Abstract
γ-Tocopherol, the major form of dietary vitamin E, is absorbed in the intestine and is secreted in chylomicrons, which are then transferred to liver lysosomes. Most γ-tocopherol is transferred to liver microsomes and is catabolized by cytochrome p450. Due to the hydrophobicity of γ-tocopherol, a binding and transfer protein is plausible, but none have yet been isolated and characterized. We recently found that a ubiquitous cytosolic protein, saposin B, binds and transfers coenzyme Q10 (CoQ10), which is an essential factor for ATP production and an important antioxidant. Here, we report that saposin B also binds γ-tocopherol, but not α-tocopherol, as efficiently as CoQ10 at pH 7.4. At acidic pH, saposin B binds γ-tocopherol preferentially to CoQ10 and α-tocopherol. Furthermore, we confirmed that saposin B selectively binds γ-tocopherol instead of CoQ10 and α-tocopherol at every pH between 5.4 and 8.0 when all three lipids are competing for binding. We detected γ-tocopherol in human saposin B monoclonal antibody-induced immunoprecipitates from human urine, although the amount of γ-tocopherol was much smaller than that of CoQ10. These results suggest that saposin B binds and transports γ-tocopherol in human cells.
Keywords: saposin B, γ-tocopherol, α-tocopherol, coenzyme Q10
Introduction
Vitamin E was discovered as a dietary factor that is essential for reproduction in 1922 [1]. It occurs as 4 tocopherols (α, β, γ, and δ) and 4 tocotrienols (α, β, γ, and δ), of which α-tocopherol (α-Toc) has the highest biological activity [2, 3]. In addition to α-Toc, γ-tocopherol (γ-Toc) is an abundant tocopherol in our diet and both tocopherols are taken up equally by the intestine and are secreted in chylomicrons together with triglyceride and cholesterol [4, 5]. Both tocopherols are transferred to peripheral tissues such as muscle and adipose during the lipoprotein lipase-dependent catabolism of chylomicrons [6]. The resulting chylomicron remnants are subsequently taken up by the liver and disassembled in lysosomes [5]. α-Toc is preferentially associated with α-Toc transfer protein (α-TTP) and is then secreted in very low density lipoprotein (VLDL) [5, 7]. VLDL is converted to LDL in circulation. Since all tissues have LDL receptors, α-Toc in LDL is thus transferred throughout the body. On the other hand, γ-Toc in liver lysosomes is transferred to the bile duct and is secreted in bile [5], or to microsomes where it is degraded to 2,7,8-trimethyl-2-(2'-carboxyethyl)6-hydroxychroman (γ-CEHC) [8] by cytochrome P450 [9, 10] and excreted in urine [8, 11]. Consequently, plasma and tissue concentrations of γ-Toc are much smaller than those of α-Toc [3].
The most important physiological role of vitamin E is thought to be as an antioxidant [2, 3], inhibiting free radical oxidation of lipids. The antioxidant activity of γ-Toc is smaller than that of α-Toc [12]. Because of this observation, and its much lower concentrations when compared with α-Toc, γ-Toc has been scarcely studied. However, γ-CEHC, a γ-Toc metabolite, is natriuretic [8, 11]. Moreover, both γ-Toc and γ-CEHC, but not α-Toc, inhibit cyclooxygenase activity and, thus, possess anti-inflammatory properties [13]. These findings have drawn more attention to γ-Toc.
One of the unsolved issues related to γ-Toc is its carrier protein, as it is insoluble to water. We recently found that a ubiquitous cytosolic protein, saposin B, binds and transfers coenzyme Q10 (CoQ10) [14], which is an essential lipid for ATP production in the mitochondria and an important antioxidant in every biomembrane. Saposin B is a heat-stable glycoprotein comprising 80 amino acids and a carbohydrate component (molecular weights, 9100 and 3000, respectively), having a total molecular weight of 12.1 kDa [15]. Saposin B is known to bind sphingolipids including ganglioside and ceramide [15], as well as glycerophospholipids such as phosphatidylcholine and phosphatidylinositol [16]. This occurs because saposin B is present as a shell-like dimer [17] possessing a hydrophobic cavity for lipid binding. We also found that saposin B binds CoQ10, and that the saposin B-CoQ10 complex is present in human cells such as sperm and the hepatoma cell line HepG2 [14]. During the course of our study on lipid affinity to saposin B, we found it binds γ-Toc as efficiently as CoQ10. Here, we discuss the possibility that saposin B is a binding/transfer protein for γ-Toc.
Materials and Methods
Chemicals
CoQ10 was a generous gift from Kaneka (Osaka, Japan). Coenzyme Q7 (CoQ7), α-Toc, and γ-Toc were kindly provided by Eisai (Tokyo, Japan). Methanol and 2-propanol were of the highest grade commercially available.
Lipid analysis
Concentrations of CoQ10, CoQ7, α-Toc and γ-Toc were determined using an HPLC system with an electrochemical detector (ECD), as reported previously [18], with minor modification. Briefly, samples were added to a 9-fold volume of HPLC grade 2-propanol, vigorously mixed and centrifuged. Supernatants thus obtained were injected onto the HPLC-ECD system. Mobile phase: 50 mM NaClO4 in methanol/2-propanol (7/3, v/v); flow rate: 1.0 ml/min; analytical column: KANTO RP-18 (L) GP, 5 µm × 150 mm × 4.6 mm (Kanto Chemical, Tokyo, Japan); post-reduction column: RC-10, 15 mm × 4 mm (IRICA, Kyoto, Japan); detector: ECD (600 mV) NANOSPACE SI-1 (Shiseido, Tokyo, Japan).
Preparation of saposin B
Saposin B containing CoQ10 was purified as described previously [14]. CoQ10-free saposin B was prepared by mixing an aqueous solution with a 10-fold volume of hexane followed by centrifugation.
Lipid binding assay
CoQ10-free saposin B (0.5 µM) in 50 mM phosphate buffer (pH 7.4) containing 150 mM NaCl was vigorously mixed for 5 min with hexane containing 10 mM lipids, such as CoQ10, CoQ7, α-Toc and γ-Toc, followed by centrifugation at 1,600 × g for 10 min. Hexane was removed and the aqueous layer was further centrifuged in Eppendorf-tubes at 15,000 × g for 10 min to separate contaminating hexane. Lipid concentrations in the aqueous layer were analyzed by HPLC-ECD. To investigate the pH dependence of the above lipids binding to saposin B, 50 mM phosphate buffer (pH 5.4, 6.4, 7.4 or 8.0) was employed. Saposin B-free buffers were used as blanks.
Immunoprecipitation
Samples placed in Protein LoBind Tubes (Eppendorf, Hamburg, Germany) were incubated with 5 µg of anti-saposin B IgG or mouse normal IgG for 1 h at room temperature. After incubation, 10 µl of protein G beads (Amersham Biosciences, Uppsala, Sweden) were added to samples, followed by further incubation for 1 h. Beads were washed three times with PBS. Lipids were extracted from beads with 2-propanol, followed by HPLC-ECD analysis. SDS-PAGE sample buffer was mixed with beads and was then subjected to Western blotting analysis of saposin B.
Western blotting
Saposin B was detected by Western blotting, as reported previously [14]. Briefly, samples were separated by electrophoresis using an SDS/polyacrylamide gel (15% acrylamide). After electrophoresis, gels were stained with silver staining kits (ATTO, Tokyo, Japan). For Western blotting analysis, proteins were transferred to PVDF membranes, which were then incubated with mouse anti-saposin B IgG for 1 h at room temperature. Proteins were visualized using HRP-conjugated secondary antibodies (Bio-Rad Japan, Tokyo, Japan).
Statistical analyses
Data were analyzed by Student t test and values are means ± SD. *, ** and *** indicate significant differences (p<0.05, 0.01 and 0.001, respectively) between the two groups.
Results and Discussion
Binding specificity of lipids to saposin B
The chemical structures of CoQ10, CoQ7, α-Toc, and γ-Toc are shown in Fig. 1. We compared the binding affinities of these lipids to saposin B by independently incubating 10 mM hexane solutions with aqueous CoQ10-free saposin B (0.5 µM) solution (pH 7.4) for 5 min, and then analyzing lipid contents in aqueous saposin B solution. Fig. 2 shows the binding affinity of lipids, which decreases in the order of CoQ10>CoQ7>>α-Toc, thus suggesting that tail length is an important factor in determining binding affinity. It is therefore surprising that saposin B binds γ-Toc as efficiently as CoQ10 (Fig. 2) despite, the much shorter tail length of γ-Toc. Saposin B binds γ-Toc much more strongly than α-Toc (Fig. 2), suggesting that the methyl group at the C-5 position of α-Toc interferes with binding to saposin B. These results suggest that saposin B has a binding site for γ-Toc.
Fig. 1.
Chemical structures of CoQ10, CoQ7, α-Toc, and γ-Toc.
Fig. 2.
Binding affinity of CoQ10, CoQ7, α-Toc, and γ-Toc to saposin B. Each lipid (10 mM) in hexane solution was incubated independently with aqueous 0.5 µM saposin B (pH = 7.4) for 5 min and amounts of lipids bound to saposin B were measured. All experiments were repeated 3–6 times and means ± SD are shown. * and *** indicate significant differences (p<0.05 and 0.001, respectively) compared to values for γ-Toc. NS: not significant.
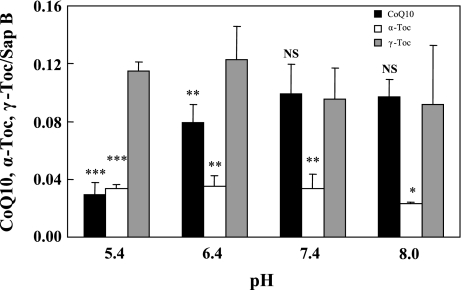
We next examined the pH dependence of binding of the three lipids (CoQ10, α-Toc and γ-Toc) to saposin B. Buffers at pH 5.4, 6.4, 7.4 and 8.0 were employed. Binding affinities of γ-Toc and α-Toc remained unchanged, irrespective of pH, and the former had greater affinity than the latter (Fig. 3), thus confirming the stronger affinity of γ-Toc when compared with α-Toc. On the other hand, binding affinity of CoQ10 to saposin B increased with pH, before leveling off at neutral and alkaline pH levels (Fig. 3), thus suggesting that binding affinity of saposin B for γ-Toc is greater than that for CoQ10 at acidic pH. As the isoelectric point (pI) of saposin B is 4.6 [15], at alkaline pH the hydrophobic cavity for lipid binding may become larger because of electric repulsion of carboxylic anions, and therefore binding of CoQ10 to saposin B also becomes more favorable. In contrast, the binding of γ-Toc to saposin B was independent of pH, which indicates a specific binding site for γ-Toc in saposin B. However, further investigation is required.
Fig. 3.
Effects of buffer pH on binding of CoQ10, α-Toc, and γ-Toc to saposin B. Each lipid (10 mM) in hexane solution was incubated independently with aqueous 0.5 µM saposin B for 5 min. All experiments were repeated 3–6 times and means ± SD are shown. *, **, and *** indicate significant differences (p<0.05, 0.01 and 0.001, respectively) compared to values for γ-Toc. NS: not significant.
Binding competition between two lipids
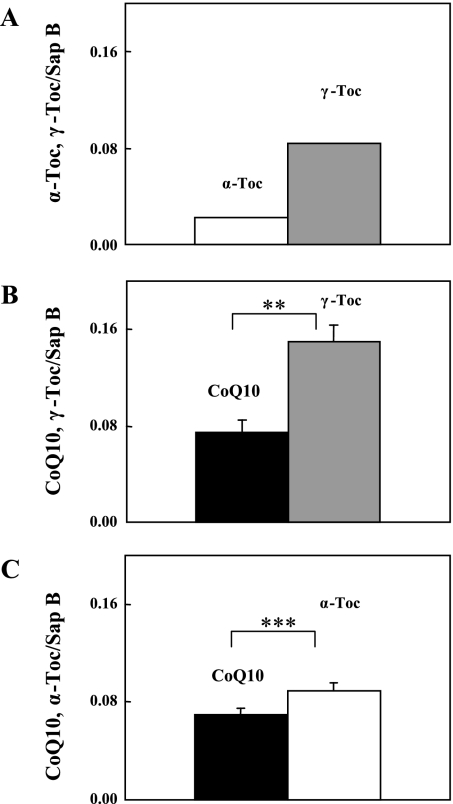
The above results prompted us to examine the binding competition between the lipids instead of independently measuring their binding affinities. γ-Toc and α-Toc were selected for the first competition. A 10 mM hexane solution of the two lipids was mixed with an aqueous saposin B (0.5 µM) solution (pH 7.4) and the resulting aqueous saposin B-bound lipids were measured. As expected, γ-Toc was preferentially associated to saposin B (Fig. 4A). In the competition between CoQ10 and γ-Toc at pH 7.4, saposin B unexpectedly bound γ-Toc in preference to CoQ10 (Fig. 4B), as the independent binding affinities of the two lipids were similar (Fig. 2). Moreover, α-Toc bound to saposin B in preference to CoQ10 (Fig. 4C), despite of the fact that saposin B affinity for α-Toc was much smaller than that for CoQ10 (Fig. 2). It has been reported that saposin B dimer binds more than one molecule of ganglioside [15] or one molecule of phosphatidylinositol plus one molecule of phosphatidylcholine or ganglioside GM1 [16]. As the molecular size of CoQ10 is similar to that of phospholipids, such as phosphatidylinositol and phosphatidylcholine, or the hydrophobic part of ganglioside, and is twice that of tocopherol, it is reasonable to assume that saposin B dimer has capacity to bind two molecules of CoQ10 or one molecule of CoQ10 plus two molecules of tocopherol. Therefore, our results suggest that association of one molecule of CoQ10 to saposin B dimer accelerates the binding of two molecules of α-Toc, rather than the association of an additional CoQ10 molecule. Binding of γ-Toc was more favorable than α-Toc, as shown in Fig. 4A, as saposin B is likely to have a specific binding site for γ-Toc. This is consistent with our other results, but further experiments are needed for confirmation.
Fig. 4.
Saposin B binding competition between (A) α-Toc and γ-Toc, (B) CoQ10 and γ-Toc, and (C) CoQ10 and α-Toc. Each lipid pair (10 mM) in hexane solution was incubated with aqueous 0.5 µM saposin B (pH = 7.4) for 5 min. All experiments were repeated 2–3 times and means ± SD are shown. ** indicates a significant difference (p<0.01) between the two groups.
Binding competition among CoQ10, α-Toc, and γ-Toc
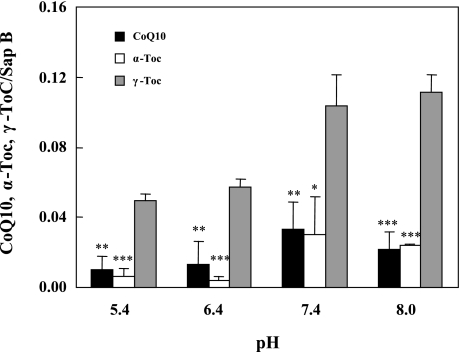
The binding competition results for two lipids (Fig. 4) predict that binding affinity to saposin B will decrease in the order of γ-Toc>CoQ10 ≈ α-Toc when the three lipids are all competing for binding. To confirm this, all three lipids at 10 mM in hexane solution were mixed with an aqueous saposin B solution at pH 5.4, 6.4, 7.4 or 8.0, and bound lipids to saposin B were quantitated. We confirmed that saposin B binds with γ-Toc significantly better than with CoQ10 and α-Toc at all pH levels tested (Fig. 5), indicating that γ-Toc is favored by saposin B. However, tissue concentrations of γ-Toc are much smaller than those of α-Toc and CoQ10, as discussed below.
Fig. 5.
Effects of buffer pH on competitive binding of CoQ10, α-Toc, and γ-Toc to saposin B. Each lipid (10 mM) in hexane solution was incubated with aqueous 0.5 µM saposin B for 5 min. All experiments were repeated 3 times and means ± SD are shown. *, **, and *** indicate significant differences (p<0.05, 0.01 and 0.001, respectively) compared to values for γ-Toc.
In vivo binding of γ-Toc to saposin B
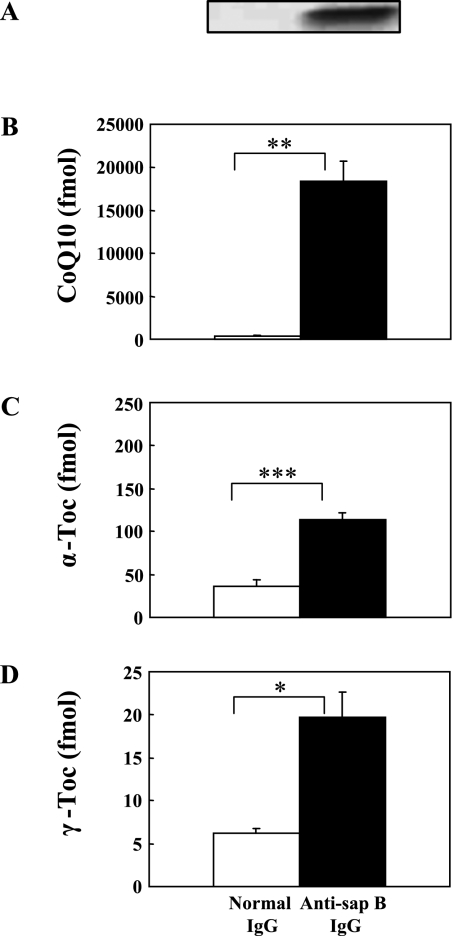
Saposin B is a housekeeping protein necessary for lysosomal sphingolipid hydrolysis, and is therefore present in all tissues [15]. To confirm the presence of the γ-Toc-saposin B complex in vivo, we performed an immunoprecipitation experiment using monoclonal antibody against human saposin B. Fig. 6 shows that saposin B in human urine bound considerable amounts of CoQ10, α-Toc, and γ-Toc. The amounts increased in the order of γ-Toc<α-Toc<<CoQ10, with their ratio being 1/5.7/1320. This order is in contrast to the binding affinity of these lipids to saposin B decreases; γ-Toc>>α-Toc, CoQ10 (Fig. 5). But this is not surprising because amount of lipids bound to saposin B in vivo reflect the concentration of lipids in tissues. In fact, the reported molar ratios of γ-Toc/α-Toc/CoQ are 1/540/1580, 1/127/1515, 1/63/1214, and 1/73/309 in mouse brain, heart, kidney and liver, respectively [19]. Unfortunately human tissue data are not available. If we assume that the molar ratio of the three lipids in human kidney is similar to that in mouse kidney (1/63/1214) and compare it with the molar ratio of saposin B bound to the three lipids in human urine (1/5.7/1350), we can conclude that human saposin B favors γ-Toc and CoQ10 as binding lipids, but not α-Toc, in vivo.
Fig. 6.
(A) Immunoprecipitates obtained with monoclonal anti-saposin B IgG and normal IgG from human urine, and bound CoQ10 (B), α-Toc (C), and γ-Toc (D). Values are means ± SD (n = 3). *, **, and *** indicate significant differences (p<0.05, 0.01, and 0.001, respectively) between the two groups.
In summary, we demonstrated for the first time that human saposin B binds γ-Toc as well as CoQ10. Saposin B is thus thought to play an important role in transferring γ-Toc from liver lysosomes to microsomes, where it is metabolized to γ-CEHC, or to the bile duct.
Acknowledgments
This work was supported by the High-Tech Research Center Project for Private Universities from the Ministry of Education, Culture, Sports, Science and Technology (to Y.Y.) and by a grant from Japanese Coenzyme Q Association (to S.Y. and Y.Y.).
References
- 1.Evans H.M., Bishop K.S. On the existence of a hitherto unrecognized factor essential for reproduction. Science. 1922;56:650–651. doi: 10.1126/science.56.1458.650. [DOI] [PubMed] [Google Scholar]
- 2.Brigellius-Flohe R., Traber M.G. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 3.Jiang Q., Christen S., Shigenaga M., Ames B.N. γ-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutri. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 4.Traber M.G., Burton G.W., Hughes L., Ingold K.U. Discrimination between forms of vitamin E by humans with and without genetic abnormalities of lipoprotein metabolism. J. Lipid Res. 1992;33:1171–1182. [PubMed] [Google Scholar]
- 5.Kayden H.J., Traber M.G. Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. J. Lipid Res. 1993;34:343–358. [PubMed] [Google Scholar]
- 6.Traber M.G., Olivecrona T., Kayden H.J. Bovine milk lipoprotein lipase transfers tocopherol to human fibroblasts during triglyceride hydrolysis in vitro. J. Clin. Invest. 1985;75:1729–1734. doi: 10.1172/JCI111883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato Y., Hagiwara K., Arai H., Inoue K. Purification and characterization of the α-tocopherol transfer protein from rat liver. FEBS Lett. 1991;288:41–45. doi: 10.1016/0014-5793(91)80999-j. [DOI] [PubMed] [Google Scholar]
- 8.Wechter W.J., Kantoci D., Murray E.D. Jr., DiAmico D.C., Jung M.E., Wang W-H. A new endogenous natriuretic factor: LLU-α. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6002–6007. doi: 10.1073/pnas.93.12.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker R.S., Sontag T.J., Swanson J.E. Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem. Biophys. Res. Commun. 2000;277:531–534. doi: 10.1006/bbrc.2000.3706. [DOI] [PubMed] [Google Scholar]
- 10.Sontag T.J., Parker R.S. Cytochrome P450 ω-hydroxylase pathway of tocopherol catabolism. J. Biol. Chem. 2002;277:25290–25296. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 11.Swanson J.E., Ben R.N., Burton G.W., Parker R.S. Urinary excretion of 2,7,8-trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman is a major route of elimination of γ-tocopherol in humans. J. Lipid Res. 1999; 40:665–671. [PubMed] [Google Scholar]
- 12.Burton G.W., Ingold K.U. Autoxidation of biological molecules. 1. The antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J. Am. Chem. Soc. 1981;103:6472–6477. [Google Scholar]
- 13.Jiang Q., Elson-Schwab I., Courtemanche C., Ames B.N. γ-Tocopherol and its major metabolite, in contrast to α-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin G-Z., Kubo H., Kashiba M., Horinouchi R., Hasegawa M., Suzuki M., Sagawa T., Oizumi M., Fujisawa A., Tsukamoto H., Yoshimura S., Yamamoto Y. Saposin B is a human coenzyme Q10-binding/transfer protein. J. Clin. Biochem. Nutr. 2008;42:167–174. doi: 10.3164/jcbn.2008024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishimoto Y., Hiraiwa M., O’Brien J.S. Saposins: structure, function, distribution, and molecular genetics. J. Lipid Res. 1992;33:1255–1267. [PubMed] [Google Scholar]
- 16.Ciaffoni F., Tatti M., Boe A., Salvioli R., Fluharty A., Sonnino S., Vaccaro A.M. Saposin B binds and transfers phospholipids. J. Lipid Res. 2006;47:1045–1053. doi: 10.1194/jlr.M500547-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Ahn V.E., Faull K.F., Whitelegge J.P., Fluharty A.L., Prive G.G. Crystal structure of saposin B reveals a dimeric shell for lipid binding. Proc. Natl. Acad. Sci. U.S.A. 2003;100:38–43. doi: 10.1073/pnas.0136947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamashita S., Yamamoto Y. Simultaneous detection of ubiquinol and ubiquinone in human plasma as a marker of oxidative stress. Anal. Biochem. 1997;250:66–73. doi: 10.1006/abio.1997.2187. [DOI] [PubMed] [Google Scholar]
- 19.Podda M., Weber C., Traber M.G., Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J. Lipid Res. 1996;37:893–901. [PubMed] [Google Scholar]