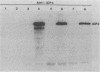
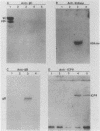
Abstract
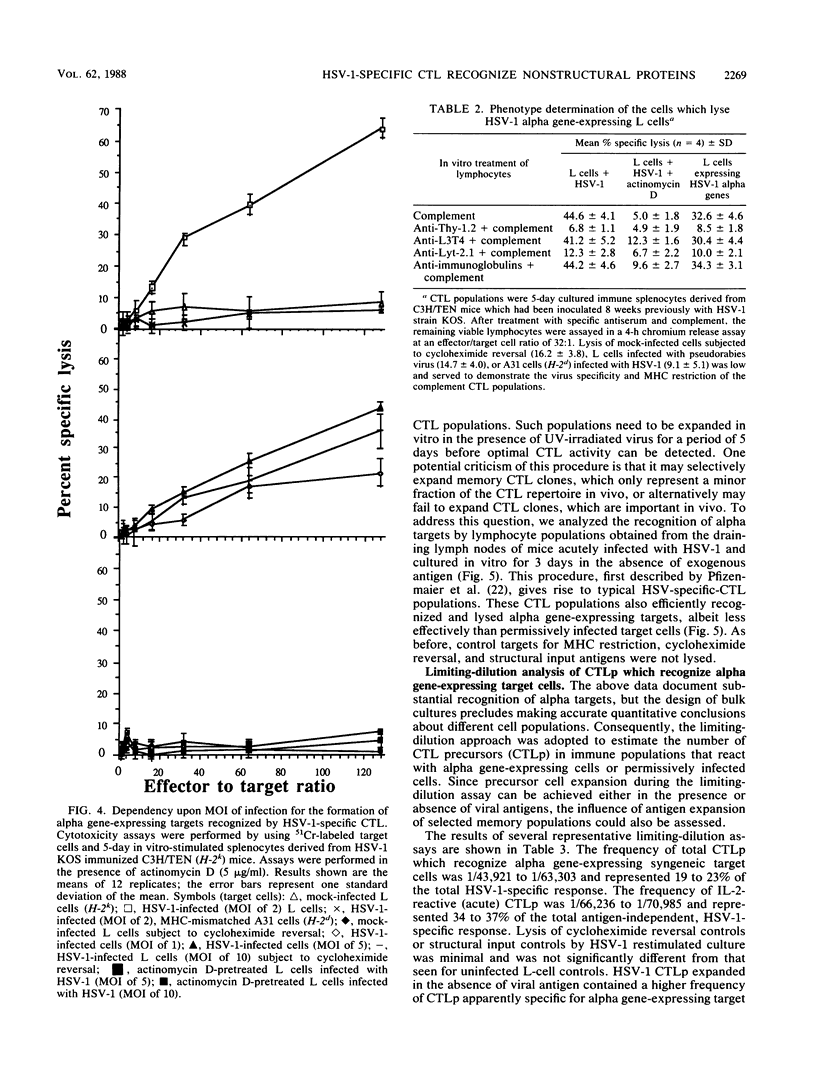
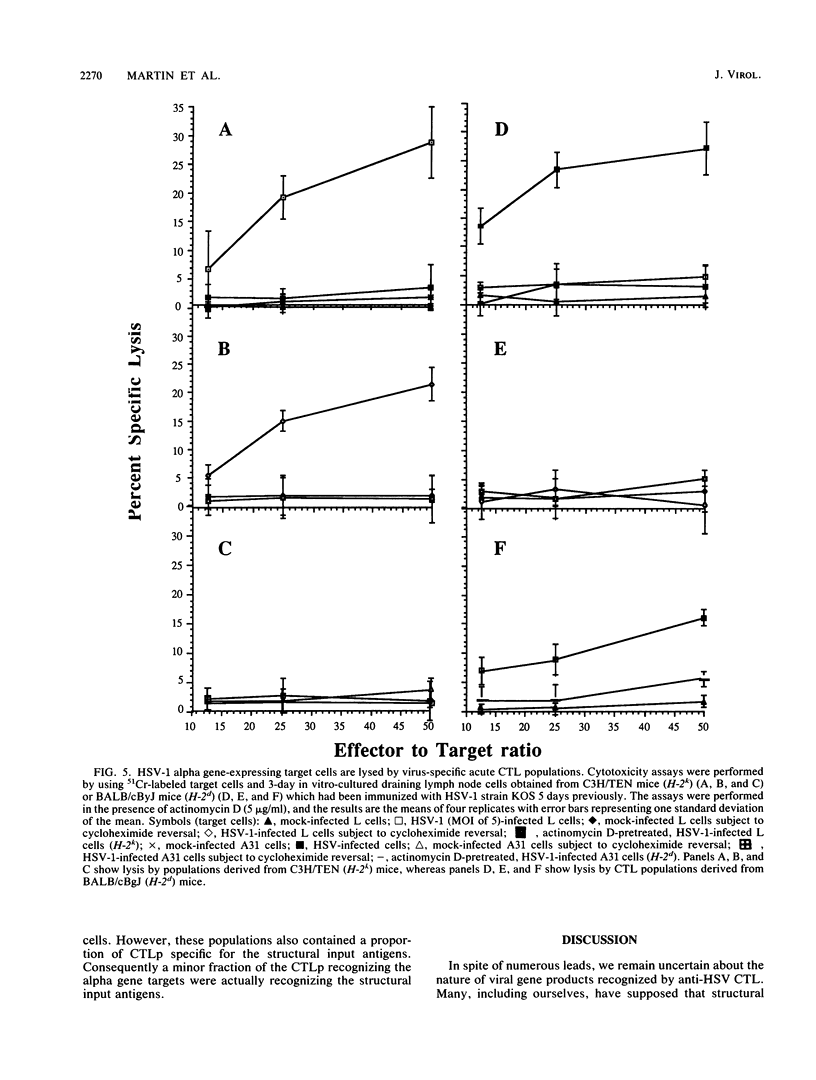
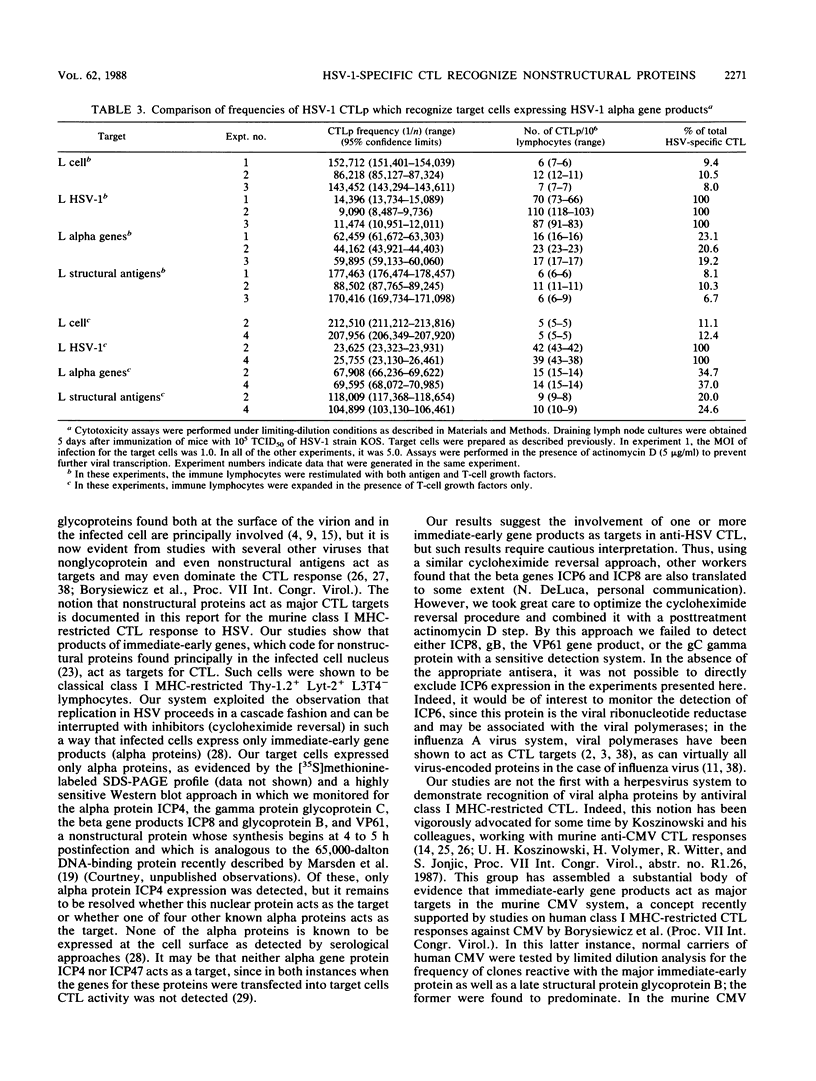
The specificity of herpes simplex virus type 1-specific cytotoxic T cells was examined with target cells expressing either input viral structural antigens or antigens resulting from permissive infection or cells from an interrupted infection in which they expressed predominantly nonstructural immediate-early proteins. These studies indicated that only an insignificant minority of cytotoxic T cells recognized the input viral antigens, whereas a significant proportion (20 to 35%) recognized target cells that expressed the immediate-early proteins despite the absence of serologically detectable viral antigens upon the infected cell surface. The finding that a significant proportion of cytotoxic T-cell populations obtained from the draining lymph nodes of mice acutely infected with herpes simplex virus type 1 also recognized immediately-early gene-expressing target cells indicates the importance of nonstructural herpes simplex virus proteins to antiviral immunity in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangham C. R., Openshaw P. J., Ball L. A., King A. M., Wertz G. W., Askonas B. A. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986 Dec 15;137(12):3973–3977. [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Gerhard W. A viral polymerase involved in recognition of influenza virus-infected cells by a cytotoxic T-cell clone. Nature. 1982 Mar 4;296(5852):75–76. doi: 10.1038/296075a0. [DOI] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Smith G. L., Moss B. Anti-influenza virus cytotoxic T lymphocytes recognize the three viral polymerases and a nonstructural protein: responsiveness to individual viral antigens is major histocompatibility complex controlled. J Virol. 1987 Apr;61(4):1098–1102. doi: 10.1128/jvi.61.4.1098-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter V. C., Rice P. L., Tevethia S. S. Intratypic and intertypic specificity of lymphocytes involved in the recognition of herpes simplex virus glycoproteins. Infect Immun. 1982 Jul;37(1):116–126. doi: 10.1128/iai.37.1.116-126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T., Courtney R. J. Virus-specific glycoproteins associated with the nuclear fraction of herpes simplex virus type 1-infected cells. J Virol. 1984 Feb;49(2):594–597. doi: 10.1128/jvi.49.2.594-597.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R. J., Benyesh-Melnick M. Isolation and characterization of a large molecular-weight polypeptide of herpes simplex virus type 1. Virology. 1974 Dec;62(2):539–551. doi: 10.1016/0042-6822(74)90414-0. [DOI] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. Preparation and characterization of specific antisera to individual glycoprotein antigens comprising the major glycoprotein region of herpes simplex virus type 1. J Virol. 1980 Sep;35(3):902–917. doi: 10.1128/jvi.35.3.902-917.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 1984 Dec 20;3(13):3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso J., Kees U., Kümel G., Kirchner H., Krammer P. H. Identification of herpes simplex virus type 1 (HSV-1) glycoprotein gC as the immunodominant antigen for HSV-1-specific memory cytotoxic T lymphocytes. J Immunol. 1985 Jul;135(1):575–582. [PubMed] [Google Scholar]
- Kast W. M., Bronkhorst A. M., de Waal L. P., Melief C. J. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. Protection by T cells is MHC-restricted and MHC-regulated; a model for MHC-disease associations. J Exp Med. 1986 Sep 1;164(3):723–738. doi: 10.1084/jem.164.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kees U., Krammer P. H. Most influenza A virus-specific memory cytotoxic T lymphocytes react with antigenic epitopes associated with internal virus determinants. J Exp Med. 1984 Feb 1;159(2):365–377. doi: 10.1084/jem.159.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U. H., Allen H., Gething M. J., Waterfield M. D., Klenk H. D. Recognition of viral glycoproteins by influenza A-specific cross-reactive cytolytic T lymphocytes. J Exp Med. 1980 Apr 1;151(4):945–958. doi: 10.1084/jem.151.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U. H., Reddehase M. J., Keil G. M., Schickedanz J. Host immune response to cytomegalovirus: products of transfected viral immediate-early genes are recognized by cloned cytolytic T lymphocytes. J Virol. 1987 Jun;61(6):2054–2058. doi: 10.1128/jvi.61.6.2054-2058.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U., Gething M. J., Waterfield M. T-cell cytotoxicity in the absence of viral protein synthesis in target cells. Nature. 1977 May 12;267(5607):160–163. doi: 10.1038/267160a0. [DOI] [PubMed] [Google Scholar]
- Lawman M. J., Courtney R. J., Eberle R., Schaffer P. A., O'Hara M. K., Rouse B. T. Cell-mediated immunity to herpes simplex virus: specificity of cytotoxic T cells. Infect Immun. 1980 Nov;30(2):451–461. doi: 10.1128/iai.30.2.451-461.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawman M. J., Rouse B. T., Courtney R. J., Walker R. D. Cell-mediated immunity against herpes simplex induction of cytotoxic T lymphocytes. Infect Immun. 1980 Jan;27(1):133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon V. R., Domber E. A., Holowczak J. A. Vaccinia virus proteins on the plasma membranes of infected cells. II. Expression of viral antigens and killing of infected cells by vaccinia virus-specific cytotoxic T cells. Virology. 1985 Aug;145(1):1–23. doi: 10.1016/0042-6822(85)90197-7. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Campbell M. E., Haarr L., Frame M. C., Parris D. S., Murphy M., Hope R. G., Muller M. T., Preston C. M. The 65,000-Mr DNA-binding and virion trans-inducing proteins of herpes simplex virus type 1. J Virol. 1987 Aug;61(8):2428–2437. doi: 10.1128/jvi.61.8.2428-2437.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Moss B., Berman P. W., Laskey L. A., Rouse B. T. Mechanisms of antiviral immunity induced by a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: cytotoxic T cells. J Virol. 1987 Mar;61(3):726–734. doi: 10.1128/jvi.61.3.726-734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizenmaier K., Starzinski-Powitz A., Röllinghoff M., Falks D., Wagner H. T-cell-mediated cytotoxicity against herpes simplex virus-infected target cells. Nature. 1977 Feb 17;265(5595):630–632. doi: 10.1038/265630a0. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Puddington L., Bevan M. J., Rose J. K., Lefrançois L. N protein is the predominant antigen recognized by vesicular stomatitis virus-specific cytotoxic T cells. J Virol. 1986 Nov;60(2):708–717. doi: 10.1128/jvi.60.2.708-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Keil G. M., Koszinowski U. H. The cytolytic T lymphocyte response to the murine cytomegalovirus. II. Detection of virus replication stage-specific antigens by separate populations of in vivo active cytolytic T lymphocyte precursors. Eur J Immunol. 1984 Jan;14(1):56–61. doi: 10.1002/eji.1830140111. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Koszinowski U. H. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature. 1984 Nov 22;312(5992):369–371. doi: 10.1038/312369a0. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Yao Q. Y., Wallace L. E. The Epstein-Barr virus as a model of virus-host interactions. Br Med Bull. 1985 Jan;41(1):75–79. doi: 10.1093/oxfordjournals.bmb.a072030. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. L., Smiley J. R., South S., Johnson D. C. Cells expressing herpes simplex virus glycoprotein gC but not gB, gD, or gE are recognized by murine virus-specific cytotoxic T lymphocytes. J Virol. 1987 Aug;61(8):2438–2447. doi: 10.1128/jvi.61.8.2438-2447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Larsen H. S., Wagner H. Frequency of cytotoxic T lymphocyte precursors to herpes simplex virus type 1 as determined by limiting dilution analysis. Infect Immun. 1983 Feb;39(2):785–792. doi: 10.1128/iai.39.2.785-792.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Norley S., Martin S. Antiviral cytotoxic T lymphocyte induction and vaccination. Rev Infect Dis. 1988 Jan-Feb;10(1):16–33. doi: 10.1093/clinids/10.1.16. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Israelsohn E. S. Generation of specific cytotoxic T cells with a fragment of the Epstein-Barr virus-encoded p63/latent membrane protein. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5384–5388. doi: 10.1073/pnas.84.15.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Skehel J. J. The influenza A virus nucleoprotein gene controls the induction of both subtype specific and cross-reactive cytotoxic T cells. J Exp Med. 1984 Aug 1;160(2):552–563. doi: 10.1084/jem.160.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]
- von Boehmer H., Hengartner H., Nabholz M., Lernhardt W., Schreier M. H., Haas W. Fine specificity of a continuously growing killer cell clone specific for H-Y antigen. Eur J Immunol. 1979 Aug;9(8):592–597. doi: 10.1002/eji.1830090804. [DOI] [PubMed] [Google Scholar]