Abstract
Immunological memory is a hallmark of adaptive immunity, and understanding T cell memory will be central to the development of effective cell-mediated vaccines. The characteristics and functions of CD4 memory cells have not been well defined. Here we demonstrate that the increased size of the secondary response is solely a consequence of the increased antigen-specific precursor frequency within the memory pool. Memory cells proliferated less than primary responding cells, even within the same host. By analyzing the entry of primary and memory cells into the cell cycle, we found that the two populations proliferated similarly until day 5; after this time, fewer of the reactivated memory cells proliferated. At this time, fewer of the reactivated memory cells made IL-2 than primary responding cells, but more made IFNγ. Both these factors affected the low proliferation of the memory cells, because either exogenous IL-2 or inhibition of IFNγ increased the proliferation of the memory cells.
Keywords: interferon gamma, interleukin 2, proliferation, LCMV, recall
Immunological memory is a defining characteristic of the adaptive immune response and can be manifested by antigen-specific B cells and CD4 and CD8 T cells (1). Work of the last 10 years has tremendously advanced our knowledge of CD8 T cell memory (2). It is now generally accepted that, as a population, memory CD8 T cells are maintained at fairly constant numbers (3) and provide protection more effectively than naïve cells (4). In contrast, the maintenance and functional capabilities of memory CD4 T cells are still controversial (5–9).
Some of the confusion about CD4 memory T cells may lie in the use of TCR transgenic (Tg) cells primed either in vitro (8) or in vivo (10). Although these cells have provided a wealth of knowledge about the early stages of CD4 T cell responses (11), their usefulness in memory cell studies is less clear, as they are often used at unnaturally high frequencies, which can lead to anomalous results (12, 13).
Although cytokines affect both the generation and survival of CD4 memory T cells (14), their production by antigen-specific memory cells after reactivation is not well defined. The classification of memory cells into T effector memory (TEM) and T central memory (TCM) (15) suggests that, whereas some memory cells may make an enhanced effector response, a second subset still have the capacity to produce IL-2 and proliferate well. Studies using TCR Tg cells have indicated that this may be the case (16, 17) but, since these experiments were done by using large numbers of transferred TCR Tg cells, such results may not reflect events in unmanipulated animals.
To avoid such issues, we have used MHC class II/peptide tetramers to examine antigen specific endogenous CD4 memory cells in intact hosts, tracking them through initial activation and contraction and during subsequent responses in vivo. Although CD4 memory T cells proliferated in response to antigen, they did not divide as efficiently as primary responding cells at later time points. This reduced proliferation was accompanied by a decrease in the percentage of cells making IL-2 and an increase in IFNγ production. Addition of IL-2 or blockage of IFNγ in vivo increased the proliferation of the memory cells to that of the primary responding cells. This work demonstrates that reactivated CD4 memory cells, via the cytokines they do and do not produce, limit their own proliferation upon antigen stimulation in vivo.
Results
Secondary Responses Are Larger and Peak Earlier than Primary Responses.
To study endogenous memory CD4 T cell responses, we immunized C57BL/6 (B6) mice with the IAb-binding peptide, 3K, and tracked the response using IAb/3K class II tetramers (18). The number of tetramer (tet)+ cells was determined as described in Materials and Methods and background staining set by the number of tet+CD44hi cells in naïve mice.
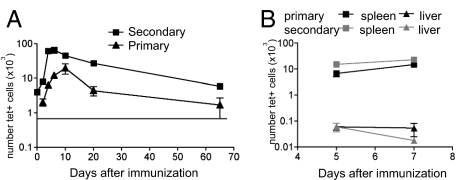
To examine the kinetics with which memory CD4 T cells responded to IAb/3K, B6 mice were primed with 3K+LPS i.v., rested for 4–20 weeks, then reimmunized. At this point, age-matched naïve mice were primed to allow us to measure the primary response. Supporting information (SI) Fig. S1 shows sample FACS plots of tet staining and the phenotype of the primary and secondary memory cells. The primary response peaked on day (d)9, whereas the secondary response peaked earlier, on d5, and the number of tet+ cells was greater in the secondary than the primary response at their respective peaks (Fig. 1A). This increased size was not due to activation of both naïve and memory cells, because the secondary response in mice that had been thymectomized (Tx) before priming was the same as in sham-Tx mice (Fig. S2).
Fig. 1.
The CD4 T cell secondary response peaks earlier and is numerically larger than the primary response. (A) B6 that had been primed with 3K+LPS 4–20 weeks earlier (squares) and naïve mice (triangles) were immunized with 3K+LPS i.v. At the indicated time, splenocytes were isolated and the number of IAb/3K tet+ cells analyzed. As a control, splenocytes from naïve B6 mice were also stained to indicate a level of background staining (gray line). Data points represent the mean +/−SEM from 4 time course experiments with 3–4 mice per group. (B) Spleens (squares) and livers (triangles) were taken at d5 and d7 of a primary (black) or secondary (gray) response to 3K+LPS and the number of tet+ cells examined. The points indicate the mean± SEM of four mice per group.
We also examined the migration of primary and secondary responding cells. At d5, we found there were more tet+ cells in all organs examined in the secondary response (Table S1). However, this reflects the total increased number of tet+ cells in the secondary response rather than an alteration in migration patterns. Moreover, we did not find a significant migration of the reactivated memory cells out of the spleen after day 5; in contrast, the number of cells present in the liver of secondary responding mice decreased between d5–7 (Fig. 1B).
Memory CD4 Cells Expand Less Efficiently than Naïve Cells to Ag Stimulation.
The larger size of the secondary response could be due to three nonmutually exclusive factors: the increased number of Ag specific cells; enhanced proliferation by the memory cells; and/or reduced death of the memory cells. To investigate this, we first needed to know the number of IAb/3K-specific cells in the naïve and memory pool.
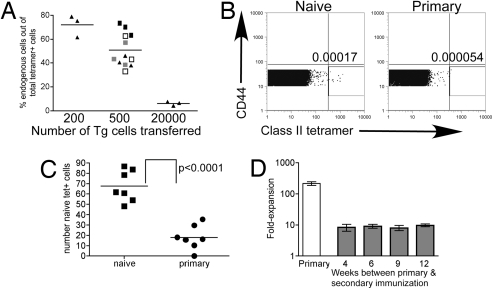
To count the number of naïve IAb/3K-specific cells, we transferred different numbers of TCR Tg cells that respond to IAb/3K into naïve congenic hosts. After immunization with 3K+LPS, both the transferred cells and the host response could be measured with tetramer and the populations distinguished by Thy expression. By transferring different numbers of cells and analyzing the percentage of total tet+ cells that were host-derived, we were able to determine when the starting population of Tg cells and host IAb/3K specific cells were equal, i.e., when the host response was 50% of the total response. This occurred after the transfer of 500 Tg cells (Fig. 2A). We and others (19, 20) have found that ≈10% of cells survive after transfer. After the transfer of 500 cells, ≈50 would be expected to respond to a subsequent immunization, suggesting there are ≈50 naive IAb/3K specific cells in a mouse.
Fig. 2.
Memory cells undergo a lower fold-expansion than naïve cells. (A) Two hundred, 500, or 2 × 104 IAb/3K-specific TCR Tg cells were transferred into naïve B6.PL mice and the recipients primed the next day with 3K+LPS i.v. Six days later, splenocytes were isolated and the number of TCR Tg and host Ag-specific CD4 T cells analyzed. The percentages of the total tet+ that were host-derived were calculated. Each point represents one mouse, and the line shows the mean of the groups. Data from one experiment after the transfer of 200 or 20,000 Tg cells, four (indicated by different symbols) after the transfer or 500 cells. (B) Total spleen and LN cells from naïve or recently primed B6 mice were stained with tetramer. Cells are gated as detailed in Materials and Methods on naïve CD4+ cells. Plots are overlays of all six samples collected from one naïve and one primed mouse. The number indicates the mean percentage of cells within the gate. (C) As in B, but the mean number of cells within the naïve-tet+ gate for each mouse is shown by each point and the line shows the mean of the group. Data are from two experiments with three to four mice per group. (D) B6 mice were either primed (white bar) or primed then immunized a second time after the indicated length of time. The fold expansion of the primary responding cells was calculated by comparing the difference between the number of tet+ cells at d9 and the estimated number of naïve Ag specific cells (70). The fold expansion of the memory cells was calculated by comparing the number of tet+ present on d5 after the second immunization to the number of memory cells in a cohort of mice that were not immunized again.
There are caveats to this indirect method of counting naïve Ag specific cells, so we directly counted the number of naïve IAb/3K-specific cells in mice by staining total spleen and lymph node (LN) cells from naïve B6 mice with tetramer and analyzing all these cells by FACS (see Materials and Methods for details). By gating on live naïve CD4 T cells (Fig. 2B), we identified ≈70 IAb/3K-specific cells per mouse (Fig. 2C). Because we did not collect every LN, and there may have been some naïve IAb/3K-specific cells in the blood, this may be an underestimate, but this number is similar to that found in the Tg cell experiment and to that described for T cells with other specificities (19–21). To set a level of background staining, we examined tetramer staining of naïve CD4 T cells from mice that had been primed with an optimal dose of 3K+LPS 7–9 days earlier. In these mice, we believe all of the Ag-specific cells capable of responding should up-regulate surface CD44 and should not be present in the naïve cell gate. We counted 20 cells per immunized mouse in this gate, suggesting that 70 IAb/3K-specific cells per mouse may be a slight overestimate.
Using this number of naïve IAb/3K-specific T cells (70) and the number of tet+ cells at the peak of the response (6–30 × 103), we calculated that the fold expansion of the primary responding cells was >100 (Fig. 2D). The fold expansion of the memory cells was calculated to be ≈20-fold by comparing the number of memory cells present before the second immunization and the number of cells at the peak. The time between the two immunizations did not affect this result (Fig. 2D), indicating that, because memory cells declined over time, the number of cells in the memory pool did not determine their fold expansion.
Reduced Fold Expansion of Memory Cells Is Intrinsic to the Cells.
Immunization results in an increase in the number of IAb/3K-specific T cells compared with that in naïve mice. Therefore, upon reexposure to the same dose of 3K+LPS, this increased frequency could result in greater competition for resources such as Ag, antigen-presenting cells (APC), or cytokines.
To test this, we used a system that allowed us to track a primary and memory response in the same host by transferring surface-marked IAb/3K memory cells from immunized to naïve mice. To obtain enough of these cells, we used cells from mice that had been immunized twice. Such cells had the same markers and proliferative capacity as secondarily responding cells (Figs. S1 and S3 and data not shown).
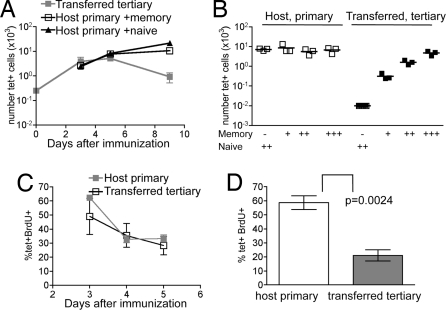
Naïve B6.PL mice were given Thy1.2+ memory cells, or control mice received the same number of cells from naïve animals. Recipients were primed with 3K+LPS, and the numbers of transferred and host IAb/3K-specific cells measured. The memory cells peaked on d5, whereas the host cells peaked, as usual, on d9 whether or not their hosts contained transferred memory cells (Fig. 3A). These data indicate that the lower fold expansion of the memory cells was not due to competition but rather to a cell intrinsic factor. Although it is possible that the memory cells competed with each other for a factor not used by primary responding T cells, we think this is unlikely. If they did, the response would be increased when the cells were present at very low frequencies. In contrast, we found that, regardless of the number of memory cells present (compare Figs. 1 and 3), the kinetics and fold expansion of the memory response were the same.
Fig. 3.
The frequency of memory cells does not affect their expansion, which is less than primary responding cells only at later stages of the response. (A) 2–3 × 107 total cells from B6 mice immunized twice with 3K+LPS (B6 memory mice) were transferred i.v. into naïve B6.PL mice and the recipients immunized the next day with 3K+LPS i.v. The mean number of either memory or primary cells in the same mouse is shown +/− SEM. As a control, the primary host response was examined in separate mice that received naïve B6 cells (closed triangles). Representative of three experiments with three to four mice per group. (B) The transfer of cells from B6 memory mice was carried out as in A at three different frequencies: +: 7 × 106 total cells, 550 tet+, ++: 20 × 106 total, 1,500 tet+, +++: 50 × 106 total, 4,000 tet+. The recipients were immunized the next day with 3K+LPS i.v. On d5, the number of tet+ primary (open squares) and memory (closed squares) cells in the spleen was examined. Each point represents a mouse and the line shows the mean. Representative of two experiments. (C) As in A, but recipients were given 1 mg of BrdU i.p. 15 h before death. The percentages of BrdU+-tet+ primary (open squares, black line) or memory cells (closed squares, gray line) were calculated. Each point represents the mean +/− SEM on each day. Results are representative of three experiments with three to four mice per group.(D) As in C, but the recipient mice received drinking water containing BrdU continuously from d5 to d7. The mean percentages +/− SEM of primary (open bars) and memory cells (gray bars) that were BrdU+ on d7 are shown. Representative of three experiments with four mice per group.
To test whether the frequency of Ag-specific cells affected their fold expansion, we transferred different numbers of cells from memory B6 mice into naïve B6.PL mice. We found that the number of tet+ donor cells correlated with the number of cells transferred (Fig. 3B). The size of the primary responses in these hosts was unaffected by the memory cells, suggesting neither the memory cells nor any regulatory cell or factor that may have been transferred, nor any any limiting factor created by the memory cells in the host, affected the size of the primary T cell response.
Memory Cells Proliferate Less at Later Stages of the Response.
The low expansion could have been due to reduced proliferation or an increase in cell death. To test this, the rates of proliferation of memory and naïve cells were examined. The memory transfer system was used for these experiments, as it allowed us to examine the responses in the same host. Memory cells from B6 or B6.PL mice were transferred into either B6.PL or B6 animals, respectively. The animals were immunized and, at various times thereafter, injected with BrdU i.p. to label cells in cycle. Fifteen hours later, T cells were analyzed by flow cytometry. For both primary responding host and memory-transferred populations, the percentage of cells in cycle dropped from 50% to 60% on d3 to 20–30% on d5 (Fig. 3C). These data suggest there was no distinction in the capacity of primary or memory cells to proliferate during the first 5 days.
The primary response peaked on d9, so these cells must continue to increase between d5 and d9. In contrast, after d5, the number of secondary responding cells started to drop. Because after i.p. injection the half-life of BrdU is very short (22), and the percentage of cells in cycle dropped dramatically by d5, we administered BrdU continuously in drinking water over a 2-day period. Between d5 and d7, 40–70% of the primary responding cells were BrdU+, but only 10–25% of the memory cells had divided (Fig. 3D). Therefore, the majority of the memory cells stopped proliferating earlier than the primary responding cells. We confirmed this was not an artifact of the transfer system, nor a difference between secondary and tertiary responding cells by giving BrdU drinking water to separate primary and secondarily responding mice and found the same result (Fig. S4).
Although Fewer Memory Cells Make IL-2, More Make IFNγ.
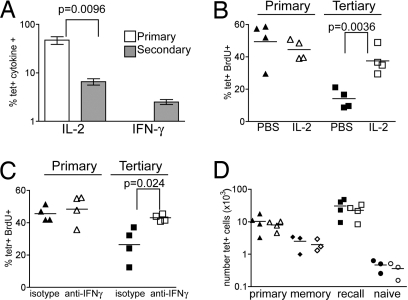
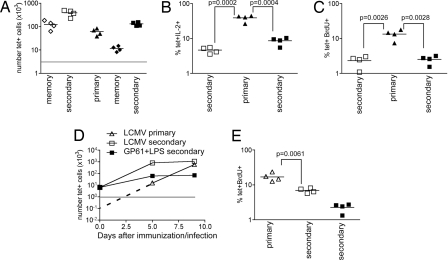
The memory T cells might curtail their response because they are less able to maintain production of growth factors, such as IL-2. The number of IL-2+ cells was the same in the primary and secondary response (Fig. S5). However, because there were more tet+ cells in the secondary response, the relative percentage of Ag-specific cells making IL-2 was reduced (Fig. 4A).
Fig. 4.
Proliferation of the memory cells is affected by their cytokine production. (A) B6 mice primed with 3K+LPS i.v. 4 weeks earlier (secondary) or naïve age-matched mice (primary) were immunized with 3K+LPS i.v. and splenocytes isolated 5 days later. Cells were either stained with tetramer to enumerate Ag-specific cells or incubated with/without peptide in the presence of Golgi plug for 6 h and the number of IL-2 or IFNγ+ cells calculated. The percentage of tetramer cells making cytokine was calculated as described in Materials and Methods. Results are representative of four experiments with three to four mice per group. Error bars are SEM. (B) Naïve recipients of IAb/3K memory cells were immunized with 3K+LPS (d0) then treated with PBS (closed symbols) or 1.5 μg of IL-2 (open symbols) i.p. on d3–6 and given BrdU+ water from d5 to d7. On d7, splenocytes were stained with tetramer and anti-BrdU and the percentage of double+ primary (triangles) and memory cells (squares) measured. The symbols indicate each mouse, and the line shows the mean. Representative of two experiments with four mice per group. (C) As in B, but memory cell recipients were given 1 mg of control rat or anti-IFNγ on d3–4 i.p. (D) Irradiated CD45.1 mice reconstituted with 50% WT CD45.1 and 50% IFNγR KO bone marrow were immunized i.v. with 3K+LPS after 8 weeks. Thirty days later, the numbers of tet+ WT or KO CD4 cells were examined in the spleens of memory mice (diamond) or in memory (squares) or naïve (triangles) mice immunized 7d previously with 3K+LPS. Tetramer staining in naïve chimeras is shown as a staining control (circles). Cells were gated on live CD4+ cells that were dump-negative and either CD45.1+ (WT, closed symbols) or CD45.2+ (KO, open symbols). Each point represents a chimera, and the line shows the mean of the group. Representative of two experiments with three to four mice per group.
This drop in the frequency of cells making IL-2 may have been caused by a switch to effector cytokine production. Because we used LPS, which induces a Th1 response, we measured IFNγ production. Ex vivo Ag stimulation did not induce IFNγ above background in the primary response (Fig. S5), but a small number of the reactivated Ag-specific memory cells were IFNγ+ (Fig. 4A).
Frequency of Cells Making IL-2 and IFNγ Affect the Proliferation of Reactivated Memory Cells.
To test whether these cytokine changes caused the reduced proliferation, the effects of additional IL-2 or blockage of IFNγ were measured. Naïve B6 mice that had been given IAb/3K-specific memory cells from B6.PL mice were immunized on d0 and treated with either IL-2 or anti-IFNγ or, as controls, PBS or rat IgG, between d3 and d6. All mice were given BrdU+ drinking water on d5–7.
Treatment with either IL-2 or anti-IFNγ restored the percentage of Ag-specific memory cells that were BrdU+ to the same level as that of the primary responding cells (Fig. 4 B and C). Notably, the primary responding cells were unaffected by either treatment. As there was little change in the number of either population between d5 and d7, we did not expect to see a large difference in the number of tet+ cells in treated mice. We did, however, find a significant increase in the number of memory tet+ cells after treatment with anti-IFNγ on one occasion and, when considered as a whole, there is a trend toward an increase in the number of memory cells after either treatment (data not shown).
IFNγ Does Not Directly Inhibit Memory Cell Proliferation.
To test whether IFNγ acted directly on the memory T cells, we made bone marrow chimeras reconstituted with 50% WT and 50% IFNγ receptor (IFNγR) KO bone marrow. These chimeras were immunized and 30 days later the secondary response of the WT and KO CD4 T cells examined. We also measured the number of primary and memory tet+ cells. There were equal numbers of WT and KO tet+ cells at all stages of the response (Fig. 4D), suggesting that IFNγ does not directly affect T cell proliferation but rather acts through another cell type.
Memory Cells Either Generated by or Reactivated with Lymphocytic Choriomeningitis Virus (LCMV) Also Proliferate Poorly.
It was possible that the low fold expansion of the memory cells was a result of poor differentiation after immunization with 3K+LPS, exemplified by the low IFNγ response. Therefore, we examined the reactivation of CD4 memory cells specific for GP61 (23) generated after infection with LCMV, an agent that induces excellent CD4 T cell IFNγ production [data not shown and (7)].
B6 mice were infected with LCMV or immunized with GP61 peptide+LPS. After 40 days, we counted the numbers of CD4 IAb/GP61tet+ cells either in these animals or in these mice or naïve mice that had been immunized 5 days earlier with GP61+LPS. Reinfection with LCMV was not used to avoid complications because of preexisting antibody or LCMV-specific CD8 T cells. The memory cells in both the LCMV- and GP61-primed mice expanded poorly in response to GP61+LPS with LCMV memory cells expanding <5-fold, whereas GP61+LPS memory cells expanded 10-fold (Fig. 5A). Whitmire et al. (19) have shown there are ≈100 GP61-specific naïve cells in B6 mice (19), and we found a similar number (160) after directly labeling total spleen and lymph node cells with IAb/GP61tet (Fig. S6). Using this number, the primary responding cells expanded >300-fold in response to GP61+LPS from d0 to d5.
Fig. 5.
Memory cells generated by or reactivated with LCMV proliferate poorly. (A) B6 mice were primed with LCMV (open squares) or GP61+LPS (closed squares) i.p. 35 days later some of each type of mouse were immunized with GP61+LPS i.p., or naïve mice were primed with GP61+LPS (closed triangles). The numbers of IAb/GP61 tet+ T cells in the spleens of these mice were examined on d5. Also examined were the numbers of memory tet+ cells in LCMV (open diamonds) or GP61+LPS (closed diamonds) mice primed 40 days previously. Each point represents a mouse, and the line shows the mean of the group. The gray line indicates the limit of detection for the tet staining. (B) As in A, but splenocytes were stimulated ex vivo with/without GP61 peptide in the presence of Golgi plug and the number of IL-2 producing cells examined. The percentage of tet+ cells making IL-2 was then calculated for each mouse. (C) As in A, but mice were given BrdU+ drinking water between d5 and d8 after immunization with GP61+LPS and the percentage of tet+ cells BrdU+ calculated. Representative of two experiments with four mice per group. (D) B6 mice primed with GP61+LPS 8 weeks earlier were infected with LCMV (open squares) or immunized with GP61+LPS (closed squares) or naïve mice were infected with LCMV (open triangles). The numbers of IAb/GP61 tet+ cells present in the spleens of the mice were examined. The dotted line indicates the hypothesized expansion of primary responding GP61-specific cells from d0 to d5; the gray line indicates the level of background staining. (E) As in D, but the mice were given BrdU+ drinking water from d7 to d9 and the percentage of IAb/GP61 tet+/BrdU+ cells analyzed on d9. Representative of two experiments with four mice per group.
We also examined the IL-2 response by primary and reactivated GP61-specific cells and found that, as for 3K+LPS memory cells, the percentage of both LCMV and GP61+LPS secondary responding cells making IL-2 was less than primary responding cells (Fig. 5B). To further confirm that LCMV memory cells responded to reactivation in a similar fashion to peptide and LPS memory cells, we examined the incorporation of BrdU. As suggested by the reduced percentage of cells making IL-2, fewer memory cells were in cycle between d5 and d8 than primary responding cells (Fig. 5C).
Reactivation with a more inflammatory Ag may allow the memory cells to proliferate to the same extent as primary responding cells. To examine this, we did the converse experiment and measured the extent of proliferation of memory cells generated with GP61+LPS to LCMV infection. Although memory cells expanded more in response to LCMV than GP61+LPS, this differences was apparent only in the first 5 days of the response (Fig. 5D). The fold expansion of the memory cells was only 200, compared with an estimated 4- to 6,000-fold expansion in the primary response. Moreover, the memory cells incorporated less BrdU than the cells in the primary response between d5 and d7 regardless of the restimulation (Fig. 5E).
Discussion
Memory responses are considered to be better than primary responses, thereby providing enhanced protection to the host. We show this may be a matter of definition and call into question the criteria used to define memory. Here, we show that CD4 memory T cells curtail their own proliferation as a result of changes in cytokine production. Our results confirm and extend other reports on this subject as in both a viral and a bacterial model, Ag-specific CD4 cells were found to expand at a similar or reduced rate to primary responding cells respectively upon recall (6, 7). However, in both systems, memory CD8 T cells could have affected the amount of Ag presentation by killing infected cells more rapidly (24), an issue, we have avoided by examining the response to a single CD4 epitope in the absence of a CD8 T cell response. Moreover, we showed that the memory cells proliferated to a reduced extent after transfer to naïve hosts in which the primary response proceeded as normal, demonstrating that neither competition for resources nor space are the causes of the reduced expansion. These data clearly show that primary and secondary responding cells are intrinsically different and explain the earlier peak of memory responses; this is neither a result of changes in Ag presentation nor T cell migration but a consequence of the reduced proliferation of the memory cells several days into the response.
In contrast to this result, Mercia et al. (25) found that TCR Tg memory cells proliferated more after transfer to naïve hosts. Our results may differ for several reasons: first, they transferred a large number of TCR Tg cells rather than studying an endogenous CD4 T cell response as we have done. This large population may affect the differentiation of memory cells (12, 13). Second, they used a persistent Ag that may have caused an anergized phenotype in the memory cells that was alleviated after transfer. To avoid this, we used soluble Ag and adjuvant. Our system was also devised so that the majority, if not all, of the Ag-specific cells would receive sufficient signals to be fully activated, avoiding the differentiation of two classes of memory T cells with distinct proliferative capacities (26). Indeed, we do not believe that the low fold expansion of the memory cells can be explained by their differentiation into TEM cells because, although they were CD62Llo, the majority of the memory cells in our experiments expressed CCR7 (Fig. S1).
The requirement for IL-2 at later stages of the response is reminiscent of findings that IL-2 sustains the expansion of and is more critical in the secondary response of CD8 T cells (27, 28). Our findings suggest that the IL-2 must be consumed in an autocrine fashion, because IL-2 from the primary host cells could not support memory cell proliferation. However, the populations may be present in separate locations of the spleen preventing the IL-2 from the primary responding cells from reaching the memory cells. The question arises of why fewer memory cells make IL-2? Perhaps IFNγ from the memory cells causes local APC to make IL-12, which in turn reduces the percentage of cells making IL-2 (29)?
Although the peptide+LPS memory cells had a reduced fold expansion, they did make more IFNγ than primary cells. The percentage of IFNγ+ Ag-specific CD4 cells in both responses is much less than that found in LCMV infection (7, 19). We excluded the possibility that this low effector cell differentiation explained the poor expansion by examining the response of CD4+ LCMV memory cells. In response to GP61+LPS reactivation, the LCMV memory cells actually responded worse than GP61+LPS memory cells. In the converse experiment, we also showed that even in the very inflammatory environment of a LCMV infection, memory cells still expanded much less than cells undergoing a primary response. This results contrasts with a recent article that found that TCR Tg cells responding to LCMV accumulated to greater numbers than primary responding TCR Tg cells in the same host (30). Our results may differ for several reasons, in particular because of the differences between endogenous T cells (studied here) and TCR Tg cells used in the other study.
Despite the low percentage of memory cells making IFNγ, we found that blocking IFNγ restored the percentage of these cells that entered cell division to that of primary responding cells. IFNγ increases the contraction of CD4 cells (31, 32) but can also enhance CD4 T cell responses (33). By examining the responses of IFNγR sufficient and deficient TCR Tg cells, Whitton and colleagues (33) have examined the effect of IFNγ from the primary response onwards. We show that IFNγ inhibits the proliferation of only the memory cells, suggesting that IFNγ may act in an opposing manner on primary and secondary responding cells. However, we found no differences in the numbers WT and IFNγR KO cells at any stages of the response suggesting that, in this system, IFNγ does not enhance the CD4 T cell responses.
We found that the IFNγ did not act directly on the memory cells; a similar result has been recently demonstrated for CD8 T cells (34). Why then were only the memory cells and not the primary responding cells in the same host inhibited by IFNγ? As suggested above, the populations may be present in different areas of the spleen so the IFNγ would only act locally. Alternatively, the timings of T cell and APC interactions may differ such that the effects of the IFNγ were limited by time. In support of this, secondarily responding CD8 T cells exit the T cell area of the spleen more rapidly than primary responding cells (35).
Effector cytokine producing CD4 memory T cells have been shown to be protective in viral, bacterial and parasitic infections (36–38), suggesting that memory CD4 cells may provide protection to the host via an enhanced effector cytokine response that directs other immune cells. Thus, inhibition of proliferation may be a direct and inevitable consequence of the effector functions of the memory cells; somehow during effective immune responses CD4 memory cells must strike the right balance between expansion and effector function.
Materials and Methods
Mice and Immunizations.
Female B6, B6.PL-Thy1a/CyJ (Thy1.1+), B6.SJL-PtprcaPep3b/BoyJ (CD45.1), and B6.129S7-Ifng KO mice were obtained from The Jackson Laboratory; 508 TCR Tg mice expressing a TCR specific for IAb/3K (39) and IFNγ receptor knockout mice were bred at the National Jewish Medical and Research Center (NJC). All mice were maintained in a specific-pathogen-free environment in accordance with institutional guidelines in the Animal Care Facility at the NJC. Mice were age matched within experiments and primed at 6–10 weeks of age. Mice were immunized with 10 μg of 3K (FEAQKAKANKAVD) or GP61 (GLNGPDIYKGVYQFKSVEFD) supplied by the Molecular Resources Center at NJC and 7 μg of LPS (Escherichia coli, Difco) i.v. or i.p. in GP61+LPS immunized mice. Mice were infected with a single dose of 2 × 105 plaque-forming units of LCMV (Armstrong) i.p. In experiments in which memory cells were transferred, donor mice were primed as above and 4–6 weeks later were immunized again. Cells were harvested 4–15 weeks later.
Cell Transfer.
Spleens and LN (brachial, axillary, inguinal, popliteal, lumbar) were taken from memory B6 or B6.PL mice or from 508 TCR Tg mice. A single cell suspension was prepared and red blood cells lysed. Cells were washed and injected i.v.
Cell Analysis.
Cells were obtained from red blood cell lysed spleens. For liver, mice were perfused, suspensions prepared by using a cell strainer, large debris removed by centrifugation and lymphocytes separated by resuspending cells in 46% Percoll and underlaying with 54% Percoll.
Flow Cytometry.
PE-labeled IAb/3K tetramer was produced as described (18). PE-labeled IAb/GP66–77 tetramer (referred to as IAb/GP61) was supplied by the National Institute of Health (NIH) tetramer core facility. Single-cell suspensions were stained with tetramer at 37°C for 2 h. Antibodies to surface markers (SI Text) were added and the cells incubated for a further 20 min at 4°C. Tet+ cells were defined by gating on live CD4 cells that were B220, F4/80, MHC class II negative (dump) negative and CD44hi.
For analysis of naïve IAb/3K or Gp61-specific cells, spleens, and LN (as above and cervical and mesenteric) were taken from naïve mice or mice primed 7–9 days earlier with 3K or Gp61+LPS i.v. Total cells were stained with tetramer and antibodies against CD44, CD62L, CD127, CD4, CD8, B220, F4/80, MHC II, and TCRcβ. Total cells were acquired in four to seven samples of 10 million events. Naïve IAb/3K cells were gated through forward and side scatter, CD4+, dump (CD8, B220, F4/80, MHC II)−, CD127+, TCR+, CD44lo, and CD62Lhi. The number of tet+ cells for each acquired sample of 10 million events was calculated and a mean for each mouse calculated.
For analysis of cell cycle entry, mice were either given 1 mg of BrdU (Sigma) i.p. 15 h before death or drinking water containing 0.8 mg/ml of BrdU for 2–3 days before death. BrdU+ drinking water was replaced daily and always protected from light. Staining was carried out as described (40) after tetramer and surface antibody staining.
For analysis of intracellular cytokine production, splenocytes were incubated ex vivo with 5 μg/ml 3K or GP61 peptide and 1 μl/ml Golgi plug (BD) for 6 h. Cells were stained with surface antibodies and then fixed and permeabilized by using the BD Cytofix/Cytoperm kit according to manufacturer's instructions before staining with anticytokine antibodies. Cells were gated on live CD44hi CD4 cells that were B220, F4/80 and MHC class II negative. Ag-specific cytokine was defined as staining above that from cells cultured in the absence of peptide and this number used to calculate the percentage of tet+ cells making cytokine in each mouse.
To examine primary or memory responses 2–5 million events were collected on a CyAn LX or CyAn ADP (Dakocytomation) and data analyzed by using FlowJo version 8 (TreeStar).
In Vivo Treatment with IL-2 or Anti-IFNγ.
Memory cell recipients were given 1.5 μg of IL-2 (R&D) i.p. on d3–6 or PBS or a total of 1 mg of anti-IFNγ (grown in FCS depleted of IgG) or rat IgG (Jackson Immunoresearch) i.p. on d3–4 of the response.
Bone Marrow Chimeras.
CD45.1 B6 mice were irradiated with 900Rads and reconstituted with 5 × 106 cells of 50:50 CD45.1 WT and CD45.2 IFNγR KO bone marrow. Chimeras were primed 8 weeks after reconstitution.
Statistics.
Statistical significance determined by using Student's two-tailed t test (GraphPad Prism V4).
Supplementary Material
Acknowledgments.
We thank Dr. Dirk Homann (University of Colorado Health Science Center, Aurora, CO) for supplying LCMV and the National Institutes of Health tetramer core facility for IAb/GP66–77 tetramer; Drs. Trine Jorgensen and Richard Willis for critical reading of the manuscript and all members of the Kappler Marrack laboratory for intellectual contributions to this project. This work was supported by National Institutes of Health Grants NIH-AI-17134, NIH-AI-18785, NIH-AI-22295, NHI-AI-52225.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807449105/DCSupplemental.
References
- 1.Kalia V, et al. Differentiation of memory B and T cells. Curr Opin Immunol. 2006;18:255–264. doi: 10.1016/j.coi.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Antia R, Ganusov VV, Ahmed R. The role of models in understanding CD8+ T-cell memory. Nat Rev Immunol. 2005;5:101–111. doi: 10.1038/nri1550. [DOI] [PubMed] [Google Scholar]
- 3.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 4.Williams MA, Bevan MJ. Effector and Memory CTL Differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 5.MacLeod M, et al. CD4 memory T cells survive and proliferate but fail to differentiate in the absence of CD40. J Exp Med. 2006;203:897–906. doi: 10.1084/jem.20050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiemann M, et al. Differences in maintenance of CD8+ and CD4+ bacteria-specific effector-memory T cell populations. Eur J Immunol. 2003;33:2875–2885. doi: 10.1002/eji.200324224. [DOI] [PubMed] [Google Scholar]
- 7.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 8.London CA, Perez VL, Abbas AK. Functional characteristics and survival requirements of memory CD4+ T lymphocytes in vivo. J Immunol. 1999;162:766–773. [PubMed] [Google Scholar]
- 9.Varga SM, Welsh RM. Stability of virus-specific CD4+ T cell frequencies from acute infection into long term memory. J Immunol. 1998;161:367–374. [PubMed] [Google Scholar]
- 10.Reinhardt RL, et al. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins MK, et al. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 12.Marzo AL, et al. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hataye J, et al. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 14.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 15.Sallusto F, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 16.Roman E, et al. CD4 effector T cell subsets in the response to influenza: Heterogeneity, migration, and function. J Exp Med. 2002;196:957–968. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moulton VR, et al. Divergent generation of heterogeneous memory CD4 T cells. J Immunol. 2006;177:869–876. doi: 10.4049/jimmunol.177.2.869. [DOI] [PubMed] [Google Scholar]
- 18.Crawford F, et al. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 19.Whitmire JK, Benning N, Whitton JL. Precursor Frequency, Nonlinear Proliferation, and Functional Maturation of Virus-Specific CD4+ T Cells. J Immunol. 2006;176:3028–3036. doi: 10.4049/jimmunol.176.5.3028. [DOI] [PubMed] [Google Scholar]
- 20.Blattman JN, et al. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kriss JP, Revesz L. The distribution and fate of bromodeoxyuridine and bromodeoxycytidine in the mouse and rat. Cancer Res. 1962;22:254–265. [PubMed] [Google Scholar]
- 23.Oxenius A, et al. Presentation of endogenous viral proteins in association with major histocompatibility complex class II: on the role of intracellular compartmentalization, invariant chain and the TAP transporter system. Eur J Immunol. 1995;25:3402–3411. doi: 10.1002/eji.1830251230. [DOI] [PubMed] [Google Scholar]
- 24.Belz GT, et al. Killer T cells regulate antigen presentation for early expansion of memory, but not naive, CD8+ T cell. Proc Natl Acad Sci USA. 2007;104:6341–6346. doi: 10.1073/pnas.0609990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merica R, et al. Antigen-experienced CD4 T cells display a reduced capacity for clonal expansion in vivo that is imposed by factors present in the immune host. J Immunol. 2000;164:4551–4557. doi: 10.4049/jimmunol.164.9.4551. [DOI] [PubMed] [Google Scholar]
- 26.Catron DM, et al. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J Exp Med. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Souza WN, Lefrancois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171:5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 28.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villarino AV, et al. Helper T cell IL-2 production is limited by negative feedback and STAT-dependent cytokine signals. J Exp Med. 2007;204:65–71. doi: 10.1084/jem.20061198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitmire JK, Eam B, Whitton JL. Tentative T cells: memory cells are quick to respond, but slow to divide. PLoS Pathog. 2008;4:e1000041. doi: 10.1371/journal.ppat.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haring JS, Harty JT. Aberrant contraction of antigen-specific CD4 T cells after infection in the absence of gamma interferon or its receptor. Infect Immun. 2006;74:6252–6263. doi: 10.1128/IAI.00847-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalton DK, et al. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J Exp Med. 2000;192:117–122. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitmire JK, Benning N, Whitton JL. Cutting edge: early IFN-gamma signaling directly enhances primary antiviral CD4+ T cell responses. J Immunol. 2005;175:5624–5628. doi: 10.4049/jimmunol.175.9.5624. [DOI] [PubMed] [Google Scholar]
- 34.Tewari K, Nakayama Y, Suresh M. Role of direct effects of IFN-gamma on T cells in the regulation of CD8 T cell homeostasis. J Immunol. 2007;179:2115–2125. doi: 10.4049/jimmunol.179.4.2115. [DOI] [PubMed] [Google Scholar]
- 35.Khanna KM, McNamara JT, Lefrancois L. In situ imaging of the endogenous CD8 T cell response to infection. Science. 2007;318:116–120. doi: 10.1126/science.1146291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tibbetts SA, et al. Effective vaccination against long-term gammaherpesvirus latency. J Virol. 2003;77:2522–2529. doi: 10.1128/JVI.77.4.2522-2529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glomski IJ, Corre JP, Mock M, Goossens PL. Cutting Edge: IFN-{gamma}-Producing CD4 T Lymphocytes Mediate Spore-Induced Immunity to Capsulated Bacillus anthracis. J Immunol. 2007;178:2646–2650. doi: 10.4049/jimmunol.178.5.2646. [DOI] [PubMed] [Google Scholar]
- 38.Anthony RM, et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huseby ES, et al. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Lenz DC, et al. IL-7 regulates basal homeostatic proliferation of antiviral CD4+T cell memory. Proc Natl Acad Sci USA. 2004;101:9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.