Abstract
Effects of astaxanthin (AX) derived from H. pluvialis on human blood rheology were investigated in 20 adult men with a single-blind method. The experimental group was 57.5 ± 9.8 years of age and the placebo group was 50.8 ± 13.1 years of age. A blood rheology test that measures whole blood transit time was conducted using heparinized blood of the volunteers by a MC-FAN apparatus (microchannel array flow analyzer). After administration of AX 6 mg/day for 10 days, the values of the experimental group were decreased from 52.8 ± 4.9 s to 47.6 ± 4.2 s (p<0.01) and a comparison of the values between the experimental (47.6 ± 4.2 s) and the placebo (54.2 ± 6.7 s) groups showed a significant difference (p<0.05). There were no adverse effects resulting from the administration of AX 6 mg/day for 10 days. Informed consent was obtained from each subject.
Keywords: astaxanthin, blood rheology, microchannel array flow analyzer, male volunteers, blood transit time
Introduction
Astaxanthin (AX), a form of carotenoid referred to as a xanthophyll, is a substance distributed widely in nature. Since being separated from lobster (Astacus gammaus L.) in 1938 by Kuhn, R. et al. [1], AX has been identified as the substance composing the red pigmentation in microorganisms [2, 3], fish [4], Crustacea [5] and other organisms. AX is the substance that gives salmon [6] and trout, two marine products enjoyed as favorite foods in Japan, their distinct color. Thus many people, especially northern peoples including Japanese who have long included seafood in their diets, have been ingesting AX for many years.
While much research concerning the physiological action of AX has been reported, in research concerning the eyes, Grangaud, R, et al. [7] have reported on the effect found on dry eye when AX was administered to rats. Tso, M.O.M., et al. [8] have reported AX was found to be effective for eye damage in rats, and that a recovery effect after application of optical stress to the eyes was found in a group administered AX when the thickness of the retina outer granular layer and rhodopsin decrease were measured and recovery from damage were compared. In addition, the xanthophyll group including AX has been reported to protect human eye lens epithelial cells from UVB-induced stress [9], and AX’s protective action also has been found to be stronger than that of α-tocopherol.
As effects of AX ingestion on the eyes, Sawaki et al. [10] reported improvement in visual acuity for depth and flicker fusion threshold, while Nagaki, Y. et al. [11] reported significant improvement in amplitude of accommodation before and after ingestion (no change in amplitude of accommodation in the control group) in a study employing VDT workers. Nakamura et al. [12] reported that as a result of administering 0 mg, 2 mg, 4 mg and 12 mg of AX to four groups, respectively, once per day for 28 days, there was improvement in far vision uncorrected visual acuity and shortened positive accommodation time for the 4 mg group and 12 mg group, respectively.
In this paper, we report on a study of the effect of AX ingestion on human blood rheology as one clarification of the action mechanism for such effects.
Materials and Methods
Study materials and equipment
Study food and placebo food
Study food capsules containing a preparation of AX 3 mg (as a free-form) derived from H. pluvialis from Fuji Chemical Industry Co., Ltd. (FHAX09) and placebo food capsules that did not contain AX (FHAX10) were used as a study food and placebo food.
Study equipment
Vacuum blood collection tubes (Terumo) to which 0.25 mL of heparin liquid (Novo Heparin, 1000 units/ml Aventis Pharma Japan) had been added beforehand were used to collect 5 ml blood samples from subjects’ elbow veins, which were used as samples for the rheology evaluations.
To perform the blood rheology evaluations, heparinized whole blood samples of approximately 200 µL were forced to flow through the microchannels of a Microchannel Array (Bloody 6-7; 8736 channels; width 7 µm, length 30 µm, depth 4.5 µm; Hitachi Haramachi Electronics Co., Ltd.) under a pressure difference of 20 cm H2O, and the transit time for 100 µl of blood was determined using a MC-FAN (Hitachi Haramachi Electronics Co., Ltd.), in accordance with the blood rheology procedure described in Kikuchi et al. [13]. The values obtained were shown by using the following equation to convert them into the values when the transit time for 100 µL isotonic saline solution is 12 s, based on the transit time for isotonic saline solution measured immediately before.
Conversion equation: Converted value = Blood transit time × 12 s. / Isotonic saline solution transit time
Subjects
The subjects were selected from individuals who had freely consented to participation in the study in accordance with the Declaration of Helsinki (individuals who had signed a consent form). Prior to undertaking the survey, the physicians in charge of the study handed the participants an explanation and consent form, and explained all necessary matters pertaining to the purpose, significance and method of the study, the anticipated results and risks, the fact the participant will not suffer any disadvantages if he/she does not consent to participation in the study, the fact the participant can withdraw at any time even after having consented to participation in the study, other related matters, and protection of the subject’s human rights, and after having verified the participants had understood the details explained, obtained consent to voluntary participation in the study in writing from the participants.
Examinations and measurements were performed at the first screening. From the subjects determined by the study physician to be suitable, 20 individuals who showed a stable blood transit time in a range of 45–70 s/100 µl were selected at a second screening and made available for the study.
Study method
To create a balance between the two groups based on the blood transit times measured at the time of the screening examinations, ten individuals each were allocated to the study food ingestion group and the placebo food ingestion group. The subjects were asked to ingest two capsules of the study food or placebo (AX 6 mg/day or 0 mg/day) once each day after dinner, and were examined and their blood samples collected at the start before ingestion and after ten days. For the blood rheology values immediately prior to ingestion, the values at the time of the second screening were adopted. On the day before the day of each visit to the study hospital, subjects were required to finish dinner by 9:00 PM and consume no food or beverages (other than water); the subjects were examined the following morning in a fasting condition, and blood was collected from a vein in the elbow area. The daily ingestion amount of 6 mg and ingestion period of ten days were set based on the results of studies by Ohno et al. to set ingestion amount [14] and verify the results [15].
Evaluation procedure
Mean value and standard deviation were determined for each evaluation item. The comparison before and after ingestion was performed by statistical analysis using a paired t test. In addition, the comparisons between the study food and placebo were verified for homoscedasticity using the F test. Student’s t test was performed when the results were homoscedastic, and the Aspin-Welch t test was performed when the results were heteroscedastic. The significance levels were 5% for the F test and 5% and 1% for the t test.
Results
Preliminary study
To establish the criteria for subject selection for the blood rheology study, a preliminary study was conducted. Seven adult males who had consented to participation of their own free will ingested two AX capsules identical to the study food once each day (AX 6 mg/day) after dinner for a ten-day period. Blood transit time measurement was performed by collecting blood samples, on the morning of the day ingestion began and on the morning of the day after the final ingestion day, and measuring transit times. The results are shown in Table 1. Because a tendency for the value to decrease after ingestion was seen for cases in which the blood rheology values before ingestion were higher than 45 s/100 µl, we decided to select individuals who showed a value within the 45–70 s/100 µl range for the human blood rheology measurement study as well.
Table 1.
Preliminary study blood rheology
| Subjects | Age | Before ingestion | After ingestion | Difference |
|---|---|---|---|---|
| 7 | 44 | 37.1 | 37.8 | 0.7 |
| 5 | 36 | 37.6 | 40.7 | 3.1 |
| 6 | 48 | 38.5 | 38 | −0.5 |
| 3 | 48 | 40.3 | 42.1 | 1.8 |
| 1 | 46 | 45.4 | 40.7 | –4.7 |
| 2 | 60 | 46.1 | 43.8 | –2.3 |
| 4 | 70 | 47.7 | 44.3 | –3.4 |
Astaxanthin (AX) 6 mg/day; measured before and after ingestion for ten days Unit: Sec/100 µL
Human blood rheology measurement study
Subjects’ backgrounds
The subjects’ physical examination backgrounds are shown in Table 2. At the start of ingestion, the subjects had a mean age of 57.5 ± 9.8 years in the study food ingestion group and 50.8 ± 13.1 years in the placebo food ingestion group. Body weight, body fat percentage, Body Mass Index (BMI), and other measures were all within normal ranges. As shown by the results, both the study food ingestion group and the placebo food ingestion group were structured to have nearly identical subject backgrounds.
Table 2.
Subjects’ backgrounds
| Item | Unit | Placebo food | Study food |
|---|---|---|---|
| Number of subjects | Persons | 10 | 10 |
| Age | Years | 50.8 ± 13.1 | 57.5 ± 9.8 |
| Height | Cm | 164.8 ± 5.9 | 166.5 ± 3.8 |
| Weight | Kg | 63.41 ± 1.3 | 66.7 ± 8.5 |
| Body fat percentage | % | 22.5 ± 5.2 | 23.9 ± 5.5 |
| BMI | — | 23.3 ± 3.2 | 24.0 ± 2.6 |
| Temperature | °C | 36.1 ± 0.4 | 35.8 ± 0.4 |
Mean value ± standard deviation
Adverse events and examination opinions
In the physician examinations implemented on the study food ingestion start day and after ingestion for ten days, no conditions corresponding to an adverse event were found. Furthermore, no anomalies of any kind were found in the subjective symptom opinions in the ingestion diaries in which the subjects themselves recorded information such as their study food ingestion circumstances or side effects for each day.
Study food ingestion circumstances and meal questionnaire
The study food ingestion circumstances were 100% for all subjects. On the meal questionnaire completed on the screening day, there were no subjects with eating habits that deserved special mention.
Blood rheology
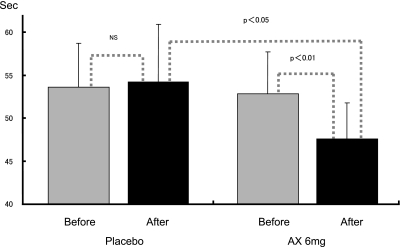
A summary of the measurement results for each subjects’ blood transit time measurements is shown in Table 3 and Fig. 1. Compared with the values before ingestion, after ingestion for ten days, the blood transit times for the study food ingestion group were shortened significantly (52.8 ± 4.9 s → 47.6 ± 4.2 s). In addition, after ingestion for ten days, compared with the placebo food ingestion group, the study food ingestion group showed significantly lower values for blood transit time (placebo food ingestion group: 54.2 ± 6.7 s; study food ingestion group: 47.6 ± 4.2 s). With ingestion of AX 6 mg/day for a ten-day period, the blood rheology of the study food ingestion group was improved significantly (p = 0.05) in both a comparison within the group (Day 0 and Day 10) and a comparison with the placebo food ingestion group.
Table 3.
Blood transit time (Unit: Sec/100 µL)
| Placebo food |
Study food |
|||||
|---|---|---|---|---|---|---|
| Subject No. | Ingestion Day 0 | Ingestion Day 10 | Subject No. | Ingestion Day 0 | Ingestion Day 10 | |
| A01 | 61.7 | 57.6 | B01 | 60 | 53.2 | |
| A02 | 58.9 | 49.8 | B02 | 57.4 | 47.5 | |
| A03 | 57 | 45.7 | B03 | 57 | 53.4 | |
| A04 | 56.4 | 49.2 | B04 | 55.7 | 44.7 | |
| A05 | 55.4 | 61 | B05 | 54.8 | 49.2 | |
| A06 | 53.2 | 57.8 | B06 | 52.3 | 42.6 | |
| A07 | 49.7 | 54.7 | B07 | 49 | 49.5 | |
| A08 | 48.5 | 66.1 | B08 | 48.4 | 50.4 | |
| A09 | 48.3 | 55 | B09 | 47.5 | 42.8 | |
| A10 | 46.5 | 45.4 | B10 | 45.4 | 42.7 | |
| M ± SD | 53.6 ± 5.1 | 54.2 ± 6.7 | 52.8 ± 4.9 | 47.6 ± 4.2**# | ||
M ± SD: mean value ± standard deviation
**: p<0.01; Significant difference compared with Day 0
#: p<0.05; Significant difference compared with the placebo food group
Fig. 1.
Summary of measurement results for each subject’s blood transit time
Discussion
AX, a form of carotenoid referred to as a xanthophyll, possesses a chemical structure with the long chain conjugate double bond and β-ionone ring at both ends that has been replaced by a keto-group and hydroxyl-group. Since being separated as a natural pigment with a reddish orange color, much research has been conducted and its widespread presence as a pigment in marine products such as shrimp, crabs, salmon and salmon roe has been clarified. Such fish and crustaceans are consumed by humans for food, and considered historically AX is a substance the human race has ingested from centuries ago. Recently the strong antioxidant effect of AX has attracted attention, and AX is being tested actively in use as a health food supplement for maintaining human health. It is believed that microorganisms such as algae, yeasts and bacilli utilize AX’s strong antioxidant effect to defend themselves from strong natural sunlight, and the fish and crustaceans that consume these microorganisms also use AX as a pigment because it is useful in protecting them from the ravages of light and stress. In addition, AX also is present abundantly in eggs spawned to produce the next generation, where it is thought to protect the eggs and be used for the protection and growth of young fish. While AX is assumed to be one of the substances with an antioxidant effect found in nature [16, 17] that shows the strongest antioxidant effect, at this point this is conjectured from its role in the world of living organisms.
Research in recent years continues to clarify that AX shows not only an antioxidant effect but various other physiological actions. On the other hand, as societies evolve into societies in which people live longer, measures to prevent the lifestyle diseases that accompany longer lives also have grown in importance. Ingestion of AX and other antioxidant substances is likely to be considered as one such measure. AX demonstrates a variety of physiological actions and is considered suitable for this purpose.
AX has attracted attention because its action is believed to work effectively against oxidation in lipid metabolism, given that AX is said to prevent overoxidation of lipids through its strong antioxidant effect and have action that controls oxidation of LDL [18], and also because it is widely distributed in the lipoprotein areas of the body [19, 20]. Such actions are though to work by preventing oxidation of biomembranes including cell membranes, and there is a possibility this will be linked to effects such as an arteriosclerosis prevention effect [21], an anti-inflammatory effect [22] and control of diabetes nephropathy (kidney disease) [23, 24].
Furthermore, AX has been reported to have an ocular accommodation improvement effect [10–15], and the dose setting study [14] and effect verification study [15] by Ohno et al. in particular provided results that strongly reconfirm the effect of AX on ocular accommodation. It is thought such promotion of recovery from asthenopia in this manner might contribute to the improvement of blood circulation in peripheral systems. Based on such conjecture, we performed a study of the effect of continuous ingestion of AX 6 mg/day for a ten-day period on blood rheology.
In the study food ingestion group, compared with the values before ingestion a decrease in blood transit time was found in eight of ten individuals after ingestion for ten days, and a significant shortening in time was shown for mean value as well. Moreover, after ingestion for ten days, blood transit time showed a significantly low value for the study food ingestion group compared with the placebo food ingestion group. A shortening of blood transit time, or in other words an improvement in blood rheology, was found from continuously ingesting AX 6 mg per day for ten days.
In the survey of the subjects conducted at the same time, no abnormal findings or adverse events that appeared to result from ingestion of the study food were found in the subjective and objective findings.
Other substances such as tocotrienol, an antioxidant derived from palm oil [25], and black vinegar [26] (which has shown antioxidant power) also have been reported as showing effects for improvement of blood rheology. Tocotrienol has been found to improve excessive sensitivity to cold (unpublished data) by improving precapillary circulation. Based on such reports, the possibility that improvement of blood rheology improves precapillary circulation is conjectured. The improvement in blood rheology from AX is conjectured to confer an antioxidant effect to the erythrocyte membrane or flexibility to the membrane, by means such as taking a position that enables it to traverse longitudinally the suitable membrane to demonstrate an antioxidant effect within and outside the lipid double membrane, in contrast to β-carotene, which is trapped in the lipid double membrane [27] or becomes difficult to convert into an excellent pro-oxidant for physical elimination of radicals [28].
Conclusion
We found a shortening of blood transit time—that is, an improvement of blood rheology—when we studied healthy adult male volunteers with a blood transit time of 45–70 s to evaluate the effect on blood rheology from continuous ingestion of AX 6 mg per day for a ten-day period.
References
- 1.Kuhn R., Soerensen N.A. The coloring matters of the lobster (Astacus gammarus L.) Z. Angew. Chem. 1938;51:465–466. [Google Scholar]
- 2.Tischer J. Carotenoids of fresh-water algae V. The carotenoids of H. pluvialis. Z. Physiol. Chem. 1938;252:25–233. [Google Scholar]
- 3.Andrewes A.G., Phaffia H.J., Starr M.P. Carotenids of Phaffa rhodozyma, a red pigmented fermenting yeast. Phyiochem. 1976;15:1003–1007. [Google Scholar]
- 4.Miki W., Fujita T. Fish and Carotenoid metabolization. Biosci. Biotechnol. Biochem. 1985;23:640–648. [Google Scholar]
- 5.De Nicola. M. Carotenoids of the carapace of the echinoderm. Ophidiaster Ophidianus. Biochem. J. 1954;56:555–558. doi: 10.1042/bj0560555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khare A., Moss G.P., Weedon B.C., Matthews A.D. Identification of astaxanthin in Scottish salmon. Comp. Biochem. Physiol. B. 1973;45:971–973. doi: 10.1016/0305-0491(73)90158-2. [DOI] [PubMed] [Google Scholar]
- 7.Grangaud R., Massonet R. Anti-xerophthalmic activity of astaxanthin esters. C. R. Seances Soc. Biol. Fil. 1954;148:1392–1394. [PubMed] [Google Scholar]
- 8.Tso M.O.M., Lam T.T. Method of retarding and ameliorating central nervous system and eye damage. U.S. Patent. 1996;5:527–533. [Google Scholar]
- 9.Chitchumroonchokchai C., Bomser J.A., Glamm J.E., Failla M.L. Xanthophylls and alpha-tocopherol decrease UVB-induced lipid peroxidation and stress signaling in human lens epithelial cells. J. Nutr. 2004;134:3225–3232. doi: 10.1093/jn/134.12.3225. [DOI] [PubMed] [Google Scholar]
- 10.Sawaki K., Yoshigi H., Aoki K., Koikawa N., Azumane A., Kaneko K., Yamaguchi M. Effect of astaxanthin on sports performance—Effect on visual function and muscle fatigue recovery in athletes. J. Clin. Ther. and Med. 2002;18:1085–1099. [Google Scholar]
- 11.Nagaki Y., Hayasaka S., Yamada T., Hayasaka Y., Sanada M., Uonomi T. Effects of astaxanthin on accommodation, critical flicker fusion, and pattern visual evoked potential in visual display terminal workers. J. Trad. Med. 2002;19:170–173. [Google Scholar]
- 12.Nakamura A., Isobe A., Otaka Y., Abematsu Y., Nakata D., Honma Sakurai., Shimada Y., Horiguchi M. Change in visual function from astaxanthin. Jpn. J. Clin. Ophthalmol. 2004;58:1051–1054. [Google Scholar]
- 13.Kikuchi Y., Sato K., Izuguchi Y. Modified cell-flow microchannels in a singlet-crystal silicon substrate and flow behavior of blood cells. Microvasc Res. 1994;47:126–139. doi: 10.1006/mvre.1994.1008. [DOI] [PubMed] [Google Scholar]
- 14.Nitta T., Ohgami K., Shiratori K., Shinmei Y., Chin S., Yoshida K., Tsukahara H., Ohno S. Effects of astaxanthin on accommodation and asthenopia—dose finding study in healthy volunteers—. J. Clin. Ther. and Med. 2005;21:543–556. [Google Scholar]
- 15.Shiratori K., Ohgami K., Nitta T., Shinmei Y., Chin S., Yoshida K., Tsukahara H., Takehara I., Ohno S. Effects of astaxanthin on accommodation and asthenopia—efficacy identification study in healthy volunteers—. J. Clin. Ther. and Med. 2005;21:637–650. [Google Scholar]
- 16.Jorgensen K., Skibsted L.H. Carotenoid scavenging of radicals. Effect of carotenoid structure and oxygen partial pressure on antioxidative activity. Z. Lebensm. Unters. Forsch. 1993;196:423–429. doi: 10.1007/BF01190806. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu N., Goto M., Miki W. Carotenoids as singlet oxygen quenchers in marine organisms. Fisheries Science. 1996;62:134–137. [Google Scholar]
- 18.Iwamoto T., Hosoda K., Hirano R., Kurata H., Matsumoto A., Miki W., Kamiyama M., Itakura H., Yamamoto S., Kondo K. Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheroscler. Theromb. 2000;7:216–222. doi: 10.5551/jat1994.7.216. [DOI] [PubMed] [Google Scholar]
- 19.Osterlie M., Bjerkeng B., Liaaen-Jensen S. Plasma appearance and distribution of astaxanthin E/'Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin. J. Nutr. Biochem. 2000;11:482–490. doi: 10.1016/s0955-2863(00)00104-2. [DOI] [PubMed] [Google Scholar]
- 20.Coral-Hinostroza G.N., Ytrestoyl T., Ruyter B., Bjerkeng B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3’R/S isomers of astaxanthin fatty acyl diesters. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004;139:99–110. doi: 10.1016/j.cca.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Li W., Hellsten A., Jacobsson L.S., Blomqvist H.M., Olsson A.G., Yuan X.M. Alpha-tocopherol and astaxanthin decrease macrophage infiltration, apoptosis and vulnerability in atheroma of hyperlipidaemic rabbits. J. Mol. Cell Cardiol. 2004;37:969–978. doi: 10.1016/j.yjmcc.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Ohgami K., Shiratori K., Kotake S., Nishida T., Mizuki N., Yazawa K., Ohno S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 2003;44:2694–2701. doi: 10.1167/iovs.02-0822. [DOI] [PubMed] [Google Scholar]
- 23.Uchiyama K., Naito Y., Hasegawa G., Nakamura N., Takahashi J., Yoshikawa T. Astaxanthin protects beta-cells against glucose toxicity in diabetic db/db mice. Redox. Rep. 2002;7:290–293. doi: 10.1179/135100002125000811. [DOI] [PubMed] [Google Scholar]
- 24.Naito Y., Uchiyama K., Aoi W., Hasegawa G., Nakamura N., Yoshida N., Maoka T., Takahashi J., Yoshikawa T. Prevention of diabetic nephropathy by treatment with astaxanthin in diabetic db/db mice. Biofactors. 2004;20:49–59. doi: 10.1002/biof.5520200105. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi J., Uonomi T., Ikushima H., Kikuchi Y. Effect of tocotrienol on blood rheology. Hemorheology and Related Research. 2002;5:31–34. [Google Scholar]
- 26.Saito K., Maruyama I. Effect of black vinegar on the blood rheology of male long distance athletes. Hemorheology and Related Research. 2002;5:31–34. [Google Scholar]
- 27.Goto S., Kogure K., Abe K., Kimata Y., Kitahama K., Yamashita E., Terada H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antioxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta. 2001;1512:251–258. doi: 10.1016/s0005-2736(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen B.R., Jorgensen K., Skibsted L.H. Triplet-triplet extinction coefficients, rate constants of triplet decay and rate constants of anthraacene triplet sensitization by laser flash photolysis of astaxanthin, B-carotene, canthaxanthin and zeaxanthin in deaerated toluene at 298K. J. Photochem. Photobiol. A. 1998;112:127–133. [Google Scholar]