Abstract
Purpose
To determine the inter- and intra-observer reproducibility of cardiac magnetic resonance (CMR)-derived measurements of RV mass, volume, and function in patients with normal and dilated ventricles.
Materials and Methods
CMR studies of 60 patients in three groups were studied: a normal RV group (n = 20) and two groups with RV dilation—atrial septal defect (n = 20) and repaired tetralogy of Fallot (n = 20). Two independent observers analyzed each study on two separate occasions. Inter- and intra-observer reproducibility of biventricular mass, volume, ejection fraction (EF), and stroke volume measurements were calculated.
Results
High intra-class correlation coefficients (ICC) were found for inter-observer (ICC 0.94 - 0.99) and intra-observer (ICC 0.96 - 0.99) comparisons of RV and left ventricular (LV) mass, volume, and stroke volume measurements. RV and LV EF measurements were less reproducible (ICC 0.79 - 0.87). RV mass measurements were significantly less correlated than the respective LV measurements. Small but statistically significant differences in correlation were noted in RV measurements across groups.
Conclusions
Except for RV mass, inter- and intra-observer reproducibility of RV size and function measurements is high and generally comparable to that in the LV in patients with both normal and dilated RV.
Keywords: magnetic resonance imaging, congenital heart disease, reproducibility, right ventricle
Accurate quantitative assessment of right ventricular (RV) size and function is important in a wide range of congenital and acquired cardiovascular diseases, including tetralogy of Fallot (TOF), atrial septal defect (ASD), anomalous pulmonary venous return, tricuspid regurgitation, and pulmonary hypertension. Chronic RV volume overload may lead to right heart failure, arrhythmia, and death. Management decisions increasingly rely on measurements of RV size and function and their trends during serial follow-up examinations (1-4). An accurate imaging modality would therefore have low inter- and intra-observer variability.
Cardiac magnetic resonance (CMR) has emerged as the reference standard imaging modality for quantitative assessment of ventricular size and function (5-9). During the past two decades CMR has been studied extensively for accuracy and reproducibility in assessing the left ventricle (LV) (10-15). However, only few studies have examined the reproducibility of CMR-derived RV measurements and none of these studies examined patients with congenital heart disease or dilated right ventricles with current imaging techniques (16-18). The goal of this study was thus to assess the inter- and intra-observer variability of RV size and function measurements by contemporary CMR techniques in patients with normal and dilated right ventricles due to volume overload, and to compare them to those of the LV.
MATERIALS AND METHODS
Subjects
Three study groups, consisting of a normal RV group (Group I) and two RV volume overload groups (Groups II and III) were defined. Group II consisted of patients with unrepaired ASD and/or partially anomalous pulmonary venous connection (PAPVC); group III consisted of patients who had undergone surgical repair of TOF and had pulmonary regurgitation. The first 20 patients with CMR studies between January 2004 and April 2006 meeting the above criteria were selected for each group. Demographic and clinical information was abstracted from medical records. Body surface area was calculated using the formula of Haycock et al. (19).
The Institutional Scientific Review Committee approved the study protocol, and its Committee on Clinical Investigation approved review of the medical records. Informed consent was waived for this de-identified study. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Cardiac Magnetic Resonance
Examinations were performed on commercially available 1.5 T scanners (GE Healthcare, Waukesha, WI; Philips Medical Systems, Best, the Netherlands) utilizing ECG-gated steady-state free precession imaging sequences (TE 1.5-2.0 msec, TR 2.8-4.0 msec, flip angle 45°, views per segment 10-20, reconstructed images per cardiac cycle 20-30) and standard protocols for morphology and function assessment according to the method described by Samyn et al. (20). Briefly, 12 contiguous short-axis slabs perpendicular to the long axis of ventricles (slice thickness 6-8 mm, interslice distance 0-2 mm) were usually obtained during brief (6-12 seconds) periods of breath holding. Additional slabs were sometimes performed to provide complete coverage of very large ventricles.
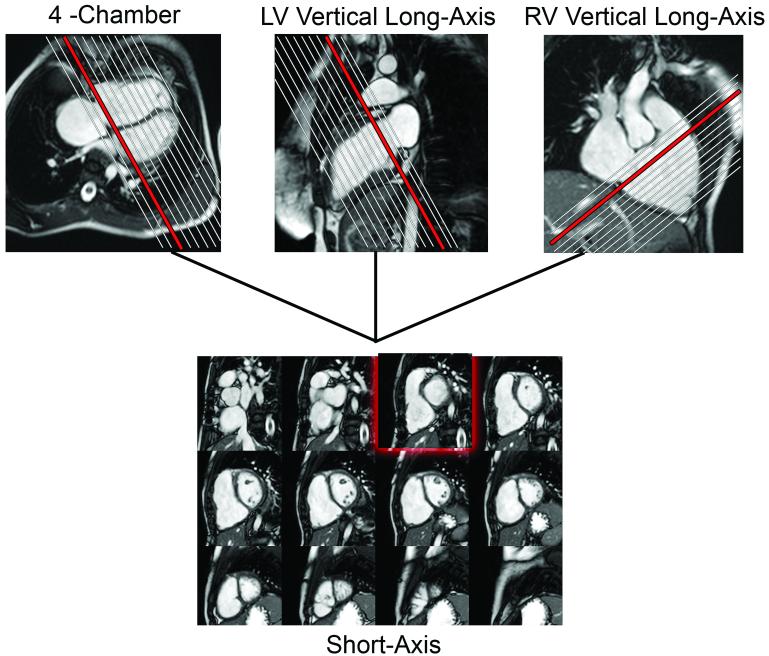
CMR data was reviewed using a commercially available computer workstation (ViewForum, Philips Medical Systems, Best, The Netherlands). Long- and short-axis cine images of the ventricles were cross-referenced to facilitate identification of basal slices to be included in the analysis (Figure 1). RV and LV end-diastolic and end-systolic volumes and masses, stroke volumes (SV), and ejection fractions (EF) were measured on a personal computer using commercially available software (MASS, Medis, Leiden, The Netherlands) as previous described (21).
Figure 1.
Use of cross-reference between ventricular long- and short-axis imaging planes to determine inclusion of basal slices in the ventricular volume analysis. When an operator selects a frame on the short-axis grid, that location is highlighted on the linked horizontal and vertical long axis images, allowing the operator to determine the location of the slab relative to the atrio-ventricular valves.
Two medical student observers independently analyzed all CMR studies. Prior to the commencement of the study protocol, each student underwent training with one-on-one instruction by an attending physician experienced in cardiac CMR followed by practice on 5 sample cases on which they were given corrections and feedback by the same attending physician. Each observer analyzed all 60 study cases twice, with a 9-day interval between repeat measurements. Each observer analyzed the studies both times in the same order. In each study, 96 contours were drawn (LV and RV endocardial and epicardial volumes at end-diastole and end-systole) for a total of 11,520 contours per observer. Contours from each set of measurements were saved in separate databases to ensure blinding of observations. The time it took the observers to analyze each study, from the moment the phase-determining cine loop was started to the moment the last contour was drawn, was recorded.
Statistical Analysis
Mean values and standard deviations among patients were calculated for all measurements. Coefficients of variability were calculated by dividing the standard deviation of the differences by the respective mean measurement. Repeatability coefficients (RC) were also calculated as described by Bland and Altman (22). The repeatability coefficient reflects the largest difference between measurements that is likely due to measurement error. Intra- and inter-observer agreement was assessed using intra-class correlation coefficients (ICC) estimated with variance components models. The ICC can be interpreted as the proportion of variability explained by subject differences as opposed to rater differences and random error. The level of agreement for each RV and LV measurement was compared using methods described by Donner (23). Bland-Altman 95% limits of agreement were calculated to assess intra- and inter-observer agreement and plots of differences versus mean values between raters are shown. A p-value < 0.05 was considered significant. Statistical analysis was performed using SAS software Version 9.1 (SAS Institute Inc., Cary, NC, USA).
RESULTS
The CMR studies of 60 patients—20 in each group—were analyzed. Table 1 summarizes patient age, gender, and BSA in the cohort and in the respective subgroups. Mean CMR measurements of ventricular size and function, along with coefficients of variability and repeatability coefficients, are also shown in Table 1. Measurements of ventricular mass did not differ between end-diastole and end-systole.
Table 1.
Demographic, CMR, and inter-rater variability data
| All Patients (n = 60) | Normal RV (n = 20) | ASD/PAPVC (n = 20) | S/P TOF (n = 20) | |
|---|---|---|---|---|
| Age (years) | 20.5 ± 13.2 | 20.6 ± 10.7 | 18.4 ± 17.1 | 22.6 ± 11.2 |
| Male gender n (%) | 34 (57%) | 12 (60%) | 8 (40%) | 14 (70%) |
| BSA (m2) | 1.6 ± 0.5 | 1.9 ± 0.5 | 1.4 ± 0.6 | 1.6 ± 0.2 |
| RV EDV index (mL/m2) | ||||
| Mean ± SD | 120 ± 46 | 82 ± 17 | 135 ± 48 | 142 ± 41 |
| Mean inter-rater difference (COV) | 8.4 ± 7.6 (6.4) | 4.4 ± 5.4 (6.6) | 11.9 ± 8.6 (6.4) | 8.9 ± 6.8 (4.8) |
| Repeatability Coefficient | 24.4 | 14.7 | 31.5 | 24.1 |
| RV ESV index (mL/m2) | ||||
| Mean ± SD | 54 ± 29 | 35 ± 9 | 58 ± 32 | 70 ± 28 |
| Mean inter-rater difference (COV) | 5.5 ± 7.1 (13.0) | 3.7 ± 5.7 (16.3) | 4.2 ± 7.1 (12.1) | 8.6 ± 7.6 (10.9) |
| Repeatability Coefficient | 19.2 | 14.6 | 18.7 | 23.3 |
| RV SV index (mL/m2) | ||||
| Mean ± SD | 65 ± 23 | 47 ± 10 | 76 ± 25 | 72 ± 19 |
| Mean inter-rater difference (COV) | 2.9 ± 7.7 (11.8) | 0.7 ± 4.0 (8.4) | 7.7 ± 8.5 (11.2) | 0.3 ± 7.6 (10.6) |
| Repeatability Coefficient | 19.2 | 9.1 | 26.4 | 17.9 |
| RV EF (%) | ||||
| Mean ± SD | 56 ± 8 | 58 ± 5 | 58 ± 10 | 51 ± 8 |
| Mean inter-rater difference (COV) | -1.2 ± 4.4 (8.0) | -2.0 ± 4.8 (8.3) | 1.1 ± 3.6 (6.2) | -2.6 ± 4.1 (8.0) |
| Repeatability Coefficient | 10.7 | 11.5 | 10.0 | 10.7 |
| RV Mass index (g/m2) | ||||
| Mean ± SD | 28 ± 12 | 19 ± 4 | 31 ± 14 | 35 ± 7 |
| Mean inter-rater difference (COV) | -1.2 ± 3.2 (11.3) | -2.1 ± 2.5 (13.4) | 0.5 ± 2.8 (9.0) | -2.0 ± 3.6 (10.1) |
| Repeatability Coefficient | 8.5 | 6.9 | 8.3 | 10.1 |
| LV EDV index (mL/m2) | ||||
| Mean ± SD | 75 ± 17 | 81 ± 16 | 64 ± 14 | 81 ± 16 |
| Mean inter-rater difference (COV) | 2.3 ± 2.7 (3.6) | 2.1 ± 2.6 (3.1) | 0.8 ± 2.1 (3.2) | 4.1 ± 2.5 (3.1) |
| Repeatability Coefficient | 8.2 | 7.7 | 5.2 | 10.7 |
| LV ESV index (mL/m2) | ||||
| Mean ± SD | 30 ± 9 | 34 ± 9 | 24 ± 7 | 32 ± 8 |
| Mean inter-rater difference (COV) | 1.9 ± 3.1 (10.5) | 1.8 ± 3.5 (10.4) | 0.3 ± 2.5 (10.5) | 3.6 ± 2.5 (7.8) |
| Repeatability Coefficient | 8.4 | 8.4 | 6.5 | 9.8 |
| LV SV index (mL/m2) | ||||
| Mean ± SD | 45 ± 11 | 47 ± 10 | 41 ± 9 | 48 ± 12 |
| Mean inter-rater difference (COV) | 0.4 ± 3.0 (6.6) | 0.3 ± 3.9 (8.2) | 0.4 ± 2.7 (6.6) | 0.5 ± 2.4 (4.9) |
| Repeatability Coefficient | 8.0 | 9.0 | 7.5 | 7.4 |
| LV EF (%) | ||||
| Mean ± SD | 61 ± 7 | 59 ± 6 | 63 ± 7 | 60 ± 7 |
| Mean inter-rater difference (COV) | -1.2 ± 3.5 (5.8) | -1.0 ± 4.0 (6.8) | -0.4 ± 3.6 (5.7) | -2.3 ± 2.8 (4.8) |
| Repeatability Coefficient | 9.4 | 9.1 | 10.3 | 8.7 |
| LV Mass index (g/m2) | ||||
| Mean ± SD | 49 ± 11 | 53 ± 12 | 44 ± 9 | 51 ± 9 |
| Mean inter-rater difference (COV) | -1.5 ± 2.6 (5.3) | -0.9 ± 2.7 (5.1) | -0.9 ± 2.3 (5.3) | -2.9 ± 2.5 (4.9) |
| Repeatability Coefficient | 7.7 | 7.7 | 6.5 | 8.9 |
Abbreviations: COV = coefficient of variability; EDV = end-diastolic volume; ESV = end-systolic volume; LV = left ventricle; RV = right ventricle; SD = standard deviation; SV = stroke volume
Intra-observer agreement for most RV and LV parameters were highly correlated between observers’ two measurements (ICC 0.956 - 0.993) and were not significantly different between the right and left ventricles (Table 2). RV and LV intra-observer correlation of EF were somewhat lower (ICC 0.874 and 0.824, respectively). Although not statistically significant, RV volume measurements tended to vary more widely than LV volume measurements as evident by the larger within-rater standard deviations. Variability of RV mass was significantly higher as compared with variability of LV mass measurements; nonetheless, absolute differences were small (for intra-observer variability, ICC 0.956 v. 0.985 and within-rater standard deviation 4.27 v. 4.22, p < 0.001).
Table 2.
Comparison between intra-class correlation coefficients of right and left ventricular measurements
| Intra-observer | Inter-observer | |||||
|---|---|---|---|---|---|---|
| ICC | P (RV v. LV) | w/in rater SD | ICC | P (RV v. LV) | w/in subject SD | |
| End-diastolic volume | ||||||
| RV | 0.991 | 8.19 | 0.977 | 13.53 | ||
| LV | 0.993 | 0.202 | 3.92 | 0.989 | 0.007 | 5.07 |
| End-systolic volume | ||||||
| RV | 0.982 | 6.62 | 0.950 | 11.32 | ||
| LV | 0.967 | 0.021 | 3.84 | 0.939 | 0.247 | 5.3 |
| Stroke volume | ||||||
| RV | 0.969 | 8.15 | 0.947 | 10.87 | ||
| LV | 0.976 | 0.194 | 4.76 | 0.973 | 0.011 | 5.01 |
| Ejection fraction | ||||||
| RV | 0.874 | 3.03 | 0.810 | 3.87 | ||
| LV | 0.824 | 0.107 | 3.02 | 0.789 | 0.342 | 3.39 |
| Mass | ||||||
| RV | 0.956 | 4.27 | 0.942 | 4.96 | ||
| LV | 0.985 | <0.001 | 4.22 | 0.981 | <0.001 | 4.82 |
Abbreviations: ICC = intra-class correlation coefficient; LV = left ventricle; RV = right ventricle; SD = standard deviation
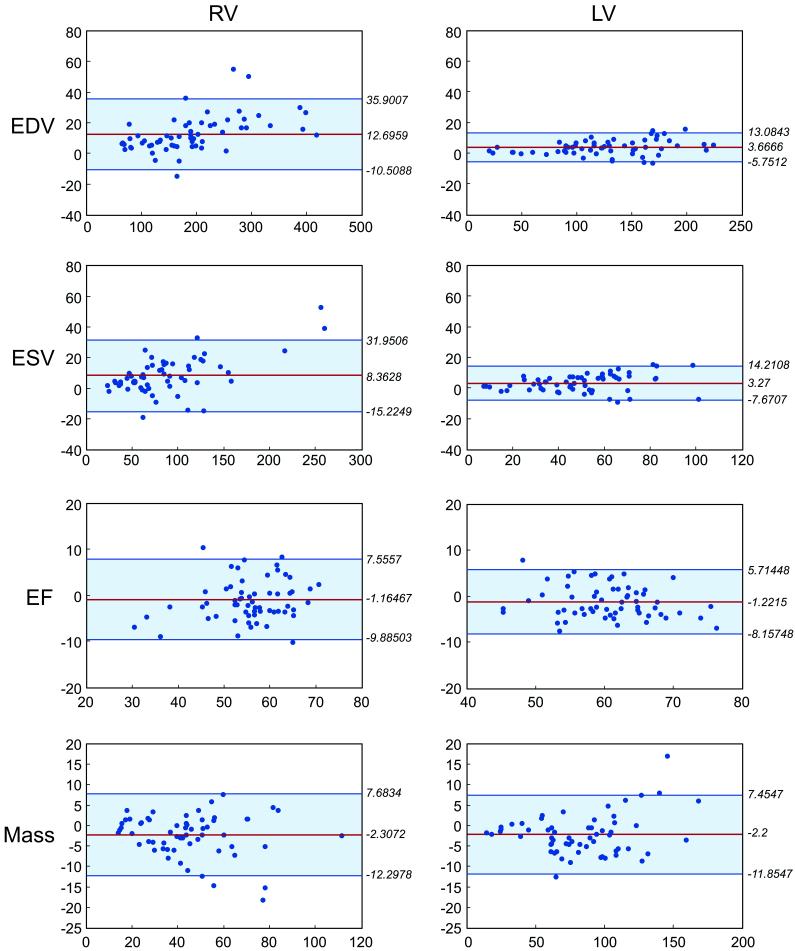
Patterns for inter-observer variability mirrored those for intra-observer variability. ICCs for all parameters were > 0.94, with the exception of RV and LV EF (0.81 and 0.789, respectively; Table 2). The only significant, albeit small, difference between RV and LV inter-observer ICC was chamber mass (ICC 0.942 v. 0.981 and within subject SD 4.96 v. 4.82, p < 0.001). Bland-Altman plots indicate no systematic error in measurements related to the absolute value of the measures (Figure 2).
Figure 2.
Bland-Altman plots of the mean difference between observers and the 95% confidence limits for RV and LV end-diastolic and end-systolic volumes, ejection fraction, and end-diastolic mass.
In sub-group analyses, several RV parameters’ ICCs were significantly different in the ASD/PAPVC group (Group II) and/or the TOF group (Group III) as compared to the normal group (Table 3). However, the absolute differences were small with the most notable difference being a higher ICC for RV EF in Group II as compared with Group I (0.872 v. 0.518, p = 0.001; within subject SD 4.14 v. 3.62). The differences between groups in LV parameters were small and, for the most part, did not reach statistical significance.
Table 3.
Comparison of intra-class correlations between patients with normal right ventricle and those with atrial septal defect or repaired tetralogy of Fallot
| Intra-Observer | Inter-Observer | |||||||
|---|---|---|---|---|---|---|---|---|
| ICC | P (v. Normal) | P (v. ASD) | w/in rater SD | ICC | P (v. Normal) | P (v. ASD) | w/in subject SD | |
| RV end-diastolic volume | ||||||||
| Normal | 0.985 | 5.76 | 0.954 | 10.14 | ||||
| ASD | 0.993 | 0.079 | 9.06 | 0.983 | 0.031 | 14.88 | ||
| TOF | 0.987 | 0.395 | 0.126 | 9.26 | 0.966 | 0.284 | 0.097 | 14.99 |
| RV end-systolic volume | ||||||||
| Normal | 0.914 | 5.99 | 0.778 | 10.4 | ||||
| ASD | 0.984 | 0.001 | 7.44 | 0.974 | 0.000 | 9.41 | ||
| TOF | 0.985 | 0.000 | 0.452 | 6.33 | 0.932 | 0.008 | 0.034 | 13.69 |
| RV stroke volume | ||||||||
| Normal | 0.968 | 5.08 | 0.953 | 6.17 | ||||
| ASD | 0.978 | 0.241 | 9.55 | 0.956 | 0.450 | 13.84 | ||
| TOF | 0.938 | 0.104 | 0.025 | 9.08 | 0.909 | 0.101 | 0.080 | 11.17 |
| RV ejection fraction | ||||||||
| Normal | 0.691 | 2.87 | 0.518 | 4.14 | ||||
| ASD | 0.874 | 0.026 | 3.59 | 0.872 | 0.001 | 3.62 | ||
| TOF | 0.91 | 0.004 | 0.251 | 2.56 | 0.817 | 0.010 | 0.229 | 3.85 |
| RV mass | ||||||||
| Normal | 0.933 | 2.7 | 0.821 | 4.72 | ||||
| ASD | 0.973 | 0.042 | 4.39 | 0.98 | 0.000 | 3.78 | ||
| TOF | 0.866 | 0.085 | 0.001 | 5.31 | 0.831 | 0.452 | 0.000 | 6.1 |
| LV end-diastolic volume | ||||||||
| Normal | 0.989 | 4.44 | 0.985 | 5.27 | ||||
| ASD | 0.997 | 0.008 | 2.65 | 0.997 | 0.001 | 2.88 | ||
| TOF | 0.983 | 0.210 | 0.001 | 4.39 | 0.965 | 0.057 | 0.000 | 6.41 |
| LV end-systolic volume | ||||||||
| Normal | 0.961 | 3.67 | 0.904 | 5.91 | ||||
| ASD | 0.957 | 0.427 | 3.88 | 0.958 | 0.055 | 3.87 | ||
| TOF | 0.943 | 0.235 | 0.295 | 3.97 | 0.883 | 0.346 | 0.023 | 5.84 |
| LV stroke volume | ||||||||
| Normal | 0.966 | 5.2 | 0.954 | 6.1 | ||||
| ASD | 0.982 | 0.117 | 4.5 | 0.983 | 0.031 | 4.44 | ||
| TOF | 0.959 | 0.362 | 0.061 | 4.56 | 0.963 | 0.340 | 0.073 | 4.28 |
| LV ejection fraction | ||||||||
| Normal | 0.864 | 2.33 | 0.762 | 3.28 | ||||
| ASD | 0.732 | 0.072 | 3.88 | 0.75 | 0.456 | 3.7 | ||
| TOF | 0.869 | 0.470 | 0.062 | 2.62 | 0.822 | 0.264 | 0.229 | 3.14 |
| LV mass | ||||||||
| Normal | 0.978 | 5.19 | 0.974 | 5.62 | ||||
| ASD | 0.993 | 0.017 | 3.22 | 0.992 | 0.014 | 3.33 | ||
| TOF | 0.964 | 0.178 | 0.001 | 4.03 | 0.941 | 0.060 | 0.000 | 5.21 |
Abbreviations: ASD = atrial septal defect; ICC = intra-class correlation coefficient; TOF = tetralogy of Fallot
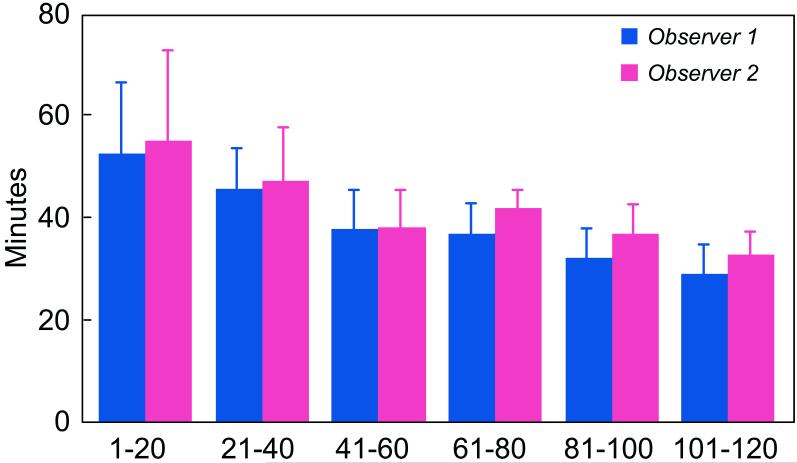
The time it took observers to complete all contours for a particular study decreased with the number of studies read (Figure 3). By the time the final study was read for the second time (i.e., the 120th study read), analysis time had decreased by 40-45% for both observers, from an average of 54 minutes to an average of 31 minutes.
Figure 3.
Average analysis time per patient as a function of observer experience.
DISCUSSION
The results of this study demonstrate that CMR-derived measurements of RV size and function have high intra- and inter-observer reproducibility both in normal and dilated ventricles. Although there were some statistically significant differences in ICC among subgroups, the absolute differences were small. Furthermore, reproducibility of most RV measurements did not differ significantly from that of LV measurements, which have been widely validated (10-15).
In contrast to the abundant literature on reproducibility of LV parameters (24,25), only few previous studies have examined reproducibility of RV measurements by CMR. None of these studies examined subjects with congenital heart disease. The study by Pattynama et al. examined reproducibility in 40 repeated scans from 2 healthy volunteers (16); that of Beygui et al. evaluated 15 healthy volunteers and 10 patients with acquired heart disease (17). A larger study by Grothues et al. examined 60 subjects of whom 40 had heart disease, but all their subjects had acquired, not congenital, heart disease (18). Our study examined 60 subjects, 2/3 of whom had congenital heart disease including both unoperated right-sided volume overload as well as patients who were post-surgical repair of TOF lesions. In addition, our study benefited from the use of current CMR techniques, including steady-state free precession pulse sequences as opposed to older gradient echo cine sequences used in the prior studies. A comparison between the inter-observer variability found in this study and in selected published reports is shown in Table 4.
Table 4.
Comparison between inter-observer coefficient of variations in this study and in selected published reports
| Present study | Grothues et al. (8) | Hudsmith et al. (25) | Karamitsos et al. (post-training) (26) | Karamitsos et al. (expert) (26) | Moon et al. (24) | |
|---|---|---|---|---|---|---|
| No. of patients | 60 | 60 | 12 | 10 | 10 | 20 |
| Diagnosis | Normal/ASD/TOF | Normal/CHF/LVH | Normal | Normal | Normal | Normal/CHF |
| CMR technique | SSFP | FLASH | SSFP | SSFP | SSFP | SSFP |
| Right ventricle | ||||||
| EDV | 6.4% | 6.2% | 9.6% | |||
| ESV | 13.0% | 14.1% | ||||
| EF | 8.0% | 8.3% | 10.7% | |||
| Mass | 11.3% | 8.7% | ||||
| Left ventricle | ||||||
| EDV | 3.6% | 2.7% | 4.6% | 2.6% | 2.6% | |
| ESV | 10.5% | 7.4% | 6.9% | 10.5% | ||
| EF | 5.8% | 3.3% | 3.7% | 2.9% | 6% | |
| Mass | 5.3% | 5.2% | 6.7% | 5.8% | 6% |
Abbreviations: ASD = atrial septal defect; CHF = congestive heart failure; EDV = end-diastolic volume; EF = ejection fraction; ESV = end-systolic volume; FLASH = fast low-angle shot; LVH = left ventricular hypertrophy; SSFP= steady-state free precession; TOF = tetralogy of Fallot
It is also worth noting that although we measured RV volumes and mass from the short-axis plane of the LV, other investigators have examined alternative imaging orientations. For example, Alfakih et al. found that RV volume measurements from the axial plane were slightly lower than those obtained from the short-axis plane (4.8% lower end-diastolic volume) and were associated with slightly lower observer variability (26). Notably, use of the axial plane precludes measurements of ventricular mass because the endocardial and epicardial boundaries of the inferior LV and RV walls are not defined in this plane. Strugnell and colleagues proposed the use of a modified RV short-axis orientation—orthogonal to the long-axis plane of the RV outflow from the pulmonary valve to the RV apex (27). Their study of 50 patients after heart transplantation concluded that compared with the standard short-axis plane, the modified RV outflow short-axis plane allowed easier identification of the tricuspid valve plane and closer agreement between RV and LV stroke volumes. In our study, identification of the atrioventricular junction on short-axis images was facilitated by simultaneous display of cross-referenced long- and short-axis cine images of the ventricles as shown in figure 1.
Greater intra- and inter-observer variability was noted in two instances. First, ICC was lower for both LV and RV measurements of EF, as would be expected when error in two independent measurements is mathematically increased by dividing them. Second, RV measurements of mass had slightly but statistically significantly lower ICC. This finding is likely related to the trabeculations and thin wall of the RV and is similar to that reported by Grothues et al. (18). In practical terms, our study found that a difference of more than 12 ml/m2 or 15% between measurements of RV end-diastolic volume index in an individual with a normal RV is less than 5% likely to be explained by inter-observer difference. In a patient with repaired TOF, the corresponding value is 34 ml/m2 or 24%. For RV ejection fraction, a change unlikely to be explained by inter-observer difference in patients with a normal RV is 6.4 EF points or 11.5% and in patients with repaired TOF, 5.5 EF points or 11%.
A secondary finding of our study is the notable decrease in contouring time as observers analyzed an increasing number of studies. Indeed, by the time the 120th study had been processed, the time required to analyze a study had decreased by as much as 45%. This rapid decrease was expected, as the medical student observers who carried out the analysis had had no prior exposure to cardiac CMR, and they were trained on only 5 sample cases each prior to commencing the study. The inexperience of the analysts implies that our results on intra- and inter-observer variability likely represent a “worst-case” scenario for the reproducibility of chamber measurements by CMR. Karamitsos et al. demonstrated that in two months of intensive training, inexperienced operators significantly improved the reproducibility of their measurements of almost all LV parameters, with the exception of LV mass (28). Thus, it could be expected that, with increasing training, the reproducibility of RV measurements that we found would improve further.
Several limitations of our study merit attention. Our data is derived from only two observers; other groups should thus reproduce these results. In addition, there was no randomization of the order in which studies were drawn from subgroups; thus, some differences in reproducibility among subgroups may be due to the increasing experience of the observers as they analyzed more studies rather than to true differences in chamber characteristics.
In conclusion, our results indicate that CMR measurements of RV size and function are highly reproducible in patients with both normal and abnormal volume load. With increasing training of operators, further improvements in reproducibility may be expected. Such high intra- and inter-observer reproducibility further establishes the utility of CMR in diagnosis and longitudinal follow-up of heart disease affecting the right ventricle.
Acknowledgement
Mr. Mooij and Mr. de Wit gratefully acknowledge the mentorship of Livia Kapusta, MD, PhD, Children’s Heart Center, Radboud University Nijmegen Medical Centre, Nijmegen, the Netherlands.
Grant Support: Christiaan F. Mooij and Cornelis J. de Wit were supported by the Radboud University Nijmegen Medical Centre, Nijmegen, the Netherlands. Drs. Geva and Powell were supported in part by the National Institutes of Health (NIH/NHLBI 1P50 HL074734-01) and by the Higgins Family Noninvasive Cardiac Imaging Research Fund.
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.Oosterhof T, van Straten A, Vliegen HW, et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116:545–451. doi: 10.1161/CIRCULATIONAHA.106.659664. [DOI] [PubMed] [Google Scholar]
- 2.Geva T. Indications and timing of pulmonary valve replacement after tetralogy of fallot repair. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006:11–22. doi: 10.1053/j.pcsu.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Knauth AL, Gauvreau K, Powell AJ, et al. Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart. 2008;94:211–216. doi: 10.1136/hrt.2006.104745. [DOI] [PubMed] [Google Scholar]
- 4.Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. 2004;43:1068–1074. doi: 10.1016/j.jacc.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 5.van den Bosch AE, Robbers-Visser D, Krenning BJ, et al. Comparison of real-time three-dimensional echocardiography to magnetic resonance imaging for assessment of left ventricular mass. Am J Cardiol. 2006;97:113–117. doi: 10.1016/j.amjcard.2005.07.114. [DOI] [PubMed] [Google Scholar]
- 6.Mannaerts HF, Van Der Heide JA, Kamp O, et al. Quantification of left ventricular volumes and ejection fraction using freehand transthoracic three-dimensional echocardiography: comparison with magnetic resonance imaging. J Am Soc Echocardiogr. 2003;16:101–109. doi: 10.1067/mje.2003.7. [DOI] [PubMed] [Google Scholar]
- 7.Sobkowicz B, Hirnle T, Haran T, Wrabec K, Mielecki T. Validation of two-dimensional echocardiography for quantifying left ventricular aneurysm: comparison with magnetic resonance imaging and evaluation during cardiac surgery. Acta Cardiol. 2002;57:73–74. [PubMed] [Google Scholar]
- 8.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 9.Poutanen T, Ikonen A, Jokinen E, Vainio P, Tikanoja T. Transthoracic three-dimensional echocardiography is as good as magnetic resonance imaging in measuring dynamic changes in left ventricular volume during the heart cycle in children. Eur J Echocardiogr. 2001;2:31–39. doi: 10.1053/euje.2000.0054. [DOI] [PubMed] [Google Scholar]
- 10.Semelka RC, Tomei E, Wagner S, et al. Normal left ventricular dimensions and function: interstudy reproducibility of measurements with cine MR imaging. Radiology. 1990;174:763–768. doi: 10.1148/radiology.174.3.2305059. [DOI] [PubMed] [Google Scholar]
- 11.Semelka RC, Tomei E, Wagner S, et al. Interstudy reproducibility of dimensional and functional measurements between cine magnetic resonance studies in the morphologically abnormal left ventricle. Am Heart J. 1990;119:1367–1373. doi: 10.1016/s0002-8703(05)80187-5. [DOI] [PubMed] [Google Scholar]
- 12.Germain P, Roul G, Kastler B, Mossard JM, Bareiss P, Sacrez A. Inter-study variability in left ventricular mass measurement. Comparison between M-mode echography and MRI. Eur Heart J. 1992;13:1011–1019. doi: 10.1093/oxfordjournals.eurheartj.a060307. [DOI] [PubMed] [Google Scholar]
- 13.Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2:271–278. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]
- 14.Bellenger NG, Marcus NJ, Rajappan K, Yacoub M, Banner NR, Pennell DJ. Comparison of techniques for the measurement of left ventricular function following cardiac transplantation. J Cardiovasc Magn Reson. 2002;4:255–263. doi: 10.1081/jcmr-120003951. [DOI] [PubMed] [Google Scholar]
- 15.Myerson SG, Bellenger NG, Pennell DJ. Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension. 2002;39:750–755. doi: 10.1161/hy0302.104674. [DOI] [PubMed] [Google Scholar]
- 16.Pattynama PM, Lamb HJ, Van der Velde EA, Van der Geest RJ, Van der Wall EE, De Roos A. Reproducibility of MRI-derived measurements of right ventricular volumes and myocardial mass. Magn Reson Imaging. 1995;13:53–63. doi: 10.1016/0730-725x(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 17.Beygui F, Furber A, Delepine S, et al. Routine breath-hold gradient echo MRI-derived right ventricular mass, volumes and function: accuracy, reproducibility and coherence study. Int J Cardiovasc Imaging. 2004;20:509–516. doi: 10.1007/s10554-004-1097-7. [DOI] [PubMed] [Google Scholar]
- 18.Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147:218–223. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–66. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 20.Samyn MM, Powell AJ, Garg R, Sena L, Geva T. Range of ventricular dimensions and function by steady-state free precession cine MRI in repaired tetralogy of Fallot: Right ventricular outflow tract patch vs. conduit repair. J Magn Reson Imaging. 2007;26:934–940. doi: 10.1002/jmri.21094. [DOI] [PubMed] [Google Scholar]
- 21.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–329. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. Measurement error. BMJ. 1996;313:744. doi: 10.1136/bmj.313.7059.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donner A. A review of inference procedures for the intraclass correlation coefficient in a one-way random effects model. Int Stat Rev. 1986;54:67–82. [Google Scholar]
- 24.Moon JC, Lorenz CH, Francis JM, Smith GC, Pennell DJ. Breath-hold FLASH and FISP cardiovascular MR imaging: left ventricular volume differences and reproducibility. Radiology. 2002;223:789–797. doi: 10.1148/radiol.2233011181. [DOI] [PubMed] [Google Scholar]
- 25.Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7:775–782. doi: 10.1080/10976640500295516. [DOI] [PubMed] [Google Scholar]
- 26.Alfakih K, Plein S, Bloomer T, Jones T, Ridgway J, Sivananthan M. Comparison of right ventricular volume measurements between axial and short axis orientation using steady-state free precession magnetic resonance imaging. J Magn Reson Imaging. 2003;18:25–32. doi: 10.1002/jmri.10329. [DOI] [PubMed] [Google Scholar]
- 27.Strugnell WE, Slaughter RE, Riley RA, Trotter AJ, Bartlett H. Modified RV short axis series--a new method for cardiac MRI measurement of right ventricular volumes. J Cardiovasc Magn Reson. 2005;7:769–774. doi: 10.1080/10976640500295433. [DOI] [PubMed] [Google Scholar]
- 28.Karamitsos TD, Hudsmith LE, Selvanayagama JB, Neubauer S, Francis JM. Operator Induced Variability in Left Ventricular Measurements with Cardiovascular Magnetic Resonance is Improved After Training. J Cardiovasc Magn Reson. 2007;9:777–783. doi: 10.1080/10976640701545073. [DOI] [PubMed] [Google Scholar]