Abstract
Effect of Calendula officinalis flower extract was investigated against experimentally induced thermal burns in rats. Burn injury was made on the shaven back of the rats under anesthesia and the animals were treated orally with different doses of the flower extract (20 mg, 100 mg and 200 mg/kg body weight). The animals treated with the extract showed significant improvement in healing when compared with the control untreated animals. The indicators of the wound healing such as collagen-hydroxyproline and hexosamine contents were significantly increased in the treated group indicating accelerated wound healing in the treated animals. The acute phase proteins—haptoglobin and orosomucoid which were increased due to burn injury were found to be decreased significantly in 200 mg/kg body weight extract treated animals. The antioxidant defense mechanism, which was decreased in the liver during burn injury, was found to be enhanced in treated animals. The lipid peroxidation was significantly lowered in the treated group when compared to control animals. Tissue damage marker enzymes- alkaline phosphatase, alanine and aspartate transaminases were significantly lowered in the treated groups in a dose dependant manner. The histopathological analyses of skin tissue also give the evidence of the increased healing potential of the extract after burn injury.
Keywords: acute phase proteins, antioxidant enzymes, Calendula officinalis, granuloma tissue, thermal injury
Introduction
Thermal injury produces profound systemic changes such as oligemic shock, anemia, renal failure and metabolic disturbances. It causes direct tissue damage as well as inflammatory reactions. Infection is another major complication of thermal injury [1]. It also leads to increased oxidant stress in the cells as seen by decreased endogenous non-enzymatic and enzymatic antioxidant activity [2]. The mediators of burn shock include histamine, serotonin, kinins, oxygen free radicals, prostaglandins, thrombaxene and interleukins [3]. Acute phase responses are initiated to restore homeostasis after trauma. Clinical studies have shown that a sustained or increased acute phase response can contribute to multiorgan failure, hyper metabolism, complications, and death [4]. There are only very few drugs, which are effective in the treatment and management of thermal burns.
Many drugs of plant origin can produce wound healing and are used in burn injury. Calendula officinalis. Linn of Family Asteraceae has been widely used in homeopathic medicine for the treatment of many diseases [5]. It has been reported to possess many pharmacological activities, which include antioxidant [6] anti-inflammatory [7] antibacterial [8], antifungal [9] and antiviral [10]. It also possess cytotoxic as well as tumor reducing potential [11].
In the present study we have evaluated the potential activity of Calendula officinalis flower extract against thermal burns in animals. The enzyme markers of tissue damage, the level of antioxidant enzymes and the acute phase proteins were evaluated and compared with the untreated thermally injured animals.
Materials and Methods
Chemicals
Nitro blue tetrazolium (NBT), 5-5'dithiobis (2-nitro benzoic acid) (DTNB) and riboflavin were purchased from Sisco Research Laboratories Pvt. Ltd., (Mumbai, India), Thiobarbaturic acid was purchased from Himedia laboratories, (Mumbai, India). Para dimethyl amino benzaldehyde was purchased from E Merck, (Mumbai, India). Ketamine hydrochloride was purchased from Neon laboratories Ltd., (Mumbai, India). Span Diagnostics Ltd., (Surat, India) supplied the biochemical kits for determining alkaline phosphatase, aspartate transaminase, alanine transaminase activities and bilirubin content. The kits for haptoglobin and orosomucoid were supplied by Orion Diagnostica, (Espoo, Finland). All other chemicals and reagents used were of analytical grade.
Preparation of the extract
Fresh Calendula flower tops were used for extraction of the active components. They were collected from Government Botanical Gardens, Ooty, Nilgiris during January 2006 and were authenticated by Dr. S. Rajan, Field Botanist, Central Council for Research in Homeopathy, Ooty, India and the voucher specimen was deposited at Amala Ayurvedic Research Centre (Voucher No: Co05). Extraction was done as per Pharmacopoeia [12]. Calendula flowers (700 g) were extracted with 450 ml ethyl alcohol by masturation. For this, the material was placed in a wide mouth bottle and the alcohol was added. The jar was stoppered and sealed to prevent evaporation. It was placed in a dark room at room temperature and shaken everyday for two weeks. Then the clear liquid was decanted and the residue was pressed out through clean linen, which was added to the decanted liquid. Volume was made upto 1 L with alcohol. 100 ml of this tincture was evaporated to dryness in a shaker water bath at 42°C. The yield was found to be 1.1 g. Dried extract (1 g) was resuspended in a known amount of distilled water and used for all experiments.
Experimental animals
Female Wistar rats (150–200 g) were obtained from Small Animal Breeding Station, (Mannuthy, Thrissur, Kerala). They were housed in well-ventilated cages and fed with normal mouse chow (Sai Durga Feeds and Food, Bangalore, India) and water ad libitum. All the animal experiments were done after approval from the Institutional Animal Ethical Committee.
Burn wound model
Burn wounds were created on the shaved dorsal part of rats after anaesthetizing them with ketamine hydrochloride (100 mg/kg body weight). The animals were immersed in water bath of 90°C for 12 s to produce thermal burns [13]. To achieve uniform area of burn the animals were kept on a box with a hole so that the body exposed through the hole will only be burned. The method was standardized and confirmed through histopathological analysis of burned skin.
Experimental protocol
Fifty four animals were used for the study. Animals were treated with the extract orally 24 h prior to burning. The animals were grouped as follows: Group I: Normal without any treatment or burning (n = 6), Group II: Burned animals without any treatment (n = 12), Group III: Burned animals treated with 20 mg/Kg body weight Calendula extract (n = 12), Group IV: Burned animals treated with 100 mg/Kg body weight Calendula extract (n = 12), Group V: Burned animals treated with 200 mg/Kg body weight Calendula extract (n = 12). Treatment with the drug was continued upto 10 consecutive days. Six animals from each group were sacrificed on 5th day and the rest of the animals were sacrificed on 10th day. Blood was collected; serum was separated for analyzing alkaline phosphatase, glutamate-pyruvate transaminase, glutamate-oxaloacetate transaminase activities, and bilirubin levels using commercially available kits. Haptoglobin and alpha-1 acid glycoprotein (orosomucoid) were estimated in serum by turbidometric method.
Granuloma tissue of the burned skin was carefully excised and washed in cold saline. A small portion of the skin was fixed in 10% formalin and sectioned for histopathological analysis. The rest of the tissue was lyophilized. These tissues were then hydrolyzed with 6N hydrochloric acid and estimated for the content of hexosamine by the method of Elson & Morgan [14] and collagen hydroxyproline by method of Newman & Logan [15].
Liver was excised out and washed in ice-cold saline and a 25% homogenate was prepared in Tris-HCl buffer (pH 7.0) and centrifuged at 10,000 rpm for 30 min at 4°C. The supernatant was collected and analyzed for glutathione content [16], protein content [17], lipid peroxidation [18], superoxide dismutase activity [19] and catalase activity [20].
Statistical analysis
The results are expressed as mean ± SD Statistical evaluation of the data was done by one-way ANOVA followed by Dunnet’s test (post-hoc) using In Stat 3 software package. The control animals were compared with the normal whereas the drug treated with that of the control values.
Results
Effect of Calendula officinalis extract on granuloma formation during thermal injury
The granuloma tissue excised from the burned skin was estimated for hydroxy proline and hexosamine contents, which are the indicators of extracellular matrix. The result obtained is shown in Table 1. There was a significant higher amount of hydroxyproline in the granuloma tissue of drug treated animals compared to controls on both the time periods. The hexosamine content was found to be decreased during thermal burn but was significantly more in drug treated groups on both 5th as well as 10th day of burning.
Table 1.
Effect of Calendula officinalis on hexosamine and collagen hydroxy proline content in the skin granuloma tissue of thermally burned animals
| Hexosamine (mg/100 mg dry weight tissue) |
Hydroxy proline (mg/gm dry weight tissue) |
||||
|---|---|---|---|---|---|
| 5th day | 10th day | 5th day | 10th day | ||
| Group I (Normal) | 6.6 ± 1.4 | — | 9.70 ± 1.85 | — | |
| Group II (Burned untreated) | 1.72 ± 0.14*** | 2.96 ± 0.27*** | 7.56 ± 2.15 | 6.99 ± 1.50 | |
| Group III (Burned + 20 mg/kg body weight drug) | 1.85 ± 0.17 | 3.08 ± 0.33 | 9.25 ± 0.93 | 11.05 ± 0.98** | |
| Group IV (Burned + 100 mg/kg body weight drug) | 1.88 ± 0.07 | 3.52 ± 0.16** | 9.54 ± 1.06 | 12.70 ± 2.1** | |
| Group V (Burned + 250 mg/kg body weight drug) | 2.20 ± 0.28** | 3.72 ± 0.17** | 12.95 ± 0.87** | 13.26 ± 1.84** | |
**: p<0.01, ***: p<0.001
There was also a significant increase in the level of acute phase proteins, haptoglobin as well as orosomucoid in the serum of burned animals on both 5th and 10th day of thermal burn (Table 2). The increase in the level was found to be significantly lowered by treatment with the extract.
Table 2.
Effect of Calendula officinalis on level of acute phase proteins in the serum of thermally burned animals
| Haptoglobin (g/l) |
Serum orosomucoid (g/l) |
||||
|---|---|---|---|---|---|
| 5th day | 10th day | 5th day | 10th day | ||
| Group I (Normal) | 5.01 ± 1.33 | — | 0.49 ± 0.23 | — | |
| Group II (Burned untreated) | 9.74 ± 4.05** | 8.97 ±1.66*** | 2.18 ± 0.94*** | 2.85 ± 0.88*** | |
| Group III (Burned + 200 mg/kg body weight drug) | 4.76 ± 1.5* | 5.03 ± 0.91*** | 0.97 ± 0.31* | 0.65 ± 0.18*** | |
*: p<0.05, ***: p<0.001
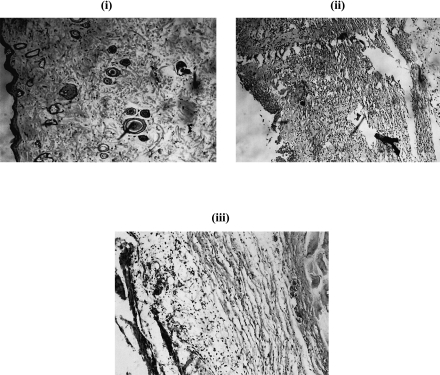
Histopathology section of the burned skin of control animals on 5th day showed denuded epidermis, diffuse infiltration of plasma cells, lymphocytes and polymorphs. The stroma also showed edema and congestion. But in treated animals only the sub epidermal region and dermis showed scattered lymphocytes. Histopathological analysis supported the healing potential of Calendula extract (Fig. 1).
Fig. 1.
Histopathological section of skin on 5th day of burning. (i) Normal skin, (ii) Skin of thermally burned animal without treatment, (iii) Skin of thermally burned animal treated with 200 mg/kg body weight Calendula extract
Effect of Calendula officinalis extract on antioxidant defense system and lipid peroxidation in the liver after thermal injury
Glutathione was found to be increased in the liver of animals with thermal burns possibly to scavenge the free radicals generated during burning. This was found to be further increased by treatment with the extract. On 5th day for extract treated animals the glutathione content was significantly higher than that of untreated animals. On 10th day the level reached almost normal level in all treated groups whereas it was significantly low in the untreated control group (Table 3).
Table 3.
Effect of Calendula officinalis on antioxidant parameters in liver of thermally burned animals
| LPO (n moles of MDA formed/mg protein) |
GSH (n moles/mg protein) |
SOD (Units/ mg protein) |
CAT (Units/mg protein) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5th day | 10th day | 5th day | 10th day | 5th day | 10th day | 5th day | 10th day | ||||
| Group I (Normal) | 0.83 ± 0.03 | — | 14.1 ± 2.5 | — | 0.63 ± 0.13 | — | 8.50 ± 1.50 | — | |||
| Group II (Burned untreated) | 3.70 ± 0.88*** | 1.18 ± 0.15 | 19.3 ± 2.9** | 9.2 ± 0.89** | 0.82 ± 0.10* | 0.54 ± 0.06 | 7.43 ±1.24 | 7.78 ± 0.89 | |||
| Group III (Burned + 20 mg/kg body weight drug) | 3.70 ± 0.57 | 0.90 ± 0.19** | 23.5 ± 2.7* | 12.0 ± 0.29** | 0.85 ± 0.12 | 0.65 ± 0.10 | 8.05 ± 0.98 | 9.24 ± 2.09 | |||
| Group IV (Burned + 100 mg/kg body weight drug) | 3.41 ± 0.90 | 0.84 ± 0.01** | 23.6 ± 2.7* | 13.5 ± 0.59** | 1.09 ± 0.22* | 0.91 ± 0.06** | 8.35 ± 1.59 | 9.27 ± 1.88 | |||
| Group V (Burned + 250 mg/kg body weight drug) | 2.68 ± 0.20 | 0.80 ± 0.15** | 23.6 ± 2.9* | 12.1 ± 1.88** | 1.11 ± 0.18* | 1.10 ± 0.22** | 10.91 ± 2.55** | 10.03 ± 2.24* | |||
*: p<0.05, **: p<0.01, ***: p<0.001
Lipid peroxidation was significantly high in the liver of control animals. Lipid peroxidation was lowered in the drug treated group (200 mg/kg body weight) on 5th day. On 10th day lipid peroxidation attained the normal level in treated animals whereas the control animals had significant high lipid peroxidation (Table 3).
Superoxide dismutase activity was found to be increased in burned animals on 5th day possibly to reduce the oxidative stress. The level was further increased in drug treated groups (p<0.05). On 10th day when control animals possessed much low level of SOD activity 100 mg and 200 mg/Kg body weight extract treated animals had significantly high SOD activity (p<0.01). Similarly catalase was also found to be enhanced in the extract (200 mg/kg body weight) treated animals (p<0.01) (Table 3).
Effect of Calendula officinalis extract on hepatic function markers after thermal injury
There was a significant change in the biochemical parameters of serum in the burned animals. Serum ALP activity was significantly increased in burned animals and was found to be significantly lowered by Calendula treatment (Table 4). Serum GPT activity was significantly increased in control animals on 10th day. In the extract treated group it was significantly (p<0.01) decreased and was almost normal. Serum GOT activity was also significantly increased on 5th day in control group of animals whereas significant reduction was found in 200 mg/Kg body weight extract treated group (p<0.01). On 10th day there was further increase in serum GOT activity in all the groups. However GOT was significantly low in treated animals (p<0.01). Bilirubin was found to be increased on 10th day of burning but reduced significantly in treated group, especially in 200 mg/kg body weight (p<0.01).
Table 4.
Effect of Calendula officinalis on hepatic function of thermally burned animals
| Serum ALP KA units |
SGPT IU/L |
SGOT IU/L |
Bilirubin mg/dL |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5th day | 10th day | 5th day | 10th day | 5th day | 10th day | 10th day | ||||
| Group I (Normal) | 14.8 ± 2.2 | — | 19.5 ± 3.4 | — | 24.5 ± 3.8 | — | 0.48 ± 0.15 | |||
| Group II (Burned untreated | 38.9 ± 8.7*** | 56.7 ± 6.8*** | 18.2 ± 0.8 | 27.0 ± 5.1** | 46.2 ± 7.2** | 108.3 ± 13.6*** | 0.53 ± 0.13 | |||
| Group III (Burned + 20 mg/kg body weight drug) | 30.6 ± 3.7 | 44.5 ± 4.7** | 17.2 ± 1.2 | 25.6 ± 1.9 | 45.8 ± 3.8 | 87.5 ± 5.2** | 0.58 ± 0.09 | |||
| Group IV (Burned + 100 mg/kg body weight drug) | 24.5 ± 5.0** | 42.9 ± 2.5** | 16.5 ± 0.5* | 19.3 ± 5.7* | 45.3 ± 10.0 | 85.0 ± 5.4** | 0.53 ± 0.11 | |||
| Group V (Burned + 250 mg/kg body weight drug) | 19.3 ± 4.7** | 37.3 ± 5.3** | 16.3 ± 1.0** | 12.6 ± 4.1** | 35.7 ± 3.2* | 80.8 ± 7.3** | 0.34 ± 0.16** | |||
*: p<0.05,**: p<0.01, ***: p<0.001
Discussion
Healing of burned tissue is a complex process, which involves re-epithelisation, granulation tissue formation and remodeling of extracellular matrix. There is also experimental evidence that indicates the involvement of superoxide radical in the pathogenesis of burn wound [21]. Earlier studies have shown that there is a close relationship between a lipid peroxidative reaction and secondary pathological changes following thermal injury [13]. It has been known that severe burning not only affects skin but also internal organs. An attack of biomolecules by ROS can result in an alteration of the structure of biological membranes of tissues. A local burn injury produces oxidant-induced organ changes as evidenced by increased lipid peroxidation in remote organs. The body’s innate mechanism to protect itself from the deleterious effect of free radicals is antioxidants. Glutathione plays an important role in the detoxification of foreign compounds, hydrogen peroxide and free radicals [22]. There was a significant increase in the glutathione content in all the burned animals, which may be through the triggering of the antioxidant system. This level was further increased by the treatment with the Calendula extract. Similar results were also found with SOD and catalase, which were increased by treatment with the extract. Results indicate the effectiveness of Calendula officinalis extract on enhancing the antioxidant defense mechanism thereby decreasing the burn injury.
The significant increase of both hexosamine and hydroxyproline content in the granuloma tissue shows the effectiveness of the extract in enhancing the collagen content in burned tissue. This could be either due to increased synthesis or decreased catabolism of collagen due to presence of flavonoids in the extract which can produce artificial cross linkage between collagen molecules.
The systemic inflammatory response after trauma leads to protein degradation, catabolism, and hyper metabolism. To restore systemic homeostasis, the liver reprioritizes its synthesis from constitutive hepatic proteins toward acute-phase proteins. A prolonged increase in the acute-phase response, however, has been shown to increase morbidity and mortality [4]. The increased level of the acute phase proteins in thermally injured animals were found to be decreased in Calendula extract treated animals. The administration of Calendula officinalis extract significantly decreased the serum level of marker enzymes of tissue damage like ALP, GOT and GPT indicating that Calendula extract reduce the injury of the internal organs during thermal burning.
Antioxidant therapy in burn injury has been used to prevent the harmful effect of oxygen free radicals [23]. The anti-inflammatory drugs are reported to be effective against wound healing [24]. Nitric oxide radical and inflammatory mediators play an important role in thermal injury [25]. Our previous study has shown that the Calendula extract is an antioxidant both in vitro and in vivo [6]. This property may be attributed to the effectiveness of Calendula extract in burn injury. Calendula extract may be act through modifying the activities of proinflammatory cytokines and inhibits cyclooxygenase-2 enzyme (unpublished data).
The phytochemical constituents of Calendula officinalis include flavonoids like lupeol, quercetin, protocatechuic acid etc. many alkaloids and triterpinoids [26]. Flavoxanthin, luteoxanthin, lycopene, auroxanthin, lutein, β-carotene etc. are the major carotenoids present in this flower [27]. Most of these constituents are reported as free radical scavengers and enhance wound healing by producing artificial cross linkage [28].
Biological activity of Calendula officinalis extract could be related to pharmacological activities of these ingredients in the extract.
References
- 1.Mokaddas E., Rotimi V.O., Sanyal S.C. In vitro activity of pipetacillin/tazobactam versus other broad antibiotics against nosocomial gram-negative pathogens isolated from burn patients. J. Chemother. 1998;10(3):208–214. doi: 10.1179/joc.1998.10.3.208. [DOI] [PubMed] [Google Scholar]
- 2.Gurel A., Armutcu F., Hosnuter M., Unalacak M., Kargi E., Altinyazar C. Caffeic acid phenethyl ester improves oxidative organ damage in rat model of thermal trauma. Physiol. Res. 2004;53:675–682. [PubMed] [Google Scholar]
- 3.Friedl H.P., Till G.O., Trentz. O., Ward P.A. Roles of histamine, complement and xanthine oxidase in thermal injury of skin. Am. J. Pathol. 1989;135:203–209. [PMC free article] [PubMed] [Google Scholar]
- 4.Jeschke M.G., Herndon D.N., Barrow R.E. Insulin like growth factor I in combination with insulin like growth factor binding protein 3 affects the hepatic acute phase response and hepatic morphology in thermally injured rats. Ann. Surgery. 2000;231(3):408–416. doi: 10.1097/00000658-200003000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zitterl-Eglseer K., Sosa S., Jurenitsch J., Schubert-Zsilavecz M., Della Loggia R., Tubaro A., Bertoldi M., Franz C. Anti-edematous activities of the main triterpendiol esters of marigold (Calendula officinalis L.) J. Ethnopharmacol. 1997;57:139–144. doi: 10.1016/s0378-8741(97)00061-5. [DOI] [PubMed] [Google Scholar]
- 6.Preethi K.C., Kuttan G., Kuttan R. Antioxidant potential of Calendula officinalis flowers in vitro and in vivo. Pharmaceutical. Biol. 2006;44(9):691–697. [Google Scholar]
- 7.Della Logia R., Tubaro A., Sosa S., Becker H., Saar S., Issac O. The role of triterpenoids in the topical anti-inflammatory activity of Calendula flowers. Planta. Med. 1994;60:516–520. doi: 10.1055/s-2006-959562. [DOI] [PubMed] [Google Scholar]
- 8.Dumenil G., Chemli R., Balausard G. Evaluation of antibacterial properties of Calendula officinalis flowers and mother homeopathic tinctures of Calendula officinalis. Ann. Pharma. Fran. 1980;38:493–499. [PubMed] [Google Scholar]
- 9.Kasiram K., Sakharkar P.R., Patil A.T. Antifungal activity of Calendula officinalis. Indian. J. Pharm. Sci. 2000;6:464–466. [Google Scholar]
- 10.Barbour E.K., Sagherian V., Talhouk S., Talhouk R., Farran M.T., Sleiman F.T., Harakeh S. Evaluation of homeopathy in broiler chickens exposed to live viral vaccines and administered Calendula officinalis extract. Med. Sci. Monit. 2004;10(8):281–285. [PubMed] [Google Scholar]
- 11.Boucaud-Maitre Y., Algernon O., Raynaud J. Cytotoxic and antitumoral activity of Calendula officinalis extracts. Pharmazie. 1988;43:220–221. [PubMed] [Google Scholar]
- 12.Committee on Pharmacopoeia of the American Institute of Homeopathy. The Homeopathic Pharmacopoeia of the United States, 6th ed. Otis Clapp & Son, Inc.; Boston: 1954. pp. 39–42. [Google Scholar]
- 13.Zhi-Bo Z., Hai-Qin D., Feng-Jun Q., Lin L., Shi C. Effect of Zn7-metallothionein on oxidative stress in liver of rats with severe thermal injury. Acta. Pharmacol. Sin. 2003;24(8):764–770. [PubMed] [Google Scholar]
- 14.Elson L.A., Morgan W.T.J. A colorimetric method for the determination of glucosamine and chondrosamine. J. Biochem. 1933;27:1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman R.T., Logan N.A. The determination of collagen and elastin in tissues. J. Biochem. 1950;184:299–301. [PubMed] [Google Scholar]
- 16.Moron M.A., De Pierre J.W., MannerVick B. Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat liver. Biochem. Biophys. Acta. 1979;582:67–68. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 17.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Ohkawa H., Oshishi N., Yagi K. Assay for lipid peroxide in animal tissue by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.McCord J.M., Fridovich I. Superoxide dismutase enzyme function for erythrocaprein. J. Biochem. 1969;244:6049–6056. [PubMed] [Google Scholar]
- 20.Aebi H. In: Catalase estimation, in Methods of Enzymatic Analysis. Bergmeyer H.U., editor. Verlag Chemic; New York: 1974. pp. 673–684. [Google Scholar]
- 21.Cetinkale O., Konukoglu D., Penel O., Kemerli GD., Yazar S. Modulating the functions of neutrophils and lipid peroxidation by FK 506 in a rat model of thermal injury. Burns. 1999;25:105–112. doi: 10.1016/s0305-4179(98)00147-8. [DOI] [PubMed] [Google Scholar]
- 22.Gul M., Kutay F.Z., Temocin S., Hanniem O. Cellular and clinical implications of glutathione. Indian. J. Exp. Biol. 2000;38:625–634. [PubMed] [Google Scholar]
- 23.Demling R.H., LaLonde C. Early postburn lipid peroxidation: effect of ibuprofen and allopurinol. Surgery. 1990;107:85–93. [PubMed] [Google Scholar]
- 24.Fei Y.M., Zainol J., Pillay A.G., Yusof N. Experimental evaluation of healing process of burn wound treated by lyophilized Aloe vera dressing. The Sciences. 2002;2(1):1–6. [Google Scholar]
- 25.Jeschke M.G., Aililow J.F., Spies M., Vita R., Hawkins H.K., Hernson D.N., Barrow R.E. Cell proliferation, apoptosis, NF-κB expression, enzyme, protein and weight changes in livers of burned rats. Am. J. Physiol. Gastrointest. Liver. Physiol. 2001;280:G1314–G1320. doi: 10.1152/ajpgi.2001.280.6.G1314. [DOI] [PubMed] [Google Scholar]
- 26.Matysik G., Wojciak-Kosior M., Paduch R. The influence of Calendula officinalis flos extract on cell cultures and the chromatographic analysis of extracts. J. Pharm. Biomed. Anal. 2005;38(2):285–292. doi: 10.1016/j.jpba.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 27.Kishimoto S., Maoka T., Sumitomo K., Ohmiya A. Analysis of carotenoid composition in petals of Calendula (Calendula officinalis L.) Biosci. Biotechnol. Biochem. 2005;69(11):2122–2128. doi: 10.1271/bbb.69.2122. [DOI] [PubMed] [Google Scholar]
- 28.Kuppast I.J., Nayak P.V. Wound-healing activity of Cordia dichotoma Forst. f. Fruits. Nat. Pro. Rad. 2006; 5:99–102. [Google Scholar]