology. 1997;86:346–363. [PubMed] [Google Scholar]
- Quintin L, Bonnet F, Macquin I, Szekely B, Becquemin P, Ghignone M. Aortic surgery: effect of clonidine on intraoperative catecholaminergic and circulatory stability. Acta Anaesthesiol Scand. 1990;34:132–137. doi: 10.1111/j.1399-6576.1990.tb03057.x. [DOI] [PubMed] [Google Scholar]
- Aho M, Lehitinen A-M, Erkola O, Kallio A, Kortilla K. The effect of intravenously administered dexmedetomidine on perioperative hemodynamics and isoflurane requirements in patients undergoing abdominal hysterectomy. Anesthesiology. 1991;74:997–1002. doi: 10.1097/00000542-199106000-00005. [DOI] [PubMed] [Google Scholar]
- Proctor L.T, Shmeling W.T, Roerig D, Kampine J.P, Warltier D.C. Oral dexmedetomidine attenuates hemodynamic responses during emergence from general anesthesia in chronically instrumented dogs. Anesthesiology. 1991;74:108–114. doi: 10.1097/00000542-199101000-00018. [DOI] [PubMed] [Google Scholar]
- Spaulding T.C, Fielding S, Venafro J.J, Lal H. Antinociceptive activity of clonidine and its potentiation of morphine analgesia. Eur J Pharmacol. 1979;57:19–25. doi: 10.1016/0014-2999(79)90335-2. [DOI] [PubMed] [Google Scholar]
- Bonnet F, Boico O, Rostaing S, Loriferne J.F, Saada M. Clonidine-induced analgesia in postoperative patients: epidural versus intramuscular administration. Anesthesiology. 1990;72:423–427. doi: 10.1097/00000542-199003000-00004. [DOI] [PubMed] [Google Scholar]
- Segal I.S, Jarvis D.J, Duncan S.R, White P.F, Maze M. Clinical efficacy of oral-transdermal clonidine combinations during the perioperative period. Anesthesiology. 1991;74:220–225. doi: 10.1097/00000542-199102000-00005. [DOI] [PubMed] [Google Scholar]
- Nelson L.E, Lu J, Guo T, Saper C.B, Franks N.P, Maze M. The α2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- Venn R.M, Bradshaw C.J, Spencer R, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anesthesia. 1999;54:1136–1142. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- Ramsay M.A, Savege T.M, Simpson B.R, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmann M.G, Trieger N, Loskota W.J, Jacobs A.W. A comparative study of psychomotor effects of intravenous agents used in dentistry. Oral Surg. 1970;30:34–40. doi: 10.1016/0030-4220(70)90007-1. [DOI] [PubMed] [Google Scholar]
- Bailey P.L, Sperry R.J, Johnson K, et al. Respiratory effects of clonidine alone and combined with morphine, in humans. Anesthesiology. 1991;74:43–48. doi: 10.1097/00000542-199101000-00008. [DOI] [PubMed] [Google Scholar]
- Segal I.S, Jarvis D.J, Duncun S.R, White P.F, Maze M. Clinical efficacy of oral-transdermal clonidine combinations during the perioperative period. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-199102000-00005. [DOI] [PubMed] [Google Scholar]
- Belleville J.P, Ward D.S, Bloor B.C, Maze M. Effects of intravenous dexmedetomidine in humans. Anesthesiology. 1992;77:1125–1133. doi: 10.1097/00000542-199212000-00013. [DOI] [PubMed] [Google Scholar]
- Unnerstall J.R, Kopajtic T.A, Kuhar M.J. Distribution of α2 agonist binding sites in the rat and human central nervous system: analysis of some functional, anatomic correlates of the pharmacologic effects of clonidine and related adrenergic agents. Brain Res Rev. 1984;7:69–101. doi: 10.1016/0165-0173(84)90030-4. [DOI] [PubMed] [Google Scholar]
- Hall J.E, Uhrich T.D, Barney J.A, Arain S.R, Ebert T.J. Sedative, amnestic, and analgesic properties of small-dose of dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Kobilka B.K, Schenin M. Gene targeting—homing in on α2 adrenoceptor–subtype function. Trends Pharmacol Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- Abraham-Inpijn L, Borgmeijer-Hoelen A, Gortzak R.A.T. Changes in blood pressure, heart rate, and electrocardiogram during dental treatment with use of local anesthesia. J Am Dent Assoc. 1988;116:531–536. doi: 10.14219/jada.archive.1988.0318. [DOI] [PubMed] [Google Scholar]
- Boakes A.J, Laurence D.R, O'Neil R, Verrill P.J. Adverse reactions to local anaesthetic-vasoconstrictor preparations. Br Dent J. 1972;133:137–140. doi: 10.1038/sj.bdj.4802884. [DOI] [PubMed] [Google Scholar]
- Kamibayashi T, Maze T. Clinical uses of α2 adrenergic agonist. Anesthesiology. 2000;93:1345–1349. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- Fuller N.P, Menke R.A, Meyers W.J. Perception of pain to three different penetrations of needles. J Am Dent Assoc. 1979;99:822–824. doi: 10.14219/jada.archive.1979.0384. [DOI] [PubMed] [Google Scholar]
- Kawaai H, Tanaka K, Yamazaki S, Sugita T, Okuaki A. A study of intravenous sedation with propofol for dental treatment and oral surgery—influences on respiration, circulation sedation, and the recovery process. Dentistry in Japan. 2000;36:120–124. [Google Scholar]