Abstract
Background
Until recently, Plasmodium knowlesi malaria in humans was misdiagnosed as P. malariae. The present objectives were to determine the geographic distribution of P. knowlesi in the human population in Malaysia and to investigate four suspected fatal cases.
Methods
Sensitive and specific nested-PCR was used to identify all Plasmodium species present in blood from i) 960 patients with malaria hospitalized in Sarawak, Malaysian Borneo from 2001-2006, ii) 54 P. malariae archival blood-films from 15 districts in Sabah, Malaysian Borneo (2003–2005) and four districts in Pahang, Peninsular Malaysia (2004–2005), and iii) suspected knowlesi fatalities. In the four latter cases, available clinical and laboratory data were reviewed.
Results
P. knowlesi DNA was detected in 266 of 960 (27·7%) of the samples from Sarawak hospitals, 41 of 49 (83·7%) from Sabah and all 5 from Pahang. Only P. knowlesi DNA was detected in archival blood films from the 4 fatal cases. All were hyperparasitemic and developed marked hepatorenal dysfunction.
Conclusions
Human infections with P. knowlesi, commonly misidentified as the more benign P. malariae, are widely distributed across Malaysian Borneo and extend to Peninsular Malaysia. Because P. knowlesi replicates every 24 hours, rapid diagnosis and prompt effective treatment are essential. In the absence of a specific routine diagnostic test for knowlesi malaria, we recommend that patients in, or who have travelled to, South-east Asia who are ill with a ‘P. malariae’ hyperparasitemia diagnosis by microscopy should receive intensive management as appropriate for severe falciparum malaria.
Keywords: Knowlesi, malaria, hyperparasitaemia, Southeast Asia
Plasmodium knowlesi is a malaria parasite of Old World monkeys [1]. Naturally-acquired knowlesi infections in humans were thought to be rare until we described a large focus of cases in the Kapit Division of Sarawak State, Malaysian Borneo [2]. In that study, all infections diagnosed as P. malariae by microscopy were P. knowlesi or other non-P. malariae species using a nested-PCR assay. P. malariae and P. knowlesi are difficult to distinguish microscopically leading to parasite species misidentification. Symptomatic malaria attributed to P. malariae infections in adults has been reported in other parts of Malaysia, suggesting that the emergence of P. knowlesi in humans may extend beyond the Kapit Division.
At the time of our initial publication in 2004 [2], P. knowlesi (reported as P. malariae) was not considered to cause severe human disease. However, during the period between November 2004 and March 2005, there were four deaths in patients with ‘P. malariae’ infections in Sarawak. Since P. malariae is normally associated with low parasitemia and an uncomplicated clinical course, this raises the possibility that P. knowlesi malaria could become severe.
In the present series of studies, we have determined the distribution of P. knowlesi in different locations in Malaysian Borneo and Peninsular Malaysia using a highly specific nested-PCR assay, and assessed all available demographic, clinical and laboratory data from the four fatal cases.
METHODS
Human blood samples
The present study was approved by the Medical Research Ethics Sub-Committee of the Malaysian Ministry of Health. In Sarawak it is government policy to hospitalize all slide-positive malaria cases regardless of clinical severity. During various periods between March 2001 and March 2006, a total of 960 blood spots on filter paper were collected from unselected patients admitted with slide-positive malaria to 12 hospitals across Sarawak; Bau, Lundu, Betong, Serian, Sibu, Sarikei, Kanowit, Kapit, Marudi, Miri, Lawas and Limbang (for locations and sample numbers see Figure 1a). Hospitalization with microscopy-positive malaria was the only criteria used for blood spot collection. The samples from Kapit exclude those previously reported [2]. Parasite identification by routine diagnostic microscopy recorded 428 (44.6%) P. vivax, 312 (32.5%) P. malariae, 216 (22.5) P. falciparum, 2 (0·2%) P. ovale and 2 (0.2%) mixed infections (Table 1). The patients were predominantly male (75·8%) with a mean age of 36·9 (range 0·2-91) years.
figure 1. Distribution and prevalence of human knowlesi malaria in Malaysia.


The inset maps of Southeast Asia show the position of Sarawak (1a), Sabah and Pahang (1b). Figure 1a shows the proportion of the different species of malaria detected at each of 12 hospitals in Sarawak from March 2001 – March 2006. The total number of samples for each location is given as (n). The administrative districts in Sabah are outlined in black (1b). Samples were not obtained from the un-shaded districts in Sabah.
Table 1.
Comparison of results for detection of Plasmodium species by PCR and microscopy.
The demographic breakdown of PCR-confirmed cases is as follows: 1.6% (n = 4) of the P. knowlesi single infection group were children (<15years) compared with 13.7% (n = 60) and 9.17% (n = 20) in the P. vivax and P. falciparum groups respectively. There were fewer men in the P. knowlesi group (69.9% male, n = 170) compared with the P. vivax (74.54% male, n = 328) and P. falciparum (82.6% male, n = 181) groups. The demographic make-up of the knowlesi group reflects that previously reported [2].
| Microscopy results | PCR Total | |||||
|---|---|---|---|---|---|---|
| PCR results | Pf | Pv | Pm | Po | Pf+Pv | |
| Pf | 167 | 18 | 33 | 0 | 0 | 219 |
| Pv | 23 | 372 | 43 | 1 | 1 | 440 |
| Pm | 0 | 0 | 1 | 0 | 0 | 1 |
| Po | 0 | 2 | 2 | 0 | 0 | 4 |
| Pk | 11 | 16 | 216 | 0 | 0 | 243 |
| Pf+Pv | 11 | 9 | 4 | 0 | 1 | 25 |
| Pf+Pm | 0 | 0 | 1 | 0 | 0 | 1 |
| Pf+Po | 1 | 0 | 0 | 0 | 0 | 1 |
| Pf+Pk | 1 | 0 | 2 | 0 | 0 | 3 |
| Pv+Pk | 2 | 8 | 9 | 0 | 0 | 19 |
| Pv+Pm | 0 | 2 | 0 | 0 | 0 | 2 |
| Pv+Po | 0 | 1 | 0 | 0 | 0 | 1 |
| Po+Pk | 0 | 0 | 1 | 0 | 0 | 1 |
| Microscopy Total | 216 | 428 | 312 | 2 | 2 | 960 |
In Malaysia there is a requirement for malaria-positive blood films taken in district hospitals and health clinics to be sent to the respective state Vector-Borne Diseases Control Programme (VBDCP) headquarters for re-examination and species confirmation by microscopy. These slides are stored for seven years. In response to our request for microscopy-confirmed archival P. malariae blood films, a total of 49 stained blood smears identified as P. malariae were obtained from 15 administrative districts in Sabah, Malaysian Borneo. Of these, 13 were from 2003, 10 from 2004 and 26 from 2005. Five archival blood films identified as P. malariae by microscopy were obtained from 4 districts in Pahang, Peninsular Malaysia (3 in 2004 and 2 in 2005). In addition, blood films taken from four fatal malaria cases and reported as showing P. malariae by microscopy were obtained from the Sarawak Health Department. DNA was extracted from all of the archival blood films received for confirmation of Plasmodium species by nested-PCR.
DNA extraction and nested-PCR examination of samples
DNA was extracted from blood spots on filter papers and whole blood as described previously [2, 3]. At least one negative control blood spot from an uninfected individual was included for every 11 patient blood spots. Positive controls for P. falciparum, P. vivax, P. malariae, P. ovale and P. knowlesi were included in all nested-PCR speciation assays and measures to prevent cross-contamination were as described previously [2]. For blood films on microscope slides, DNA was extracted by moistening the blood film with one drop of Tris-EDTA (TE) buffer, pH 8, and scraping the film of blood into a microcentrifuge tube containing 100 μl TE buffer. Ten μl of 10 mg/ml Proteinase K (Amresco, USA) and 100 μl of lysis buffer (5 mM EDTA, 0·5% Sodium dodecyl sulfate, 200 mM NaCl and 100 mM Tris-Cl, pH 8) was added to the tube and incubated in a thermomixer at 56 °C with shaking at 900 rpm for 10 min. An equal volume of phenol-chloroform isoamyl alcohol (Amresco, USA) was then added to each sample, followed by vigorous mixing for 15 sec and centrifugation for 2 min at 14,000 rpm. After transferring the aqueous phase into a new microcentrifuge tube, the organic phase was re-extracted by adding 100 μl TE buffer, with further mixing and centrifugation. The aqueous phase from the second extraction was pooled with that from the first and the DNA was ethanol precipitated as previously described [4]. The air-dried DNA pellet was dissolved in 50 μl TE buffer. DNA samples were analysed using both genus and species-specific nested-PCR assays capable of detecting all Plasmodium species and then specifically detecting P. falciparum, P. vivax, P. malariae, P. ovale and P. knowlesi as described previously [2].
Fatal cases
Permission to review the case notes from four fatal cases was obtained from the Sarawak State Health Department.
RESULTS
Incidence and distribution of human P. knowlesi infections
PCR analysis of 960 samples from malaria patients from across Sarawak revealed that 266 (27.7%) were infected with P. knowlesi (Table 1). These had been misdiagnosed by microscopy, with 228 (85·7%) being reported as ‘P. malariae’ but only four (0.4%) containing P. malariae DNA by nested-PCR (Table 1). Overall 53 (5·5%) of the patients had mixed species infections by PCR, including 23 with P. knowlesi. P. knowlesi infections were detected in samples from 11 of the 12 hospitals in the study, with the proportion of cases ranging between 0 and 77% and a relative preponderance in the central and northern region of Sarawak (Figure 1a). For hospitals where more than 50 malaria blood samples were obtained, the highest proportion of P. knowlesi infections were observed at Kapit (41%), Sarikei (59%) and Kanowit (71%).
Official figures for microscopy-confirmed cases of malaria at government hospital and health clinics in Sarawak from 2000 to March 2006 reveal that 1,731 (14·3%) of the total 12,082 malaria cases were reported as ‘P. malariae’ by routine microscopy. In our 960 samples from 12 hospitals and the 208 samples from our previous study in Kapit [2], P. malariae DNA was detected in only 4 samples by PCR, only two of which were P. malariae by microscopy while 266 (27.7%) had P. knowlesi (Table 1). The hospital records for the four patients with PCR-confirmed P. malariae show that all had recently returned to Sarawak after working overseas; three from Papua New Guinea (one returned 5, another 13 and the other 23 days prior to hospitalization) and one from Irian Jaya (returned 5 days prior to hospital admission). Furthermore, of the 312 present cases reported as P. malariae by microscopy, 228 (73.1%) were single or mixed P. knowlesi infections by PCR. Of the remainder, 82 (26.3%) were either single or mixed infections of P. falciparum, P. vivax or P. ovale by PCR (Table 1). Only two of the four PCR-positive P. malariae samples were correctly identified as P. malariae by microscopy, and one of these was a mixed infection with P. falciparum (Table 1). These results suggest that there are no indigenous cases of P. malariae in Sarawak and that parasites identified as P. malariae by microscopy are mainly P. knowlesi or other non-P. malariae species.
To test for the presence of P. knowlesi in other Malaysian states, DNA was extracted from blood films recorded as P. malariae by routine microscopy. P. knowlesi DNA was detected in 41 (83·6%) of the blood films from Sabah (32 single P. knowlesi infections and 7 mixed with P. vivax , 1 mixed with P. falciparum and 1 with P. malariae). P. knowlesi infections were detected in samples from 14 of the administrative districts of Sabah (Figure 1b). P. malariae DNA was detected in only 8 slides (6 as single infections and 2 mixed with P. vivax or P. knowlesi). Six of these were from the Kudat District in northern Sabah (five were children aged 7-15 years from two particular villages who had not travelled outside Sabah). All five ‘P. malariae’ blood films obtained from four districts in Pahang, Peninsular Malaysia contained only P. knowlesi DNA.
Fatal cases
The four fatal cases were identified through malaria notification procedures and investigation by the Sarawak State Department of Health. All available documentation was reviewed retrospectively to establish the diagnosis of malaria, comorbid conditions, clinical course and cause of death. The results of laboratory tests are shown in Table 2. Due to restricted local availability of pathology services (including microbiological testing) and/or socio-religious considerations, blood cultures were not performed and post mortem examinations were not conducted. All patients were healthy prior to presentation. P. knowlesi was detected by PCR as the sole Plasmodium species present in the blood films used to make the initial diagnosis of malaria. High parasitemia was a common feature as illustrated in Figure 2. None of the patients was enrolled in a research study and the primary cause of death was reported as malaria in only one of these cases.
Table 2.
Admission details of the four fatal cases of knowlesi malaria.
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Time to death (hours) | 35 | 141 | 5 | 316 |
| Blood pressure (mmHg) | 120/90 | 124/66 | 81/51 | 132/67 |
| Pulse ( /min ) | 88 | 132 | 84 | 84 |
| Axillary temperature (°C) | 36.8 | 38 | 37 | 36 |
| Hemoglobin (g/dL) [Females 12·0 – 16·0] [Males 13·5 – 17·5] |
10·6 | 15·2 | 15·4 | 11·9 |
| White cell count (/μL) [4,500 – 11,000] |
16,700 | 6,600 | 13,400 | 11,400 |
| Platelets (/ μL) [150,000 – 450,000] |
22,000 | 25,000 | 24,000 | 24,000 |
| Serum creatinine (μmol/L) [63 – 133] |
500 | Not available |
Not available |
557 |
| Serum urea (mmol/L) [1·0 – 8·3] |
60·9 |
26·6 |
19 |
25·6 |
| Total serum bilirubin (μmol/L)[<17] |
79 | 300 | N/A | 490 |
| Conjugated serum bilirubin (μmol/L) [<1·7] |
59 | 187 | N/A | 350 |
| Serum aspartate aminotransferase (U/L) [<37] |
122 | 163 | N/A | 87 |
| Serum alanine aminotransferase (U/L) [<40] |
104 | 77 | N/A | 82 |
| Serum albumin (g/L) [35-60] |
24 | 15 | N/A | 28 |
| Serum alkaline phosphatase ( U/L) [39 – 117] |
160 | 77 | N/A | 151 |
Data are from tests performed within 24 hours of first presentation in each case. Laboratory reference ranges are given in square brackets.
figure 2.
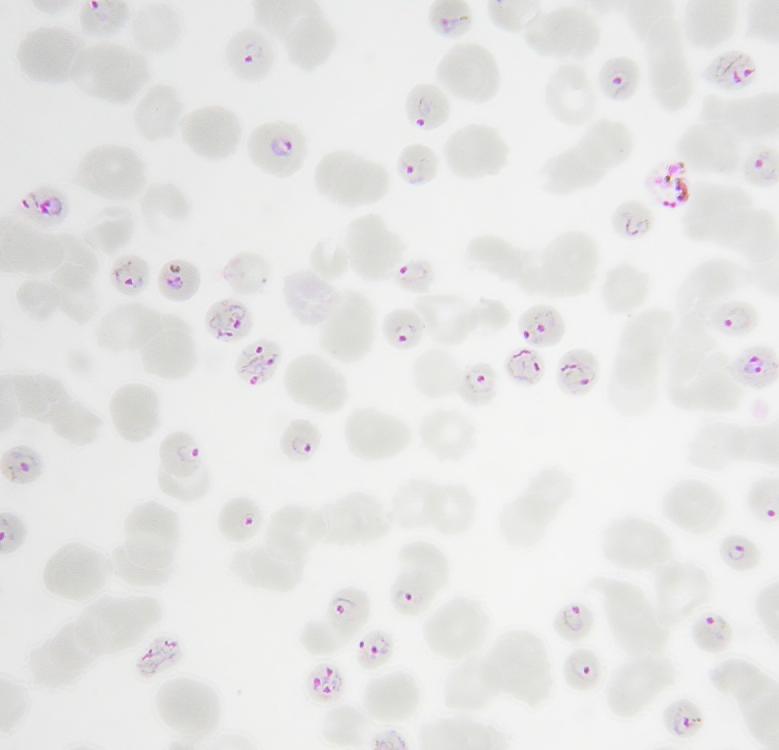

Admission thin blood film microscopy from two PCR-confirmed P. knowlesi single infections in humans. Parts a) and b) demonstrate hyperparasitemia and a parasite morphology most often confused with P. malariae.
Case 1, a 66-year-old woman presented to her local health post on Day 1 with a 3-day history of epigastric pain, diarrhea, vomiting, fever and rigors. Malaria was not considered as a possible diagnosis. She was commenced on intravenous rehydration and oral metronidazole but became hypotensive overnight and was transferred to the district hospital on Day 2 where the main physical examination finding was epigastric tenderness. A diagnosis of acute gastroenteritis was made and intravenous cimetidine was commenced. She remained anuric and responded poorly to fluid challenges and intravenous furosemide. On the morning of Day 3, she was noted to be jaundiced and a blood film for malaria parasites was ordered. She continued to have abdominal pain and diarrhea. She was started on broad-spectrum intravenous antibiotic therapy to cover intra-abdominal sepsis and, since mid-stream urine microscopy revealed large numbers of pus cells, urosepsis. The microscopist reported that the thick blood film contained 204,800 P. malariae parasites per μl whole blood (a retrospective re-count of this slide revealed 764,720 parasites/μl). Chloroquine and sulfadoxine/pyrimethamine were added to her treatment but she became hypotensive and unrousable. She was intubated and inotropic support was commenced. Her blood pressure and conscious state did not improve and she died early on Day 4. The cause of death was reported as complicated malaria.
Case 2, a 69-year-old man, presented to a district hospital on Day 1 with a 7-day history of fever and rigors, 3 days of diarrhea, and severe abdominal pain over the previous 24 hours. He also complained of weakness, dyspnea and cough. He was alert and orientated, but was dehydrated, jaundiced and tachypneic. There were bi-basal crackles on chest auscultation. Abdominal examination revealed generalised tenderness and guarding. His blood film was reported as P. malariae positive (75,000 parasites/μl). Chest X-ray showed free air under the diaphragm but no evidence of pneumonia. He was diagnosed with a perforated viscus and severe malaria. He was transferred to a central hospital. Intravenous rehydration, antibiotics and quinine were started and, at emergency laparotomy, a perforated gastric ulcer was repaired. He required ventilation, inotropic support and blood transfusion. Hemodialysis was started on Day 3 because of renal failure. He became aparasitaemic but developed refractory hypotension on Day 5 and died the following morning.
Case 3, a 39-year-old man, was admitted to a district hospital with a 3-day history of headache, fever and chills in association with vomiting, abdominal pain and syncope. On examination, he was jaundiced, hypotensive and had generalised abdominal tenderness and a palpable spleen. Malaria was suspected and a blood film was reported as showing 112,000 P. malariae parasites/μl. A diagnosis of severe malaria was made. He responded initially to rapid intravenous rehydration, oxygen, chloroquine and sulfadoxine/pyrimethamine but remained anuric. Two hours after admission, his blood pressure became unrecordable. Further fluid resuscitation including plasma expanders was administered and inotropic support was started. His blood pressure responded but, two hours later, became unrecordable again and he developed a metabolic acidosis. He died of cardiorespiratory failure.
Case 4, a 40-year-old man, presented to a district hospital on Day 1 with a 7-day history of fever, chills and rigors in association with abdominal pain, headache and vomiting. He was dehydrated, jaundiced and drowsy. His chest was clear on auscultation but he had hepatomegaly on abdominal palpation. Malaria was suspected and a thick film was reported as showing P. malariae ‘++++’ (this grading indicates >10 parasites in every high-power microscopy field) A diagnosis of severe malaria with hyperparasitemia and acute renal failure was made. He was rehydrated and given oral chloroquine, sulfadoxine, pyrimethamine and primaquine, and intravenous quinine was started. He was transferred to a central hospital on Day 2 and commenced intravenous antibiotic therapy. On Day 4, he developed acute respiratory distress syndrome, was intubated and ventilated, and hemodialysis was started. He experienced frequent hypoglycemic episodes. He remained ventilator-dependent and a tracheostomy was performed on Day 7. He died on Day 13 after developing cardiorespiratory failure complicated by a tracheostomy site hemorrhage.
DISCUSSION
The entry of new viral pathogens from non-human reservoirs to the human population, particularly in China and South-east Asia, has had a significant impact on disease surveillance, public health awareness and the understanding of events leading to pathogen host switch [5-9]. Previously a focus of entry of P. knowlesi in the human population in the Kapit Division of Sarawak was described [2]. Here we report that human knowlesi malaria is not restricted to this relatively small geographic area. It is encountered throughout Malaysian Borneo (in Sarawak and Sabah) and in Peninsular Malaysia (Pahang) but is being incorrectly diagnosed as P. malariae. The distribution may even be more widespread as a human P. knowlesi infections have been found in Thailand [10] and in China in a logging-camp worker who had recently returned from Myanmar [11]. Our finding that P. knowlesi and not P. malariae is a significant cause of potentially severe malaria in Malaysia has important implications for clinical management and control strategies in the local setting, as well as for physicians attending patients who have visited areas in South-east Asia within the range of long and pigtailed macaques, the natural hosts for P. knowlesi [1].
P. knowlesi is unusual among malaria parasites of primates in that it exhibits a degree of relaxed host specificity being permissive in humans under natural and experimental conditions [2, 12], rhesus monkeys (M. mulatta) [12, 13] and the olive baboon [14]. However, P. knowlesi transmission is vector-restricted to the Anophleles leucosphyrus group [15] of which An. latens has been incriminated as the vector of knowlesi malaria in the Kapit Division [16]. These mosquitoes are equally attracted to monkeys and humans, feeding predominantly in the forest and forest-fringe after dusk [16]. Mosquito transmission of P. knowlesi from human-to-human has been successful under experimental conditions [12] and knowlesi patients in Sarawak carry gametocytes. Vector restriction to a mosquito preferring the forest fringe habitat is probably the factor that prevents full-blown emergence and spread of this potentially virulent malaria parasite in humans.
All four PCR-confirmed fatal cases of P. knowlesi were hyperparasitemic and all presented with severe abdominal pain and a history of fever and chills. The marked hepatorenal dysfunction in these fatal cases is a feature of severe falciparum malaria, as is the refractory hypotension seen in Case 3 which appears similar to ‘algid’ malaria [17] [19]. The recurrent hypoglycemia experienced by Case 4 is likely to have resulted from quinine therapy [18]. Although the clinical and laboratory data for these four cases is unavoidably incomplete, the large parasite burden and the compounding effect of a 24 hour asexual replication cycle strongly suggest that P. knowlesi is a potentially life-threatening pathogen. Post-mortem material could not be obtained which limits assessment of the underlying pathophysiological mechanisms.
The trophozoite, schizont and gametocyte stages of P. knowlesi are morphologically indistinguishable from P. malariae by microscopy and uncomplicated cases of P. knowlesi respond to chloroquine, but that is where the similarity ends [2]. P. malariae is associated with a relatively low parasitaemia and a benign clinical course [17]. The 24-hour asexual lifecycle of P. knowlesi is the shortest of all of the human and non- human primate malarias [13, 20]. Daily schizont rupture, with attendant fever spikes and a potentially rapid increase in parasitaemia, is unprecedented in human malaria and even a short delay in accurate diagnosis, treatment and adjunctive management could increase the risk of complications. In addition, the misdiagnosis of knowlesi malaria in the ill patient and the assumption that ‘malariae’ malaria is benign may cause the clinician to look for alternative causes of emerging vital organ dysfunction. As well as delayed and inappropriate management, there are epidemiological implications, specifically those relating to underestimation of the incidence of severe knowlesi malaria.
Because of this situation and the overwhelmingly low incidence of P. malariae compared to P. knowlesi in Malaysia, we strongly recommend that symptomatic malaria with hyperparasitemia and parasite morphology resembling that of P. malariae be diagnosed as P. knowlesi in Malaysia and probably in other areas of South-east Asia inhabited by the non-human primate hosts. Detailed prospective studies to define the spectrum of disease in P. knowlesi malaria are underway which should help identify clinical and laboratory markers of disease severity.
The present results demonstrate that P. knowlesi malaria in humans is not as rare as previously thought, is widely distributed in Malaysia and can be fatal. In addition, single infections have been recently reported from Thailand [10] and Myanmar [11] and it is likely that further studies in these and other South-east Asian countries will uncover a more extensive distribution. On the strength of the evidence presented here, directives on the diagnosis and treatment of malaria acquired in regions of Southeast Asia that are within the range of long and pigtailed macaques should include up-to-date guidelines for the diagnosis, treatment and management of knowlesi malaria.
ACKNOWLEDGEMENTS
This study was funded by The Wellcome Trust, United Kingdom and University Malaysia Sarawak. Authors Janet Cox-Singh, No conflict; Timothy M. E. Davis, No conflict; Kim-Sung Lee, No conflict; Sunita S. G. Shamsul, No conflict; Asmad Matusop, No conflict; Shanmuga Ratnam, No conflict; Hasan A. Rahman, No conflict; David J Conway, No conflict; Balbir Singh, No conflict. We thank the Directors and staff of the hospitals in Sarawak at Bau, Lundu, Betong, Serian, Sibu, Sarikei, Kanowit, Kapit, Marudi, Miri, Lawas and Limbang for their assistance throughout the duration of the project; the staff of the Vector-Borne Diseases Control Programmes in Sarawak, Sabah and Pahang for collecting and tracing blood samples and to all those who provided their blood samples.
This study was funded by The Wellcome Trust, United Kingdom and University Malaysia Sarawak.
Footnotes
No financial disclosure or conflict of interest to report.
Financial disclosures: None reported.
Contributor Information
Janet Cox-Singh, Malaria Research Centre, Faculty of Medicine and Health Sciences, University Malaysia Sarawak, Kuching, Sarawak, Malaysian Borneo.
Timothy M. E. Davis, University of Western Australia, School of Medicine and Pharmacology, Fremantle Hospital, Fremantle, Western Australia, Australia.
Kim-Sung Lee, Malaria Research Centre, Faculty of Medicine and Health Sciences, University Malaysia Sarawak, Kuching, Sarawak, Malaysian Borneo.
Sunita S. G. Shamsul, Malaria Research Centre, Faculty of Medicine and Health Sciences, University Malaysia Sarawak, Kuching, Sarawak, Malaysian Borneo.
Asmad Matusop, Sarawak Health Department, Kuching, Sarawak, Malaysian Borneo.
Shanmuga Ratnam, Disease Control Unit, Sabah Health Department, Kota Kinabalu, Sabah, Malaysian Borneo.
Hasan A. Rahman, Pahang State Health Department, Kuantan, Pahang, Malaysia.
David J Conway, London School of Hygiene & Tropical Medicine, London, United Kingdom.
Balbir Singh, Malaria Research Centre, Faculty of Medicine and Health Sciences, University Malaysia Sarawak, Kuching, Sarawak, Malaysian Borneo.
REFERENCES
- 1.Garnham PCC. Malaria Parasites and other Haemosporidia. Oxford: Blackwell Scientific Publications; 1966. [Google Scholar]
- 2.Singh B, Kim Sung L, Matusop A, et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363(9414):1017–24. doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- 3.Cox-Singh J, Mahayet S, Abdullah MS, Singh B. Increased sensitivity of malaria detection by nested polymerase chain reaction using simple sampling and DNA extraction. Int J Parasitol. 1997;27(12):1575–7. doi: 10.1016/s0020-7519(97)00147-1. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel FM, Brent R, Kingston RE, et al. Current Protocols in Molecular Biology. John Wiley & Sons Inc.; 2003. [Google Scholar]
- 5.Bellini WJ, Harcourt BH, Bowden N, Rota PA. Nipah virus: an emergent paramyxovirus causing severe encephalitis in humans. J Neurovirol. 2005;11(5):481–7. doi: 10.1080/13550280500187435. [DOI] [PubMed] [Google Scholar]
- 6.Becker NG, Glass K, Li Z, Aldis GK. Controlling emerging infectious diseases like SARS. Math Biosci. 2005;193(2):205–21. doi: 10.1016/j.mbs.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Bauch CT, Lloyd-Smith JO, Coffee MP, Galvani AP. Dynamically modeling SARS and other newly emerging respiratory illnesses: past, present, and future. Epidemiology. 2005;16(6):791–801. doi: 10.1097/01.ede.0000181633.80269.4c. [DOI] [PubMed] [Google Scholar]
- 8.Fauci AS, Touchette NA, Folkers GK. Emerging infectious diseases: a 10-year perspective from the National Institute of Allergy and Infectious Diseases. Emerg Infect Dis. 2005;11(4):519–25. doi: 10.3201/eid1104.041167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longini IM, Jr., Nizam A, Xu S, et al. Containing pandemic influenza at the source. Science. 2005;309(5737):1083–7. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 10.Jongwutiwes S, Putaporntip C, Iwasaki T, Sata T, Kanbara H. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerg Infect Dis. 2004;10(12):2211–3. doi: 10.3201/eid1012.040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu HM, Li J, Zheng H. [Human natural infection of Plasmodium knowlesi] Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2006;24(1):70–1. [PubMed] [Google Scholar]
- 12.Chin W, Contacos PG, Collins WE, Jeter MH, Alpert E. Experimental mosquito-transmission of Plasmodium knowlesi to man and monkey. Am J Trop Med Hyg. 1968;17(3):355–8. doi: 10.4269/ajtmh.1968.17.355. [DOI] [PubMed] [Google Scholar]
- 13.Chin W, Contacos PG, Coatney GR, Kimball HR. A Naturally Acquired Quotidian-Type Malaria in Man Transferable to Monkeys. Science. 1965;149:865. doi: 10.1126/science.149.3686.865. [DOI] [PubMed] [Google Scholar]
- 14.Ozwara H, Langermans JA, Maamun J, et al. Experimental infection of the olive baboon (Paplio anubis) with Plasmodium knowlesi: severe disease accompanied by cerebral involvement. Am J Trop Med Hyg. 2003;69(2):188–94. [PubMed] [Google Scholar]
- 15.Collins WE, Contacos PG, Guinn EG. Studies on the transmission of simian malarias. II. Transmission of the H strain of Plasmodium knowlesi by Anopheles balabacensis balabacensis. J Parasitol. 1967;53(4):841–4. [PubMed] [Google Scholar]
- 16.Vythilingam I, Tan CH, Asmad M, Chan ST, Lee KS, Singh B. Natural transmission of Plasmodium knowlesi to humans by Anopheles latens in Sarawak, Malaysia. Trans R Soc Trop Med Hyg. 2006;100:1087–8. doi: 10.1016/j.trstmh.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 17.White NJ. Malaria. In: Cook CG, editor. Manson's Tropical Diseases. Twentieth ed. London: W.B. Saunders Company Ltd; 1996. pp. 1087–164. [Google Scholar]
- 18.Davis TM. Antimalarial drugs and glucose metabolism. Br J Clin Pharmacol. 1997;44(1):1–7. doi: 10.1046/j.1365-2125.1997.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Severe falciparum malaria World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 20.Knowles R, Das Gupta BM. A study of monkey-malaria and it's experimental transmission to man. The Indian Medical Gazette. 1932 Jun;:301–21. [PMC free article] [PubMed] [Google Scholar]


