Abstract
The yeast Ca2+ adenosine triphosphatase Pmr1, located in medial-Golgi, has been implicated in intracellular transport of Ca2+ and Mn2+ ions. We show here that addition of Mn2+ greatly alleviates defects of pmr1 mutants in N-linked and O-linked protein glycosylation. In contrast, accurate sorting of carboxypeptidase Y (CpY) to the vacuole requires a sufficient supply of intralumenal Ca2+. Most remarkably, pmr1 mutants are also unable to degrade CpY*, a misfolded soluble endoplasmic reticulum protein, and display phenotypes similar to mutants defective in the stress response to malfolded endoplasmic reticulum proteins. Growth inhibition of pmr1 mutants on Ca2+-deficient media is overcome by expression of other Ca2+ pumps, including a SERCA-type Ca2+ adenosine triphosphatase from rabbit, or by Vps10, a sorting receptor guiding non-native luminal proteins to the vacuole. Our analysis corroborates the dual function of Pmr1 in Ca2+ and Mn2+ transport and establishes a novel role of this secretory pathway pump in endoplasmic reticulum-associated processes.
INTRODUCTION
Compared with the knowledge on mechanisms that distribute distinctive sets of proteins into specific organelles along the eukaryotic secretory pathway, relatively little is known about how the ionic milieu within these organelles contribute to their function. This scarcity of information stems, in part, from the lack of convenient tools with which to individually alter the intracellular distribution of specific ions. A23187, an ionophore widely used to dissipate Ca2+ gradients in vivo, binds Mn2+, Zn2+, and other heavy metals with 100- to 1000-fold higher affinity than Ca2+ and fails to sufficiently discriminate between Mg2+ and Ca2+ (Pfeiffer and Lardy, 1976). This note of caution is underscored by a recent study in Saccharomyces cerevisiae demonstrating that Mn2+ can effectively replace Ca2+ ions to promote growth of yeast cells. In fact, Mn2+ ions may be the physiological mediator of processes previously thought to uniquely require Ca2+ (Loukin and Kung, 1995).
Despite this ambiguity, the Ca2+ concentration in the endoplasmic reticulum (ER) has been implicated in retention of resident luminal proteins (Booth and Koch, 1989), in export of secretory proteins (for review, see Sambrook, 1990), in protein folding and degradation (reviewed in Lodish and Kong, 1990; Wileman et al., 1991; Gething and Sambrook, 1992), and in the association of the ER chaperone BiP with misfolded proteins (Suzuki et al., 1991). Recent studies have demonstrated that some misfolded luminal proteins are exported from the ER into the cytosol for ubiquitination and subsequent degradation by the proteasome (for review, see Brodsky and McCracken, 1997; Sommer and Wolf, 1997). Yeast CpY*, a mutant form of carboxypeptidase Y (CpY), is subjected to this degradative route, and the ubiquitin-conjugating enzymes (Ubc6, Ubc7) have been identified (Hiller et al., 1996). In contrast, other non-native luminal proteins appear to exit the yeast ER via the secretory pathway and interact with Vps10, a transmembrane receptor normally engaged in sorting of soluble hydrolases, including CpY, from the Golgi to the vacuole (Marcusson et al., 1994; Cooper and Stevens, 1996). Apparently, Vps10 exerts a dual role in intracellular targeting, i.e., it participates in the sorting of specific vacuolar enzymes and in a more general salvage pathway guiding non-native luminal proteins to the vacuole (Hong et al., 1996). It is not known whether Ca2+ ions in the ER affect export of substrates for degradation by the proteasome. Similarly, it is unclear how intralumenal Ca2+ influences the Vps10-mediated salvage pathway.
Accumulation of unfolded proteins in the ER, experimentally induced by the addition of tunicamycin to block glycosylation or by treatment with reducing agents such as DTT, activates a universal signal transduction cascade that allows eukaryotic cells to alter the conditions in the ER (Lee, 1987; Kozutsumi et al., 1988). This unfolded protein response (UPR), best understood in the yeast Saccharomyes cerevisiae (for review, see Shamu et al., 1994; Nunnari and Walter, 1996; Shamu, 1997), coordinates the transcription of genes encoding ER-resident chaperones with the synthesis of glycerophospholipids destined for the ER membrane (Cox et al., 1997). Inactivation of UPR components, like Ire1 (a transmembrane kinase of the ER and/or inner nuclear membrane) or Hac1 (a transcription factor binding to UPR-regulated promoters), leads to a complete block of the UPR. Such yeast mutants are sensitive to DTT and tunicamycin and fail to grow on media lacking inositol, a precursor in the synthesis of phosphatidylinositol.
Within the Golgi complex, high Ca2+ concentrations are found in all cisternae along the cis–trans axis (Pezzati et al., 1997). The available evidence for a role of Ca2+ in important Golgi functions, including luminal and membrane protein traffic (Carnell and Moore, 1994), cargo condensation, and precursor processing (Chanat and Huttner, 1991; Fuller et al., 1989; Oda, 1992), suggests that Golgi Ca2+ transport and storage are specifically regulated, independently from the control of ER Ca2+. As inferred from the in vitro requirement for Mn2+ by numerous enzymes involved in oligosaccharide addition in animal cells and yeast, the Golgi complex hosts reactions that depend on Mn2+ (Sharma et al., 1974; Nakajima and Ballou, 1975; Parodi, 1979; Sugiura et al., 1982; Elhammer and Kornfeld, 1986; Haselbeck and Schekman, 1986). Depletion of manganese in vivo inhibits O-linked and N-linked protein glycosylation in mammalian cells (Kaufman et al., 1994). Similar results were obtained with thapsigargin, a potent inhibitor of the SERCA-type Ca2+-adenosine triphosphatases present in sarco/endoplasmic reticulum membranes, suggesting a role of these ion pumps in maintaining intralumenal Ca2+ and Mn2+ concentrations (Kaufman et al., 1994).
In yeast, the only Ca2+ pump known to reside in the early secretory pathway (ER, Golgi) is Pmr1 (Rudolph et al., 1989; Antebi and Fink, 1992; Cunningham and Fink, 1994, 1996; Sorin et al., 1997). In subcellular fractionation and immunofluorescence microscopy, Pmr1 almost entirely coincides (> 98%) with the steady-state pool of Emp47, a lectine-like transmembrane protein of the medial-Golgi, which recycles, in contrast to Pmr1, between the ER and the Golgi (Schröder et al., 1995). These findings, consistent with previous work demonstrating a Golgi-like distribution for Pmr1 (Antebi and Fink, 1992), imply that only minute amounts of Pmr1 reside in the ER membrane. Since no other candidate gene that could encode an ATP-driven Ca2+ pump of the ER, is present in the genome of Saccharomyces cerevisiae, the yeast ER appears to lack a spezialized Ca2+ pump (Sorin et al., 1997). This raises interesting questions on how yeast cells achieve the appropriate distribution of Ca2+ and Mn2+ ions within organelles of the early secretory pathway, which is thought to critically depend on the adequate presence of both ions.
Inactivation of Pmr1 leads to conspicuous defects, compatible with the loss of an ion pump shared by Ca2+ and Mn2+. pmr1 mutants display a subtle CpY sorting defect and are blocked in pheromone maturation; both defects are fully reversed by the addition of extracellular Ca2+ (Antebi and Fink, 1992). However, pmr1 mutants also accumulate Mn2+ and are hypersensitive to this metal cation (Lapinskas et al., 1995). Finally, pmr1 mutants are sensitive to EGTA, an effective chelator of Ca2+, Mn2+, and other divalent metal ions (Rudolph et al., 1989). In this study, we provide evidence for a dual role of Pmr1 in the transport of Ca2+ and Mn2+ into the secretory pathway and show that both ions, albeit interchangeable to support growth, exert distinct functions. Furthermore, we demonstrate that Pmr1-mediated ion transport, despite the prominent location of this ion pump in the medial-Golgi, is an important determinant of processes hosted in the lumen of the ER.
MATERIALS AND METHODS
Yeast Strains and Growth Conditions
Yeast strains used in this study are shown in Table 1. Standard yeast culture medium was prepared as described (Sherman et al., 1986). Media containing the ion chelator bis-(O-aminophenoxy)-ethane-N,N,N′,N′,-tetraacetic acid (BAPTA) used in the analysis of CpY sorting were derived from standard defined synthetic medium SD (Wickersham, 1951.) without the salts of calcium, manganese, zinc, copper, and iron; Ca2+ pantothenate was replaced by the sodium salt. Ammonium sulfate was replaced by ammonium chloride (37 mM), potassium sulfate was replaced by the chloride salt, phosphate was reduced to 200 μM, and the pH was adjusted to pH 6.5 with 50 mM potassium 2-[N-morpholino]ethane sulfonic acid (MES). All components were used at the highest purity available (Ultrapure, Merck, Darmstadt, Germany). Growth medium contained ammonium sulfate (200 μM), which was omitted in the medium (1 M sorbitol; 0.1% glucose) used for spheroblasting and labeling. Before the addition of BAPTA, both media were analyzed for their metal content (calcium, manganese, iron, copper, and zinc) by inductively coupled plasma spectroscopy. From the total metal concentrations, the necessary additions of calcium, manganese, and zinc (all as chlorides) and K-BAPTA were calculated (MAXChelator by Chris Patton, Stanford University, Stanford, CA) to keep the concentrations of free zinc (2.5 × 10−10 M), free iron (6.3 × 10−21 M), and free BAPTA (1.8 × 10−3 M) constant, but achieve desired concentrations for calcium and manganese. Binding constants used in the calculations are described by Loukin and Kung (1995). Manganese and calcium concentrations in the media used are given in the legend to Figure 1.
Table 1.
Yeast strains
| Strain | Genotype | Source |
|---|---|---|
| YGD57 | YR98 pmr1-Δ1::LEU2/pKC46 [2μ, PMC1] | This study |
| YR98 | MATα ade2 his3-Δ200 leu2-3,112 lys2-Δ201 ura3-52 (AA255) | Rudolph et al., 1989 |
| YR122 | YR98 pmr1-Δ1::LEU2 (AA274) | Rudolph et al., 1989 |
| YR123 | YR98 pmr1-Δ2::HIS3 (AA298) | Fink lab |
| YR437 | W303-1B pmr1::HIS3 pmc1::TRP1 cnb1::LEU2 (K616) | K. Cunningham |
| YR439 | YR98 pmr1-Δ1::LEU2/YEp24 | This study |
| YR441 | YR98 pmr1-Δ1::LEU2/br327 [2μ PMR1] | This study |
| YR469 | W303-1B pmr1::HIS3 pmc1::TRP1 cnb1::LEU2/YEp24 | This study |
| YR472 | W303-1B pmr1::HIS3 pmc1::TRP1 cnb1::LEU2/br327 [2μ PMR1] | This study |
| YR477 | W303-1B pmr1::HIS3 pmc1::TRP1 cnb1::LEU2/br349 [2μ VPS10] | This study |
| YR480 | YR98 pmr1-Δ1::LEU2/br356 [2μ VPS10] | This study |
| YR537 | YR98 pmr1-Δ1::LEU2 vps10::HIS3 | This study |
| YR547 | YR98 pmr1-Δ1::LEU2 vps10::HIS3/YEp24 | This study |
| YR549 | YR98 pmr1-Δ1::LEU2 vps10::HIS3/br358 [SUC2::VPS10-ΔN] | This study |
| YR550 | YR98 pmr1-Δ1::LEU2 vps10::HIS3/br375 [2μ VPS10-1385] | This study |
| YR551 | YR98 pmr1-Δ1::LEU2 vps10::HIS3/br356 [2μ VPS10] | This study |
| YR657 | YR98 pmr1-Δ1::LEU2/pKC11 [2μ, PMR1] | This study |
| YR663 | YR98 pmr1-Δ1::LEU2/br434 [CEN PMA1::SERCA1A::ADC1] | This study |
| YR664 | YR98 pmr1-Δ1::LEU2/br435 [2μ PMA1::SERCA1A::ADC1] | This study |
| YRP023 | W303 prc1-1 pmr1::HIS3 | This study |
| W303-1B | MATα ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 | K. Cunningham |
| W303-1C | W303 prc1-1 | Knop et al., 1996 |
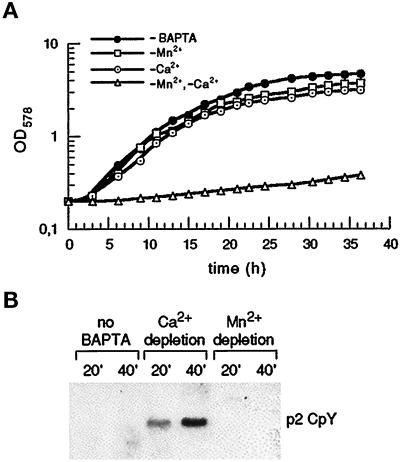
Figure 1.
Growth and vacuolar sorting of S. cerevisiae in ion-depleted media. (A) Growth in Ca2+- or Mn2+-depleted media. Wild-type strain YR98 was inoculated into media containing BAPTA to buffer the free ion concentrations of Ca2+ and Mn2+ as described. Media: −Mn2+ medium (1.4 × 10−6 M Ca2+, 1.6 × 10−13 M Mn2+), −Ca2+ medium (8.4 × 10−10 M Ca2+, 2.0 × 10−8 M Mn2+), −Mn2+, −Ca2+ medium (8.4 × 10−10 M Ca2+, 1.8 × 10−11 M Mn2+). For the BAPTA-free control medium (−BAPTA), total ion concentrations (5.7 × 10−6 M Ca2+; 7.3 × 10−8 M Mn2+) were determined. (B) Depletion of Ca2+, but not Mn2+, induces partial secretion of CpY in wild type. PMR1 cells (YR98), pregrown in the defined media given in panel A, were converted to spheroblasts and labeled with 35S-methionine maintaining the defined free ion concentrations. After 20 min and 40 min, supernatants were analyzed for the presence of total CpY by immunoprecipitation, SDS-PAGE, and autoradiography. All samples showed a single band migrating with the molecular mass (69 kDa) of the Golgi form, p2 CpY. Each lane corresponds to 3.5 OD of cells.
Plasmids
Plasmids used in this study are shown in Table 2. Plasmid br434 was constructed by inserting the PMA1 promoter fragment (HindIII/blunt–BamHI) from pSPPMA1C (Supply et al., 1993) into the pRS316 polylinker (EagI/blunt, BamHI). ADC1 transcription terminator sequences (BamHI–HindIII) from pAAH5 (Ammerer, 1983) were added into the SalI site (blunt-ended), and the SERCA1a cDNA (Moutin et al., 1994) was added into the EcoRI site (blunt-ended). Plasmid br435 was constructed by moving the expression casette (ApaI/blunt–SacI) from br434 into the KpnI(blunt)/SacI sites of YEplac195 (Gietz and Sugino, 1988).
Table 2.
Plasmids
| Plasmid | Source | |
|---|---|---|
| br327 | 2μ PMR1, isolate from library in Yep24 (Carlson and Botstein, 1982) | This study |
| br349 | 2μ VPS10, isolate from library in YEp24 | This study |
| br356 | VPS10 (6-kb BglII fragment in pRS426; pEMY10-2) | E. Marcusson/S. Emr |
| br358 | SUC2::VPS10-ΔN (pEMY101-pSEYc350) | E. Marcusson/S. Emr |
| br375 | VPS10-1385 (pEMY10-7) | E. Marcusson/S. Emr |
| br434 | CEN PMA1::SERCA1A::ADC1 | This study |
| br435 | 2μ PMA1::SERCA1A::ADC1 | This study |
| pKC11 | 2μ PMR1 (4.38-kb PvuII-SpeI fragment, in B2205) | K. Cunningham |
| pKC46 | 2μ PMC1 (4.9-kb HindIII-HindIII fragment, in B2205) | K. Cunningham |
Analysis of CpY Sorting
Cultures (15 ml) in the defined growth medium were inoculated at 0.1 optical density (OD) units/ml and grown to 1–1.5 U/ml of OD at 600 nm. Cells were washed twiced with defined labeling medium and resuspended at 3.5 OD units/400 μl. Cells were treated with DTT (10 min), washed, resuspended, and converted to spheroblasts (zymolyase 100T) as described (Vida et al., 1990; Horazdovsky and Emr, 1993). After washing, spheroblasts were incubated in defined labeling medium for 30 min in the presence of protease inhibitors, BSA and α2-macroglobulin. After the addition of 35S-methionine (15 μCi/OD cells), aliquots (400 μl) were withdrawn at the times indicated and separated into supernatant and pellets. Processing of samples for immunoprecipitation of CpY (commercially available anti-CpY antibody; Molecular Probes, Eugene, OR), SDS-PAGE, and autoradiography was as described previously (Vida et al., 1990; Horazdovsky and Emr, 1993).
Analyis of CpY* Degradation and Deg1-β-Galactosidase Activity
To assay CpY* degradation by pulse-chase analysis, cells were grown at 30°C in complete synthetic medium containing 2% glucose. For each time point, cells corresponding to 2.5 U of OD at 600 nm (0.75 × 108 to 1 × 108 cells) were taken and labeled with 62.5 μCi 35S-methionine. Labeling and chase conditions, as well as all other experimental procedures, e.g., cell lysis, immunoprecipitation, and SDS-PAGE, were performed as described previously (Finger et al., 1993). To monitor β-galactosidase activity in strains expressing Deg1-β-galactosidase, cells were grown as described for the analysis of CpY* degradation. After cycloheximide was added to a final concentration of 0.5 mg/ml at zero time (t = 0) to the logarithmically growing culture, cells corresponding to 0.3 U of OD at 600 nm (0.9 × 107 to 1.2 × 107 cells) were taken for each time point, mixed with lysis buffer (0.6% Triton-X 100, 0.75% M O-nitrophenyl β-d-galactopyranoside, 2.25% β-mercaptoethanol, 0.15 M Tris/HCl, pH 7.5), and kept at −80°C for 30 min. After a 60- to 90-min incubation at 37°C, 75 μl 1 M NaHCO3 were added to the samples, debris was removed by centrifugation (20,000 × g, 3 min), and the OD405 was determined.
RESULTS
Mn2+ Can Replace Ca2+ to Promote Growth, but Is Unable to Sustain Vacuolar Protein Sorting
As a basis for subsequent studies, we first examined the growth of our wild-type strain in media depleted for either Ca2+ or Mn2+. Following the published procedure (Loukin and Kung, 1995), we used BAPTA, a chelator of divalent cations (Tsien, 1980), to manipulate the free concentrations of Ca2+ and Mn2+ in liquid culture media, maintaining fixed free concentrations for other essential cations (see MATERIALS AND METHODS). As shown in Figure 1A, a Mn2+-depleted medium (free Mn2+ ≈ 0.16 pM) with a concentration of ≈1.4 μM free Ca2+ allows growth comparable to the BAPTA-free control medium. Similarly, efficient growth is observed in a Ca2+-depleted medium (free Ca2+ ≈ 0.84 nM) with a concentration of free Mn2+ of ≈ 20 nM. The growth observed in both media is dependent on the presence of free Ca2+ or Mn2+, since simultaneous depletion of both ions completely inhibits growth (Figure 1A). Thus, our strain grows well at very low Ca2+ (≈ 0.84 nM) or Mn2+ (≈ 0.16 pM), in very good agreement with the data reported for another strain of S. cerevisiae (Loukin and Kung, 1995).
Since mutants lacking the Golgi Ca2+ adenosine triphosphatase Pmr1 partially secrete the vacuolar hydrolase CpY (Antebi and Fink, 1992), we wished to evaluate the individual effects of Ca2+ and Mn2+ ions on this sorting reaction. Wild-type cells were grown in BAPTA-free control medium and in BAPTA media depleted for either Ca2+ or Mn2+. To monitor CpY sorting, cells were converted to spheroplasts and labeled with 35S-methionine, maintaining the defined ionic conditions during all incubations. After 20 and 40 min, CpY secreted due to missorting was recovered from the culture supernatants by immunoprecipitation and analyzed by SDS-PAGE. As seen in Figure 1B, cells growing in control medium (no BAPTA, left panel in Figure 1B) with regular concentrations of total Ca2+ (≈5.7 μM) and Mn2+ (≈73 nM) fail to secrete CpY into the medium. Depletion of free Mn2+ from this medium has no apparent effect on sorting: CpY is not secreted into the medium (Figure 1B, right panel). However, the reduction of free Ca2+ to ≈ 0.84 nM, a concentration demonstrated to allow effficient growth (see Figure 1A, −Ca2+), significantly impairs vacuolar sorting. Approximately 15–20% of total CpY is secreted into the medium; the molecular mass of the secreted material (69 kDa) is characteristic for the Golgi-associated p2 form of CpY (Figure 1B, middle panel). Analysis of intracellular CpY under both conditions indicates that the bulk of CpY reaches the vacuole and is properly processed (Dürr and Rudolph, unpublished observations). Although we have not explored the kinetics of intracellular transport under both conditions, our data allow a firm conclusion: Ca2+ ions are required to sustain accurate sorting of CpY in wild-type cells.
Mn2+ Ions Stimulate Protein Glycosylation
pmr1 strains produce and secrete a form of invertase essentially lacking high-mannose outer chains (Rudolph et al., 1989; Antebi and Fink, 1992). Due to this reduction in outer-chain glycosylation, invertase isolated from pmr1 mutants (Figure 2A; two panels, pmr1::LEU2 and pmr1::HIS3) migrates on native gels significantly faster than invertase isolated from a wild-type strain (Figure 2A; left panel, PMR1). However, we have found that the presence of Mn2+ ions (450 μM) during induction of invertase synthesis in low-glucose medium strongly stimulates invertase glycosylation in the pmr1 mutants. As demonstrated in Figure 2A, invertase secreted from pmr1 cells in Mn2+-rich medium migrates similarly to the heterogeneous, high-molecular mass material seen with wild-type cells. In contrast, even high concentrations of Ca2+ (20 mM) exert only a modest effect on invertase glycosylation, in complete agreement with previous work (Antebi and Fink, 1992). This finding strongly suggests that the known defect in N-linked glycosylation in pmr1 cells primarily results from an insufficient supply of Mn2+ ions.
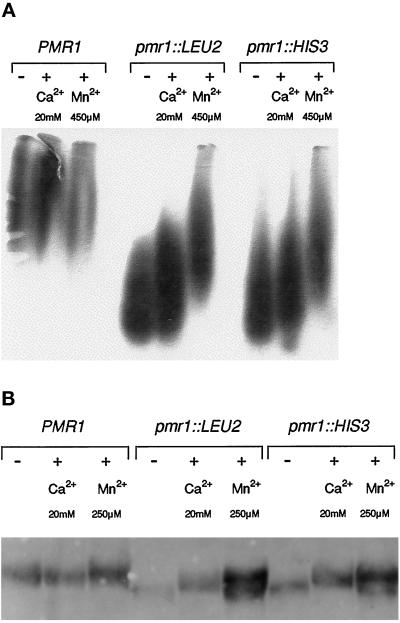
Figure 2.
Mn2+ ions effectively stimulate glycosylation in pmr1 mutants. (A) N-glycosylated invertase secreted by wild-type (PMR1) and pmr1 mutant cells in unsupplemented medium (−), and after stimulation with Ca2+ or Mn2+ ions. Strains were pregrown in YPD (5% glucose), transferred to YPD (5% glucose, 1 h), supplemented with Ca2+ or Mn2+, and induced for invertase production in YPD (0.1% glucose, 1 h) supplemented with Ca2+ or Mn2+ as indicated. Control cells were grown and induced for invertase in unsupplemented media (−). Analysis of external invertase by native-gel electrophoresis and subsequent activity staining was as described (Rudolph et al., 1989). (B) O-glycosylated chitinase secreted by wild-type and pmr1 cells upon stimulation with Ca2+ or Mn2+. Cultures were grown overnight in YPD medium supplemented with Ca2+ or Mn2+ as indicated; control cells were grown in unsupplemented YPD medium (−). Secreted chitinase was affinity-purified using chitin powder (Guthrie and Fink, 1991) and analyzed by SDS-PAGE and Western blotting as described (Immervoll et al., 1995). Strains: YR98 (PMR1), YR122 (pmr1-Δ1::LEU2), YR123 (pmr1-Δ2::HIS3)
Similarly, we analyzed the gel mobility of secreted chitinase, a protein exclusively O-glycosylated (Kuranda and Robbins, 1991) and a convenient indicator of O-glycosylating activity in vivo (Immervoll et al., 1995; Gentzsch and Tanner, 1996). As shown in Figure 2B, chitinase secreted from pmr1 mutants growing in regular YPD medium migrates faster than chitinase produced by a PMR1 wild-type strain (Figure 2B; see lanes marked “−”). Apparently, pmr1 cells are also defective in O-linked glycosylation. Interestingly, this increased mobility can, in part, be reversed by supplementing the medium with Mn2+ (250 μM), which causes pmr1 cells to produce two forms of chitinase: a high-molecular mass form with essentially wild-type mobility, and a form migrating even faster than chitinase produced by pmr1 in the absence of Mn2+. In contrast, addition of Ca2+ (20 mM) to the pmr1 cultures yields only one form of chitinase with intermediate mobility, indicating that Ca2+ is unable to restore production of fully glycosylated chitinase in pmr1 cells. Taken together with the results on invertase, our findings suggests that the Pmr1 ion pump functions in vivo to supply the Mn2+ ions required for N-linked and O-linked glycosylation.
Two Ca2+ Pumps, Pmc1 and SERCA1a, Restore Growth of pmr1 on Ca2+-Deficient and EGTA-Containing Media
pmr1 null mutants fail to grow on Ca2+-deficient media (Rudolph et al., 1989). In a search for yeast genes that, upon overexpression, could compensate this defect, we transformed a pmr1 mutant with a YEp24-based genomic library (Carlson and Botstein, 1982). In addition to PMR1, we found two distinct groups of library plasmids able to promote growth under these conditions. All plasmids also alleviated the hypersensitivity of the pmr1 strain to EGTA. The first group of plasmids was found to harbor the PMC1 gene, which encodes a Ca2+ pump in the yeast vacuolar membrane (Cunningham and Fink, 1994). To confirm that PMC1 was solely responsible for the suppression, we transformed the pmr1 mutant with the 2μ-based plasmid pKC46, carrying PMC1 on a 4.9 kilobase (kb) HindIII fragment (gift from K. Cunningham). As evident from Figure 3A, this plasmid allowed vigorous growth of pmr1 cells in the presence of EGTA, indicating that the vacuolar Ca2+ pump Pmc1 can indeed compensate the loss of Pmr1 during growth on Ca2+-deficient or EGTA-containing media.
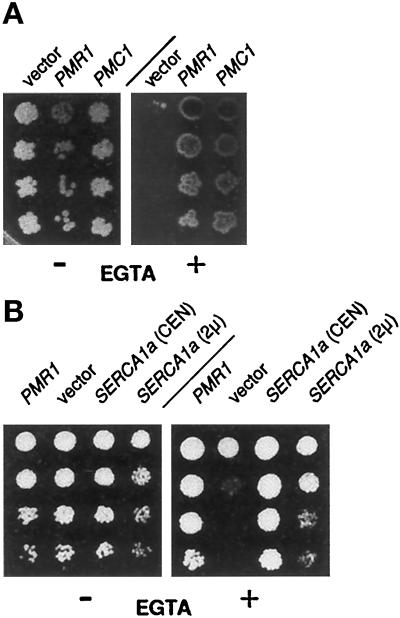
Figure 3.
Expression of Ca2+ pumps restores growth of a pmr1 mutant on EGTA-containing media. (A) Expression of the vacuolar ion pump Pmc1 in pmr1 cells. Serial fivefold dilutions of saturated cultures were spotted onto complete medium lacking uracil to maintain selection for the plasmids. Addition (+) or omission (−) of 6.5 mM EGTA is indicated. Plates were photographed after 3 d incubation at 30°C. Strains (from left to right): YR439 (pmr1; 2μ-vector); YR657 (pmr1, 2μ-PMR1) and YGD57 (pmr1, 2μ-PMC1). (B) Expression of a sarcoplasmic Ca2+ pump, SERCA1a (rabbit). Conditions as in panel A; strains are (from left to right): YR441 (pmr1, 2μ-PMR1), YR439 (pmr1, 2μ-vector), YR663 (pmr1, CEN-SERCA1a), and YR664 (pmr1, 2μ-SERCA1a).
To confirm that suppression was indeed related to the activity of Pmc1 as a Ca2+ pump, we also tested SERCA1a from rabbit, a sarcoplasmic Ca2+ pump, for its ability to suppress the EGTA hypersensitivity of the pmr1 mutant. To express SERCA1a in yeast, we inserted the SERCA1a cDNA (Moutin et al., 1994) into a single-copy plasmid behind the strong, constitutive PMA1 promoter and upstream of ADC1 sequences to ensure transcription termination. As shown in Figure 3B, a single-copy plasmid with this casette (CEN-SERCA1a) strongly supports growth of pmr1 cells on EGTA plates. A 2μ-based derivative (2μ-SERCA1a) harboring the same PMA1::SERCA1a::ADC1 casette is much less effective (see Figure 3B), indicating that SERCA1a overexpression may exert toxic effects on pmr1 cells. The data show that SERCA1a is functionally expressed and replaces Pmr1 under these conditions. To find suppression by two different Ca2+ pumps, Pmc1 and SERCA1a, a highly Ca2+-specific ion pump, strongly suggests that changes in intracellular Ca2+ flux are sufficient to relieve the growth inhibition of pmr1 on EGTA-containing media.
Vps10, a Receptor for Vacuolar Sorting, Also Suppresses EGTA hypersensitivity of pmr1
The second group of plasmids, which restored growth of pmr1 on Ca2+-deficient media, contained a copy of the VPS10 gene. Transformation of the pmr1 mutant with a plasmid solely containing VPS10 (gift from E. Marcusson) confirmed that VPS10 was indeed responsible for the suppression (shown in Figure 4A). The suppression of pmr1 by elevated expression of Vps10 is not mediated through the vacuolar pump Pmc1, since suppression was still observed in a pmr1 pmc1 cnb1 mutant (Figure 4B). The known functions of Vps10 in vacuolar sorting and in salvage of malfolded luminal proteins suggest that the pmr1 growth defect in Ca2+-deficient media may reside in the lumen of the secretory pathway.
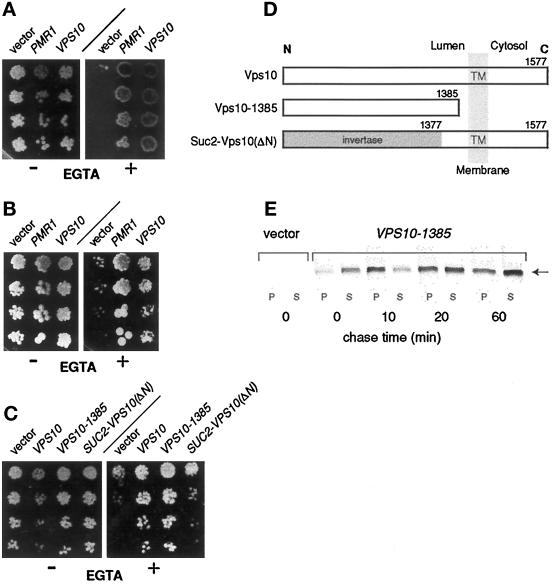
Figure 4.
Suppression of EGTA hypersensitivity in pmr1 cells by the sorting receptor Vps10 is mediated through its luminal domain. (A) Suppression by wild-type Vps10. Serial fivefold dilutions of saturated cultures were spotted onto complete medium lacking uracil. Addition (+) or omission (−) of 6.5 mM EGTA is indicated. Plates were photographed after 3 d incubation at 30°C; strains are (left to right): YR439 (pmr1, 2μ-vector), YR657 (pmr1, 2μ-PMR1), and YR480 (pmr1, 2μ-VPS10). (B) Vps10-mediated suppression does not require Pmc1. Conditions are as in panel A. Strains (from left to right): YR469 (pmr1 pmc1 cnb1, 2μ-vector), YR472 (pmr1 pmc1 cnb1, 2μ-PMR1), and YR477 (pmr1 pmc1 cnb1, 2μ-VPS10). (C) The luminal domain of Vps10 is necessary and sufficient for suppression. Same conditions as in panel A; strains are (from left to right): YR547 (pmr1, 2μ-vector), YR551 (pmr1, 2μ-VPS10), YR550 (pmr1, 2μ-VPS10–1385), and YR549 (pmr1, 2μ-SUC::VPS10-ΔN). (D) Vps10 derivatives used in the analysis. The transmembrane topology of Vps10 and its derivatives is depicted; numbers indicate the Vps10 residues present in each derivative. (E) Vps10–1385 is secreted by pmr1 cells during growth in EGTA-containing medium. Cells were cultured in synthetic complete medium, converted to spheroblasts, and labeled (15 μCi/OD cells) with 35S-methionine as described (Horazdovsky and Emr, 1993), but EGTA (3 mM) was present during these steps. At the times indicated, cells and media supernatants were analyzed for the presence of Vps10–1385 by immunoprecipitation, SDS-PAGE, and autoradiography. The band corresponding to Vps10–1385 in SDS-PAGE is marked (arrow). Strains: YR547 (pmr1, 2μ-vector) and YR550 (pmr1, 2μ-VPS10–1385).
To test whether Vps10 was indeed resolving a luminal defect, we examined two Vps10 derivatives (see Figure 4D) for their ability to suppress pmr1. A truncated form, Vps10–1385, consists of the first 1385 residues encompassing essentially the luminal domain of Vps10, but lacks the transmembrane segment and the cytoplasmic tail. The second variant, Suc2-Vps10(ΔN), represents an Invertase-Vps10 fusion protein, wherein the luminal domain of Vps10 is replaced by invertase sequences (gift from E. Marcusson). To avoid interference from wild-type Vps10 expressed from the chromosomal VPS10 locus, these plasmids were transformed into a pmr1 vps10 double mutant. As shown in Figure 4C, Vps10–1385 mediates suppression similar to wild-type Vps10, whereas replacement of the luminal part by invertase in Suc2-Vps10(ΔN) essentially abolishes suppression. Evidently, the luminal domain of Vps10 is necessary and sufficient to allow vigorous growth of the pmr1 vps10 strain on EGTA. As shown in Figure 4E, pmr1 cells secrete the bulk of Vps10–1385 in intact form during growth in EGTA-containing medium, i.e., under conditions where growth is dependent on Vps10–1385. Taken together, our data show that Vps10-mediated suppression resolves a growth-limiting step located within the lumen of early secretory organelles, either in the ER or in Golgi compartments. One of the known functions of Vps10, the salvage of malfolded proteins to the vacuole (Hong et al., 1996), suggests that pmr1 cells may have a defect in protein folding and/or in the degradation of malfolded luminal proteins, thereby increasing the need for Vps10 function.
DER5, a Gene Involved in ER-Associated Protein Degradation, Is Identical to PMR1
In a genetic approach to dissect components of ER-associated protein degradation, we previously isolated different der mutants unable to degrade CpY*, a mutant form of carboxypeptidase Y (Finger et al., 1993; Knop et al., 1996). One mutant in this collection, der5, showed phenotypes similar to pmr1, such as hypersensitivity to EGTA and Mn2+. Cloning of DER5, DNA sequencing, and tetrad analysis of a diploid heterozygous for the der5 mutation and a LEU2-marked pmr1 allele demonstrated that DER5 is identical to PMR1 (our unpublished observations). Therefore, we introduced a pmr1::LEU2 null allele (Rudolph et al., 1989) into our wild-type strain engineered to express CpY* and used this pmr1 null mutant together with its congenic parent for further comparisons.
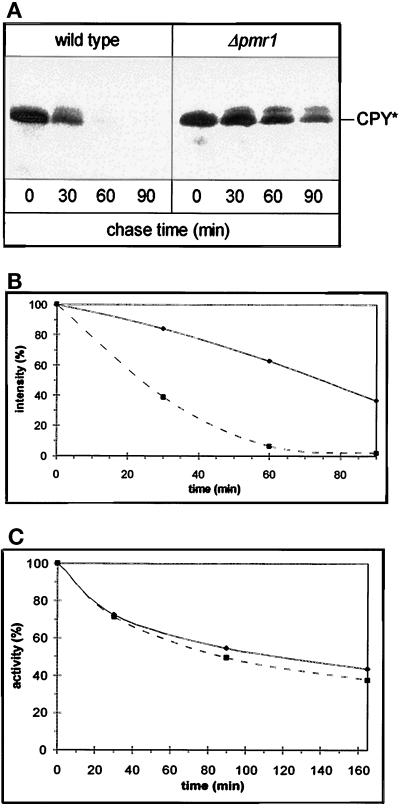
The wild-type strain degrades CpY* with a half-time of 20 min (Finger et al., 1993). However, introduction of the pmr1::LEU2 mutation drastically reduces the rate of CpY* degradation as demonstrated by the pulse-chase analysis shown in Figure 5A. Quantitation of these results reveals that the half-life of CpY* is increased nearly fourfold in the pmr::LEU2 strain (Figure 5B). To gain further insight into the nature of this degradation defect, we introduced into our strains a plasmid directing synthesis of a purely cytosolic protein, Deg1-β-galactosidase. As was demonstrated for CpY* (Hiller et al., 1996), Deg1-β-galactosidase is degraded by the proteasome exclusively via an UBC6-, UBC7-dependent mechanism (Chen et al., 1993). As shown in Figure 5C, the kinetics of degradation for this substrate, as reflected in the decrease in β-galactosidase activity, are virtually identical in both strains. Since Deg1-β-galactosidase is not stabilized in the pmr1 mutant, the ubiquitin–proteasome pathway for degradation of CpY* appears to be functional in the pmr1 mutant. Thus, the observed stabilization of CpY* suggests that loss of Pmr1 function diminishes export of CpY* from the ER into the cytosol.
Figure 5.
Pmr1 is required for ER-associated degradation. (A) CpY* is stabilized in the pmr1 mutant. Pulse-chase analysis of CpY* was performed using the congenic strains W303–1C (wild type, ▪) and YRP023 (Δpmr1, ♦). Cells were grown at 30°C. At the indicated chase times, cells were lysed, and CpY* was immunoprecipitated. Antigenic material was separated by SDS-PAGE; the band corresponding to CpY* is indicated. (B) Quantification of the results shown in panel A using a Molecular Dynamics imaging system. (C) The Ubc6/Ubc7-dependent proteasome degradation system is functional in pmr1. β-Galactosidase activity was tested after alkaline lysis of transformants of W303–1C (PMR1, ▪) and YRP023 (Δpmr1, ♦) expressing the plasmid-encoded fusion protein Deg1-β-galactosidase (Chen et al., 1993).
Loss of PMR1 Causes Phenotypes Characteristic for Perturbations in the UPR
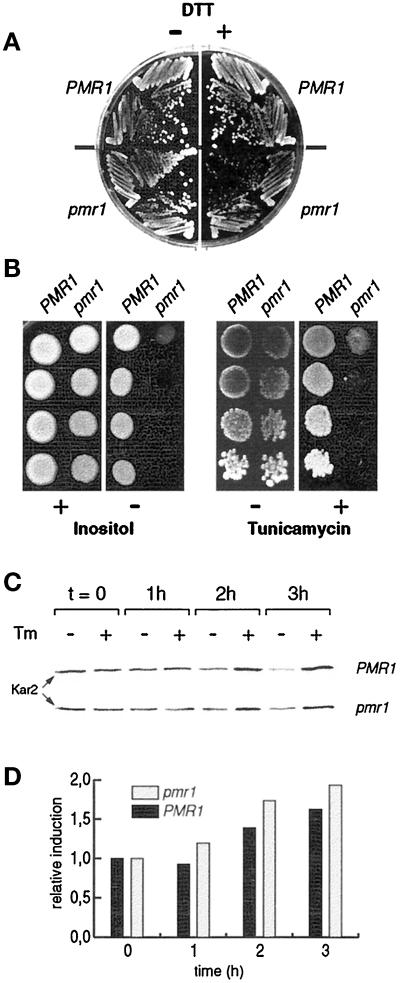
Based on the reduced capability of a pmr1 mutant to clear malfolded CpY* from the ER, we hypothesized that pmr1 cells might be particularly sensitive to an increase in misfolded proteins within the ER. To test this idea, we examined the growth of pmr1 cells in the presence of DTT and tunicamycin, two drugs known to induce accumulation of malfolded proteins in the ER. As demonstrated in Figure 6, A and B, the pmr1 mutant is hypersensitive to both drugs. Similar phenotypes have been reported for mutants defective in the UPR pathway, which coordinately regulates synthesis of ER chaperones and ER membrane biogenesis in response to several stress signals. Mutants blocked in UPR also exhibit, in addition to hypersensitivity toward DTT and tunicamycin, auxotrophy for inositol (for review, see Shamu et al., 1994). As demonstrated in Figure 6B (left panel), pmr1 cells also require inositol. According to these data, pmr1 cells could have some general defect in the UPR response.
Figure 6.
Mutations in PMR1 affect the response to induced accumulation of malfolded proteins in the ER. (A) The pmr1 mutant is sensitive to DTT. PMR1 (YR98) and pmr1 (YR122) cells were streaked onto YPD media, supplemented with 0.2% DTT (+) or without addition of DTT (−). Plates were photographed after 3 d incubation at 30°C. (B) The pmr1 mutant displays inositol auxotrophy and hypersensitivity to tunicamycin. Serial fivefold dilutions of saturated cultures were spotted onto synthetic media (left panel) containing (+) or lacking (−) inositol and onto YPD media containing (+) or lacking (−) tunicamycin (0.3 μg/ml, right panel). Plates were photographed after 3 d incubation at 30°C. Strains: YR98 (PMR1) and YR122 (pmr1). (C) Induction of Kar2 after treatment with tunicamycin. Tunicamycin (1 μg/ml) was added to cells growing in YPD (OD600 = 0.8). After 1 h, 2 h, and 3 h, cell extracts were prepared and analyzed for the presence of Kar2 by SDS-PAGE and Western blotting. Each lane corresponds to 20 μg of total protein. Strains: YR98 (PMR1) and YR122 (pmr1). (D) PhosphoImager quantitation of the results shown in panel C. For each strain, signals are expressed as ratio of relative induction; the values at t = 0 were arbitrarily set to 1. Strains: YR98 (PMR1) and YR122 (pmr1).
To examine the nature of this defect in more detail, we monitored induction of Kar2 in wild-type and pmr1 cells in response to tunicamycin. As shown in Figure 6, C and D, Kar2 is induced in the pmr1 mutant, throughout a 3-h challenge with tunicamycin, to a level even slightly higher than in wild type. The modest induction of Kar2 in these experiments might be due to the genetic background of our strains or reflect experimental conditions. However, we obtained very similar kinetics with reporter plasmids containing UPR elements, the DNA sequences mediating transcriptional activation during UPR (Mori et al., 1992; Strayle and Rudolph, unpublished observations). Our findings indicate that the apparent sensitivity of pmr1 cells to experimentally induced accumulation of malfolded proteins in the ER, if indeed related to UPR function, is not due to an immediate, complete block in signaling through the UPR cascade.
DISCUSSION
S. cerevisiae can grow at very low Ca2+ concentrations (< 10−9 M) if a sufficient supply of free Mn2+ is available. Vice versa, growth at very low Mn2+ concentrations (< 10−12 M) is sustained by free Ca2+ (Loukin and Kung, 1995). This apparent interchangeability of Ca2+ and Mn2+, which we confirmed for the wild-type strain used in the present study, implies that both ions have access to secretory organelles expected to host vital processes dependent on one of the two cations. We have provided evidence that the Golgi-resident ion pump Pmr1 operates as a common transporter for Ca2+ and Mn2+ in vivo. Mn2+ ions are required for the addition of complex carbohydrates onto N- and O-glycosylated proteins in the Golgi, whereas intralumenal Ca2+ is a prerequisite for accurate vacuolar sorting. Most important, we have demonstrated that Pmr1-mediated ion transport also affects processes in the ER. In particular, we have shown that CpY*, a malfolded protein normally degraded in the cytosol after export from the ER, is stabilized in pmr1 mutants due to an intralumenal defect. Furthermore, we have demonstrated that pmr1 mutants are sensitive to conditions inducing accumulation of malfolded proteins in the ER. We propose that the cation content of the yeast ER is critically determined by the secretory pathway pump Pmr1, which also appears to be necessary for an appropriate response of the ER to stress conditions.
The function of Pmr1 as an ATP-driven Ca2+ pump was recently demonstrated by assaying Ca2+ uptake into purified Golgi-derived vesicles (Sorin et al., 1997). Genetically, the role of Pmr1 in Ca2+ transport is manifest in pmr1 mutants in several Ca2+-related phenotypes, one of which is a partial defect in CpY sorting. In media with approximately 200 μM Ca2+, pmr1 cells mis-sort and secrete a small fraction (< 7%) of the Golgi form of CpY, normally routed to the vacuole in wild-type cells. Addition of Ca2+ (10 mM) fully restores CpY sorting (Antebi and Fink, 1992). In addition, the free cytosolic Ca2+ in pmr1 cells is known to be elevated relative to wild type, and cytosolic Ca2+ increases even further upon addition of extracellular Ca2+ (Halachmi and Eilam, 1996). Therefore, the reversibility of the CpY-sorting defect in pmr1 cells by external Ca2+ suggests that a low intralumenal Ca2+ content within some secretory organelle(s), rather than an elevated cytosolic Ca2+ concentration, is causing mis-sorting of CpY. This view is now corroborated by our finding that severe Ca2+ depletion in wild-type cells induces the same effect on CpY sorting as a pmr1 mutation, i.e., partial secretion of CpY. These data also provide a clear demonstration that chelators can effectively induce cation depletion in secretory organelles of growing yeast cells.
The aberrant secretion of CpY from wild-type cells is not caused by the Mn2+ ions present in the Ca2+-depleted medium to support growth, since chelator-free medium with a similar concentration of Mn2+ allows for accurate sorting. In addition, the growth rates in Ca2+- or Mn2+-depleted media are very similar, suggesting that either cation alone does not drastically alter bulk flow through the secretory pathway. Based on the ER-associated changes we observed in pmr1 cells (see Figure 5), it seems likely that Ca2+ depletion leads to an increased production or reduced turnover of malfolded proteins, which might then utilize the Vps10-mediated salvage pathway to the vacuole and could thereby compete with CpY for binding to the Vps10 receptor. Likewise, the biogenesis or function of Vps10 might be compromised under low intralumenal Ca2+ conditions. Alternatively, luminal Ca2+ could directly promote specific sorting steps, such as partitioning of receptor–cargo complexes into a budding vesicle or binding of CpY to Vps10, but the Ca2+ dependence of these reactions has not been explored in vitro (for binding of CpY to Vps10, see Cooper and Stevens, 1996).
A role of Pmr1 in Mn2+ transport was first suggested based on changes in intracellular Mn2+ distribution observed in pmr1 cells, which appear to possess an increased cytosolic Mn2+ level (Lapinskas et al., 1995). The present study extends this observation by showing that pmr1 cells display Mn2+-related defects in glycosylation reactions that take place in the lumen of the Golgi. We have shown that chitinase, a solely O-glycosylated secretory protein, is produced by pmr1 cells in an aberrant form migrating during SDS-PAGE significantly faster than chitinase isolated from a wild-type strain. A similar behavior was previously reported for N-glycosylated invertase, which in pmr1 cells lacks the complex carbohydrate chains normally added in the Golgi (Antebi and Fink, 1992). As we have shown, addition of Mn2+ stimulates pmr1 cells to produce high-molecular mass forms of chitinase and invertase. Since the mannosyltransferase activities responsible for carbohydrate addition onto N- and O-glycosylated precursors in the yeast Golgi require Mn2+ in vitro (Sharma et al., 1974; Nakajima and Ballou, 1975; Parodi, 1979; Haselbeck and Schekman, 1986), we assume that the altered molecular mass in the different forms of chitinase and invertase reflects changes in carbohydrate content, although this has only been demonstrated for invertase produced by pmr1 cells (Antebi and Fink, 1992).
Glycosylation reactions in the Golgi complex of mammalian cells display similar Mn2+ requirements (Sugiura et al., 1982; Elhammer and Kornfeld, 1986). In one study, defects in O- and N-linked glycosylation were generated in vivo by A23187-induced cation depletion and shown to be partially reversible by the addition of Mn2+, but not Ca2+. Interestingly, Mn2+ addition in the presence of A23187 produced two distinct populations of secreted molecules (macrophage colony-stimulating factor): one form carried fully restored complex N-linked and O-linked carbohydrates; the other one lacked O-linked oligosaccharides, but displayed high-mannose N-linked carbohydrates (Kaufman et al., 1994). Our results with chitinase produced upon Mn2+ addition are very similar: pmr1 cells also secrete two populations of chitinase under these conditions. This “all or none” behavior in glycosylation could reflect secretion of chitinase from different secretory compartments or point to some other defect within the secretory pathway, presumably caused by a partial Ca2+ depletion in the absence of Pmr1. The appearance of a single, intermediate form of chitinase upon addition of Ca2+ to pmr1 cells favors this explanation. Likewise, Mn2+ addition does not restore full glycosylation onto invertase in pmr1 cells, and the sole addition of Ca2+ appears to have a slight stimulatory effect. Thus, the faithful addition of carbohydrates onto O- and N-glycosylated proteins seems to require an intricate balance of intralumenal Ca2+ and Mn2+ levels within the secretory pathway. We have also noticed that the two pmr1 strains, derived from a common his3 leu2 parent strain by transformation, produce the two chitinase forms in different amounts (see Figure 2B). The particulary strong response to Mn2+ observed in the pmr1::LEU2 strain could be due to the his3 mutation, which is complemented in the pmr1::HIS3 strain. Histidine is a fairly good chelator of Mn2+ with a dissociation constant of 20 nM (see Hughes and Poole, 1991, and references therein), and is stored in high amounts by yeast vacuoles (Kitamoto et al., 1988), which also accumulate divalent cations, including Ca2+, Mg2+, Mn2+, and other heavy metals (Okorokov et al., 1978; Ohsumi and Anraku, 1983; Bode et al., 1995). The ability to use the full capacity of the endogenous biosynthetic pathway to supply histidine for sequestration of Mn2+ into the vacuole might, to some extent, attenuate the high Mn2+ conditions in pmr1::HIS3 cells, and thereby indirectly affect chitinase glycosylation.
Two sets of data, one of which relates to the failure of pmr1 cells to effectively degrade CpY*, indicate that Pmr1-mediated ion transport affects intralumenal processes hosted in a secretory compartment outside the Golgi, presumably within the ER. The observed stabilization of CpY* appears to reflect an intralumenal defect, since the same pmr1 strain is able to degrade cytosolic Deg1-β-galactosidase. Deg1-β-galactosidase is, like CpY* (Hiller et al., 1996), an Ubc6-/Ubc7-dependent substrate of the proteasome due to the presence of the Deg1 domain, which is responsible for ubiquitination of Matα2 by the ubiquitin-conjugating enzymes Ubc6 and Ubc7 (Chen et al., 1993). Overexpression of the luminal domain of Vps10 (Vps10–1385), which we demonstrated to suppress EGTA hypersensitivity in pmr1 strains, does not affect the high steady-state level of CpY* in pmr1 cells, indicating that CpY* accumulation is not indirectly caused by a potentially Ca2+-controlled redistribution of malfolded proteins between Vps10-dependent and -independent degradative pathways (Plemper, Strayle, Wolf, and Rudolph, unpublished data). In contrast to this, expression of the SERCA1a Ca2+ pump, which in PMR1 wild-type cells accumulates in proliferating ER membranes (Catty, unpublished data), reduces the steady-state level of CpY* in pmr1 cells (Plemper, Strayle, Wolf, and Rudolph, unpublished data). These findings suggest that a sufficient level of ER Ca2+ is necessary to accomplish export of CpY* into the cytosol for degradation by the proteasome. A requirement for Kar2, the yeast BiP homolog, in CpY* export has been demonstrated (Plemper et al., 1997). Thus, the observed effects of Pmr1 on CpY* degradation could result from cation-dependent functions of the Kar2 chaperone. Remarkably, mammalian BiP was shown to undergo autophosphorylation, which in vitro is stimulated by Ca2+ and inhibited by Mn2+, and the resulting BiP isoforms display altered properties in protein binding and oligomerization in vivo (Hendershot et al., 1988; Leustek et al., 1991; Carlino et al., 1992). Similar, cation-dependent changes in Kar2 phosphorylation could reduce the level of Kar2 available for CpY* export and presumably affect other Kar2-mediated reactions, including protein folding.
The second set of data pointing to an ER-related function of Pmr1 concerns the EGTA hypersensitivity of pmr1 cells. EGTA and BAPTA both lower the divalent cation content of secretory organelles in animal cells, and the partial mis-sorting of CpY we observed here with wild-type cells provides direct evidence for a similar effect of chelators on yeast cells. The strong suppression of EGTA hypersensitivity by Vps10–1385, an entirely luminal polypeptide secreted under these conditions, further confirms the inferred intralumenal nature of the EGTA-induced growth inhibition in pmr1 cells. Based on the function of Vps10 in salvage of non-native luminal proteins to the vacuole (Hong et al., 1996), we hypothesize that EGTA-induced cation depletion in some secretory organelle(s) of pmr1 cells might generate malfolded proteins, which above a certain threshold could cause growth inhibition. As shown here, accumulation of CpY* is already occurring during growth of pmr1 cells in a normal ionic milieu, indicating that the ER of pmr1 cells is particularly sensitive to cation depletion. Thus, we suspect that the EGTA-induced growth inhibition originates in this compartment. It is not clear, however, whether reduced export of misfolded proteins from the ER to the cytosol, a prerequisite for degradation of CpY* and perhaps other malfolded ER proteins, is responsible for the growth inhibtion by EGTA. Alternatively, a low cation content in the ER could compromise protein folding to an extent exceeding the capacity of the Vps10 salvage pathway, which might also be affected under these conditions. Nevertheless, expression of either Ca2+ pump, yeast Pmc1, or heterologous SERCA1a restores growth of pmr1 cells in the presence of EGTA, suggesting that the mechanisms underlying EGTA hypersensitivity are Ca2+-dependent.
Interestingly, the loss of Pmr1 leads to phenotypes also observed in yeast mutants with a blocked UPR, which coordinates synthesis of ER-resident chaperones and ER membrane biogenesis under a variety of ER stress conditions (Cox et al., 1997; see Shamu, 1997 and references therein). As we have shown, induction of Kar2 after tunicamycin challenge proceeds unperturbed for at least 3 h in pmr1 cells, suggesting that the regulatory branch in the UPR to increase chaperone synthesis is functional. It needs to be tested whether pmr1 cells are unable to sustain prolonged induction of chaperone synthesis or fail to induce INO1, the gene encoding inositol-1-phosphate synthase required for synthesis of phosphatidylinositol. It is also possible that an altered cation distribution in pmr1 cells indirectly affects phospholipid biosynthetic enzymes requiring Mg2+, Mn2+, or, as in one case, Ca2+ (see Paltauf et al., 1992 and references therein). Based on our study, which emphasizes the importance of Pmr1-mediated ion transport for early secretory organelles, including the ER, we also suspect that the Pmr1 ion pump might be directly required during UPR to uphold a favorable ionic milieu within an expanding ER.
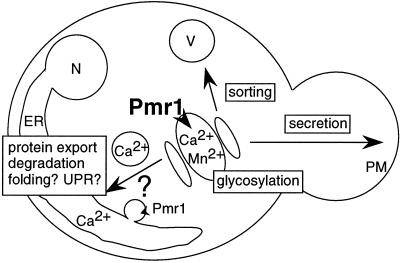
As this study has shown, the Pmr1 ion pump sustains a variety of processes hosted in different compartments of the secretory pathway (Figure 7). Transport of Mn2+ ions by Pmr1 is required for the addition of complex carbohydrates onto N- and O-glycosylated proteins in the Golgi. Within the ER, the export of malfolded proteins like CpY* and, presumably, other cation-dependent intralumenal processes rely on the Pmr1 ion pump to provide an adequate milieu in the lumen of this compartment. It remains unclear whether Ca2+ and Mn2+ ions both support these ER-associated processes. Even under conditions of extreme Ca2+ depletion, wild-type cells appear to retain about 3% of their normal total Ca2+ content (Loukin and Kung, 1995), a level perhaps sufficient to support strictly Ca2+-dependent processes in the ER. Another interesting question is how Pmr1 exerts its function in different secretory organelles (see Figure 7). Pmr1 could simultaneously operate in the Golgi and in the ER if the very low amount of Pmr1 in the ER membrane could transport sufficient ions into this compartment. Alternatively, Pmr1 activity might be restricted to the medial-Golgi, where Ca2+ and Mn2+ ions would enter the secretory pathway to be appropriately distributed into other organelles, including the ER. Such remote control of the ER cation content by a distant medial-Golgi ion pump, which presumably lacks the high Ca2+ specificity of SERCA-type ER pumps, would provide yeast cells with a mechanism to evade high Mn2+ conditions in the ER, despite the use of a single pump for Ca2+ and Mn2+ transport. The hypersensitivity of pmr1 cells toward Mn2+ is partially relieved by Vps10–1385 (Klee, Strayle, and Rudolph, unpublished observation), suggesting that an unbalanced intralumenal Mn2+ level perturbs processes within the early secretory pathway. Future studies on Pmr1, sometimes referred to as “the yeast secretory pathway pump,” together with the use of pmr1 cells to express SPCA, a putative rat homolog (Gunteski-Hamblin et al., 1992), should ultimately provide a paradigm to understand the function of the secretory pathway pump in all eukaryotes.
Figure 7.
Model for the function of the secretory pathway pump Pmr1. Pmr1 pumps Ca2+ and Mn2+ into the secretory pathway, predominantly at the medial-Golgi (Pmr1, boldface). Both ions could either uniformly enter transport vesicles or be selectively recruited (or excluded). Such mechanisms could serve to enrich Ca2+ in the ER, or to reduce Mn2+, despite the use of a single pump to transport both ions. In the ER, which contains only a small amount of Pmr1 in the membrane (Pmr1, small font), Pmr1 activity is required to export malfolded proteins into the cytosol for degradation, but other processes are likely to be affected (folding, UPR). In the Golgi, Mn2+ is required for protein glycosylation, whereas Ca2+, directly or indirectly, sustains vacuolar sorting. Thus, Pmr1 could control the secretory pathway at several stages. Reduced Pmr1 activity could induce the secretion of nonnative proteins, otherwise retained for ER-associated degradation or salvaged by the vacuole; the first isolation of a pmr1 mutant in a screen for “supersecretion” of heterologous proteins from yeast supports this view (Smith et al., 1985). Abbreviations used: N, nucleus; ER, endoplasmic reticulum; V, vacuole; PM, plasma membrane.
ACKNOWLEDGMENTS
We are indepted to Dr. Stephen Loukin for his advice on media preparations. We thank E. Marcusson, S. Emr, and K. Cunningham for sharing results and materials before publication, and T. Stevens, R. Schekman, M. Genztsch, W. Tanner, and M. Rose for plasmids or antibodies. J.S. receives a stipend from the Studienstiftung des Deutschen Volkes, Bonn, Germany. This work was supported by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie, Zentrales Schwerpunktprojekt Bioverfahrenstechnik, Universität Stuttgart, Stuttgart, Germany, and by the Fonds der Chemischen Industrie, Frankfurt, Germany.
REFERENCES
- Ammerer G. Expression of genes in yeast using the ADCI promoter. Methods Enzymol. 1983;101:192–201. doi: 10.1016/0076-6879(83)01014-9. [DOI] [PubMed] [Google Scholar]
- Antebi A, Fink GR. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol Biol Cell. 1992;3:633–654. doi: 10.1091/mbc.3.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode HP, Dumschat M, Garotti S, Fuhrmann GE. Iron sequestration by the yeast vacuole - A study with vacuolar mutants of Saccharomyces cerevisiae. Eur J Biochem. 1995;228:337–342. [PubMed] [Google Scholar]
- Booth C, Koch GL. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1989;59:729–737. doi: 10.1016/0092-8674(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Brodsky JL, McCracken AA. ER-associated and proteasome-mediated protein degradation: how two topologically restricted events came together. Trends Cell Biol. 1997;7:151–156. doi: 10.1016/S0962-8924(97)01020-9. [DOI] [PubMed] [Google Scholar]
- Carlino A, Toledo H, Skaleris D, DeLisio R, Weissbach H, Brot N. Interactions of liver Grp78 and Escherichia coli recombinant Grp78 with ATP: multiple species and disaggregation. Proc Natl Acad Sci USA. 1992;89:2081–2085. doi: 10.1073/pnas.89.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Botstein D. Two differentially regulated mRNAs with different 5′ ends encode secreted and intracellular forms of yeast invertase. Cell. 1982;28:145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Carnell L, Moore HP. Transport via the regulated secretory pathway in semi-intact PC12 cells: role of intra-cisternal calcium and pH in the transport and sorting of secretogranin II. J Cell Biol. 1994;127:693–705. doi: 10.1083/jcb.127.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanat E, Huttner WB. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991;115:1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Johnson P, Sommer T, Jentsch S, Hochstrasser M. Multiple ubiquitin-conjugating enzymes participate in the in vivo degradation of the yeast MATα2 repressor. Cell. 1993;74:357–369. doi: 10.1016/0092-8674(93)90426-q. [DOI] [PubMed] [Google Scholar]
- Cooper AA, Stevens TH. Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J Cell Biol. 1996;133:529–541. doi: 10.1083/jcb.133.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JS, Chapman RE, Walter P. The unfolded protein response coordinates the production of endoplasmic reticulum protein and endoplasmic reticulum membrane. Mol Biol Cell. 1997;8:1805–1814. doi: 10.1091/mbc.8.9.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol. 1994;124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhammer A, Kornfeld S. Purification and characterization of UDP-N-acetylgalactosamine: polypeptide N-acetylgalactosaminyltransferase from bovine colostrum and murine lymphoma BW5147 cells. J Biol Chem. 1986;261:5249–5255. [PubMed] [Google Scholar]
- Finger A, Knop M, Wolf DH. Analysis of two mutated vacuolar proteins reveals a degradation pathway in the endoplasmic reticulum or a related compartment of yeast. Eur J Biochem. 1993;218:565–574. doi: 10.1111/j.1432-1033.1993.tb18410.x. [DOI] [PubMed] [Google Scholar]
- Fuller RS, Brake A, Thorner J. Yeast prohormone processing enzyme (KEX2 gene product) is a Ca2+-dependent serine protease. Proc Natl Acad Sci USA. 1989;86:1434–1438. doi: 10.1073/pnas.86.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzsch M, Tanner W. The PMT gene family: protein O-glycosylation in Saccharomyces cerevisiae is vital. EMBO J. 1996;15:5752–5759. [PMC free article] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Gunteski-Hamblin A, Clarke DM, Shull GE. Molecular cloning and tissue distribution of alternatively spliced messenger RNAs encoding possible mammalian homologues of the yeast secretory pathway calcium pump. Biochemistry. 1992;31:7600–7608. doi: 10.1021/bi00148a023. [DOI] [PubMed] [Google Scholar]
- Guthrie C, Fink GR. Guide to yeast genetics and molecular biology. Methods Enzymol. 1991;194:682–697. [PubMed] [Google Scholar]
- Halachmi D, Eilam Y. Elevated cytosolic free Ca2+ concentrations and massive Ca2+ accumulation within vacuoles, in yeast mutant lacking PMR1, a homolog of Ca2+-ATPase. FEBS Lett. 1996;392:194–200. doi: 10.1016/0014-5793(96)00799-5. [DOI] [PubMed] [Google Scholar]
- Haselbeck A, Schekman R. Interorganelle transfer and glycosylation of yeast invertase in vitro. Proc Natl Acad Sci USA. 1986;83:2017–2021. doi: 10.1073/pnas.83.7.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot LM, Ting J, Lee AS. Identity of the immunoglobulin heavy-chain-binding protein with the 78,000-dalton glucose-regulated protein and the role of posttranslational modifications in its binding function. Mol Cell Biol. 1988;8:4250–4256. doi: 10.1128/mcb.8.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller MM, Finger A, Schweiger M, Wolf DH. ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science. 1996;273:1725–1728. doi: 10.1126/science.273.5282.1725. [DOI] [PubMed] [Google Scholar]
- Hong E, Davidson AR, Kaiser CA. A pathway for targeting soluble misfolded proteins to the yeast vacuole. J Cell Biol. 1996;135:623–633. doi: 10.1083/jcb.135.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horazdovsky BF, Emr SD. The VPS16 gene product associates with a sedimentable protein complex and is essential for vacuolar protein sorting in yeast. J Biol Chem. 1993;268:4953–4962. [PubMed] [Google Scholar]
- Hughes MN, Poole RK. Metal speciation and microbial growth - the hard (and soft) facts. J Gen Microbiol. 1991;137:725–734. [Google Scholar]
- Immervoll T, Gentzsch M, Tanner W. PMT3 and PMT4, two new members of the protein-O-mannosyltransferase gene family of Saccharomyces cerevisiae. Yeast. 1995;11:1345–1351. doi: 10.1002/yea.320111403. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ, Swaroop M, Murtha-Riel P. Depletion of manganese within the secretory pathway inhibits O-linked glycosylation in mammalian cells. Biochemistry. 1994;33:9813–9819. doi: 10.1021/bi00199a001. [DOI] [PubMed] [Google Scholar]
- Kitamoto K, Yoshizawa K, Ohsumi Y, Anraku Y. Dynamic aspects of vacuolar and cytosolic amino acid pools of Saccharomyces cerevisiae. J Bacteriol. 1988;170:2683–2686. doi: 10.1128/jb.170.6.2683-2686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M, Finger A, Braun T, Hellmuth K, Wolf DH. Der1, a novel protein specifically required for endoplasmic reticulum degradation in yeast. EMBO J. 1996;15:753–763. [PMC free article] [PubMed] [Google Scholar]
- Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- Kuranda MJ, Robbins PW. Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J Biol Chem. 1991;266:19758–19767. [PubMed] [Google Scholar]
- Lapinskas PJ, Cunningham KW, Liu XF, Fink GR, Culotta VC. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol Cell Biol. 1995;15:1382–1388. doi: 10.1128/mcb.15.3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS. Coordinated regulation of a set of genes by glucose and calcium ionophores in mammalian cells. Trends Biochem Sci. 1987;12:20–23. [Google Scholar]
- Leustek T, Toledo H, Brot N, Weissbach H. Calcium-dependent autophosphorylation of the glucose-regulated protein, Grp78. Arch Biochem Biophys. 1991;289:256–261. doi: 10.1016/0003-9861(91)90469-y. [DOI] [PubMed] [Google Scholar]
- Lodish HF, Kong N. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J Biol Chem. 1990;265:10893–10899. [PubMed] [Google Scholar]
- Loukin S, Kung C. Manganese effectively supports yeast cell-cycle progression in place of calcium. J Cell Biol. 1995;131:1025–1037. doi: 10.1083/jcb.131.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcusson EG, Horazdovsky BF, Cereghino JL, Gharakhanian E, Emr SD. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell. 1994;77:579–586. doi: 10.1016/0092-8674(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Mori K, Sant A, Kohno K, Normington K, Gething MJ, Sambrook JF. A 22 bp cis-acting element is necessary and sufficient for the induction of the yeast KAR2 (BiP) gene by unfolded proteins. EMBO J. 1992;11:2583–2593. doi: 10.1002/j.1460-2075.1992.tb05323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutin MJ, Cuillel M, Rapin C, Miras R, Anger M, Lompre AM, Dupont Y. Measurements of ATP binding on the large cytoplasmic loop of the sarcoplasmic reticulum Ca2+-ATPase overexpressed in Escherichia coli. J Biol Chem. 1994;269:11147–11154. [PubMed] [Google Scholar]
- Nakajima T, Ballou CE. Yeast manno-protein biosynthesis: solubilization and selective assay of four mannosyltransferases. Proc Natl Acad Sci USA. 1975;72:3912–3916. doi: 10.1073/pnas.72.10.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari J, Walter P. Regulation of organelle biogenesis. Cell. 1996;84:389–394. doi: 10.1016/s0092-8674(00)81283-0. [DOI] [PubMed] [Google Scholar]
- Oda K. Calcium depletion blocks proteolytic cleavages of plasma protein precursors which occur at the Golgi and/or trans-Golgi network. Possible involvement of Ca2+-dependent Golgi endoproteases. J Biol Chem. 1992;267:17465–17471. [PubMed] [Google Scholar]
- Ohsumi Y, Anraku Y. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1983;258:5614–5617. [PubMed] [Google Scholar]
- Okorokov LA, Letrikevich SB, Lichko LP, Mel’nikova EV. Vacuolar pool of magnesium in cells of the yeast Saccharomyces cereviciae. Biol Bull Acad Sci USSR. 1978;5:638–640. [PubMed] [Google Scholar]
- Paltauf F, Kohlwein SD, Hery SA. In: In The Molecular and Cellular Biology of the Yeast Saccharomyces. Jones EW, Pringle J, Broach JR, editors. Vol. 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1992. pp. 415–500. [Google Scholar]
- Parodi AJ. Biosynthesis of yeast mannoproteins. Synthesis of mannan outer chain and of dolichol derivatives. J Biol Chem. 1979;254:8343–8352. [PubMed] [Google Scholar]
- Pezzati R, Bossi M, Podini P, Meldolesi J, Grohovaz F. High-resolution calcium mapping of the endoplasmic reticulum-Golgi- exocytic membrane system. Electron energy loss imaging analysis of quick frozen-freeze dried PC12 cells. Mol Biol Cell. 1997;8:1501–1512. doi: 10.1091/mbc.8.8.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer DR, Lardy HA. Ionophore A23187: the effect of H+ concentration on complex formation with divalent and monovalent cations and the demonstration of K+ transport in mitochondria mediated by A23187. Biochemistry. 1976;15:935–943. doi: 10.1021/bi00650a001. [DOI] [PubMed] [Google Scholar]
- Plemper RK, Böhmler S, Bordallo J, Sommer T, Wolf DH. Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature. 1997;388:891–895. doi: 10.1038/42276. [DOI] [PubMed] [Google Scholar]
- Rudolph HK, Antebi A, Fink GR, Buckley CM, Dorman TE, LeVitre J, Davidow LS, Mao JI, Moir DT. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989;58:133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- Sambrook JF. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell. 1990;61:197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- Schröder S, Schimmöller F, Singer-Krüger B, Riezman H. The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret1–1 mutation in α-COP. J Cell Biol. 1995;131:895–912. doi: 10.1083/jcb.131.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamu CE. Splicing together the unfolded-protein response. Curr Biol. 1997;7:R67–70. doi: 10.1016/s0960-9822(06)00038-8. [DOI] [PubMed] [Google Scholar]
- Shamu CE, Cox JS, Walter P. The unfolded-protein-response pathway in yeast. Trends Cell Biol. 1994;4:56–60. doi: 10.1016/0962-8924(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Sharma CB, Babczinski P, Lehle L, Tanner W. The role of dolicholmonophosphate in glycoprotein biosynthesis in Saccharomyces cerevisiae. Eur J Biochem. 1974;46:35–41. doi: 10.1111/j.1432-1033.1974.tb03594.x. [DOI] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Hicks J. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
- Smith RA, Duncan MJ, Moir DT. Heterologous protein secretion from yeast. Science. 1985;229:1219–1224. doi: 10.1126/science.3939723. [DOI] [PubMed] [Google Scholar]
- Sommer T, Wolf DH. ER-degradation: reverse protein flow of no return. FASEB J. 1997;11:1227–1233. doi: 10.1096/fasebj.11.14.9409541. [DOI] [PubMed] [Google Scholar]
- Sorin A, Rosas G, Rao R. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J Biol Chem. 1997;272:9895–9901. doi: 10.1074/jbc.272.15.9895. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Kawasaki T, Yamashina I. Purification and characterization of UDP-GalNAc:polypeptide N-acetylgalactosamine transferase from an ascites hepatoma, AH 66. J Biol Chem. 1982;257:9501–9507. [PubMed] [Google Scholar]
- Supply P, Wach A, Thinès-Sempoux D, Goffeau A. Proliferation of intracellular structures upon overexpression of the PMA2 ATPase in Saccharomyces cerevisiae. J Biol Chem. 1993;268:19744–19752. [PubMed] [Google Scholar]
- Suzuki CK, Bonifacino JS, Lin AY, Davis MM, Klausner RD. Regulating the retention of T-cell receptor alpha chain variants within the endoplasmic reticulum: Ca2+-dependent association with BiP. J Cell Biol. 1991;114:189–205. doi: 10.1083/jcb.114.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Vida TA, Graham TR, Emr SD. In vitro reconstitution of intercompartmental protein transport to the yeast vacuole. J Cell Biol. 1990;111:2871–2884. doi: 10.1083/jcb.111.6.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham LJ. Taxonomy of Yeasts. United States Department of Agriculture Technical Bulletin, 1029. Washington DC: US Department of Agriculture; 1951. [Google Scholar]
- Wileman T, Kane LP, Carson GR, Terhorst C. Depletion of cellular calcium accelerates protein degradation in the endoplasmic reticulum. J Biol Chem. 1991;266:4500–4507. [PubMed] [Google Scholar]