Abstract
Helicobacter pylori (H. pylori) is one of the most widespread human pathogens, and plays major roles in chronic gastritis and gastric cancer. CD74 of gastric epithelial cells has recently been identified as an adhesion molecule to urease in H. pylori. In this study, we found that CD74 is highly expressed in a constitutive manner in NCI-N87 human gastric carcinoma cells at both the protein and mRNA levels as compared with Hs738St./Int fetal gastric cells. Subsequently, a novel cell-based ELISA able to rapidly screen the suppressive agents of CD74 expression was established. NCI-N87 cells were treated separately with 25 different food phytochemicals (4–100 µM) for 48 h and subjected to our novel assay. From those results, a citrus coumarin, bergamottin, was indicated to be the most promising compound with an LC50/IC50 value greater than 7.1, followed by luteolin (>5.4), nobiletin (>5.3), and quercetin (>5.1). Our findings suggest that these CD74 suppressants are unique candidates for preventing H. pylori adhesion and subsequent infection with reasonable action mechanisms.
Keywords: Helicobacter pylori, adhesion, CD74, cell-based ELISA, bergamottin
Introduction
Helicobacter pylori (H. pylori), which infects over half of all people in the world, is one of the most widespread human pathogens and responsible for chronic gastritis, and gastric and duodenal ulcers [1, 2]. In 1994, the organism was classified into group I, “carcinogenic to humans,” by the World Health Organization/International Agency for Research on Cancer (WHO/IARC) [3]. Although triple therapy using two antibiotics (amoxicillin and clarithromycin) and a proton pump inhibitor is widely employed for the treatment of H. pylori, antibiotic resistance to clarithromycin leads to treatment failure, especially in Asian countries [4, 5].
A number of food extracts and components have been shown to relieve the risk of damage from H. pylori infection. In Mongolian gerbils, green tea catechins, such as (–)-epigallocatechin-3-gallate (EGCG), strongly inhibited H. pylori urease activity in vitro and suppressed H. pylori-induced gastritis [6], while garlic and its diallyl sulfur compounds showed potential effects on H. pylori elimination [7]. However, these phytochemicals are yet to be proven effective enough for clinical use on account of their broad range of biological activities, thus more specific molecular targeting is indispensable to conquer this problem.
The adhesion of H. pylori to gastric cells is one of the critical steps in gastritis, which leads to release of the definitive virulence factor cytotoxin-associated antigen A (CagA) [8]. However, the adhesion mechanisms of H. pylori to gastric epithelial cells are not fully understood. Recently, CD74 was detected on gastric epithelial cells and shown to be an adhesion molecule to urease in H. pylori [9], a 5–10% bacterial whole protein that is expressed in all strains [10]. This enzyme catalyzes the hydrolysis of urea to produce ammonia and carbon dioxide, while its most crucial role is to buffer the bacteria from the acidic environment of the stomach [11]. Therefore, it is considered that H. pylori urease is essential for bacterial colonization. Urease consists of two subunits, i.e., the α-subunit at approximately 24 kDa and β-subunit at approximately 68 kDa [10]. Beswick et al. suggested that the urease β-subunit binds to CD74 on gastric epithelial cells and induces nuclear factor-kappa B (NF-κB) activation, thereby stimulating interleukin-8 (IL-8) production [9, 12]. This raises the possibility that CD74-mediated H. pylori adhesion is a critical step for gastritis and resultant carcinogenesis.
In this study, we investigated the levels of constitutive CD74 expression in N87 gastric carcinoma cells and Hs738St./Int fetal gastric cells, then established a novel cell-based ELISA for CD74 semi-quantification. Our results identified bergamottin, a coumarin-related compound in citrus fruit, as the most promising agent after screening a total of 25 food phytochemicals.
Materials and Methods
Chemicals
RPMI1640, Dulbecco’s modified eagle medium (DMEM), and fetal bovine serum (FBS) were purchased from Gibco BRL (Grand Island, NY). Oligonucleotide primers were synthesized by Sigma-Aldrich (Tokyo, Japan). TRIzol® reagent was from Qiagen (Hilden, Germany). An RNA polymerase chain reaction (PCR) Kit (AMV, Ver. 2.1) came from TaKaRa Bio (Shiga, Japan). Nonspecific IgG, used as a negative control, was purchased from Dako (Glostrup, Denmark). Antibodies were purchased from the following sources: rabbit anti-CD74 was from Santa Cruz Biotechnology Inc. (Santa Cruz, CA), α-tublin came from Calbiochem (San Diego, CA), and anti-rabbit IgG was obtained from Dako (Glostrup, Denmark). 1'-Acetoxychavicol acetate (ACA) [13], zerumbone [14], auraptene [15], nobiletin [16], and ar-turmerone [17] were isolated as previously described. PEITC (phenetyl isothiocyanate) and BUITC (butenyl isothiocyate) were purchased from Tokyo Chemical Industry (Tokyo, Japan). Both PEGLS and BUGLS were purified using a method reported by Barillari et al. [18], then used following a few modifications. Barbarea verna seeds were extracted with boiling water, then the extract was obtained by centrifugation, deproteinized by addition of 1 M Zn(OAc)2, and centrifuged, after which the supernatant was subjected to chromatography with a DEAE-Sephadex A-25. The concentrated fraction was extracted with boiling methanol and the extract was centrifuged. The supernatant was added to chilled ethanol and gluconasturtiin (PUGLS) was obtained as a white powder after centrifugation. Gluconapin (BUGLS) was isolated from Brassica rapa L. (Yamato-mana) seeds in the same manner described above. All other chemicals were purchased from Wako Pure Chemicals (Osaka, Japan), unless specified otherwise.
Cell culture
NCI-N87 gastric carcinoma and Hs738St./Int fetal gastric cells were obtained from American Type Culture Collection (Rockville, MD), and grown in RPMI 1640 and DMEM, respectively, supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 µg/ml) at 37°C under a humidified atmosphere of 95% air and 5% CO2.
Western blotting
Cells (3.4 × 105 cells/1.7 ml on a 35 mm-dish) were incubated for 13 h and washed twice with PBS, then treated with lysis buffer [protease and phosphatase inhibitors cocktail (Sigma), 10 mM Tris (pH 7.4), 1% sodium dodecyl sulfate (SDS), 1 mM sodium vanadate (V)] and the lysates were sonicated. Denatured proteins (30 µg) were separated using SDS-polyacrylamide gel electrophoresis on a 10% polyacrylamide gel and transferred onto Immobilon-P Transfer Membrane (Millipore, MA). After blocking for 1 h at room temperature in Block Ace (Dainippon Pharmaceutical, Osaka, Japan), the membranes were first incubated with each antibody at a dilution of 1:1000, followed by a second incubation performed with horseradish peroxidase-conjugated secondary anti-rabbit IgG at a dilution of 1:2000. The blots were developed using ECL Western Blotting detection reagent (Amersham Biosciences, Buckinghamshire, UK) and the band intensities were analyzed using NIH Image. Relative levels of each protein were corrected by α-tublin as the internal control.
Reverse transcription-PCR
Cells (1.0 × 106 cells/5 ml on a 60-mm dish) were incubated for 13 h, then washed twice with PBS. Total RNA was extracted using TRIzol® reagent. cDNA was synthesized using 1 µg of total RNA and an RNA PCR Kit (AMV). PCR amplification was then performed with a thermal cycler (PTC-100TM, MJ Research, Watertown, MA), under the following conditions: CD74; forward (5'-TgACCAg CgCgACCTTATCT-3') and reverse (5'-gAgCAggTgCATCACATggT-3') primers (0.05 µM each, product size 384 bp and 560 bp) for 30 cycles, with 60 s of denaturation at 94°C, 90 s of annealing at 54°C, and 60 s of primer extension at 72°C, and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH); forward (5'-GTGAAGGTCGGAGTCAACG-3') and reverse (5'-GGTGAAGACGCCAGTGGACTC-3') primers (0.05 µM each, product size 300 bp) for 23 cycles, with 30 s of denaturation at 95°C, 60 s of annealing at 58°C, and 60 s of primer extension at 72°C. Amplified cDNA was subjected to electrophoresis on 3% agarose gels and stained with SYBR® Gold. Image analysis was performed using NIH image. Relative levels of each protein were corrected by GAPDH transcript, which served as the internal control.
Cell-based ELISA
N87 Gastric carcinoma cells (4 × 104 cells/200 µl) were seeded onto a 96-well microplate with a clear bottom (IWAKI, Tokyo) and pre-incubated for 13 h. After incubation, the medium on the plate was replaced with serum-free RPMI1640 containing the samples dissolved in dimethyl sulfoxide (DMSO, final 0.5%, v/v). After 48 h of incubation, the medium were removed, and the cells were fixed by replacing the medium with 95% ethanol and 5% acetic acid for 7 min at room temperature. Subsequently, the solvents were replaced with 1% formaldehyde in PBS for 5 min at room temperature. After washing with washing buffer (0.05% Tween 20 in PBS), quenching buffer (0.6% H2O2 in wash buffer) was added and incubation was performed for 20 min at room temperature, then blocking buffer (10% BSA in PBS) was added and incubation performed for 1 h at 37°C. After washing, the plate was incubated with rabbit anti-CD74 or nonspecific IgG antibody (5 µg/ml, each) for 1 h, and, after washing, horseradish-peroxidase (HRP) conjugated secondary antibody was added at a dilution of 1:2000 for 1 h. A color reaction was initiated by adding substrate solution [o-phenylethylenediamine (OPD) in citrate buffer (1 mg/ml, each)] for 15 min and terminated with a stop solution (1 N sulfuric acid in PBS). Visible absorption at 492 nm was recorded using a microplate reader (Thermo Fisher Scientific, Waltham, MA).
Cell viability
Cell viability was measured using 3-(4,5-dimethyltiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) [19]. After incubation, the cells were washed twice with PBS, then 110 µl of serum-free RPMI1640 containing 10 µl of MTT solutions (5 mg/ml) were added to each culture, followed by another incubation at 37°C for 2 h. Next, 200 µl of DMSO was added and the culture was sonicated for 5 min, after which 500 µl of HCl/2-propanol (3.4 µl/ml) was added to each well. Visible absorbance was measured at 570 nm and 630 nm using a microplate reader. Cell viability above 70% was recognized as significant.
Results
Expression of CD74 in human gastric carcinoma and fetal gastric cells
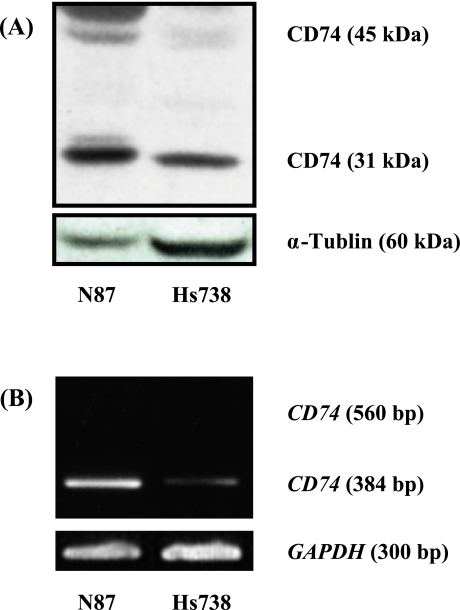
Initially, we examined the constitutive expression of CD74 using western blotting and RT-PCR. As shown in Fig. 1, we found significantly high levels of constitutive CD74 expression in NCI-N87 human gastric carcinoma cells at the protein and mRNA levels of 14- and 6-fold, respectively as compared to those in Hs738St./Int fetal gastric cells.
Fig. 1.
Constitutive expression of CD74 in NCI-N87 gastric carcinoma cells (N87) and Hs738St./Int fetal gastric cells (Hs738). High levels of constitutive CD74 expression in NCI-N87 human gastric carcinoma cells at the protein and mRNA levels were observed as compared with in Hs738St./Int fetal gastric cells. The intensity of each band was analyzed by Western blotting (A) and RT-PCR (B), as described in the Materials and Methods section. The experiments were repeated twice independently.
Screening of selected food factors for their effects on CD74 expression using novel cell-based ELISA with N87 cells
A total of 25 food factors (Fig. 2) were selected based on their previously reported anti-inflammatory in vitro and in vivo activities [17, 20–22]. Each was subjected to our novel cell-based ELISA established in the present study. As shown in Table 1, 65% of the tested compounds exhibited strong cytotoxicity [cell viability (CV) >70%] at a concentration of 100 µM after 48 h. Subsequently, some of the compounds, such as curcumin, ACA, and ursolic acid, continued to induce cytotoxicity (20 µM, CVs = 12%, 20%, and 38%, respectively). Notably, silymarin (100 µM) and bergamottin (20 µM) markedly inhibited CD74 expression [inhibitory rates (IRs) = 91% and 74%, respectively) without considerable cytotoxicity. Further, (−)-catechin, gallic acid, zerumbone, auraptene, nobiletin, genistein, quercetin, and luteolin (20 µM) were moderately suppressive (IRs = 40–65%), while none of the compounds showed marked suppression at a concentration of 20 µM. As summarized in Table 2, the IC50 values for zerumbone, auraptene, nobiletin, quercetin, luteolin, and bergamottin were lower (IC50 = 14–20 µM) than for other compounds. Marginal cytotoxicity was observed with some compounds, including (−)-catechin and gallic acid. Based on the LC50/IC50 ratios, we identified bergamottin as the most promising CD74 suppressant (LC50/IC50 >7.1), followed by in order by luteolin, nobiletin, and quercetin (LC50/IC50 >5.4, >5.3, and >5.1, respectively).
Fig. 2.
Chemical structures of selected food factors.
Table 1.
Suppressive effects of selected compounds on constitutive CD74 expression in NCI-N87 gastric carcinoma cells
| 4 µM |
20 µM |
100 µM |
||||||
|---|---|---|---|---|---|---|---|---|
| IR (%) | CV (%) | IR (%) | CV (%) | IR (%) | CV (%) | |||
| ACA | 16 ± 1.6 | 92 ± 3.4 | NT | 20 ± 0.2 | NT | 6.1 ± 0.3 | ||
| Ascorbic acid | –12 ± 3.5 | 97 ± 1.7 | 5.4 ± 3.8 | 94 ± 0.3 | 6.8 ± 1.8 | 86 ± 5.8 | ||
| Auraptene | 2.4 ± 2.0 | 96 ± 2.7 | 50 ± 0.4 | 79 ± 4.5 | NT | 18 ± 0.4 | ||
| Bergamottin | 8.6 ± 4.2 | 93 ± 0.0 | 74 ± 8.2 | 79 ± 0.0 | NT | 59 ± 2.1 | ||
| BITC | –19 ± 0.6 | 96 ± 3.4 | NT | 56 ± 7.9 | NT | 6.1 ± 1.3 | ||
| BUGLS | –15 ± 0.0 | 88 ± 6.9 | −19 ± 2.5 | 80 ± 3.5 | 4.0 ± 1.0 | 79 ± 5.6 | ||
| BUITC | –9.0 ± 0.4 | 71 ± 5.8 | 2.3 ± 0.8 | 73 ± 3.3 | NT | 44 ± 2.4 | ||
| Caffeine | 10 ± 7.7 | 100 ± 2.6 | 22 ± 6.9 | 100 ± 2.2 | 41 ± 9.2 | 87 ± 1.9 | ||
| (−)-Catechin | −6.4 ± 2.0 | 97 ± 2.5 | 40 ± 2.1 | 97 ± 3.3 | 65 ± 0.8 | 89 ± 5.9 | ||
| Curucumin | 21 ± 8.0 | 89 ± 1.1 | NT | 12 ± 1.9 | NT | 14 ± 6.8 | ||
| EGCG | −110 ± 8.3 | 78 ± 6.3 | −149 ± 3.8 | 77 ± 3.1 | −217 ± 4.0 | 78 ± 5.0 | ||
| Ferulic acid | −5.1 ± 4.3 | 96 ± 2.3 | 15 ± 5.8 | 93 ± 0.7 | 20 ± 3.8 | 82 ± 5.9 | ||
| Gallic acid | 15 ± 2.4 | 97 ± 0.2 | 57 ± 3.7 | 73 ± 2.4 | 47 ± 8.3 | 97 ± 2.1 | ||
| Genistein | 8.6 ± 7.8 | 100 ± 0.4 | 49 ± 7.2 | 84 ± 1.5 | NT | 55 ± 4.6 | ||
| Luteolin | 4.0 ± 5.1 | 99 ± 2.9 | 55 ± 1.2 | 81 ± 4.7 | NT | 61 ± 1.5 | ||
| Nobiletin | 1.8 ± 2.9 | 96 ± 0.3 | 54 ± 5.0 | 93 ± 0.5 | NT | 58 ± 5.0 | ||
| PEGLS | −11 ± 4.4 | 99 ± 1.4 | −25 ± 1.3 | 88 ± 1.7 | −13 ± 3.8 | 90 ± 1.4 | ||
| PEITC | −17 ± 2.3 | 97 ± 4.8 | NT | 43 ± 1.8 | NT | 37 ± 2.2 | ||
| Quercetin | 3.7 ± 0.0 | 100 ± 3.0 | 51 ± 4.7 | 100 ± 3.0 | NT | 51 ± 0.5 | ||
| Rutin | −4.3 ± 2.8 | 97 ± 1.2 | −3.0 ± 0.0 | 100 ± 1.1 | 11 ± 4.5 | 80 ± 4.7 | ||
| Silymarin | −11 ± 3.6 | 100 ± 3.2 | 23 ± 1.9 | 100 ± 1.4 | 91 ± 9.2 | 76 ± 2.2 | ||
| Tangeretin | −3.3 ± 3.3 | 98 ± 2.7 | 21 ± 3.1 | 100 ± 0.5 | NT | 63 ± 3.1 | ||
| ar-Turmerone | 22 ± 0.5 | 97 ± 4.1 | 39 ± 1.8 | 100 ± 3.2 | NT | 57 ± 1.3 | ||
| Ursolic acid | 35 ± 6.1 | 91 ± 1.6 | NT | 38 ± 1.1 | NT | 9.7 ± 0.0 | ||
| Zerumbone | 6.6 ± 0.8 | 100 ± 0.4 | 57 ± 0.2 | 77 ± 0.3 | NT | 16 ± 1.1 | ||
Data are shown as the mean ± average deviation, IR; Inhibition rate, CV; Cell viability, NT; Not tested, ACA; 1'-acetoxychavicol acetate, BITC; benzyl isothiocyanate, BUGLS; gluconapin, BUITC; butenyl isothiocyanate, EGCG; (−)-epigallocatechin-3-gallate, PEGLS; gluconasturtiin, PEITC; phenetyl isothiocyanate
Table 2.
The IC50 and LC50 values of 25 compounds for constitutive CD74 expression in NCI-N87 gastric carcinoma cells
| IC50 | LC50 | LC50/IC50 | Grade | |
|---|---|---|---|---|
| Bergamottin | 14.2 | >100 | >7.1 | +++ |
| Luteolin | 18.4 | >100 | >5.4 | ++ |
| Nobiletin | 18.8 | >100 | >5.3 | ++ |
| Quercetin | 19.8 | >100 | >5.1 | ++ |
| Genistein | 20.5 | >100 | >4.9 | + |
| Zerumbone | 17.7 | 60.5 | 3.4 | + |
| ar-Turmerone | 30.8 | >100 | >3.2 | + |
| PEITC | <20.0 | 61.3 | >3.1 | + |
| Auraptene | 19.8 | 59.2 | 3.0 | + |
| Tangeretin | 39.5 | >100 | >2.5 | − |
| BITC | <20.0 | 44.3 | >2.2 | − |
| Curucumin | <20.0 | 44.3 | >2.2 | − |
| Ursolic acid | <20.0 | 35.4 | >1.8 | − |
| Silymarin | 57.2 | >100 | >1.7 | − |
| (−)-Catechin | 69.2 | >100 | >1.4 | − |
| ACA | <20.0 | 25.0 | >1.3 | − |
| Gallic acid | 96.4 | >100 | >1.0 | − |
| Ascorbic acid | >100 | >100 | >1.0 | − |
| BUGLS | >100 | >100 | >1.0 | − |
| Caffeine | >100 | >100 | >1.0 | − |
| EGCG | >100 | >100 | >1.0 | − |
| Ferulic acid | >100 | >100 | >1.0 | − |
| PEGLS | >100 | >100 | >1.0 | − |
| Rutin | >100 | >100 | >1.0 | − |
| BUITC | >100 | 81.5 | >0.8 | − |
The mean values of IC50 and LC50 were obtained from duplicate experiments. IC50; 50% inhibitory concentration, LC50; 50% cell lethal concentration. Grade of LC50/IC50; − <3.0, + 3.0–4.9, ++ 5.0–6.9, +++ >7.0.
ACA; 1'-acetoxychavicol acetate, BITC; benzyl isothiocyanate, BUGLS; gluconapin, BUITC; butenyl isothiocyanate, EGCG; (–)-Epigallocatechin-3-gallate, PEGLS; gluconasturtiin, PEITC; phenetyl isothiocyanate
Discussion
Several adhesins on the cell surface of H. pylori have been identified, including BabA, SabA, and AlpA/B. BabA and SabA bind to Lewis B blood group antigen [23] and Sialyl Lewis X [24], respectively, while the ligands to AlpA/B receptors remain unknown [25]. Although they are associated with the attachment of H. pylori, those receptors are not recognized as signaling molecules responsible for activating the immune system in host cells. On the other hand, cell surface-associated urease, recently identified as an adhesin of H. pylori, binds to MHC class II molecules for inducing apoptosis [26]. Further, Beswick et al. reported that the MHC class II invariant chain, i.e., CD74, plays an essential role in binding to the urease β subunit of H. pylori [9, 12].
CD74 is a type II integral membrane protein that functions in signaling pathways for malignant B-cell proliferation and survival [27, 28]. It is expressed in cancer cells as well as stomach [29], renal [30], and bladder [31] tissues, and serves as a marker of tumor progression [32]. Conversely, preclinical studies using anti-CD74 antibodies have shown that CD74 is an effective therapeutic target for B-cell malignancy, such as non-Hodgkin lymphoma and multiple myeloma [33]. It should be pointed out that H. pylori binding to CD74 on gastric cells stimulated the NF-κB pathway that leads to IL-8 production [9, 12]. Interestingly, CD74 was also reported to serve as a receptor for macrophage migration inhibitory factor (MIF), a proinflammatory cytokine with versatile functions [34–38]. MIF binds to the CD74/CD44 complex for activating proliferative and proinflammatory signaling molecules, such as extracellular signaling-regulated kinase [39]. Collectively, targeting CD74 suppression may not only inhibit H. pylori adhesion, but also mitigate resultant proinflammatory events.
A variety of food phytochemicals and synthetic drugs have been reported to inhibit H. pylori adhesion to gastric epithelium cells [40]. For example, tea catechins were shown to decrease H. pylori colonization in Mongolian gerbils by inhibiting urease [6]. In the present study, however, EGCG, the most active principle in tea, did not suppress CD74 expression, but rather enhanced it, while (−)-catechin was suppressive only at a high concentration (100 µM). Although ascorbic acid reduced H. pylori colonization and subsequent inflammation in Mongolian gerbils [41], it (even at 100 µM) did not suppress CD74 in our study. On the other hand, a notable CD74 suppressant, citrus auraptene (LC50/IC50 = 3.0), has been reported to reduce H. pylori colonization in vivo [42]. It is tempting to speculate that this reduction is due, at least in part, to CD74 modulation. Interestingly, quercetin (LC50/IC50 >5.1), a potent anti-oxidative and anti-inflammatory agent in onions, reduced N-methyl-N’-nitro-N-nitrosoguanidine-induced inflammation in H. pylori-infected human gastric mucosal cells in a previous study, though the effects on colonization were not addressed [43].
Bergamottin, a furanocoumarin occurring in citrus fruit, was identified as the most promising suppressant in the present study (LC50/IC50 >7.1). This compound has been well described to inhibit cytochrome P450 (CYP) 3A4 [44], CYP1A1 [45], and other CYPs [46]. In addition, bergamottin reduced the formation of DNA adducts in MCF-7 cells induced by benzo[a]pyrene and 7, 12-dimethylbenz [a]anthracene [47], skin tumors in mice [48], and NO generation [49], while it induced leukemia differentiation [50]. Furthermore, some case-control studies have suggested that citrus fruit intake reduces the risk of gastric cancer [51–54]. In this context, it should be noted that several citrus compounds, i.e., bergamottin, nobiletin, auraptene, and tangeretin, exhibited significant activity in the present assays (Table 2), though their action mechanisms to suppress CD74 expression remain unknown. Nonetheless, it may be helpful to indicate that the a CD74 cytosolic fragment is released for stimulating Syk and Akt, which, in turn, directly activate NF-κB and its co-activator, TAFII105 [27, 55]. Along a similar line, cell-surface CD74 initiates a signaling cascade resulting in NF-κB activation for inducing IL-8 production. Furthermore, H. pylori and the urease β-subunit were reported to activate NF-κB for increasing CD74 expression at mRNA and protein levels in NCI-N87 and Kato III gastric carcinoma cells [9, 12, 56]. It is also interesting to note that CagA-positive H. pylori contributes to the production of MIF, though its mechanism of action has not been reported [57]. In addition, several reports have suggested that MIF up-regulates the NF-κB pathway [28, 58–60]. Taken together, NF-κB activation is considered to have a major role in CD74 expression. Another study found that genistein (LC50/IC50 >4.9) decreased NF-κB activation in MKN45 gastric carcinoma cells co-cultured with H. pylori [61], which might be partly related to modulation of CD74 expression. Also, luteolin (LC50/IC50 >5.4) [62], nobiletin (LC50/IC50 >5.3) [20], quercetin (LC50/IC50 >5.1) [63], and zerumbone (LC50/IC50 = 3.4) [64] have been reported to markedly inhibit NF-κB in several cell lines. Thus, the relevance of NF-κB activation in regard to CD74 expression in NCI-N87 cells should be addressed in the near future.
In conclusion, we established a novel ELISA system for identifying CD74 suppressive agents using NCI-N87 gastric cancer cells. The present results suggest that several compounds including bergamottin are candidates for treatment of H. pylori infection and additional in vivo evaluations are warranted.
Acknowledgements
This study was supported by a Grant-in-Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan (A.M.), and a Grant-in-Aid from the Prefecture Collaboration of Regional Entities for the Advancement of Technological Excellence, Japan Science and Technology (A.M. and K.W.).
Reference
- 1.Parsonnet J., Friedman G.D., Vandersteen D.P., Chang Y., Vogelman J.H., Orentreich N., Sibley R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 2.Nomura A., Stemmermann G.N., Chyou P.H., Kato I., Perez-Perez G.I., Blaser M.J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N. Engl. J. Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 3.Infection with Helicobacter pylori. IARC Monogr. Eval. Carcinog. Risks Hum. 1994;61:177–240. [PMC free article] [PubMed] [Google Scholar]
- 4.Debets-Ossenkopp Y.J., Sparrius M., Kusters J.G., Kolkman J.J., Vandenbroucke-Grauls C.M. Mechanism of clarithromycin resistance in clinical isolates of Helicobacter pylori. FEMS Microbiol. Lett. 1996;142:37–42. doi: 10.1111/j.1574-6968.1996.tb08404.x. [DOI] [PubMed] [Google Scholar]
- 5.Maeda S., Yoshida H., Matsunaga H., Ogura K., Kawamata O., Shiratori Y., Omata M. Detection of clarithromycin-resistant helicobacter pylori strains by a preferential homoduplex formation assay. J. Clin. Microbiol. 2000;38:210–214. doi: 10.1128/jcm.38.1.210-214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsubara S., Shibata H., Ishikawa F., Yokokura T., Takahashi M., Sugimura T., Wakabayashi K. Suppression of Helicobacter pylori-induced gastritis by green tea extract in Mongolian gerbils. Biochem. Biophys. Res. Commun. 2003;310:715–719. doi: 10.1016/j.bbrc.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 7.Iimuro M., Shibata H., Kawamori T., Matsumoto T., Arakawa T., Sugimura T., Wakabayashi K. Suppressive effects of garlic extract on Helicobacter pylori-induced gastritis in Mongolian gerbils. Cancer Lett. 2002;187:61–68. doi: 10.1016/s0304-3835(02)00401-9. [DOI] [PubMed] [Google Scholar]
- 8.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer. 2004;4:688–694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 9.Beswick E.J., Pinchuk I.V., Minch K., Suarez G., Sierra J.C., Yamaoka Y., Reyes V.E. The Helicobacter pylori urease B subunit binds to CD74 on gastric epithelial cells and induces NF-kappaB activation and interleukin-8 production. Infect. Immun. 2006;74:1148–1155. doi: 10.1128/IAI.74.2.1148-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beswick E.J., Suarez G., Reyes V.E. H. pylori and host interactions that influence pathogenesis. World J. Gastroenterol. 2006;12:5599–5605. doi: 10.3748/wjg.v12.i35.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall B.J., Barrett L.J., Prakash C., McCallum R.W., Guerrant R.L. Urea protects Helicobacter (Campylobacter) pylori from the bactericidal effect of acid. Gastroenterology. 1990;99:697–702. doi: 10.1016/0016-5085(90)90957-3. [DOI] [PubMed] [Google Scholar]
- 12.Beswick E.J., Bland D.A., Suarez G., Barrera C.A., Fan X., Reyes V.E. Helicobacter pylori binds to CD74 on gastric epithelial cells and stimulates interleukin-8 production. Infect. Immun. 2005;73:2736–2743. doi: 10.1128/IAI.73.5.2736-2743.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakami A., Ohura S., Nakamura Y., Koshimizu K., Ohigashi H. 1'-Acetoxychavicol acetate, a superoxide anion generation inhibitor, potently inhibits tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in ICR mouse skin. Oncology. 1996;53:386–391. doi: 10.1159/000227593. [DOI] [PubMed] [Google Scholar]
- 14.Murakami A., Takahashi M., Jiwajinda S., Koshimizu K., Ohigashi H. Identification of zerumbone in Zingiber zerumbet Smith as a potent inhibitor of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation. Biosci. Biotechnol. Biochem. 1999;63:1811–1812. doi: 10.1271/bbb.63.1811. [DOI] [PubMed] [Google Scholar]
- 15.Murakami A., Kuki W., Takahashi Y., Yonei H., Nakamura Y., Ohto Y., Ohigashi H., Koshimizu K. Auraptene, a citrus coumarin, inhibits 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion in ICR mouse skin, possibly through suppression of superoxide generation in leukocytes. Jpn. J. Cancer Res. 1997;88:443–452. doi: 10.1111/j.1349-7006.1997.tb00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami A., Nakamura Y., Torikai K., Tanaka T., Koshiba T., Koshimizu K., Kuwahara S., Takahashi Y., Ogawa K., Yano M., Tokuda H., Nishino H., Mimaki Y., Sashida Y., Kitanaka S., Ohigashi H. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000;60:5059–5066. [PubMed] [Google Scholar]
- 17.Murakami A., Ishida H., Kobo K., Furukawa I., Ikeda Y., Yonaha M., Aniya Y., Ohigashi H. Suppressive effects of Okinawan food items on free radical generation from stimulated leukocytes and identification of some active constituents: implications for the prevention of inflammation-associated carcinogenesis. Asian Pac. J. Cancer Prev. 2005;6:437–448. [PubMed] [Google Scholar]
- 18.Barillari J., Gueyrard D., Rollin P., Iori R. Barbarea verna as a source of 2-phenylethyl glucosinolate, precursor of cancer chemopreventive phenylethyl isothiocyanate. Fitoterapia. 2001;72:760–764. doi: 10.1016/s0367-326x(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Murakami A., Shigemori T., Ohigashi H. Zingiberaceous and citrus constituents, 1'-acetoxychavicol acetate, zerumbone, auraptene, and nobiletin, suppress lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 murine macrophages through different modes of action. J. Nutr. 2005;135:2987S–2992S. doi: 10.1093/jn/135.12.2987S. [DOI] [PubMed] [Google Scholar]
- 21.Kwon K.H., Murakami A., Ohigashi H. Suppressive effects of natural and synthetic agents on dextran sulfate sodium-induced interleukin-1beta release from murine peritoneal macrophages. Biosci. Biotechnol. Biochem. 2004;68:436–439. doi: 10.1271/bbb.68.436. [DOI] [PubMed] [Google Scholar]
- 22.Kim H.W., Murakami A., Williams M.V., Ohigashi H. Suppressive effects of selected antioxidants on the activated leukocytes-induced mutagenesis in the co-culture assay systems. Biosci. Biotechnol. Biochem. 2004;68:238–242. doi: 10.1271/bbb.68.238. [DOI] [PubMed] [Google Scholar]
- 23.Hennig E.E., Mernaugh R., Edl J., Cao P., Cover T.L. Heterogeneity among Helicobacter pylori strains in expression of the outer membrane protein BabA. Infect. Immun. 2004;72:3429–3435. doi: 10.1128/IAI.72.6.3429-3435.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unemo M., Aspholm-Hurtig M., Ilver D., Bergstrom J., Boren T., Danielsson D., Teneberg S. The sialic acid binding SabA adhesin of Helicobacter pylori is essential for nonopsonic activation of human neutrophils. J. Biol. Chem. 2005;280:15390–15397. doi: 10.1074/jbc.M412725200. [DOI] [PubMed] [Google Scholar]
- 25.Odenbreit S., Faller G., Haas R. Role of the alpAB proteins and lipopolysaccharide in adhesion of Helicobacter pylori to human gastric tissue. Int. J. Med. Microbiol. 2002;292:247–256. doi: 10.1078/1438-4221-00204. [DOI] [PubMed] [Google Scholar]
- 26.Fan X., Gunasena H., Cheng Z., Espejo R., Crowe S.E., Ernst P.B., Reyes V.E. Helicobacter pylori urease binds to class II MHC on gastric epithelial cells and induces their apoptosis. J. Immunol. 2000;165:1918–1924. doi: 10.4049/jimmunol.165.4.1918. [DOI] [PubMed] [Google Scholar]
- 27.Starlets D., Gore Y., Binsky I., Haran M., Harpaz N., Shvidel L., Becker-Herman S., Berrebi A., Shachar I. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107:4807–4816. doi: 10.1182/blood-2005-11-4334. [DOI] [PubMed] [Google Scholar]
- 28.Binsky I., Haran M., Starlets D., Gore Y., Lantner F., Harpaz N., Leng L., Goldenberg D.M., Shvidel L., Berrebi A., Bucala R., Shachar I. IL-8 secreted in a macrophage migration-inhibitory factor- and CD74-dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc. Natl. Acad. Sci. U. S. A. 2007;104:13408–13413. doi: 10.1073/pnas.0701553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishigami S., Natsugoe S., Tokuda K., Nakajo A., Iwashige H., Aridome K., Hokita S., Aikou T. Invariant chain expression in gastric cancer. Cancer Lett. 2001;168:87–91. doi: 10.1016/s0304-3835(01)00503-1. [DOI] [PubMed] [Google Scholar]
- 30.Young A.N., Amin M.B., Moreno C.S., Lim S.D., Cohen C., Petros J.A., Marshall F.F., Neish A.S. Expression profiling of renal epithelial neoplasms: a method for tumor classification and discovery of diagnostic molecular markers. Am. J. Pathol. 2001;158:1639–1651. doi: 10.1016/S0002-9440(10)64120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer-Siegler K.L., Leifheit E.C., Vera P.L. Inhibition of macrophage migration inhibitory factor decreases proliferation and cytokine expression in bladder cancer cells. BMC Cancer. 2004;4:34. doi: 10.1186/1471-2407-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chamuleau M.E., Souwer Y., Van Ham S.M., Zevenbergen A., Westers T.M., Berkhof J., Meijer C.J., van de Loosdrecht A.A., Ossenkoppele G.J. Class II-associated invariant chain peptide expression on myeloid leukemic blasts predicts poor clinical outcome. Cancer Res. 2004;64:5546–5550. doi: 10.1158/0008-5472.CAN-04-1350. [DOI] [PubMed] [Google Scholar]
- 33.Stein R., Mattes M.J., Cardillo T.M., Hansen H.J., Chang C.H., Burton J., Govindan S., Goldenberg D.M. CD74: a new candidate target for the immunotherapy of B-cell neoplasms. Clin. Cancer Res. 2007;13:5556s–5563s. doi: 10.1158/1078-0432.CCR-07-1167. [DOI] [PubMed] [Google Scholar]
- 34.Bacher M., Metz C. N., Calandra T., Mayer K., Chesney J., Lohoff M., Gemsa D., Donnelly T., Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc. Natl. Acad. Sci. U. S. A. 1996;93:7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calandra T., Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roger T., David J., Glauser M.P., Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;414:920–924. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- 37.Kleemann R., Hausser A., Geiger G., Mischke R., Burger-Kentischer A., Flieger O., Johannes F.J., Roger T., Calandra T., Kapurniotu A., Grell M., Finkelmeier D., Brunner H., Bernhagen J. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature. 2000;408:211–216. doi: 10.1038/35041591. [DOI] [PubMed] [Google Scholar]
- 38.Hudson J.D., Shoaibi M.A., Maestro R., Carnero A., Hannon G.J., Beach D.H. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J. Exp. Med. 1999;190:1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi X., Leng L., Wang T., Wang W., Du X., Li J., McDonald C., Chen Z., Murphy J.W., Lolis E., Noble P., Knudson W., Bucala R. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25:595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashi S., Sugiyama T., Amano K., Isogai H., Isogai E., Aihara M., Kikuchi M., Asaka M., Yokota K., Oguma K., Fujii N., Hirai Y. Effect of rebamipide, a novel antiulcer agent, on Helicobacter pylori adhesion to gastric epithelial cells. Antimicrob. Agents Chemother. 1998;42:1895–1899. doi: 10.1128/aac.42.8.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Y.Q., Girgensone I., Leanderson P., Petersson F., Borch K. Effects of antioxidant vitamin supplements on Helicobacter pylori-induced gastritis in Mongolian gerbils. Helicobacter. 2005;10:33–42. doi: 10.1111/j.1523-5378.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 42.Takeda K., Utsunomiya H., Kakiuchi S., Okuno Y., Oda K., Inada K., Tsutsumi Y., Tanaka T., Kakudo K. Citrus auraptene reduces Helicobacter pylori colonization of glandular stomach lesions in Mongolian gerbils. J. Oleo Sci. 2007;56:253–260. doi: 10.5650/jos.56.253. [DOI] [PubMed] [Google Scholar]
- 43.Arabski M., Kazmierczak P., Wisniewska-Jarosinska M., Morawiec Z., Morawiec-Bajda A., Klupinska G., Drzewoski J., Chojnacki J., Blasiak J. Helicobacter pylori infection can modulate the susceptibility of gastric mucosa cells to MNNG. Cell Mol. Biol. Lett. 2006;11:570–578. doi: 10.2478/s11658-006-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He K., Iyer K.R., Hayes R.N., Sinz M.W., Woolf T.F., Hollenberg P.F. Inactivation of cytochrome P450 3A4 by bergamottin, a component of grapefruit juice. Chem. Res. Toxicol. 1998;11:252–259. doi: 10.1021/tx970192k. [DOI] [PubMed] [Google Scholar]
- 45.Baumgart A., Schmidt M., Schmitz H.J., Schrenk D. Natural furocoumarins as inducers and inhibitors of cytochrome P450 1A1 in rat hepatocytes. Biochem. Pharmacol. 2005;69:657–667. doi: 10.1016/j.bcp.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 46.Lin H.L., Kent U.M., Hollenberg P.F. The grapefruit juice effect is not limited to cytochrome P450 (P450) 3A4: evidence for bergamottin-dependent inactivation, heme destruction, and covalent binding to protein in P450s 2B6 and 3A5. J. Pharmacol. Exp. Ther. 2005;313:154–164. doi: 10.1124/jpet.104.079608. [DOI] [PubMed] [Google Scholar]
- 47.Kleiner H.E., Reed M.J., DiGiovanni J. Naturally occurring coumarins inhibit human cytochromes P450 and block benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene DNA adduct formation in MCF-7 cells. Chem. Res. Toxicol. 2003;16:415–422. doi: 10.1021/tx025636d. [DOI] [PubMed] [Google Scholar]
- 48.Cai Y., Kleiner H., Johnston D., Dubowski A., Bostic S., Ivie W., DiGiovanni J. Effect of naturally occurring coumarins on the formation of epidermal DNA adducts and skin tumors induced by benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene in SENCAR mice. Carcinogenesis. 1997;18:1521–1527. doi: 10.1093/carcin/18.8.1521. [DOI] [PubMed] [Google Scholar]
- 49.Murakami A., Gao G., Kim O. K., Omura M., Yano M., Ito C., Furukawa H., Jiwajinda S., Koshimizu K., Ohigashi H. Identification of coumarins from the fruit of Citrus hystrix DC as inhibitors of nitric oxide generation in mouse macrophage RAW 264.7 cells. J. Agric. Food Chem. 1999;47:333–339. doi: 10.1021/jf980523e. [DOI] [PubMed] [Google Scholar]
- 50.Kawaii S., Tomono Y., Katase E., Ogawa K., Yano M. Isolation of furocoumarins from bergamot fruits as HL-60 differentiation-inducing compounds. J. Agric. Food Chem. 1999;47:4073–4078. doi: 10.1021/jf990155u. [DOI] [PubMed] [Google Scholar]
- 51.Buiatti E., Palli D., Decarli A., Amadori D., Avellini C., Bianchi S., Biserni R., Cipriani F., Cocco P., Giacosa A., Marubini E., Puntoni R., Vindigni C., Fraumeni J. Jr., Blot W. A case-control study of gastric cancer and diet in Italy. Int. J. Cancer. 1989;44:611–616. doi: 10.1002/ijc.2910440409. [DOI] [PubMed] [Google Scholar]
- 52.Boeing H., Frentzel-Beyme R., Berger M., Berndt V., Göres W., Körner M., Lohmeier R., Menarcher A., Männl H.F., Meinhardt M., Müller R., Ostermeier H., Paul F., Schwemmle K., Wagner K.H., Wahrendorf J. Case-control study on stomach cancer in Germany. Int. J. Cancer. 1991;47:858–864. doi: 10.1002/ijc.2910470612. [DOI] [PubMed] [Google Scholar]
- 53.Ramon J.M., Serra L., Cerdo C., Oromi J. Dietary factors and gastric cancer risk. A case-control study in Spain. Cancer. 1993;71:1731–1735. doi: 10.1002/1097-0142(19930301)71:5<1731::aid-cncr2820710505>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez C.A., Pera G., Agudo A., Bueno-de-Mesquita H.B., Ceroti M., Boeing H., Schulz M., Del Giudice G., Plebani M., Carneiro F., Berrino F., Sacerdote C., Tumino R., Panico S., Berglund G., Siman H., Hallmans G., Stenling R., Martinez C., Dorronsoro M., Barricarte A., Navarro C., Quiros J.R., Allen N., Key T.J., Bingham S., Day N.E., Linseisen J., Nagel G., Overvad K., Jensen M.K., Olsen A., Tjonneland A., Buchner F.L., Peeters P.H., Numans M.E., Clavel-Chapelon F., Boutron-Ruault M.C., Roukos D., Trichopoulou A., Psaltopoulou T., Lund E., Casagrande C., Slimani N., Jenab M., Riboli E. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Int. J. Cancer. 2006;118:2559–2566. doi: 10.1002/ijc.21678. [DOI] [PubMed] [Google Scholar]
- 55.Matza D., Wolstein O., Dikstein R., Shachar I. Invariant chain induces B cell maturation by activating a TAF(II)105-NF-kappaB-dependent transcription program. J. Biol. Chem. 2001;276:27203–27206. doi: 10.1074/jbc.M104684200. [DOI] [PubMed] [Google Scholar]
- 56.Barrera C.A., Beswick E.J., Sierra J.C., Bland D., Espejo R., Mifflin R., Adegboyega P., Crowe S.E., Ernst P.B., Reyes V.E. Polarized expression of CD74 by gastric epithelial cells. J. Histochem. Cytochem. 2005;53:1481–1489. doi: 10.1369/jhc.4A6552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beswick E.J., Pinchuk I.V., Suarez G., Sierra J.C., Reyes V.E. Helicobacter pylori CagA-dependent macrophage migration inhibitory factor produced by gastric epithelial cells binds to CD74 and stimulates procarcinogenic events. J. Immunol. 2006;176:6794–6801. doi: 10.4049/jimmunol.176.11.6794. [DOI] [PubMed] [Google Scholar]
- 58.Daun J.M., Cannon J.G. Macrophage migration inhibitory factor antagonizes hydrocortisone-induced increases in cytosolic IkappaBalpha. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1043–1049. doi: 10.1152/ajpregu.2000.279.3.R1043. [DOI] [PubMed] [Google Scholar]
- 59.Cao W.G., Morin M., Metz C., Maheux R., Akoum A. Stimulation of macrophage migration inhibitory factor expression in endometrial stromal cells by interleukin 1, beta involving the nuclear transcription factor NFkappaB. Biol. Reprod. 2005;73:565–570. doi: 10.1095/biolreprod.104.038331. [DOI] [PubMed] [Google Scholar]
- 60.Cao W.G., Morin M., Sengers V., Metz C., Roger T., Maheux R., Akoum A. Tumour necrosis factor-alpha up-regulates macrophage migration inhibitory factor expression in endometrial stromal cells via the nuclear transcription factor NF-kappaB. Hum. Reprod. 2006;21:421–428. doi: 10.1093/humrep/dei315. [DOI] [PubMed] [Google Scholar]
- 61.Nozawa Y., Nishihara K., Peek R.M., Nakano M., Uji T., Ajioka H., Matsuura N., Miyake H. Identification of a signaling cascade for interleukin-8 production by Helicobacter pylori in human gastric epithelial cells. Biochem. Pharmacol. 2002;64:21–30. doi: 10.1016/s0006-2952(02)01030-4. [DOI] [PubMed] [Google Scholar]
- 62.Kim J.A., Kim D.K., Kang O.H., Choi Y.A., Park H.J., Choi S.C., Kim T.H., Yun K.J., Nah Y.H., Lee Y.M. Inhibitory effect of luteolin on TNF-alpha-induced IL-8 production in human colon epithelial cells. Int. Immunopharmacol. 2005;5:209–217. doi: 10.1016/j.intimp.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 63.Natarajan K., Manna S.K., Chaturvedi M.M., Aggarwal B.B. Protein tyrosine kinase inhibitors block tumor necrosis factor-induced activation of nuclear factor-kappaB, degradation of IkappaBalpha, nuclear translocation of p65, and subsequent gene expression. Arch. Biochem. Biophys. 1998;352:59–70. doi: 10.1006/abbi.1998.0576. [DOI] [PubMed] [Google Scholar]
- 64.Eguchi A., Kaneko Y., Murakami A., Ohigashi H. Zerumbone suppresses phorbol ester-induced expression of multiple scavenger receptor genes in THP-1 human monocytic cells. Biosci. Biotechnol. Biochem. 2007;71:935–945. doi: 10.1271/bbb.60596. [DOI] [PubMed] [Google Scholar]