Abstract
A chemo- and regioselective copper-catalyzed cross-coupling reaction for effective amination of 2-chlorobenzoic acids with aniline derivatives has been developed. The method eliminates the need for acid protection and produces a wide range of N-aryl anthranilic acid derivatives in up to 99%. The amination was found to proceed with both electron-rich and electron-deficient aryl chlorides and anilines and also utilizes sterically hindered anilines such as 2,6-dimethylaniline and 2-tert-butylaniline. The conformational isomerism of appropriately substituted N-aryl anthranilic acids has been investigated in the solid state. Crystallographic analysis of seven anthranilic acid derivatives showed formation of two distinct supramolecular architectures exhibiting trans-anti- and unprecedented trans-syn-dimeric structures.
Introduction
Cross-coupling reactions resulting in carbon-heteroatom bond formation have received increasing attention in recent years.1 Significant progress has been reported for Pd-catalyzed amination reactions proceeding with aryl halides under mild conditions.2 Alternatively, copper-mediated cross-coupling reactions utilizing anilines,3 amides,4 imidazoles5 and other heterocycles,6 β-amino alcohols,7 and aliphatic amines have also been developed.8
Copper-mediated amination of 2-chlorobenzoic acid was first accomplished by Ullmann.9 Since then, various efforts to improve the efficiency of this reaction involving the use of ultrasound-assisted methods have been reported.10 We have recently reported a copper-catalyzed amination protocol for the synthesis of substituted N-aryl anthranilic acids, which were further utilized as precursors for ring construction of 9-chloro- and 9-bromoacridines.11 N-Aryl anthranilic acids, such as flufenamic and mefenamic acid, are important non-steroidal anti-inflammatory drugs (NSAIDs) and promising candidates for the therapy of neurodegenerative and amyloid diseases (Figure 1).12 They are also important precursors for the synthesis of substituted acridines which have been used as antimalarial and anticancer drugs.13 Buchwald et al. developed an effective Pd-catalyzed amination procedure that is applicable to aryl chlorides with free carboxylic acid groups in meta- or para-position.14 A complementary copper-catalyzed amination reaction that proceeds with ortho-chlorobenzoic acid albeit with only moderate yields has been reported.15 As a result, N-aryl anthranilic acid derivatives are usually prepared through Ullmann-Jourdan reaction or Buchwald-Hartwig amination with alkyl 2-iodobenzoates and subsequent hydrolysis.16 We wish to report a general synthetic procedure providing substituted N-aryl anthranilic acids in high to excellent yields via Cu-catalyzed amination of ortho-chlorobenzoic acids. Through isothermal evaporation, we were able to obtain single crystals of a series of N-aryl anthranilic acid derivatives. Crystallographic analysis revealed two distinctive packing motifs exhibiting either a syn- or an anti-arrangement of anthranilic acid dimers which are stabilized by two centrosymmetric C=O···H-O hydrogen bonds. The unprecedented interdependence of the dimeric arrangement and crystal structure of substituted anthranilic acids is closely related to supramolecular conformational isomerism.
Figure 1.
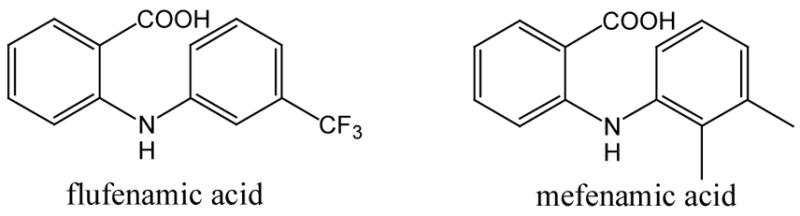
Structures of anthranilic acid derivatives used as NSAIDs and for the treatment of amyloidogenic diseases.
Results and Discussion
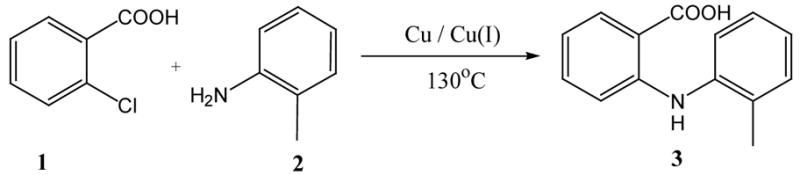
For initial screening of suitable reaction conditions, we decided to study formation of N-(2-methylphenyl)anthranilic acid, 3, from 2-chlorobenzoic acid, 1, and 2-methylaniline, 2, through copper-catalyzed amination using potassium carbonate as the base and 2-ethoxyethanol as solvent. The desired anthranilic acid 3 was obtained in moderate yields with catalytic amounts of CuI, Cu2O or Cu (Table 1, entries 1–3). However, results improved significantly in the presence of both Cu and Cu2O and 3 was produced in 71% and 76% after 5 and 24 hours, respectively (Table 1, entries 5 and 7). Variation of the Cu/Cu2O-ratio and replacement of Cu2O by CuI did not further increase yields (Table 1, entries 4, 6–9).
Table 1.
Copper-catalyzed amination of 2-chlorobenzoic acida
 | |||
|---|---|---|---|
| entry | catalyst (mol %) | reaction time (h) | yield (%)b |
| 1 | CuI (4%) | 24 | 55 |
| 2 | Cu2O (4%) | 24 | 56 |
| 3 | Cu (9%) | 24 | 55 |
| 4 | Cu (9%), CuI (4%) | 24 | 51 |
| 5 | Cu (9%), Cu2O (4%) | 24 | 76 |
| 6 | Cu (12%), Cu2O (4%) | 5 | 66 |
| 7 | Cu (9%), Cu2O (4%) | 5 | 71 |
| 8 | Cu (6%), Cu2O (4%) | 5 | 51 |
| 9 | Cu (4%), Cu2O (4%) | 5 | 51 |
Reaction conditions: 2-Chlorobenzoic acid (1.38 g, 8.83 mmol), 1.05 equiv. of 2-methylaniline, 2.0 equiv. of potassium carbonate in 3 mL of 2-ethoxyethanol at 130°C.
Isolated yields.
We then decided to study the effect of solvent and base on the amination reaction. Anthranilic acid 3 was obtained in 75% and 76%, respectively, using Cu/Cu2O as the catalyst in n-butanol or 2-ethoxyethanol in the presence of two equivalents of potassium carbonate, while only poor yields were obtained in water and ethylene glycol (Table 2, entries 1–4). We were pleased to find that yields of 3 could be enhanced to 90% when diethylene glycol or 2-ethoxyethanol was used as the solvent (Table 2, entries 5 and 12). The Cu/Cu2O-catalyzed amination of 2-chlorobenzoic acid proved to depend significantly on the base employed. Comparison of the results obtained with Na2CO3, Cs2CO3, K3PO4, NaOAc, tert-BuOK, and 2,2,6,6-tetramethylpiperidine (TMP) shows that yields of 3 obtained in diethylene glycol vary from 5–90% (Table 2, entries 5–11). We found that anthranilic acid 3 was produced in only 5–30% in the presence of cesium carbonate, 2,2,6,6-tetramethylpiperidine and tert-BuOK (Table 2, entries 6, 8 and 11). By contrast, anthranilic acid 3 was formed in 90% and 82% through Cu/Cu2O-catalyzed amination of 1 with 2 using K2CO3 or Na2CO3 in diethylene glycol (Table 2, entries 5 and 7). We also observed that the amination proceeds with excellent yields with one equivalent of sodium or potassium carbonate in 2-ethoxyethanol (Table 2, entries 12–14).
Table 2.
Screening of solvents and basesa
 | ||||
|---|---|---|---|---|
| entry | base | solvent | T (°C) | yield (%)b |
| 1 | K2CO3 | n-butanol | 117 | 75 |
| 2 | K2CO3 | water | 100 | 35 |
| 3 | K2CO3 | ethylene glycol | 130 | 40 |
| 4 | K2CO3 | 2-ethoxyethanol | 130 | 76 |
| 5 | K2CO3 | diethylene glycol | 130 | 90 |
| 6 | Cs2CO3 | diethylene glycol | 130 | 5 |
| 7 | Na2CO3 | diethylene glycol | 130 | 82 |
| 8 | tert-BuOK | diethylene glycol | 130 | 11 |
| 9 | K3PO4 | diethylene glycol | 130 | 70 |
| 10 | NaOAc | diethylene glycol | 130 | 66 |
| 11 | TMP | diethylene glycol | 130 | 30 |
| 12 | Na2CO3 | 2-ethoxyethanol | 130 | 92d |
| 13 | K2CO3 | 2-ethoxyethanol | 130 | 75c |
| 14 | K2CO3 | 2-ethoxyethanol | 130 | 83c,d |
Reaction conditions: 2-Chlorobenzoic acid (1.38 g, 8.83 mmol), 1.05 equiv. of 2-methylaniline, 9 mol% of Cu, 4 mol% of Cu2O, 2.0 equiv. of base, 3 mL of solvents, 130°C for 24 hours.
Isolated yields.
Aniline was used.
1.0 equiv. of base was used.
The copper-catalyzed C-N bond formation procedure was then applied to a variety of aryl amines and chlorobenzoic acids to produce a series of N-arylanthranilic acids (Table 3). We found that the presence of one bulky ortho-substituent does not impede effective amination (Table 3, entries 2–5). For example, 2-tert-butylaniline, 8, and 2-phenylaniline, 10, gave N-(2-tert-butylphenyl)anthranilic acid, 9, in 86% and N-(2-biphenyl)anthranilic acid, 11, in 85% yield (Table 3, entries 4 and 5). Incorporation of two ortho-substituents to aniline, however, was found to decrease yields, e.g. N-(2,6-dimethylphenyl)anthranilic acid, 13, was obtained in only 65% (Table 3, entry 6). Coupling of 1-aminonaphthalene or 1-aminopyrene gave the corresponding products 21 and 23 in 96% and 73% yields (Table 3, entries 10 and 11). Importantly, no significant electronic effects were observed in the reaction of substituted anilines and chlorobenzoic acids. For example, amination of 1 with 4-methoxyaniline, 24, and 4-nitroaniline, 26, proceeded with 84% and 87%, respectively, and electron-deficient chlorobenzoic acid 32 gave anthranilic acid 33 in 99% yield (Table 3, entries 12, 13 and 16). The copper-catalyzed amination was found to proceed with high regio- and chemoselectivity. Amination only occurs when the halide is located in ortho-position to the carboxylic acid moiety. For example, coupling of 2,4-dichlorobenzoic acid, 34, with 4-methoxyaniline, 24, gave anthranilic acid 35 in 86% and amination of 5-bromo-2-chlorobenzoic acid, 36, produced 37 in 85% yield (Table 3, entries 17 and 18). Aryl halide bonds located in the aniline moiety are also not affected. The coupling reaction between 2-chlorobenzoic acid and 3-chloroaniline, 28, gave 29 in 99% yield (Table 3, entry 14). The ortho-carboxylate group has been known to effectively accelerate homogeneous copper-catalyzed exchange reactions.17 Accordingly, our amination procedure affords high regioselectivity and tolerates various functionalities thus complementing other methods that allow effective amination of meta- and para-chlorobenzoic acids.
Table 3.
Arylation of aryl chlorides with aryl aminesa
 | ||||
|---|---|---|---|---|
| entry | aryl chloride | amine | product | Yield (%)b |
| 1 |
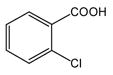
1 |

4 |
5 |
83 |
| 2 |

1 |
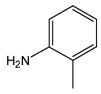
2 |

3 |
92 c |
| 3 |

1 |

6 |
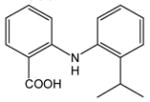
7 |
73 |
| 4 |

1 |

8 |

9 |
86 |
| 5 |

1 |
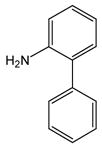
10 |

11 |
85 |
| 6 |

1 |

12 |

13 |
65 |
| 7 |

1 |

14 |

15 |
80 |
| 8 |

1 |

16 |

17 |
85 |
| 9 |

1 |
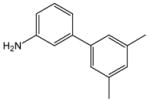
18 |

19 |
99 |
| 10 |

1 |
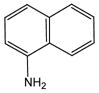
20 |

21 |
96 |
| 11 |

1 |

22 |

23 |
73 |
| 12 |

1 |

24 |

25 |
84 |
| 13 |

1 |
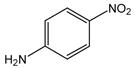
26 |

27 |
87 |
| 14 |

1 |

28 |

29 |
99 |
| 15 |

1 |
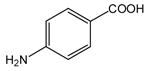
30 |

31 |
98d |
| 16 |

32 |
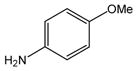
24 |
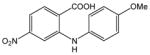
33 |
99 |
| 17 |
34 |

24 |

35 |
86 |
| 18 |

36 |
24 |
37 |
85 |
Reaction conditions: 2-Chlorobenzoic acid (1.38 g, 8.83 mmol), 1.05 equiv. of amine, 1.0 equiv. of K2CO3, 9 mol% of Cu, 4 mol% of Cu2O, 3 mL of 2-ethoxyethanol and 130°C for 24 hours.
Isolated yields.
Na2CO3 was used as base.
2.0 equiv. of base were used
Carboxylic acids are known to form centrosymmetric dimers in solution and in the solid state in order to minimize the overall dipole moment.18 In the case of substituted N-aryl anthranilic acids, dimer formation could lead to two geometrical arrangements isolable in the solid state, namely cis- and probably favored trans-isomers exhibiting the N-aryl substituents on the same or on opposite sides. In addition, both trans- and cis-isomers could exhibit either syn- or anti-orientation having substituted N-aryl groups either pointing up or down relative to the anthranilic plane as shown for N-(2-methylphenyl)anthranilic acid, 3 (Figure 2).
Figure 2.

Possible arrangements of dimers of 3.
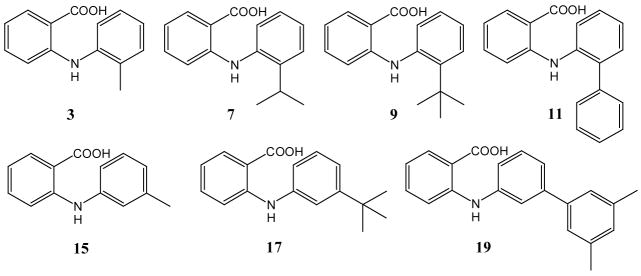
Supramolecular isomerism has been defined as the existence of different types of crystal architectures produced from the same molecular building blocks and is closely related to molecular isomerism and polymorphism.19 The desire to understand and rationally design solid-state structures has resulted in remarkable advances in crystal engineering and supramolecular synthesis.20 The possibility of four isomeric dimeric structures of anthranilic acids has led us to the study of solid-state conformational isomerism. We therefore employed appropriately substituted anthranilic acids 3, 7, 9, 11, 15, 17, 19, 21, and 29 in crystallization experiments. Through isothermal evaporation from methylene chloride solutions, we were able to grow single crystals of seven anthranilic acids (Figure 3). Anthranilic acids 3 and 11 formed monoclinic systems while the others gave triclinic crystals (Table 4). As expected, dimeric N-arylanthranilic acid structures were obtained in all cases. The dimeric arrangement of 3, 7, 9, 11, and 15 are of the centrosymmetric trans-anti-type. A pair of C=O···H-O hydrogen bonds and C=O···H-N hydrogen forces both anthranilic rings into the same plane. The unit cell of the single crystal of 3 contains eight molecules forming four centrosymmetric dimers (Figure 4). Through a Cambridge Structural Database (CSD) search we found five additional N-phenylanthranilic acid derivatives showing acid dimers with the same trans-anti-arrangement in the solid state.21
Figure 3.
Structures of N-phenylanthranilic acid derivatives employed in single crystal analysis.
Table 4.
Crystallographic parameters of anthranilic acid derivatives
| compound name | 3 | 7 | 9 | 11 | 15 | 17 | 19 |
|---|---|---|---|---|---|---|---|
| formula | C14H13NO2 | C16H17NO2 | C17H19NO2 | C19H15NO2 | C14H13NO2 | C17H19NO2 | C21H19NO2 |
| mol wt | 227.25 | 255.31 | 269.33 | 289.32 | 227.25 | 269.33 | 317.37 |
| temp (K) | 173 | 183 | 173 | 183 | 173 | 163 | 173 |
| system | Monoclinic | Triclinic | Triclinic | Monoclinic | Triclinic | Triclinic | Triclinic |
| space group | C2/c | P-1 | P-1 | P21/c | P-1 | P-1 | P-1 |
| a (Å) | 11.7804(16) | 7.4585(10) | 7.4748(12) | 5.7231(8) | 4.6299(14) | 10.9634(7) | 10.5769(16) |
| b (Å) | 7.4503(10) | 7.5891(10) | 9.3856(15) | 17.929(2) | 8.332(2) | 11.7048(7) | 11.4108(17) |
| c (Å) | 26.627(3) | 13.6653(19) | 11.0319(17) | 14.4300(19) | 15.336(4) | 22.9688(14) | 14.162(2) |
| α(°) | 90 | 79.998(2) | 106.582(3). | 90° | 98.466(4) | 88.8990(10) | 80.856(3) |
| β(°) | 100.168(2) | 85.875(2) | 91.207(3) | 101.064(2) | 92.198(4) | 89.0970(10) | 82.334(3) |
| γ(°) | 90 | 62.141(2) | 99.212(3) | 90 | 101.337(4) | 82.0050(10) | 84.982(3) |
| V (Å3) | 2300.3(5) | 673.42(16) | 730.4(2) | 1453.2(3) | 572.3(3) | 2918.0(3) | 1668.5(4) |
| Z | 8 | 2 | 2 | 4 | 2 | 8 | 4 |
| ρ (g/cm3) | 1.312 | 1.259 | 1.225 | 1.322 | 1.319 | 1.226 | 1.263 |
| θ range (°) | 1.55–27.99 | 1.51–27.99 | 1.93–28.00 | 1.83–27.99 | 2.52–28.00 | 1.76–28.00 | 1.95–27.99 |
| index ranges | −15 ≤ h ≤ 15 | −9 ≤ h ≤ 9 | −9 ≤ h ≤ 9 | −7 ≤ h ≤ 7 | −76≤ h ≤ 6 | −14 ≤ h ≤ 14 | −13 ≤ h ≤ 13 |
| −9 ≤ k ≤ 9 | −9 ≤ k ≤ 10 | −12 ≤ k ≤ 12 | −23 ≤ k ≤ 22 | −10 ≤ k ≤ 11 | −15 ≤ k ≤ 15 | −14 ≤ k ≤ 14 | |
| −35 ≤ l ≤ 34 | −17 ≤ l ≤ 18 | −14 ≤ l ≤ 14 | −18 ≤ l ≤ 19 | −20 ≤ l ≤ 19 | −30 ≤ l ≤ 29 | −18 ≤ l ≤ 18 | |
| reflns collected | 9959 | 6018 | 6614 | 12467 | 4861 | 25329 | 14927 |
| unique reflns | 2714 | 3054 | 3338 | 3401 | 2552 | 13122 | 7547 |
| R(int) | 0.0410 | 0.0282 | 0.0231 | 0.0430 | 0.0211 | 0.0345 | 0.0309 |
| refined params | 155 | 174 | 192 | 199 | 155 | 733 | 445 |
| GOF on F2 | 1.020 | 0.914 | 1.060 | 1.027 | 0.980 | 1.045 | 1.032 |
| R1 [I>2σ (I)] | 0.0483 | 0.0462 | 0.0414 | 0.0463 | 0.0442 | 0.0618 | 0.0460 |
| wR2 (all data) | 0.1409 | 0.1228 | 0.1073 | 0.1127 | 0.1140 | 0.2060 | 0.1154 |
Figure 4.
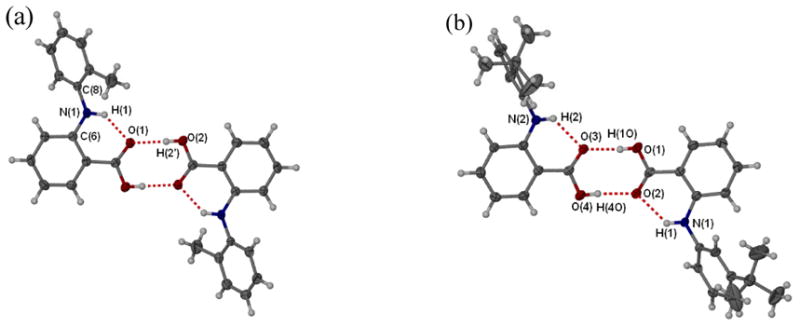
ORTEP perspectives of anthranilic acid derivatives: (a) N-(2-methylphenyl)anthranilic acid 3 and (b) N-(3-tert-butylphenyl)anthranilic acid 17. Selected interatomic distances [Å] and angles [°]: (a) O(1)···O(2) 2.224, N(1)···O(1) 2.636, O(2)-H(2′)···O(1) 172.79, N(1)-H(1)···O(1) 138.32; (b) O(1)···O(3) 2.643, O(2)···O(4) 2.707, N(1)···O(2) 2.733, N(2)···O(3) 2.692, O(4)-H(4O)···O(2) 164.42, N(1)-H(1)···O(2) 132.77.
The dihedral angle, α, between the 2-methylphenyl moiety and the anthranilic ring of 3 was determined as 49.1° (Figure 5). Changing the ortho-substituent from methyl to iso-propyl, tert-butyl and phenyl did not affect the supramolecular architecture and molecular isomerism. However, the dihedral angle, α, was found to change from 49.1° (3) to 62.5° (7), 62.8° (9) and 77.1° (11) (Figure 5). While the dihedral angle increases with the bulkiness of the ortho-substituent, the almost perpendicular arrangement of the phenyl group is stabilized by additional π-π interactions between adjacent dimers (supporting information).
Figure 5.
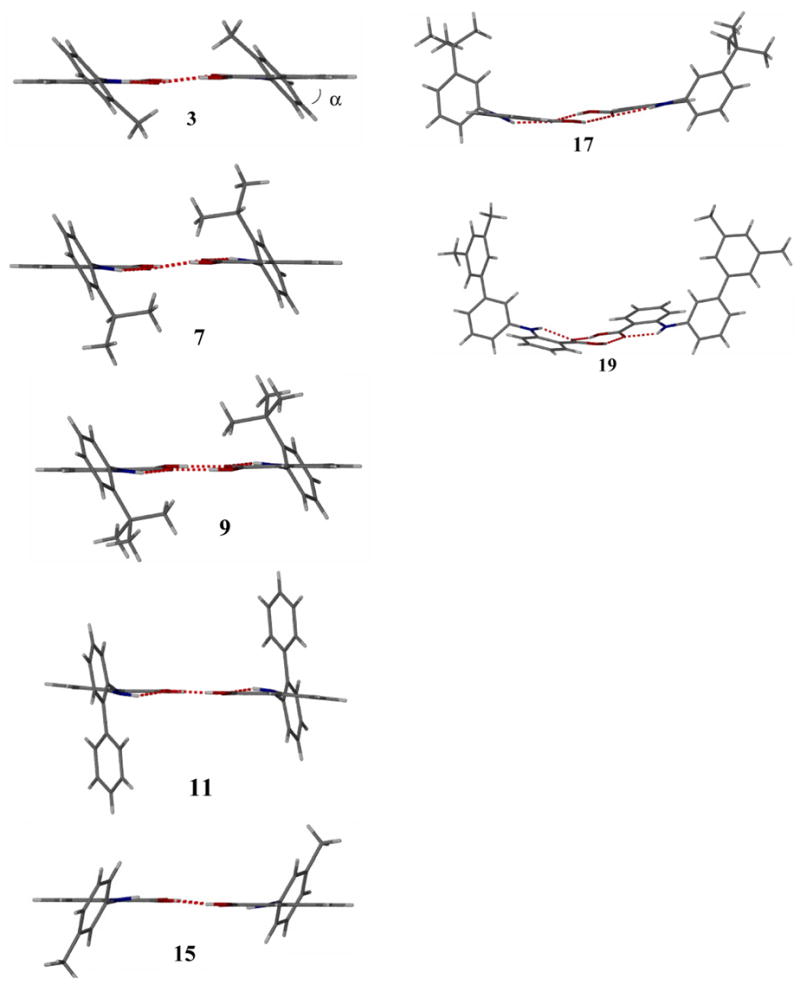
X-ray crystal structures of anthranilic acid 3, 7, 9, 11, 15, 17, and 19 exhibiting trans-anti- (left) and trans-syn-dimeric conformations (right).
The trans-anti-dimers of 3, 7, 11, and 15 show one-dimensional π-π interactions between the anthranilic aromatic rings (Figure 6). Anthranilic acid 3 formed π-stacking rods with a closest contact distance of 3.46 Å. Anthranilic acids 7, 11, and 15 have a similar packing arrangement and the corresponding closest contact distance was determined as 3.50 Å, 3.40 Å, and 3.46 Å, respectively. However, the bulky tert-butyl group of anthranilic 9 impedes effective π-stacking which is replaced by van der Waals interactions (supporting information).
Figure 6.
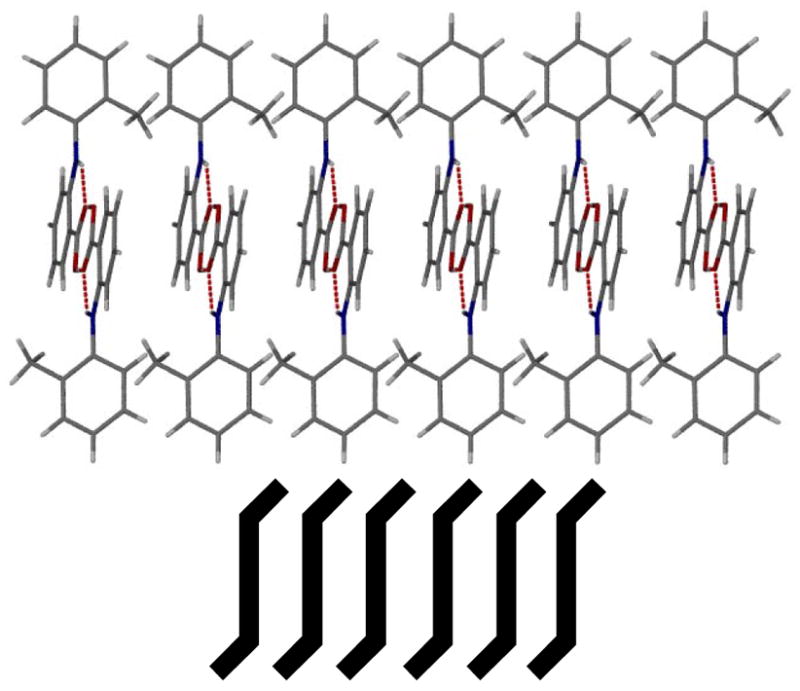
One-dimensional π-stacking of anthranilic acid 3 (top) and schematic illustration of the trans-anti-dimers and packing motif (bottom).
Surprisingly, anthranilic acids 17 and 19 crystallized as non-centrosymmetric trans-syn-dimers (Figure 5). The conformational isomerism is accompanied by a quite distinct crystal architecture. In contrast to formation of π-stacking rods observed with anthranilic acids 3, 7, 11, and 15, the crystals of 17 and 19 show one-dimensional chains stabilized by π-π-interactions between the tert-butylphenyl and 3,5-dimethylbiphenyl rings, respectively. For example, the solid-state structure of anthranilic acid 17 has a closest contact between the tert-butylphenyl rings of 3.48 Å (Figure 7). The π-stacking of the trans-syn-dimers results in infinite chains along the b axis. Empty space within one chain is filled with a complementary chain thus providing one-dimensional intertwined rods. The unexpected solid-state structures of the anthranilic acids studied resemble supramolecular conformational isomerism. While the crystal structures of acids 3, 7, 9, 11, and 15 show a previously reported packing motif exhibiting trans-anti-dimers, anthranilic acids 17 and 19 crystallized as dimeric trans-syn-conformers providing an unprecedented supramolecular architecture.
Figure 7.

Formation of intertwined rods in the crystal structure of anthranilic acid 17 (top) and schematic illustration of the packing of the trans-syn-dimers (bottom).
Conclusion
We have developed a highly regioselective Cu/Cu2O-catalyzed cross-coupling reaction for effective C-N bond formation with 2-chlorobenzoic acids and aniline derivatives. The reaction complements existing methods, tolerates a variety of functional groups and gives high to excellent yields with both electron-deficient and electron-rich reagents. Crystallographic analysis of seven substituted N-aryl anthranilic acids showed two distinct supramolecular structures due to formation of trans-anti- and trans-syn-dimers in the solid state.
Experimental Section
General procedure for the Cu-catalyzed amination of aryl halides
A mixture of aniline (9.30 mmol), aryl halide (8.83 mmol), K2CO3 (8.83 mmol), Cu powder (0.78 mmol), and Cu2O (0.35 mmol) in 3 mL of 2-ethoxyethanol was refluxed at 130°C under inert atmosphere for 24 hours. The cooled reaction mixture was poured into 30 mL of water. Charcoal was then added and the solution was filtered through celite. The crude product was obtained by precipitation upon acidification of the filtrate with diluted HCl. The residue was dissolved in 100 mL of 5 % aqueous Na2CO3 solution, filtered through celite and the final product was isolated by precipitation upon careful pH adjustment.
N-(2-Methylphenyl)anthranilic acid (3).12a
Compound 3 was obtained from 2-chlorobenzoic acid, 1, and 2-methylaniline, 2, as an off-white powder in 92% yield. 1H-NMR (300 MHz, CDCl3) δ = 2.29 (s, 3H), 6.72 (bs, 1H), 6.85 (d, J = 8.2 Hz, 1H), 7.12 (dd, J = 7.2 Hz, 7.4 Hz, 1H), 7.20–7.34 (m, 5H), 8.05 (d, J = 7.2 Hz, 1H), 9.18 (bs, 1H). 13C-NMR (75 MHz, DMSO-d6) δ = 17.6, 113.4, 116.9, 122.6, 124.0, 126.6, 130.9, 131.3, 134.0, 138.7, 148.6.
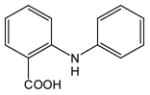
N-Phenylanthranilic acid (5).22
Anthranilic acid 5 was obtained from 2-chlorobenzoic acid, 1 and aniline, 4 as a white solid in 83% yield. 1H-NMR (300 MHz, CDCl3) δ = 6.75 (dd, J = 7.9 Hz, 8.3 Hz, 1H), 7.13 (dd, J = 7.3 Hz, 8.6 Hz, 1H), 7.20–7.50 (m, 5H), 8.05 (d, J = 7.6 Hz, 1H), 9.33 (bs, 1H). 13C-NMR (75 MHz, CDCl3) δ = 111.0, 114.7, 117.8, 123.8, 124.7, 130.0, 133.2, 135.8, 140.9, 149.5, 174.3.
N-(2-Isopropylphenyl)anthranilic acid (7).12a
Amination of 2-chlorobenzoic acid, 1, and 2-isopropylaniline, 6, gave anthranilic acid 7 as a white powder in 73% yield. 1H-NMR (300 MHz, CDCl3) δ = 1.22 (d, J = 6.9 Hz, 6H), 3.21 (sept, J = 6.9 Hz, 1H), 4.68 (bs, 1H), 6.68 (dd, J = 7.2 Hz, 7.4 Hz, 1H), 6.81 (d, J = 8.2 Hz, 1H), 7.22–7.40 (m, 4H), 8.1 (dd, J = 1.7 Hz, 8.2 Hz, 1H), 9.18 (bs, 1H). 13C-NMR (75 MHz, DMSO-d6) δ = 23.6, 28.4, 112.0, 113.6, 117.0, 125.7, 126.1, 127.1, 127.3, 132.5. 135.0, 137.9, 143.6, 149.8, 171.0.
N-(2-tert-Butylphenyl)anthranilic acid (9).23
Anthranilic acid 9 was produced from 2-chlorobenzoic acid, 1, and 2-tert-butylaniline, 8, as white crystals in 86% yield. 1H-NMR (300 MHz, CDCl3) δ = 1.41 (s, 9H), 6.65 (dd, J = 7.0 Hz, 7.9 Hz, 1H), 7.15 (d, J = 8.6 Hz, 1H), 7.17–7.28 (m, 4H), 7.47–7.50 (m, 1H), 8.02 (d, J = 8.1 Hz, 1H), 9.21 (bs, 1H). 13C-NMR (75 MHz, CDCl3) δ = 31.3, 35.7, 110.0, 114.8, 116.7, 126.6, 127.6, 128.1, 129.8, 133.1, 135.9, 139.4, 147.1, 151.4, 173.7.
N-(2-Biphenyl)anthranilic acid (11).24
Acid 11 was obtained from 2-chlorobenzoic acid, 1, and 2-aminobiphenyl, 10, as a white solid in 85% yield. 1H-NMR (300 MHz, CDCl3) δ = 4.74 (bs, 1H), 6.72 (dd, J = 7.2 Hz, 7.7 Hz, 1H), 7.20–7.52 (m, 11H), 7.94 (d, J = 8.3 Hz, 1H), 9.18 (bs, 1H). 13C-NMR (75 MHz, CDCl3) δ = 111.4, 114.7, 117.7, 123.7, 124.8, 128.0, 128.6, 129.0, 129.7, 131.7, 133.1, 135.6, 136.9, 138.2, 139.5, 149.3, 174.2.
N-(2,6-Dimethylphenyl)anthranilic acid (13).25
Amination of 2-chlorobenzoic acid, 1, and 2,6-dimethylaniline, 12, afforded acid 13 as a white powder in 65% yield. 1H-NMR (300 MHz, CDCl3) δ = 2.21 (s, 6H), 6.22 (d, J = 8.5 Hz, 1H), 6.66 (dd, J = 7.1 Hz, 7.1 Hz, 1H), 7.10–7.20 (m, 3H), 7.24 (dd, J = 7.6 Hz, 7.8 Hz, 1H), 8.04 (d, J = 7.6 Hz, 1H), 8.88 (bs, 1H). 13C-NMR (75 MHz, CD3OD) δ = 18.5, 112.0, 115.7, 126.3, 128.3, 132.8, 134.1, 135.6, 135.9, 137.0, 149.9, 173.8.
N-(3-Methylphenyl)anthranilic acid (15).25
Compound 15 was obtained from 2-chlorobenzoic acid, 1, and 3-methylaniline, 14, as a white solid in 80% yield. 1H-NMR (300 MHz, CDCl3) δ = 2.39 (s, 3H), 6.79 (bs, 1H), 6.97 (d, J = 7.31 Hz, 1H), 7.00–7.15 (m, 2H), 7.25–7.30 (m, 2H), 7.38 (m, 1H), 8.10 (m, 1H), 9.25 (bs, 1H). 13C-NMR (75 MHz, DMSO-d6) δ = 21.0, 113.9, 117.3, 118.3, 122.0, 123.8, 129.2, 132.5, 134.0, 138.9, 140.5, 147.5.
N-(3-tert-Butylphenyl)anthranilic acid (17)
Acid 17 was produced by amination of 2-chlorobenzoic acid, 1, and 3-tert-butylaniline, 16, as a white solid in 85% yield. 1H NMR(300 MHz, CDCl3) δ =1.34 (s, 9H), 6.74 (dd, J = 0.7 Hz, 7.4 Hz, 1H), 7.10 (d, J = 7.3 Hz, 1H), 7.16–7.37 (m, 5H), 8.05 (dd, J = 1.5 Hz, J = 8.1 Hz, 1H), 9.29 (bs, 1H). 13C-NMR(75 MHz, DMSO-d6) δ = 31.0, 34.4, 112.4, 113.5, 117.2, 118.7, 118.9, 120.3, 129.0, 132.0, 134.1, 140.1, 147.4, 152.2, 170.1. Anal. calcd. for C17H20ClNO2: C, 66.77; H,6.59; N, 4.58. Found: C, 67.27; H, 7.16; N, 4.93. (The HCl salt was formed by addition of hydrogen chloride (diethyl ether, 2.0 M) for elemental analysis.)
N-[3-(3′,5′-Dimethylbiphenyl)]anthranilic acid (19).26
Anthranilic acid 19 was obtained from 2-chlorobenzoic acid, 1, and 3-amino-3′,5′-dimethylbiphenyl, 18, as an off-white solid in 99% yield. 1H-NMR (300 MHz, CDCl3) δ = 2.39 (s, 6H), 4.43 (s, 2H), 6.77 (dd, J = 7.1 Hz, 7.6 Hz, 1H), 7.02 (s, 1H), 7.22–7.50 (m, 8H), 8.06 (d, J = 8.0 Hz, 1H). 13C-NMR (75 MHz, CDCl3) δ = 21.7, 113.7, 114.4, 115.9, 117.5, 121.9, 122.1, 123.1, 125.2, 128.7, 129.3, 129.8, 133.9, 135.3, 138.5, 140.9, 143.0, 149.1. Anal. calcd. for C21H19NO2: C, 79.50; H, 5.99; N, 4.42. Found: C, 79.10; H, 6.23; N, 4.32.
N-(1-Naphthyl)anthranilic acid (21).27
Anthranilic acid 21 was obtained from 2-chlorobenzoic acid, 1 and 1-aminonaphthalene, 20, as an off-white solid in 96% yield. 1H-NMR (300 MHz, CDCl3) δ = 6.73 (dd, J = 7.3 Hz, 7.6 Hz, 1H), 6.85(d, J = 8.6 Hz, 1H), 7.23–7.29 (m, 1H), 7.46–7.51 (m, 4H), 7.75 (d, J = 7.6 Hz, 1H), 7.89–7.92 (m, 1H), 8.08(m, 2H), 9.60 (bs, 1H). 13C-NMR (75 MHz, CDCl3) δ =110.5, 114.2, 117.5, 122.8, 123.5, 126.5, 126.5, 127.1, 129.1, 130.8, 133.1, 135.5, 136.0, 136.9, 151.2, 174.2.
N-(1-Pyrenyl)anthranilic acid (23)
Amination of 2-chlorobenzoic acid, 1, and 1-aminopyrene, 22, afforded anthranilic acid 23 as an off-white solid in 73% yield. 1H NMR (300 MHz, DMSO-d6) δ = 6.94 (dd, J = 7.4 Hz, 7.4 Hz, 1H), 7.14 (d, J = 8.3 Hz, 1H), 7.43 (dd, J = 8.0 Hz, 7.4 Hz, 1H), 8.09–8.41 (m, 10H), 10.7 (bs, 1H). 13C NMR (75 MHz, DMSO-d6): δ = 114.2, 118.1, 122.0, 122.1, 124.8, 124.9, 125.4, 125.7, 125.8, 126.4, 126.7, 127.2, 128.0, 128.2, 131.4, 131.7, 133.6, 134.6, 135.2, 148.8, 172.7. Anal. calcd. for C23H15NO2: C, 81.88; H, 4.48; N, 4.15. Found: C, 81.63; H, 4.74; N, 4.32.
N-(4-Methoxyphenyl)anthranilic acid (25).16
Anthranilic acid 25 was obtained from 2-chlorobenzoic acid, 1, and 4-methoxyaniline, 24, as a white powder in 84% yield. 1H-NMR (300 MHz, CDCl3) δ = 3.83 (s, 3H), 6.68 (dd, J = 7.3 Hz, 7.3 Hz, 1H), 6.93 (dd, J = 8.6 Hz, 8.6 Hz 3H), 7.18 (d, J = 8.6 Hz, 2H), 7.26–7.32 (m, 1H), 8.0 (d, J = 8.6 Hz, 1H)), 9.60 (bs, 1H). 13C-NMR (75 MHz, DMSO-d6) δ = 55.2, 111.4, 112.8, 114.8, 116.3, 125.1, 131.9, 133.0., 134.2, 148.9, 156.1, 170.2.
N-(4-Nitrophenyl)anthranilic acid (27).16
Anthranilic acid 27 was produced from 2-chlorobenzoic acid, 1, and 4-nitroaniline, 26, as a yellow solid in 87% yield. 1H-NMR (300 MHz, CD3OD) δ = 7.22 (dd, J = 7.1 Hz, 7.8 Hz, 1H), 7.50 (m, 2H), 7.68–7.78 (m, 2H), 8.26 (d, J = 8.1 Hz, 1H), 8.36-8.36 (m, 2H). 13C-NMR (75 MHz, CD3OD) δ = 118.1, 118.2, 118.8, 122.2, 127.1, 133.7, 135.3, 142.6, 145.4, 150.0, 171.5.
N-(3-Chlorophenyl)anthranilic acid (29).28
Amination of 2-chlorobenzoic acid, 1 and 3-chloroaniline, 28, gave anthranilic acid 29, as an off-white solid in 99% yield. 1H-NMR (300 MHz, CDCl3) δ = 6.82 (dd, J = 7.3 Hz, 7.6 Hz, 1H), 7.08 (d, J = 7.8 Hz, 1H), 7.14 (d, J = 7.8 Hz, 1H), 7.26–7.30 (m, 3H), 7.40 (dd, J = 7.6 Hz, 7.8 Hz, 1H), 8.06 (d, J = 7.3 Hz, 1H), 9.32 (bs, 1H). 13C-NMR (75 MHz, CDCl3) δ = 114.2, 114.7, 118.5, 118.8, 119.9, 122.1, 130.9, 132.0, 133.9, 134.0, 142.6, 145.8, 169.9.
N-(4-Carboxyphenyl)anthranilic acid (31).27
Anthranilic acid 31 was obtained from 2-chlorobenzoic acid, 1, and 4-aminobenzoic acid, 30, using two equivalents of K2CO3 as a white solid in 98% yield. 1H-NMR (300 MHz, CD3OD) δ = 7.00–7.06 (m, 1H), 7.37 (d, J = 8.8 Hz, 2H), 7.56–7.59 (m, 2H), 7.98 (d, J = 8.8 Hz, 2H), 8.04 (d, J = 8.3 Hz, 1H), 9.91 (bs, 1H). 13C-NMR (75 MHz, CD3OD) δ = 115.3, 116.2, 117.9, 119.6, 123.5, 131.2, 132.0, 134.1, 144.6, 145.6, 167.1, 169.7.
N-(4-Methoxyphenyl)-4-nitroanthranilic acid (33).26
Anthranilic acid 33 was obtained from 2-chloro-4-nitrobenzoic acid, 32, and 4-methoxyaniline, 24, as a yellow solid in 99% yield. 1H-NMR (300 MHz, CD3OD) δ = 3.98 (s, 3H), 5.07 (bs, 1H), 7.05–7.20 (m, 3H), 7.32 (d, J = 8.5 Hz, 2H), 7.40 (d, J = 8.8 Hz, 1H), 8.05 (s, 1H). 13C-NMR (75 MHz, CD3OD:DMSO-d6 = 10:1) δ = 55.0, 116.1, 116.2, 121.6, 126.7, 126.9, 132.5, 134.4, 135.3, 149.8, 158.6, 170.6.
N-(4-Methoxyphenyl)-4-chloroanthranilic acid (35).26
Compound 35 was produced from 2,4-dichlorobenzoic acid, 34, and 4-methoxyaniline, 24, as an off-white powder in 86% yield. 1H-NMR (300 MHz, CDCl3) δ = 3.83 (s, 3H), 6.87 (d, J = 9.2 Hz, 1H), 6.92 (d, J = 8.9 Hz, 2H), 7.15 (d, J = 8.9 Hz, 2H), 7.19–7.24 (m, 1H), 7.95 (d, J = 2.5 Hz, 1H), 9.05 (bs, 1H). 13C-NMR (75 MHz, CDCl3) δ = 56.2, 111.52, 115.4, 121.2, 126.9, 132.1, 133.1, 133.5, 135.5, 149.5, 157.7, 173.1.
N-(4-Methoxyphenyl)-4-bromoanthranilic acid (37)
Amination of 4-bromo-2-chlorobenzoic acid, 36, and 4-methoxyaniline, 24, afforded anthranilic acid 37 as an off-white solid in 85% yield. 1H-NMR (300 MHz, CDCl3) δ = 3.83 (s, 3H), 6.82 (d, J = 9.0 Hz, 1H), 6.92 (d, J = 8.5 Hz, 2H), 7.15 (d, J = 8.5 Hz, 2H), 7.30–7.35 (m, 1H), 8.10 (s, 1H), 9.10 (bs, 1H). 13C-NMR (75 MHz, DMSO-d6) δ = 55.8, 113.5, 115.4, 120.1, 125.8, 131.4, 133.3, 133.7, 134.2, 148.3, 157.0, 169.8. Anal. calcd. for C14H12BrNO3: C, 52.20; H, 3.75; N, 4.35. Found: C, 52.08; H, 3.72; N, 4.78.
Supplementary Material
Single crystal structures, NMR spectra, and CIF files. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
Funding from the National Science Foundation (CAREER Award, Grant CHE-0347368) and the donors of the Petroleum Research Fund, administered by the American Chemical Society (Grant PRF40897-G4), is gratefully acknowledged.
References and Notes
- 1.(a) Lindley J. Tetrahedron. 1984;40:1433–1456. [Google Scholar]; (b) Subramanian V, Batchu VR, Barange D, Pal M. J Org Chem. 2005;70:4778–4783. doi: 10.1021/jo050440e. [DOI] [PubMed] [Google Scholar]; (c) Klapars A, Parris S, Anderson KW, Buchwald SL. J Am Chem Soc. 2004;126:3529–3533. doi: 10.1021/ja038565t. [DOI] [PubMed] [Google Scholar]; (d) Beletskaya IP, Bessmertnykh AG, Averin AD, Denat F, Guilard R. Eur J Org Chem. 2005:281–305. [Google Scholar]
- 2.(a) Hamann BC, Hartwig JF. J Am Chem Soc. 1998;120:7369–7370. [Google Scholar]; (b) Hartwig JF, Kawatsura M, Hauck SI, Shaughnessy KH, Alcazar-Roman LM. J Org Chem. 1999;64:5575–5580. doi: 10.1021/jo990408i. [DOI] [PubMed] [Google Scholar]; (c) Wolfe JP, Buchwald SL. J Org Chem. 1997;62:6066–6068. [Google Scholar]; (d) Old DW, Wolfe JP, Buchwald SL. J Am Chem Soc. 1998;120:9722–9723. [Google Scholar]; (e) Hartwig JF. Angew Chem, Int Ed Engl. 1998;37:2046–2067. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]; (f) Wolfe JP, Wagaw S, Marcoux JF, Buchwald SL. Acc Chem Res. 1998;31:805–818. [Google Scholar]
- 3.(a) Gajare AS, Toyota K, Yoshifuji M, Ozawa F. Chem Comm. 2004:1994–1995. doi: 10.1039/b408232j. [DOI] [PubMed] [Google Scholar]; (b) Ferreira ICFR, Queiroz MJRP, Kirsch G. Tetrahedron. 2002;58:7943–7949. [Google Scholar]; (c) Gujadhur RK, Bates CG, Venkataraman D. Org Lett. 2001;42:4791–4793. doi: 10.1021/ol0170105. [DOI] [PubMed] [Google Scholar]; (d) Goodbrand HB, Hu NX. J Org Chem. 1999;64:670–674. [Google Scholar]
- 4.Klapars A, Huang X, Buchwald SL. J Am Chem Soc. 2002;124:7421–7428. doi: 10.1021/ja0260465. [DOI] [PubMed] [Google Scholar]
- 5.(a) Son SU, Park IK, Park J, Hyeon T. Chem Comm. 2004:778–779. doi: 10.1039/b316147a. [DOI] [PubMed] [Google Scholar]; (b) Choudary BM, Sridhar C, Kantam ML, Venkanna GT, Sreedhar B. J Am Chem Soc. 2005;127:9948–9949. doi: 10.1021/ja0436594. [DOI] [PubMed] [Google Scholar]
- 6.(a) Klapars A, Antilla JC, Huang X, Buchwald SL. J Am Chem Soc. 2001;123:7727–7729. doi: 10.1021/ja016226z. [DOI] [PubMed] [Google Scholar]; (b) Zhang H, Cai Q, Ma D. J Org Chem. 2005;70:5164–5173. doi: 10.1021/jo0504464. [DOI] [PubMed] [Google Scholar]
- 7.Job GE, Buchwald SL. Org Lett. 2002;4:3703–3706. doi: 10.1021/ol026655h. [DOI] [PubMed] [Google Scholar]
- 8.(a) Okano K, Tokuyama H, Fukuyama T. Org Lett. 2003;5:4987–4990. doi: 10.1021/ol035942y. [DOI] [PubMed] [Google Scholar]; (b) Kwong FY, Buchwald SL. Org Lett. 2003;5:793–796. doi: 10.1021/ol0273396. [DOI] [PubMed] [Google Scholar]; (c) Ma D, Cai Q, Zhang H. Org Lett. 2003;5:2453–2455. doi: 10.1021/ol0346584. [DOI] [PubMed] [Google Scholar]; (d) Lu Z, Twieg RJ, Huang SD. Tetrahedron Lett. 2003;44:6289–6292. [Google Scholar]; (e) Maes BUW, Loones KTJ, Hostyn S, Diels G, Rombouts G. Tetrahedron. 2004;60:11559–11564. [Google Scholar]; (f) Ezquerra J, Pedregal C, Lamas C, Barluenga J, Perez M, Garcia-Martin MA, Gonzalez JM. J Org Chem. 1996;61:5804–5812. [Google Scholar]; (g) Cacchi S, Fabrizi G, Goggiamani A, Zappia G. Org Lett. 2001;3:2539–2541. doi: 10.1021/ol016208m. [DOI] [PubMed] [Google Scholar]; (h) Artamkina GA, Sergeev AG, Beletskaya IP. Tetrahedron Lett. 2001;42:4381–4394. [Google Scholar]
- 9.Ullmann F. Ber dt chem Ges. 1903;36:2382–2384. [Google Scholar]
- 10.(a) Hanoun JP, Tensglia A. Synth Commun. 1995;25:2443–2448. [Google Scholar]; (b) Docampo Palacios ML, Pellon Comdom RF. Synth Commun. 2003;33:1771–1775. [Google Scholar]
- 11.(a) Wolf C, Mei X. J Am Chem Soc. 2003;125:10651–10658. doi: 10.1021/ja0358145. [DOI] [PubMed] [Google Scholar]; (b) Mei X, Wolf C. J Org Chem. 2005;70:2299–2305. doi: 10.1021/jo0479361. [DOI] [PubMed] [Google Scholar]; (c) Mei X, Wolf C. J Am Chem Soc. 2004;126:14736–14737. doi: 10.1021/ja0459781. [DOI] [PubMed] [Google Scholar]; (d) Mei X, Wolf C. Chem Comm. 2004:2078–2079. doi: 10.1039/b407718k. [DOI] [PubMed] [Google Scholar]
- 12.(a) Oza VB, Petrassi HM, Purkey HE, Kelly JW. Bioorg Med Chem Lett. 1999;9:1–6. doi: 10.1016/s0960-894x(98)00696-9. [DOI] [PubMed] [Google Scholar]; (b) Green NS, Palaninathan SK, Sacchettini JC, Kelly JW. J Am Chem Soc. 2003;125:13404–13414. doi: 10.1021/ja030294z. [DOI] [PubMed] [Google Scholar]; (c) Oza VB, Smith C, Raman B, Koepf EK, Lashuel HA, Petrassi HM, Chiang KP, Powers ET, Sachettinni J, Kelly JW. J Med Chem. 2002;45:321–332. doi: 10.1021/jm010257n. [DOI] [PubMed] [Google Scholar]; (d) Baures PW, Oza VB, Peterson SA, Kelly JW. Bioorg Med Chem. 1999;7:1339–1347. doi: 10.1016/s0968-0896(99)00066-8. [DOI] [PubMed] [Google Scholar]; (e) Klabunde T, Petrassi HM, Oza VB, Raman P, Kelly JW, Saccettini JC. Nature Struct Biol. 2000;7:312–321. doi: 10.1038/74082. [DOI] [PubMed] [Google Scholar]
- 13.(a) Girault S, Grellier P, Berecibar A, Maes L, Mouray E, Lemiere P, Debeu MA, Davioud-Charvet E, Sergheraert C. J Med Chem. 2000;43:2646–4654. doi: 10.1021/jm990946n. [DOI] [PubMed] [Google Scholar]; (b) Demeunynck M, Charmantray F, Martelli A. Curr Pharm Des. 2001;7:1703–1724. doi: 10.2174/1381612013397131. [DOI] [PubMed] [Google Scholar]; (c) Brana MF, Cacho M, De Pascual-Teresa B, Ramos A. Curr Pharm Des. 2001;7:1745–1780. doi: 10.2174/1381612013397113. [DOI] [PubMed] [Google Scholar]; (d) Cain BF, Seelye RN, Atwee GJ. J Med Chem. 1974;17:922–928. doi: 10.1021/jm00255a003. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Anderson KW, Zim D, Jiang L, Klapars A, Buchwald SL. J Am Chem Soc. 2003;125:6653–6655. doi: 10.1021/ja035483w. [DOI] [PubMed] [Google Scholar]
- 15.Kwong FY, Klapars A, Buchwald SL. Org Lett. 2002;4:581–584. doi: 10.1021/ol0171867. [DOI] [PubMed] [Google Scholar]
- 16.Csuk R, Barthel A, Raschke C. Tetrahedron. 2004;60:5737–5750. [Google Scholar]
- 17.Couture C, Paine AJ. Can J Chem. 1985;63:111–120. [Google Scholar]
- 18.Desiraju GR. Angew Chem, Int Ed Engl. 1995;34:2311–2327. [Google Scholar]
- 19.(a) Moulton B, Zaworotko MJ. Chem Rev. 2001;101:1629–1658. doi: 10.1021/cr9900432. [DOI] [PubMed] [Google Scholar]; (b) Swift JA, Pivovar AM, Reynolds AM, Ward MD. J Am Chem Soc. 1998;120:5887–5894. [Google Scholar]
- 20.(a) Desiraju GR. Crystal Engineering: The Design of Organic Solids. Elsevier; Amsterdam: 1989. [Google Scholar]; (b) Reddy DS, Craig DC, Desiraju GR. J Am Chem Soc. 1996;118:4090–4093. [Google Scholar]; (c) Desiraju GR. Acc Chem Res. 2002;35:565–573. doi: 10.1021/ar010054t. [DOI] [PubMed] [Google Scholar]; (d) Gavezzotti A. Acc Chem Res. 1994;27:309–314. [Google Scholar]
- 21.N-(2-Methoxyphenyl)anthranilic acid, N-(2-carboxyphenyl)anthranilic acid, N-(2′-carboxy-5′-chlorophenyl)-4-chloroanthranilic acid, N-(2-methyl-3-chlorophenyl)anthranilic acid and N-(3-methyl-2,6-dichlorophenyl)anthranilic acid.
- 22.Scherrer RA, Beatty HR. J Org Chem. 1980;45:2127–2131. [Google Scholar]
- 23.Denny WA, Twigden SJ, Baguley BC. Anti-Cancer Drug Des. 1986;1:125–132. [PubMed] [Google Scholar]
- 24.Graboyes H, Anderson EL, Levinson SH, Resnick TM. J Heterocyclic Chem. 1975;12:1225–1231. [Google Scholar]
- 25.Thilo D, Von Kaulla KN. J Med Chem. 1970;13:503–510. doi: 10.1021/jm00297a037. [DOI] [PubMed] [Google Scholar]
- 26.Dokorou V, Demertzis MA, Jasinski JP, Kovala-Demertzi DJ. Organomet Chem. 2004;689:317–325. [Google Scholar]
- 27.Szczepankiewicz BG, Liu G, Hajduk PJ, Abad-Zapatero C, Pei Z, Xin Z, Lubben TH, Trevillyan JM, Stashko MA, Ballaron SJ, Liang H, Huang F, Hutchins CW, Fesik SW, Jirousek MR. J Am Chem Soc. 2003;125:4087–4096. doi: 10.1021/ja0296733. [DOI] [PubMed] [Google Scholar]
- 28.Dheyongera JP, Geldenhuys WJ, Dekker TG, Van der Schyf CJ. Bioorg Med Chem. 2005;13:689–698. doi: 10.1016/j.bmc.2004.10.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Single crystal structures, NMR spectra, and CIF files. This material is available free of charge via the Internet at http://pubs.acs.org.