Abstract
Oxidative stress stimulates two opposite signaling pathways leading to cell death and cell survival. Preferential selection of survival signals leads to the protection of cells against damage induced by reactive oxygen species, whereas preferential acceleration of death signals can be used to advantage in tumor therapy with oxidizing agents such as ionizing radiation and anticancer drugs. In vitro and in vivo experiments using cultured mammalian cells and experimental animals showed that ERK was included in survival signals and SAPK and p38 MAPK in death signals in oxidative stress. The activation of SAPK/JNK and subsequent expression of death receptor Fas on the cell surface caused the induction of cell death. The results mean that the acceleration of the activation of SAPK/JNK might lead to the enhancement of cell death by oxidizing agents like ionizing radiation and anticancer drugs. In fact, when cultured mammalian cells were exposed to ionizing radiation with 2-nitroimidazole derivatives having electrophilicity, the lethal effect of ionizing radiation was found to be enhanced together with the activation of SAPK/JNK and the enhancement of Fas expression. The activation of both survival and death signals was suppressed by the antioxidants N-acetylcystein and Trolox, suggesting that both signaling pathways are redox-regulated.
Keywords: oxidative stress, ROS, SAPK/JNK, ERK, N-acetylcystein
Introduction
Oxidative stress is thought to be a phenomenon that induces various health disturbances and diseases by enhancing oxidation of biologically important molecules in vivo. Oxidation reactions by reactive oxygen species (ROS) are regarded as a trigger of the oxidative stress. Several enzymes like superoxide dismutase (SOD), glutathione peroxidase and catalase serve as protective antioxidants against oxidative stress. The ingestion of vitamins/minerals, carotinoids and polyphenols is also effective to keep the body healthy. However, recent studies indicate that ROS and free radicals have not only such negative effects but also positive effects on cells by stimulating their proliferation and protein synthesis [1, 2]. Thus, ROS are also inferred to be related to the immortalization and proliferation of tumor cells. Therefore, oxidative stress is now a general term for various phenomena induced by the loss of balance between the generation and the elimination of ROS in vivo. It is important to elucidate the mechanisms of signaling pathways of cell death and cell survival to determine the roles ROS play in these contradictory phenomena.
Signal Transduction Pathways Leading to Apoptotic Cell Death Induced by ROS
Two types of cell death, necrosis and apoptosis, are well known. Necrosis is pathologically induced cell death, and apoptosis is programmed cell death under certain physiological conditions and plays an essential role for vertebrate development, cell differentiation and homeostasis. Therefore, its execution is strictly regulated through signal transduction pathways. Apoptotic signals are normally maintained in the OFF state, but unnecessary cell death is induced in tissues and organs when the signals are in the ON state for some reason. It is of interest to clarify how ROS induce apoptosis by changing signaling pathways from OFF to ON. Various causes induce apoptosis. For example, cerebral ischemia-reperfusion induces neuronal apoptotic cell death in the CA1 region of the hippocampus. Apoptosis is also observed in virus-infected cells. A typical oxidizing agent, ionizing radiation, is known to induce apoptosis in lymphoid cells, but rarely in fibroblast cells [3] and especially in p53-decifient or -mutated cells [4]. When cultured Chinese hamster V79 fibroblast cells are exposed to X rays, little apoptotic cell death is induced. However, hydrogen peroxide H2O2 induces apoptosis in this cell line as well as bovine aortic vascular endothelial cells (BAEC) in a concentration-dependent manner [5, 6]. An increase of [Ca2+]i is essential in the H2O2-induced apoptosis in these cell lines since the addition of chelating agents to culture medium suppresses the induction of apoptosis. A chelator of extracellular calcium ions suppresses the induction of apoptosis in BAEC [7], but apoptosis is observed in the presence of the extracellular calcium chelator in the case of Chinese hamster V79 cells. The depletion of calcium ions in the intracellular calcium store, ER, by the treatment of Chinese hamster V79 cells with thapsigargin suppresses the increase of [Ca2+]i in cells exposed to a few mM H2O2, but the increase is not suppressed when they are exposed to more than 10 mM H2O2, indicating the release of calcium ions from calcium-binding proteins [8]. In fact, Hoyal et al. reported the release of calcium ions from calcium-binding proteins comprising the cytoskeleton after the treatment with H2O2 [9].
Apoptosis is induced by the activation of caspase 8 through the up-regulation of the death receptor Fas and by the activation of caspase 9 through the activation of p53 and the subsequent release of cytochrome c from mitochondria, as well as by the final activation of caspase 3 [10]. Suhara et al. reported that H2O2 induced up-regulation of Fas in human endothelial cells [11]. We observed that the activation of SAPK/JNK and the subsequent activation of c-jun were induced by the exposure of Chinese hamster V79 cells to H2O2 [8, 12]. The protein synthesis inhibitor cycloheximide suppresses the activation of SAPK/JNK and up-regulation of Fas [13], indicating that the activation of SAPK/JNK is closely related to the Fas expression on the cell surface. The activation of caspase 8 in BAEC exposed to H2O2 was also observed [14]. The increase of [Ca2+]i is essential for the activation of SAPK/JNK and the release of cytochrome c, but the release of cytochrome c from mitochondria does not depend on the activation of SAPK/JNK [8]. Therefore, ROS induce two Ca2+-dependent pathways, the activation of SAPK/JNK and the release of cytochrome c. p38 MAPK, another MAP kinase leading to apoptosis, is also activated by treatment with H2O2, but its activation is independent of the increase of [Ca2+]i [8]. The facts mentioned above mean that ROS, as a whole, induce two signaling pathways, an increase of [Ca2+]i and subsequent activation of SAPK/JNK, and the release of cytochrome c followed by the activation of p38 MPAK leading to apoptotic cell death. Recently, we proved that the induction of apoptosis was strongly interfered with by inhibitor of apoptosis proteins (IAP) such as survivin closely assembling with proteins leading to cell cycle arrest at the G2/M phase in X-irradiated Chinese hamster cells, but this interference was canceled by inhibiting the synthesis of the proteins related to the G2/M arrest by employing an anticancer drug targeting RNA synthesis [15].
Signal Transduction Pathways Leading to Cell Survival Induced by ROS
ERK (extracellular signal-regulated kinases), a member of MAPK family, and PKB (protein kinase B, Akt) are known as kinases leading to cell survival [16, 17]. However, the upstream kinase PI3K (phosphoinositide 3-kinase) plays different roles in Chinese hamster V79 fibroblasts and endothelial cells when exposed to H2O2. We have found that ROS accelerates the activation of not only PI3K but also PKB, suggesting that ROS bring about the activation of survival signaling pathways [8, 14]. The increase of [Ca2+]i is not required for the activation of PI3K in BAEC but is required for the activation of Chinese hamster V79 cells. The result is contrastive to apoptotic signaling pathways exclusively requiring an increase of [Ca2+]i. The activation of PI3K through phosphorylation of insulin receptor substance 1 (IRS-1) is dependent on the increase of [Ca2+]i in H2O2-treated V79 cells. Furthermore, it has been found that the H2O2-induced activation of SAPK/JNK is regulated by PI3K in this cell line. Wortmannin, a PI inhibitor, augments the induction of apoptosis and the activation of capases 3 and 9 as well as the suppression of the activation of PKB in H2O2-exposed BAEC [14].
In Vivo Experiments to Prove the Induction of Both Survival and Death Signaling Pathways by ROS
Ischemia-reperfusion-induced neuronal cell death in the CA1 region of the gerbil hippocampus is regarded as a good model of oxidative stress [18, 19]. We have found that transient ischemia induces the activation of SAPK/JNK and p38 MPAK leading to apoptosis and ERK leading to survival, suggesting that oxidative stress induces both death and survival signaling pathways even in vivo [20]. Inferring from in vitro experiments using cultured mammalian cells, the condition determining whether the survival pathway or the apoptotic one predominates depends on the concentration of ROS. When the spin trapping agent PBN, which has scavenging activity for hydroxyl radicals (•OH) and, therefore, functions as an antioxidant, is intraperitoneally injected into the gerbil, the suppression of the activation of SAPK/JNK and p38 MAPK relating to death signaling and the promotion of the activation of ERK related to survival signaling are simultaneously observed together with protection against neuronal cell death in the CA1 region of the hippocampus. These results are consistent with those obtained from in vitro experiments using cultured mammalian cells as described in the previous section. Interestingly, PBN administration up-regulates the expression of heat shock proteins HSPs 27 ad 70 in the gerbil hippocampal region. Direct evidence for the participation of SAK/JNK in the death signaling pathway was obtained by using another in vivo model of oxidative stress [21]. Malonate, which produces lesions similar to those of focal ischemia-reperfusion by reversible inhibition of succinate dehydrogenase in mitochondria, was injected into the left striatum in the rat brain with or without the simultaneous injection of a cell permeable peptide JNK inhibitor, (L)-HIV-TAT48–57-PP-JBD20. This JNK inhibitor consists of a carboxyl-terminal sequence derived from the JNK-binding domain (JBD) and an amino-terminal peptide containing the HIV-TAT48–57 sequence that imparts cell permeability [22, 23]. The JBD20 sequence is derived from the JBD of JNK-interacting protein-1, known as a JNK scaffolding protein [24]. Rat brains were noninvasively examined by the magnetic resonance imaging (MRI) method with the technique of apparent diffusion coefficient (ADC) mapping under 7.05 T. The increase in ADC values of hyperintense regions corresponding to the malonate-induced ischemic core and the decease in ADC values in their surrounding hypointense regions corresponding to edematous alterations were found to be suppressed by the addition of the cell permeable peptide JNK inhibitor. Since an increase of the ADC value indicates the occurrence of vasogenic edema accompanying ischemia and a decrease of the ADC value reflects histological alterations, breakdown of the energy metabolism and tissue acidosis, the suppression of both the increase and decrease in ADC values suggested that the inhibition of JNK surely protected the brain tissue against ischemia-induced injury, proving that the SAPK/JNK was responsible for the death signaling pathway.
Redox Regulation of Cell Death and Cell Survival
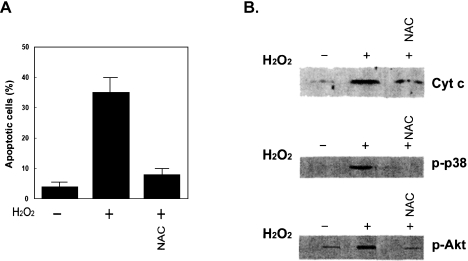
When the intracellular condition is changed into the reducing state by adding antioxidizing agents such as N-acetylcystein (NAC), Trolox (a vitamin E derivative), catechin, PBN, etc., both apoptosis and survival signaling pathways induced by ROS are suppressed [6, 14, 20, 25–27]. In contrast, when the cellular condition is changed to the oxidizing state with 2-nitroimidazole derivatives, electron affinic compounds, both signaling pathways are accelerated [28–30]. These results suggest that both death and survival signaling pathways are redox regulated. In vitro evidence for the redox regulation was obtained by observing the suppressive effects of NAC not only on the activation of PKB (Akt) but also on the activation of p38 MAPK and Bax and the release of cytochrome c from mitochondria (Fig. 1), as well as the activation of caspases 9 and 3 in H2O2-exposed BAEC [6, 14]. The induction of apoptosis in H2O2-exposed BAEC is also suppressed by NAC. A similar suppressive effect of NAC is obtained on the activation of SAPK/JNK [30]. The fact that the change of the intracellular condition into the reducing state by antioxidizing agents like NAC results in protection against H2O2-induced apoptosis seems to be in conflict with the fact that both survival and death signaling pathways are simultaneously suppressed by antioxizing agents. The suppression of death signaling may act more predominantly for cell survival than the suppression of survival signaling. When PBN-administered rats were employed in in vivo experiments, PBN acted protectively against neuronal cell death induced by ischemia/reperfusion [20]. In this case, the suppressive effect of PBN administration on the survival signal, ERK, was relatively mild, whereas the suppressive effect on the death signals, p38 MAPK and SAPK/ JNK, was strong, resulting in a shift in the balance in neuronal cells between death and survival to survival. Suppressive effects of NAC on the increase of [Ca2+]i upstream of the release of cytochrome c and the activation of SAPK/JNK were also reported by Donaldson et al. [31].
Fig. 1.
(A) Effect of the antioxidizing agent NAC on the induction of apoptosis, and (B) the effects of NAC on the release of cytochrome c from mitochondria and the activation of p38 MAPK and PKB (Akt) in H2O2-exposed BAEC. BAEC (Cell Systems, Kirkland, WA) were exposed to 1 mM H2O2 for 1 h in the presence or absence of 15 mM NAC. Apoptotic cells were analyzed by flow cytometry. Release of cytochrome c was assessed by combining SDS-PAGE and immunobloting of cytosol fraction of cells with a specific antibody for cytochrome c. In the case of measurements of phosphoryalted p38 MAPK (p-p38 MAPK) and phosphorylated Akt (p-Akt), cell lysates were analyzed by immunobloting with anti-p-p38 MAPK and anti-p Akt after BAEC were exposed to 1 mM H2O2 for 30 min.
Ionizing radiation produces strong oxidants, OH radicals, by degrading H2O and, therefore brings about the oxidation of cellular biological molecules. We have found that the lethal effects of ionizing radiation are enhanced under intracellular conditions changed to the oxidizing state with electron affinic compounds (2-nitroimidazole derivatives) [13, 26, 28–30, 32]. ROS intranuclearly produced by ionizing radiation cause DNA damage that activates the signaling pathways to apoptosis, but this pathway depends on the p53 genomic status. Therefore, p53-mutated cells such as many tumor cells tend toward resistance to ionizing radiation [33]. Our studies showed that ionizing radiation could induce apoptosis through the activation of SAPK/JNK independently of DNA damage and, therefore, the p53 genomic status. Furthermore, the oxidizing condition induced by an electron affinic compound enhances the expression of SAPK/JNK followed by enhanced induction of apoptosis in X-irradiated human leukemia cell line MOLT-4 and HL60 (Fig. 2) [28, 30]. Biologically important molecules like DNA, proteins and lipids are oxidized by the formation of their radicals due to attack by OH radicals and the subsequent addition of O2 molecules to the radical sites to form peroxyradicals. For example, the transformation of the thymine base in DNA to thymine glycol and urea through these reactions is known as typical radiation-induced oxidation reactions [34]. Oxidation reactions hardly proceed under hypoxic conditions producing the reducing state. 2-Nitroimidazole derivatives with electrophilicity were proved to take part in the reactions in a fashion similar to O2 [35]. The enhancement of the expression of Fas after the activation of SAPK/JNK in the presence of this compound under hypoxia was also observed, but the activation of ERK and PKB (Akt), serving as survival signals, was not changed in the presence of this compound, suggesting that the 2-nitroimidazole derivative exclusively activated the death signaling pathways.
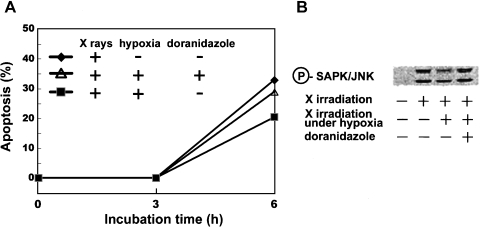
Fig. 2.
Since a few percent of cells inside tumors are known to be generally under hypoxia, human leukemia MOLT-4 cells (RIKEN Cell Bank, Tsukuba, Japan) were exposed to 15 Gy of X rays under not only oxygenated condition but also hypoxic condition. The hypoxic condition was achieved by a specially designed gas-exchangeable chamber in which plastic dish containing cells was placed. Cells were treated with 5 mM doranidazole, a 2-nitroimidazole derivative, prepared by Pola Chemical Industries (Yokohama, Japan) by incubating for 25 min under the hypoxic condition before X irradiation. After post-irradiation incubation under both oxygenated and hypoxic conditions for 0–6 h, apoptotic cells were detected with fluorescence microscopy of cells stained with propidium iodide. The time-dependent increase in the induction of apoptosis was observed, and the hypoxic condition induced apoptosis to a lesser extent (closed square) than the oxygenated condition did (closed diamond). Addition of doranidazole to hypoxic cells made the cellular condition oxidizing and brought about enhancement of the induction of apoptosis (opened triangle). Immunoblot analysis of SAPK/JNK and phosphorylated SAPK/JNK were carried out with their individual antibodies after cells were incubated under oxygenated or hypoxic condition with 5 mM doranidazole for 1.5 h. Apoptotic signaling SAKP/JNK was found to be activated by X irradiation. Hypoxia reduced the extent of its activation and the addition of doranidazole restored its activation to a level similar to that observed after X irradiation under the oxygenated condition.
Primary Target Molecules of ROS Leading to Both Cell Survival and Death
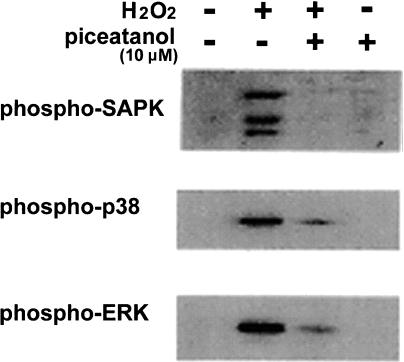
The primary effect of ROS leading to cell survival or cell death is thought to be the reversible oxidation of proteins. Thiols in proteins are considered to act as redox-sensitive sites. The activities of both protein tyrosine phosphatases (PTPs) and protein tyrosine kinases (PTKs) are reported to be modified by the oxidation of cystein residues by H2O2 [36–38]. This modification can be reversed by reducing agents such as NAC. The inhibition of PTPs and the activation of PTKs likely bring about high levels of intracellular phosphoproteins, leading to the induction of signal transduction. We observed that an inhibitor of PTKs, piceatannol [39], suppressed the activation (phosphorylation) of SAPK/JNK, p38 MAPK and ERK in H2O2-treated Chinese hamster V79 cells (Fig. 3), indicating that the inhibition of PTKs simultaneously affects both surviving (ERK) and death (SAPK/JNK and p38 MAPK) signaling pathways. This means that PTKs are present upstream from these survival and death signaling molecules. Proteins, including PTPs and PTKs (or their interaction), having cysteins as active redox sites are thought to be the target of oxidation by ROS and this oxidation can be reversed by reducing agents such as NAC.
Fig. 3.
The effects of an inhibitor of PTKs, piceatannol, on the activation (phosphorylation) of SAPK/JNK, p38 MAPK and ERK in H2O2-treated Chinese hamster V79 cells. Cells were incubated with 10 mM H2O2 in the presence or absence of 10 µM piceatannol for 15 min. Immunoblot analysis of phosphorylated SAPK/JNK, phosphorylated p-38 MAPK and phosphorylated ERK was carried out with their individual antibodies.
Effects of Orally Administered Antioxidants on ROS-Associated Tissue Damage
It is important for humans to determine in concrete experimental animal models whether orally administered antioxidants can protect against ROS-associated tissue damage. For this purpose, Rooibos tea (extract from fermented leaves and fine stems of the indigenous South African leguminous shrub, Aspalathus linearis) was orally administered to Wistar rats ad libitum from 3 to 21 months of age and then their brains were examined by MRI under 7.05 T. This tea contains many flavonoids as antioxidants [40]. After the MRI observation, the amounts of age-related lipid peroxides accumulated in the frontal, occipital and parietal cortexes as well as those in the caudate putamen, hippocampus, midbrain, cerebellum and pons/medulla were biochemically measured [27]. Significant differences in the MRI signal intensities and the age-related accumulation of lipid peroxides between controls and Rooibos tea-administered rats were found in the frontal cortex, hippocampus and cerebellum with the protective effects. The finding that in the frontal cortex, known as the region in which functional decline takes place most rapidly in the brain with aging was maintained normally by oral administration of antioxidants was important. The protective effect of orally administered pure (−)catechin against ischemia-reperfusion-induced cell death of the hippocampal CA1 region in the gerbil was also examined [25]. (−)Catechin in aqueous solution was given ad libitum to Mongolian gerbils for 15 days and the bilateral common carotid arteries of the gerbils were occluded for 5 min. The gerbils were again allowed free access to aqueous solution of (−)catechin for 7 days and then their brains were histologically examined. The protective effect of (−)catechin uptake against delayed neuronal death of pyramidal cells in the hippocampal CA1 region was clearly demonstrated. With the aid of the ESR-spin-trapping technique, it was proved that the superoxide scavenging activities of brain homogenates obtained from (−)catechin-treated gerbils were higher than those of catechin-untreated gerbils, suggesting that the signaling pathway to cell death induced by ROS produced by ischemia-reperfusion might be effectively suppressed by the antioxidant activity of (−)catechin. In another report, several flavonoids including (−)catechin were shown to have activity to inhibit protein kinase C from participating in the signal transduction to cell death [41]. When similar experiments using a fermented grain food mixture (Antioxidant Biofactor, AOBTM) were carried out, increased superoxide scavenging activities of brain homogenates from orally AOBTM-administered Mongolian gerbils were observed, and the suppressive effects on the induction of apoptosis by AOBTM uptake were also shown by TUNEL assay. These results suggested that apoptosis signaling pathways were suppressed by AOBTM-administration [42].
These experiments provide conclusive proof that oral administration of antioxidants is useful for humans because antioxidants orally administered to experimental animals could protect against ROS-associated tissue damage.
Conclusions
Using in vitro and in vivo models, we have shown that oxidative stress stimulates two opposite signaling pathways leading to cell death and cell survival. H2O2-induced survival and death signaling pathways were both simultaneously suppressed by antioxizing agents such as NAC. This seems to be in conflict with the exclusive protection against H2O2-induced apoptosis by antioxidants. This may be explained by the fact that the suppression of death signaling acts more predominantly for cell survival than the suppression of survival signaling does. Therefore, the suppression of ROS-induced death signals by treating cells or individuals with antioxidants would be effective for the protection against ROS-related cell death as well as individual health disturbances and diseases. Acceleration of death signals by some compounds with electrophilicity can be used to advantage in tumor therapy with oxidizing agents like ionizing radiation and anti-cancer drugs. In fact, 2-nitroimidazole derivatives have been shown to accelerate the activation of SAPK/JNK and the subsequent enhancement of Fas expression, and to result in the enhancement of the induction of cell death in cultured mammalian cells [13, 26, 28–30, 32]. The enhancement of radiation-induced cell death by 2-nitroimidazole derivatives has been confirmed in vivo, suggesting that these compounds are clinically promising [42, 43]. Preferential acceleration of survival and death signaling pathways by the use of antioxidants or compounds with electrophilicity can be used to advantage for protection against oxidative damage and in tumor therapy, respectively.
References
- 1.Burton R.H. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Rad. Biol. Med. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- 2.Irani K., Xia Y., Zweier J.L., Sollot S.J., Der C.J., Fearon E.R., Sundaresan M., Finkel T., Goldschmidt-Clermont P.J. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 3.Radford I.R. Initiation of ionizing radiation-induced apoptosis: DNA damage-mediated or does ceramide have a role? Int. J. Radiat. Biol. 1999;75:521–528. doi: 10.1080/095530099140168. [DOI] [PubMed] [Google Scholar]
- 4.Lu-Hesselmann J., Abend M., van Beuningen D. Comparison of endogeneous TP53 genomic status with clonogenicity and different modes of cell death after X irradiation. Radiat. Res. 2004;161:39–47. doi: 10.1667/rr3092. [DOI] [PubMed] [Google Scholar]
- 5.Hiraoka W., Fuma K., Kuwabara M. Concentration-dependent modes of cell death in Chinese hamster V79 cells after treatment with H2O2. J. Radiat. Res. 1997;38:95–102. doi: 10.1269/jrr.38.95. [DOI] [PubMed] [Google Scholar]
- 6.Niwa K., Inanami O., Yamamori T., Ohta T., Hamasu T., Kirino T., Kuwabara M. Roles of protein kinase Cδ in the accumulation of p53 and the induction of apoptosis in H2O2-treated bovine endothelial cells. Free Rad. Res. 2002;36:1147–1153. doi: 10.1080/1071576021000016409. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu S., Nomoto M., Naito S., Yamamoto T., Momose K. Stimulation of nitric oxide synthase during oxidative endothelial cell injury. Biochem. Pharmcol. 1998;55:77–83. doi: 10.1016/s0006-2952(97)00399-7. [DOI] [PubMed] [Google Scholar]
- 8.Inanami O., Ohta T., Ito S., Kuwabara M. Elevation of intracellular calcium ions is essential for the H2O2-induced activation of SAPK/JNK but not for that of p38 and ERK in Chinese hamster V79 cells. Antiox. Redox Signal. 1999;1:501–508. doi: 10.1089/ars.1999.1.4-501. [DOI] [PubMed] [Google Scholar]
- 9.Hoyal C.R., Thomas A.P., Forman H.J. Hydrogenperoxide-induced increases in intracellular calcium due to annexin VI translocation and inactivation of plasma membrane Ca2+-ATPase. J. Biol. Chem. 1996;271:29205–29210. doi: 10.1074/jbc.271.46.29205. [DOI] [PubMed] [Google Scholar]
- 10.Slee E.A., Adrain C., Martin S.J. Serial killers: ordering capase activation events in apoptosis. Cell Death Differ. 1999;8:1067–1074. doi: 10.1038/sj.cdd.4400601. [DOI] [PubMed] [Google Scholar]
- 11.Suhara T., Kukuo K., Sugimoto T., Morimoto S., Nakahashi T., Hara S., Shimizu M., Ogihara T. Hydrogen peroxide induces up-regulation of Fas in human endothelial cells. J. Immunol. 1998;160:4042–4047. [PubMed] [Google Scholar]
- 12.Inanami O., Takahashi K., Yishito A., Kuwabara M. H2O2-induced activation of SAPK/JNK regulated by phosphatidylinositol 3-kinase in Chinese hamster V79 cells. Antiox. Redox Signal. 1999;1:113–121. doi: 10.1089/ars.1999.1.1-113. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K., Inanami O., Hayashi M., Kuwabara M. Protein synthesis-dependent apoptotic signaling pathway in X-irradiated human leukemia cell line MOLT-4. Int. J. Radiat. Biol. 2002;78:115–124. doi: 10.1080/09553000110076472. [DOI] [PubMed] [Google Scholar]
- 14.Niwa K., Inanami O., Yamamori T., Ohta T., Hamasu T., Kuwabara M. Redox regulation of PI3K/Akt and p53 in bovine endothelial cells exposed to hydrogen peroxide. Antiox. Redox Signal. 2003;5:713–722. doi: 10.1089/152308603770380016. [DOI] [PubMed] [Google Scholar]
- 15.Iizuka D., Inanami O., Matsuda A., Kashiwakura I., Asanuma T., Kuwabara M. X irradiation induces the apoptotic state independent of the loss of clonogenic ability in Chinese hamster V79 cells. Radiat. Res. 2005;164:36–44. doi: 10.1667/rr3393. [DOI] [PubMed] [Google Scholar]
- 16.Xia Z., Dickens M., Raingeaud J., Daivis R.J., Greenberg M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 17.Kops G.J., Burgering B.M. Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J. Mol. Med. 77:651–656. doi: 10.1007/s001099900050. [DOI] [PubMed] [Google Scholar]
- 18.Phillis J.W., Sen S., Cao X. Amflutizole, a xanthine oxidase inhibitor, inhibits free radical generation in the ischemia/reperfused rat cerebral cortex. Neurosci. Lett. 1994;169:188–190. doi: 10.1016/0304-3940(94)90388-3. [DOI] [PubMed] [Google Scholar]
- 19.Lafon-Cazal N., Pletrl S., Culcasl M., Bockaert L. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- 20.Tsuji M., Inanami O., Kuwabara M. Neuroprotective effect of α-phenyl-N-tert-butylnitrone in gerbil hippocampus is mediated by the mitogen-activated protein kinase pathway and heat shock proteins. Neurosci. Lett. 2000;282:41–44. doi: 10.1016/s0304-3940(00)00844-2. [DOI] [PubMed] [Google Scholar]
- 21.Asanuma T., Inanami O., Tabu K., Waki K., Kon Y., Kuwabara M. Protection against malonate-induced ischemic brain injury in rat by a cell-permeable peptidic c-Jun N-terminal kinase inhibitor, (L)-HIV-TAT48–57-PP-JBD20, observed by the apparent diffusion coefficient mapping magnetic resonance imaging method. Neurosci. Lett. 2004;359:57–60. doi: 10.1016/j.neulet.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Barr R.K., Kendrick T.S., Bogojevitch M.A. Identification of the critical features of a small peptide inhibitor of JNK activity. J. Biol. Chem. 2002;277:10987–10997. doi: 10.1074/jbc.M107565200. [DOI] [PubMed] [Google Scholar]
- 23.Vives E., Richard J.P., Rispal C., Lebleu B. TAT peptide internalization: seeking the mechanism of entry. Curr. Prot. Pept. Sci. 2003;4:41–44. doi: 10.2174/1389203033487306. [DOI] [PubMed] [Google Scholar]
- 24.Yasuda J., Whitmarsh A.J., Cavanagh J., Sharma M., Davis R.J. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol. Cell Biol. 1999;19:7245–7252. doi: 10.1128/mcb.19.10.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inanami O., Watanabe Y., Syuto B., Nakano M., Tsuji M., Kuwabara M. Oral administration of (–)catechin protects against ischemia-reperfusion-induced neuronal death in the gerbil. Free Rad. Res. 1998;29:359–365. doi: 10.1080/10715769800300401. [DOI] [PubMed] [Google Scholar]
- 26.Inanami O., Takahashi K., Kuwabara M. Attenuation of caspase-3-dependent apoptosis by Trolox post-treatment of X-irradiated MOLT-4 cells. Int. J. Radiat. Biol. 1999;75:155–163. doi: 10.1080/095530099140609. [DOI] [PubMed] [Google Scholar]
- 27.Inanami O., Asanuma T., Inukai N., Jin T., Shimokawa S., Kasai N., Nakano M., Satom F., Kuwabara M. The suppression of age-related accumulation of lipid peroxides in rat brain by administration of Rooibos tea (Aspalathus linearis) Neurosc. Lett. 1995;196:85–88. doi: 10.1016/0304-3940(95)11853-o. [DOI] [PubMed] [Google Scholar]
- 28.Inanami O., Sugihara K., Okui T., Hayashi M., Tsujitani M., Kuwabara M. Hypoxia and etanidazole alter radiation-induced apoptosis in HL60 cells but not in MOLT-4 cells. Int. J. Radiat. Biol. 2002;78:267–274. doi: 10.1080/09553000110105695. [DOI] [PubMed] [Google Scholar]
- 29.Kuwabara M., Takahashi K., Inanami O. Induction of apoptosis through activation of SAPK/JNK followed by the expression of death receptor Fas in X-irradiated cells. J. Radiat. Biol. 2003;44:203–209. doi: 10.1269/jrr.44.203. [DOI] [PubMed] [Google Scholar]
- 30.Hamasu T., Inanami O., Tsujitani M., Yokoyama K., Takahashi E., Kashiwakura I., Kuwabara M. Post-irradiation hypoxic incubation of X-irradiated MOLT-4 cells reduces apoptotic cell death by changing the intracellular redox state and modulating SAPK/JNK pathways. Apoptosis. 2005;10:557–567. doi: 10.1007/s10495-005-1888-x. [DOI] [PubMed] [Google Scholar]
- 31.Donaldson K., Stone V., Borm P.J.A., Jimenez L.A., Gilmour P.S., Schins R.P.F., Knaapen A.M., Rahman I., Faux S.P., Brown D.M., MacNee W. Oxidative stress and calcium signaling in the adverse effects of environmental paricles (PM10) Free Rad. Biol. Med. 2003;34:1369–1382. doi: 10.1016/s0891-5849(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K., Inanami O., Kuwabara M. Effects of intracellular calcium chelator BAPTA-AM on radiation-induced apoptosis regulated by activation of SAPK/JNK and caspase-3 in MOLT-4 cells. Int. J. Radiat. Biol. 1999;75:1099–1105. doi: 10.1080/095530099139566. [DOI] [PubMed] [Google Scholar]
- 33.Lu-Hesselmann J., Abend M., van Beuningen D. Comparison of endogeneous TP53 genomic status with clonogenicity and different modes of cell death after X irradiation. Radiat. Res. 2004;161:39–47. doi: 10.1667/rr3092. [DOI] [PubMed] [Google Scholar]
- 34.von Sonntag C. Chemical Basis of Radiation Biology. Taylor & Francis; London: 1987. pp. 10–13. Chapters 7, 15, [Google Scholar]
- 35.Kuwabara M., Iida Y., Inanami O., Sawamura S., Yokoyama K., Tsujitani M. Radiation-chemical properties of the hypoxic cell radiosensitizer doranidazole (PR-350) J. Radiat. Res. 2002;43:77–88. doi: 10.1269/jrr.43.77. [DOI] [PubMed] [Google Scholar]
- 36.Moran L.K., Gutteridge J.M., Quinlan G.J. Thiols in cellular redox signaling and control. Curr. Med. Chem. 2001;8:763–772. doi: 10.2174/0929867013372904. [DOI] [PubMed] [Google Scholar]
- 37.Kamata H., Hirata H. Redox regulation of cellular signaling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 38.Chiarugi P. Reactive oxygen species as mediators of cell adhesion. Ital. J. Biochem. 2003;52:28–32. [PubMed] [Google Scholar]
- 39.Geahlen R.L, McLaughlin J.L. Piceatannol (3,4,3',5'-tetrahydroxy-trans-stilbene) is a naturally occurring protein-tyrosine kinase inhibitor. Biochem. Biophys. Res. Commun. 1989;165:241–245. doi: 10.1016/0006-291x(89)91060-7. [DOI] [PubMed] [Google Scholar]
- 40.Bramati L., Aquilano F., Pietta P. Unfermented rooibos tea: Quantitative characterization of flavonoids by HPLC-UV and determination of the total antioxidant activity. J. Agric. Food Chem. 2003;51:7472–7474. doi: 10.1021/jf0347721. [DOI] [PubMed] [Google Scholar]
- 41.Polya G.M., Foo L.Y. Inhibition of eukaryote signal-regulated protein kinases by plant-derived catechin-related compounds. Phytochem. 1994;35:1399–1405. doi: 10.1016/s0031-9422(00)86864-8. [DOI] [PubMed] [Google Scholar]
- 42.Shibamoto Y., Kubota T., Kishii K., Tsujitani M. Radiosensitivity of human pancreatic cancer cells in vitro and in vivo, and the effect of a new hypoxic cell sensitizer doranidazole (PR-350) Radiother. Oncol. 2000;56:265–270. doi: 10.1016/s0167-8140(00)00181-x. [DOI] [PubMed] [Google Scholar]
- 43.Nemoto K., Shibamoto Y., Ohmagari I. Phase Ia study of a hypoxic cell sensitizer doranidazole (PR-350) in combination with conventional radiotherapy. Anticancer Drugs. 2001;12:1–6. doi: 10.1097/00001813-200101000-00001. [DOI] [PubMed] [Google Scholar]