Abstract
In the Diabetes Prevention Program, treatment of subjects with impaired glucose tolerance with metformin >3.2 years reduced the risk of developing type 2 diabetes by 30% compared with placebo. This study describes the mechanisms of this effect. In proportional hazards regression models with 2,155 subjects, changes in weight, the insulinogenic index (IGR), fasting insulin, and proinsulin were predictive of diabetes, though to different degrees within each group. The mean change in weight, fasting insulin, and proinsulin, but not IGR, differed between groups during the study. The 1.7-kg weight loss with metformin versus a 0.3-kg gain with placebo alone explained 64% of the beneficial metformin effect on diabetes risk. Adjustment for weight, fasting insulin, proinsulin, and other metabolic factors combined explained 81% of the beneficial met-formin effect, but it remained nominally significant (P = 0.034). After the addition of changes in fasting glucose, 99% of the group effect was explained and is no longer significant. Treatment of high-risk subjects with metformin results in modest weight loss and favorable changes in insulin sensitivity and proinsulin, which contribute to a reduction in the risk of diabetes apart from the associated reductions in fasting glucose.
In the Diabetes Prevention Program (DPP), lifestyle intervention reduced the risk of confirmed type 2 diabetes by 55% relative to placebo (1), most of this effect being explained by the resulting changes in body weight (2). Treatment with metformin led to reductions in body weight and favorable changes in other metabolic factors and reduced the risk of diabetes versus placebo by 30% (1,3).
Univariate analyses show that changes in weight and metabolic factors over the 1st year of follow-up are associated with diabetes risk (3). This study evaluates the association between changes in weight, metabolic factors, and other factors, over the complete 3.2 years of follow-up, with the risk of diabetes among those treated with metformin versus placebo and the extent to which these factors explain the beneficial effects of metformin on the risk of diabetes.
RESEARCH DESIGN AND METHODS
The DPP was a randomized clinical trial of 3,819 participants with elevated fasting plasma glucose (FPG) and impaired glucose tolerance (IGT) followed for an average of 3.2 years; 1,079 were assigned to lifestyle intervention, 1,073 to metformin, and 1,082 to placebo. Eligibility criteria and study design were previously described (4). Elevated FPG confers high risk of diabetes among those with IGT (5,6).
Measurements
All laboratory measurements were performed centrally: FPG and A1C every 6 months and plasma glucose, total immunoreactive insulin, and proinsulin annually during a 75-g oral glucose tolerance test (OGTT). Diabetes onset was detected by an OGTT at an annual visit, or by a semi-annual fasting glucose level, with confirmation on a second test within 6 weeks (4) using the American Diabetes Association criteria (5). Insulin sensitivity was measured by the fasting insulin (7,8); insulin release was measured by the insulinogenic index: IGR = (I30 − I0)/(G30 − G0) using timed OGTT insulin and glucose (9).
Weight was measured every 6 months; waist and hip circumference were measured annually. A modified block food frequency questionnaire was administered at baseline and 1 year (10,11) and nutrition intake provided by the Nutrition Data System (University of Minnesota, Nutrition Coordinating Center). Analyses herein used percent of calories from fat. The Modifiable Activity Questionnaire provided annualized MET hours per week of leisure activity (12). The Low-Level Physical Activity Recall provided habitual physical activity for the preceding week (13). Both were administered annually.
Statistics
Analyses were conducted using SAS. Mean differences between groups were tested using the t test, within groups using the paired t test, and differences in proportions using the contingency χ2 test. Spearman’s partial correlations were adjusted for sex (14). The normal errors longitudinal regression model (15) assessed the differences between groups in the mean change from baseline up to the time of diabetes onset or the last visit, adjusting for the baseline value.
The Peto-Breslow discrete-time Cox proportional hazards model assessed covariate effects on the diabetes onset risk (hazard) (16,17). The Wald test provided P values and R2 values for individual covariates (except in Table 3), and the likelihood ratio test tested those for the combined model. Madalla’s partial R2 described the proportion of variation in risk of diabetes explained by a covariate (17), expressed as a percentage. The portion of the beneficial metformin effect explained by another factor(s) was obtained as the percentage change in the group R2 value without, and then with, adjustment for the other factor(s). Effects nominally significant at P ≤ 0.01 are cited, with no adjustment for multiple tests, corresponding to R2 ≥ 0.64% in either group.
TABLE 3.
Metformin versus placebo treatment group effect on the risk (hazard) of diabetes onset before and after adjustment for each time-dependent covariate individually and in combination
| Metformin versus placebo group effect* |
Covariate effect† |
Percent group R2 explained
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariate (unit) | Coefficient | SE | Likelihood ratio χ2 | P | HR (risk reduction) | R2 (%) | HR | R2 (%) | Univariate (%) | Jointly (%)‡ | Jointly (%)§ |
| None | −0.43922 | 0.09021 | 23.7041 | <0.0001 | 0.645 (35.5%) | 1.150 | |||||
| Body weight (kg) | −0.26896 | 0.09257 | 8.4414 | 0.0037 | 0.764 (23.6%) | 0.409 | 1.116 | 4.734 | 64.4 | 29.6 | 7.9 |
| Fasting insulin (pmol/l) | −0.38359 | 0.09090 | 17.8070 | <0.0001 | 0.681 (31.9%) | 0.863 | 1.005 | 2.245 | 24.9 | 0.7 | 0.2 |
| Proinsulin (pmol/l) | −0.33344 | 0.09175 | 13.2076 | 0.0003 | 0.716 (28.4%) | 0.639 | 1.041 | 3.671 | 44.4 | 8.5 | 1.0 |
| IGR (pmol/mmol) | −0.43174 | 0.09032 | 22.8478 | <0.0001 | 0.649 (35.1%) | 1.108 | 0.987∥ | 0.616 | 3.7 | 2.3 | 0.2 |
| Leisure activity (MET h/week) | −0.44076 | 0.09029 | 23.8320 | <0.0001 | 0.644 (35.6%) | 1.156 | 0.999 | 0.007 | 0.0 | 0.0 | 0.0 |
| All the above | −0.19843 | 0.09367 | 4.4879 | 0.0341 | 0.820 (18.0%) | 0.217 | 81.1 | ||||
| Fasting glucose (mmol/l) | −0.20607 | 0.09172 | 5.0475 | 0.0247 | 0.814 (18.6%) | 0.244 | 2.478 | 12.993 | 78.8 | 17.4 | |
| All the above | −0.05508 | 0.09462 | 0.3389 | 0.5605 | 0.946 (5.4%) | 0.016 | 98.6 | ||||
Models adjusted for baseline age, sex, ethnicity, fasting glucose, 2-h glucose, A1C, recreational activity, percent calories from fat, and the baseline value of all time-dependent covariates.
R2 values are based on likelihood ratio tests.
Covariate effect adjusted for baseline covariates in the combined groups, not adjusted for treatment group.
Not adjusted for fasting glucose over time.
Adjusted for fasting glucose over time. For example, if weight was removed from the model, the remaining covariates, including glucose, would explain 98.6−7.9 (90.7%) of the beneficial metformin effect.
HR for an increase of 10 units.
RESULTS
Baseline characteristics
There were no significant differences between treatment groups at baseline (Table 1): 68% were women, the mean age was 51 years, and 55% were Caucasian, 21% African American, 15% Hispanic, 5% American Indian, and 4% Asian American, by self-report.
TABLE 1.
Metformin and placebo group characteristics at baseline (in the combined groups) and, on average, up to the time of diabetes onset or the final visit during 3.2 years of follow-up
| Baseline
|
Follow-up
|
|||
|---|---|---|---|---|
| Metformin and placebo combined* | Placebo
(change from baseline) |
Metformin
(change from baseline) |
P | |
| n | 2,155 | 1,082 | 1,073 | |
| Weight (kg) | 94.30 ± 0.43 | 0.29 ± 0.16 | −1.72 ± 0.15† | <0.001 |
| BMI (kg/m2) | 34.02 ± 0.14 | 0.11 ± 0.06 | −0.62 ± 0.06† | <0.001 |
| Waist circumference (cm) | 105.02 ± 0.31 | −0.23 ± 0.18 | −1.63 ± 0.19† | <0.001 |
| Waist-to-hip ratio | 0.9238 ± 0.0018 | −0.0037 ± 0.0015 | −0.0078 ± 0.0015 | 0.036 |
| Fat per day (g) | 82.00 ± 1.03 | −11.66 ± 0.93† | −13.23 ± 0.91† | 0.230 |
| Calories per day (kcal) | 2,121.21 ± 22.14 | −252.36 ± 20.10† | −287.32 ± 19.83† | 0.216 |
| Percent of calories from fat | 34.05 ± 0.15 | −0.77 ± 0.18† | −0.84 ± 0.17† | 0.783 |
| MAQ: leisure MET h/week | 16.73 ± 0.59 | 1.01 ± 0.54 | 1.26 ± 0.61† | 0.751 |
| LoPAR: recreational MET h/week | 66.96 ± 0.89 | 2.46 ± 0.95† | 1.06 ± 0.86 | 0.244 |
| A1C (%) | 5.91 ± 0.01 | 0.11 ± 0.01† | 0.03 ± 0.01† | <0.001 |
| Glucose (mmol/l) | ||||
| Fasting | 5.92 ± 0.01 | 0.13 ± 0.01† | −0.08 ± 0.01† | <0.001 |
| 30-min postload | 9.44 ± 0.03 | 0.36 ± 0.04† | 0.25 ± 0.04† | 0.043 |
| 120-min postload | 9.15 ± 0.02 | −0.23 ± 0.06† | −0.31 ± 0.06† | 0.302 |
| Insulin (pmol/l) | ||||
| Fasting | 161.19 ± 1.94 | 6.02 ± 2.45† | −13.01 ± 2.17† | <0.001 |
| 30-min postload | 606.54 ± 8.49 | 2.92 ± 9.34 | −36.67 ± 8.32† | <0.001 |
| Fasting proinsulin (pmol/l) | 18.29 ± 0.31 | 0.80 ± 0.39† | −2.45 ± 0.34† | <0.001 |
| IGR (pmol/mmol)‡ | 133.92 ± 2.17 | −6.94 ± 3.89 | −12.87 ± 2.39† | 0.190 |
Data are means ± SE.
There were no significant differences (P < 0.10) between the metformin and placebo participants in any variables at baseline.
Mean change from baseline within a group is significantly different from zero at P < 0.05.
IGR 3 (I30 − I0)/(G30 − G0) using the baseline (0) and 30-min insulin and glucose values from the OGTT. MAQ, Modifiable Activity Questionnaire; LoPAR, Low-Level Physical Activity Recall.
Treatment effects
Among the 1,082 placebo-treated participants, 76.5% complied at least 80% with the medication regimen versus 71.5% of the 1,073 metformin-treated participants (1) (P < 0.001), with 85.4 and 81.8%, respectively, taking at least some coded study medication (P = 0.160). The estimated cumulative incidence of diabetes at 3 years was 28.9% in placebo-treated participants versus 21.9% with metformin, with a corresponding risk (hazard) reduction of 30% (95% CI 16–41%, P < 0.0001) with metformin. In the placebo group, 41.1% of the 304 diabetes cases were initially diagnosed by an FPG value compared with 28.9% of the 225 cases in the metformin group, 40.5 vs. 56.0% were diagnosed by a 120-min postload glucose, and 18.4 vs. 15.1% were diagnosed on both fasting and 2-h glucose values (P = 0.0016).
Table 1 also presents the average change from baseline for time-dependent quantitative factors up to the time of diabetes onset or the last visit, adjusted for the baseline value. On average over the study, metformin-treated subjects lost 1.72 kg from baseline, versus a gain of 0.29 kg among those treated with placebo, and also showed greater reductions in other measures of adiposity, with no differences between groups in measures of activity or dietary change.
The fasting glucose rose from baseline in the placebo group but fell in the metformin group (P < 0.0001). The 30-min glucose rose somewhat less in the metformin group, and the 120-min glucose fell in both groups. There was no group difference in levels of IGR. Metformin also significantly improved the fasting insulin (insulin sensitivity) and also improved the 30-min insulin and fasting proinsulin levels compared with placebo.
Intercorrelations among measures did not preclude fitting multivariate models. Interestingly, there was no correlation between the baseline weight and the change in weight over time or between weight change and change in IGR ( |r| ≤ 0.04 in both groups). Changes in FPG over time had modest correlation with weight over time (0.29 in metformin, 0.28 in placebo) but not with IGR (−0.03, −0.07, respectively).
Baseline models
In initial models (not shown) within each group, among the measures of adiposity, weight and waist circumference had equal, if not stronger, effects than did BMI on risk of onset of diabetes. Neither BMI nor height had a significant effect when adjusted for weight or waist circumference.
In the placebo group, a baseline model including sex, age, ethnicity, body weight, fasting glucose, 120-min glucose, A1C, fasting insulin, and fasting proinsulin, the IGR, recreational activity, leisure activity, and percent calories from fat explained R2 = 20.2% (P < 0.0001 on 13 degrees of freedom [df]). The dominant baseline factor was the fasting glucose with R2 = 8.9%, followed by 120-min glucose (1.7%), A1C (1.7%), and IGR (0.8%). Other effects were not nominally significant.
The model in the metformin group had R2 = 13.4% (P < 0.0001 on 13 df). Fasting glucose was much less important than in the placebo group, with R2 only 1.4%, whereas the 120-min glucose was more important with R2 = 2.9%. The IGR and A1C were nominally significant with R2 = 1.4% and 1.5%, respectively. Demographic factors and baseline dietary or leisure activity measures were not significant in either group.
Individual time-dependent covariate models
Table 2 separately presents the effects of individual time-dependent covariates on diabetes risk in each group, adjusted for baseline factors. Within both groups, weight loss was the principal measure of the effect of adiposity on risk of diabetes, with R2 = 5.8% with placebo and 3% with metformin therapy. These R2 values were at least as great as those for the percentage change in weight, slightly more than change in BMI, and substantially more than waist circumference, as well as waist-to-height ratio and waist-to-hip ratio (not shown), the latter not significant in either group.
TABLE 2.
Effect of current mean changes in time dependent covariates over time on the risk (hazard) of diabetes onset
| Placebo group
|
Metformin group
|
|||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | R2 (%) | HR | 95% CI | P | R2 (%) | |
| Univariate time-dependent covariate (unit)* | ||||||||
| Weight (kg) | 1.108 | 1.080–1.137 | <0.0001 | 5.8 | 1.113 | 1.072–1.155 | <0.0001 | 3.0 |
| Change in weight (%) | 1.099 | 1.074–1.125 | <0.0001 | 5.8 | 1.093 | 1.056–1.130 | <0.0001 | 2.5 |
| Waist (cm) | 1.066 | 1.031–1.102 | 0.0001 | 1.4 | 1.033 | 0.994–1.074 | 0.0952 | 0.3 |
| BMI (kg/m2) | 1.348 | 1.249–1.454 | <0.0001 | 5.5 | 1.331 | 1.203–1.474 | <0.0001 | 2.9 |
| Fasting insulin (pmol/l) | 1.004 | 1.003–1.006 | <0.0001 | 2.4 | 1.006 | 1.003–1.008 | <0.0001 | 2.0 |
| IGR (10 pmol/mmol) | 0.991 | 0.985–0.998 | 0.0090 | 0.6 | 0.912 | 0.878–0.948 | <0.0001 | 2.1 |
| Proinsulin (pmol/l) | 1.038 | 1.029–1.048 | <0.0001 | 6.2 | 1.044 | 1.026–1.062 | <0.0001 | 2.3 |
| Fasting glucose (mmol/l) | 2.500 | 2.256–2.771 | <0.0001 | 25.3 | 2.695 | 2.310–3.145 | <0.0001 | 14.3 |
| Multivariate time-dependent covariate (unit)† | ||||||||
| Weight (kg) | 1.099 | 1.068–1.132 | <0.0001 | 3.90 | 1.087 | 1.045–1.131 | <0.0001 | 1.66 |
| Fasting insulin (pmol/l) | 1.002 | 1.000–1.004 | 0.1276 | 0.22 | 1.003 | 1.001–1.006 | 0.0124 | 0.61 |
| IGR (10 pmol/mmol) | 0.991 | 0.985–0.998 | 0.0138 | 0.58 | 0.907 | 0.873–0.941 | <0.0001 | 2.55 |
Univariate models are adjusted for age, sex, ethnicity, fasting glucose, 2-h glucose, A1C, and the baseline value of the covariate (except for percent change in weight). The effects of recreational activity, leisure activity, and percent calories from fat over time were not significant at P ≤ 0.01 in either group.
Multivariate models are also adjusted for age, sex, and ethnicity; for baseline weight, recreational activity, percent calories from fat, and proinsulin levels; and for baseline and on-study levels of leisure activity. None of these effects were nominally significant at P ≤ 0.01 in either group.
Within the placebo group, increases in proinsulin had a slightly stronger effect on risk (R2 = 6.2%). Increases in fasting insulin had R2 = 2.4%, and decreases in IGR had R2 = 0.6%. Within the metformin group, similar changes in proinsulin levels had R2 = 2.3%, fasting insulin 2.0%, and IGR 2.1%. Within both groups, the effect of a decrease in IGR on the risk of diabetes, when also adjusted for changes in fasting insulin, was unchanged and vice versa. Changes in leisure exercise, recreational activity, and dietary factors were not significant in either group.
Within each group, fasting glucose over time is the strongest predictor of the risk of diabetes, there being ~150–170% increase in risk per millimole per liter increase in FPG, with a higher R2 value in the placebo than in the metformin group, 25 vs. 14%, respectively.
Multivariate models
Table 2 also presents the effects of the major covariates in a single model but without fasting glucose over time so as to assess the effects of other mechanisms on diabetes risk.
The placebo group model explains R2 = 27.2% of the variation in diabetes risk. Baseline fasting glucose (not shown) was the principal factor, there being a 4.1-fold increase in diabetes risk per millimole per liter greater baseline glucose level with partial R2 = 10.2% (adjusted for other factors). There is a 10% increase (9% decrease) in risk per kilogram mean increase (decrease) in body weight from baseline with R2 = 3.9%. The increase in proinsulin explains R2 of ~2%. The baseline 2-h glucose, baseline A1C, and baseline IGR are also nominally significant, each with R2 < 1.5%.
The metformin group model has R2 = 19.8%. The fasting and 2-h glucose at baseline and change in weight over time are nominally significant, each with R2 < 2.7%. The IGR at baseline has R2 = 3.1% (not shown) and the current value during follow-up R2 = 2.6%; neither effect diminished by the multivariate adjustment. The baseline insulin was nominally significant, and the change in fasting insulin approached significance.
Within each group, the hazard ratio (HR) per kilogram increase in body weight is reduced slightly after adjustment for other factors, but the strength of the effect is reduced substantially (R2 = 5.8 and 3.0% [unadjusted] 3.9 and 1.7% [adjusted] in the placebo and metformin groups, respectively), principally due to adjustment for metabolic factors. This suggests that changes in metabolic factors mediate part of the effect of weight change on diabetes risk.
In each group, the effect of changes in IGR remains largely unchanged after adjustment for the other metabolic factors (model not shown). However, the effect of the fasting insulin over time is markedly reduced (R2 = 2.4 vs. 0.4% in placebo and 2.0 vs. 1.0% in metformin), as is that of proinsulin over time (R2 = 6.2 vs. 3.4% in placebo and 2.3 vs. 1.3% in metformin). Additional adjustment for weight change (Table 2) further dilutes the proinsulin effect (R2 = 3.4 vs. 2.0% in placebo and 1.3 vs. 0.6% in metformin), but the other metabolic factors are largely unaffected.
There were no significant interactions among covariates in the placebo group. In the metformin group, there was an interaction between the on-study change in the IGR and that of proinsulin and insulin, each with R2 < 1.1%, such that as the proinsulin or the insulin decreased, the effect of a change in IGR on risk increased. While statistically significant, these interactions have negligible effect on the overall relationship of diabetes risk with changes in IGR.
Risk gradients
Figure 1 presents risk gradients to depict the partial effect of each covariate on the absolute risk of diabetes within each group, adjusted for other factors, based on the multivariate models in Table 2, with symbols for the quartiles of the distribution of the covariate. The slope of the risk gradient for a covariate (on the log scale) is determined by the covariate’s adjusted HR.
FIG. 1. Hazard rate per 100 participant-years as a function of the following.
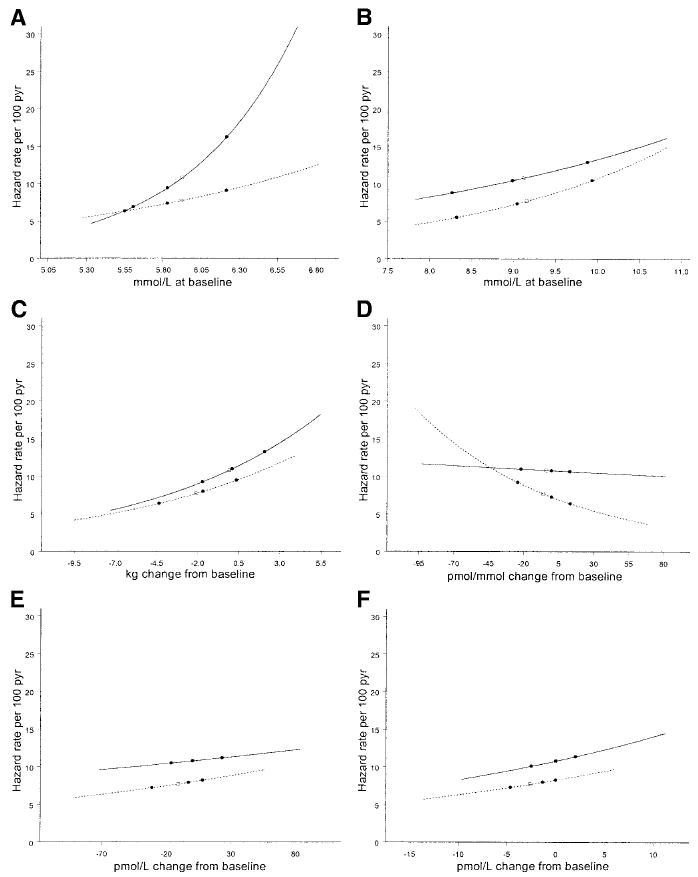
A: Baseline fasting glucose. B: Baseline 120-min glucose. C: Change in weight over time. D: Change in the IGR over time. E: Change in fasting insulin over time. F: Change in proinsulin over time. The average risk in the group at the mean of the covariate is designated as □; the risk at the quartiles of the distribution as ●. The solid lines represent the placebo group, and the dotted lines represent the metformin group.
The distributions (quartiles) of the baseline fasting glucose did not differ between groups (Fig. 1A). However, the diabetes HR per unit change in the baseline fasting glucose level is significantly (P < 0.0001) greater in the placebo than in the metformin group, and, thus, the risk gradients differ. The distributions of the baseline 120-min postload glucose, and the risk gradients (Fig. 1B), did not differ.
Weight loss was greater in the metformin than in the placebo group, and the risk gradient is somewhat, but not significantly, greater in the placebo than the metformin group (Fig. 1C). While there was little difference between groups in the levels of the IGR during the study (Table 1), changes in IGR in the placebo group had a negligible (though significant) effect on risk, whereas in the metformin group, the risk increases as the index (insulin secretion) decreases (Fig. 1D), with the HRs differing significantly between groups (P < 0.0001).
Although the fasting insulin levels during the study differed significantly between groups (Table 1), the risk increases in both groups at the same rate as the insulin levels increase (insulin sensitivity decreases) (Fig. 1E). At every level of insulin, the risk is less in the metformin than in the placebo group. The risk gradients for change in proinsulin are similar within each group, and there were greater decreases in proinsulin in the metformin than in the placebo group (Fig. 1F).
Explaining the metformin effect
The preceding analyses describe the effects of covariates on risk of diabetes within each group. Additional models (Table 3) within both groups combined assessed the extent to which these factors, individually and in combination, represented the mechanism(s) by which metformin reduced the risk of diabetes. For a factor to explain the beneficial metformin effect versus placebo in these models, the factor must differ between groups during treatment (Table 1) and must also be associated with diabetes risk within both groups combined (Table 3).
Adjusting only for baseline covariates, metformin is associated with an HR of 0.645, or a 35.5% risk reduction versus placebo, with a χ2 test value 23.70 and a corresponding R2 = 1.15%. Other than leisure activity, each of the principal time-dependent covariates was highly significant when evaluated individually. Adjustment for change in body weight alone explains 64% of the beneficial metformin versus placebo effect (group R2). Proinsulin alone explains 44% and fasting insulin explains 25% of the metformin-placebo group effect. While changes in IGR have an effect on diabetes risk within both groups, the on-study changes in IGR did not differ substantially between groups (Table 1), and, thus, the changes in IGR explain only 4% of the beneficial metformin-placebo effect. However, the group effect remained nominally statistically significant after adjustment for each of these factors individually, so that no one covariate alone completely explained the beneficial effect of metformin versus placebo on diabetes risk.
In a joint multivariate model (Table 3), all on-study covariates (other than glucose) simultaneously explained 81% of the beneficial metformin-placebo effect. Weight remains the primary covariate, explaining 30% of the metformin-placebo effect, indicating that some, but not all, of the group effect explained by weight is also explained by its intercorrelation with other factors. However, in this joint model, the group effect remains nominally significant, indicating that these factors together do not capture all of the mechanisms by which metformin affects diabetes risk.
In an additional model, the change in fasting glucose alone explained 79% of the metformin-placebo group effect, and the group effect is no longer significant. A model with all covariates including fasting glucose explained 99% of the metformin-placebo group effect, with the dominant factors being fasting glucose, which contributes to explaining 17% of the joint covariate effects on the metformin-placebo effect, and weight, which contributes to explaining 8% of this effect; each was adjusted for the other, as well as other factors.
DISCUSSION
In the DPP, metformin versus placebo reduced the risk of developing type 2 diabetes by 30% over an average of 3.2 years of follow-up in 2,155 individuals with IGT who were at high risk of developing diabetes due to having elevated fasting glucose and being overweight (1). Metformin was associated with modest weight loss and favorable changes in insulin sensitivity and insulin secretion over the 1st year of follow-up, each of which were shown to be associated with a reduction in the risk of diabetes in univariate analyses (3). Herein, we assess the joint effects of weight loss and changes in insulin resistance and secretion over the complete follow-up period, without and with adjustment for changes in blood glucose, on diabetes risk in the metformin and placebo groups. We also assess the extent to which changes in these factors explain the metformin versus placebo group difference in diabetes risk.
Metformin effects on glucose
Excess hepatic glucose production in the fasting state is a prominent feature of uncontrolled type 2 diabetes and the major cause of fasting hyperglycemia. During the DPP, metformin significantly decreased fasting glucose, but not the 120-min glucose, compared with placebo. This was in keeping with the known effects of metformin to suppress hepatic glucose production, leading to reduced fasting glucose, with a lesser effect on the 2-h glucose, which is more dependent on peripheral uptake of glucose (18-20). As a result, proportionately fewer metformin-treated participants were diagnosed with diabetes on the basis of the fasting glucose than those treated with placebo (29 vs. 41%, respectively). This suggests, therefore, that metformin reduces the risk of type 2 diabetes by suppressing the abnormal fasting hepatic glucose production as diabetes develops.
The baseline fasting glucose is the strongest predictor of diabetes onset in the placebo group, explaining R2 = 10.2% of the variation in risk, whereas the baseline 2-h glucose explained only R2 = 1.4% (Table 2). However, in the metformin group, the baseline fasting glucose is far less predictive of diabetes onset due to the reductions in fasting glucose with metformin treatment, explaining only R2 = 1.2%, which is less in fact than the baseline 2-h glucose with an R2 = 2.7%.
Further, the metformin effect on risk, compared with placebo (Fig. 1A), increases as the fasting glucose increases, with minimal effect at the lower levels of glucose, as shown previously in subgroup analyses (1). This suggests that metformin might be less effective in individuals with normal fasting glucose, even if they had IGT, although this could not be assessed in the DPP.
However, the reduction in diabetes risk with metformin is not wholly attributable to an acute pharmacologic effect on glucose levels. While a higher proportion of metformin-treated participants than placebo-treated participants were diagnosed with diabetes after the 1- to 2-week washout of study medication usage (7.2 vs. 5.0%, respectively, P = NS), the overall risk reduction with metformin after the washout remained significant (21).
Weight
Metformin produced a significant average weight loss of 1.72 kg versus a gain of 0.29 kg in the placebo group, an effect not observed with other oral antihyperglycemic agents or insulin.
In both groups, among the various measures of adiposity (Table 2), the absolute change in body weight was a stronger determinant of risk than waist circumference or waist-to-hip ratio and slightly stronger than BMI. Further analyses showed that in both groups, the effects of change in weight did not differ among the ethnic groups, either by sex or categories of age.
The effect of weight change on diabetes risk is similar in the two groups (Fig. 1C), and the relative risks (Table 2) correspond to ~9% risk reduction per kilogram weight loss, with R2 < 4%. These are less than that observed in the lifestyle group (2) with a 16% risk reduction (95% CI 13–19%) per kilogram weight loss, and R2 = 5.7%. Thus, a given amount of weight loss achieved through dietary changes and modest exercise in the lifestyle group has a greater reduction in diabetes risk than the same amount of weight loss achieved through metformin therapy or placebo. This could in part be due to the greater magnitude of weight loss with lifestyle therapy (or differential loss of fat or intra-abdominal fat or change in muscle mass) or other beneficial effects of the lifestyle intervention.
The net 2-kg difference in weight between metformin versus placebo therapy appears to be a pharmacologic effect of metformin since there were no differences between groups in measures of exercise, caloric intake, or fat intake.
Insulin resistance and secretion
While metformin primarily reduces plasma glucose concentrations, it has important but lesser effects on peripheral insulin sensitivity (19) and secretion (Table 1).
In both groups, fasting insulin individually had modest effects on diabetes risk but small effects after adjustment for other factors (Table 2). The effect of the IGR on diabetes risk was greater in the metformin group before and after adjustment for other factors (Table 2). Since the groups did not differ substantially in the IGR values over time (Table 1), this factor explained little of metformin effect on diabetes risk (Table 3).
After adjustment for these metabolic factors, the effect of change in body weight was reduced from R2 = 5.8 to 3.9% in the placebo group, and from R2 = 2.5 to 1.7% in the metformin group, suggesting that part of the effect of weight loss on risk in each group is mediated by accompanying increases in insulin secretion (IGR).
Proinsulin
Fasting proinsulin had an effect as strong, if not stronger, than did fasting insulin on the risk of diabetes (Table 2). Proinsulin is the precursor of insulin that is cleaved within the β-cell and released at the time of exocytosis from the cell (22,23). The association of proinsulin with diabetes risk could arise because it has a longer half-life than insulin, and β-cell products are released in pulses. Thus, proinsulin may have provided a better estimate of insulin sensitivity than the fasting insulin itself. However, proinsulin release relative to insulin is greater in subjects with type 2 diabetes compared with healthy subjects due to differences in β-cell function (23), and proinsulin may be less relevant in groups with different degrees of glucose intolerance.
Explaining the metformin effect
Differences between the treatment groups in weight, metabolic factors, and fasting glucose levels over time explained 99% of the reduction in risk with metformin versus placebo (Table 3). The group differences in changes in fasting glucose over time alone explained 79% of the beneficial effect of metformin on diabetes risk versus placebo, followed by weight loss (64% alone) and proinsulin (44% alone). The percent explained by each covariate was reduced when all covariates were used jointly. Thus, for example, group differences in weight explain 7.9% of this group effect when also adjusted for fasting glucose and other factors. Likewise, the percent explained by changes in glucose is reduced from 79 to 17% after adjustment for weight and other factors. Thus, some of the beneficial metformin effect explained by each factor alone was also reflected in the fractions explained by other covariates.
Limitations
Fasting insulin did not show strong effects on diabetes risk in these analyses. While more precise measurements of insulin sensitivity are available, the DPP did not collect the data necessary to compute them, other than homeostasis model assessment (HOMA). Fasting insulin, however, has been shown to be strongly related to insulin resistance (7). We have also computed the HOMA measure of insulin resistance [(fasting insulin × fasting glucose/18)/22.5]; however, HOMA showed no superiority to fasting insulin in these analyses.
Likewise, it is well documented that insulin release as measured by the IGR is a major factor in the determination of glucose tolerance (24) and thus is an appropriate measure of insulin secretory capacity.
Metformin may also have effects on endothelial function, peripheral insulin resistance, and vascular function, among others, that may influence diabetes risk that were not measured in the DPP and could not be assessed in these analyses.
In conclusion, treatment of subjects with IGT and at high risk of diabetes using metformin produced modest weight loss and favorable changes in insulin sensitivity and proinsulin relative to the placebo group. Further, these factors are strongly associated with the risk of diabetes onset; favorable effects of metformin on these factors, particularly the nearly 2 kg difference in change in weight with metformin versus placebo, in turn contribute to the reduction in the risk of diabetes. While the reduction in fasting glucose with metformin explains most of the reduction in risk with metformin, weight and other factors explain 81% of the metformin versus placebo group effect, and all factors including fasting glucose explain 99% of this treatment effect.
Acknowledgments
We gratefully acknowledge the commitment and dedication of the participants of the DPP. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study, as well as the collection, management, analysis, and interpretation of the data. The Southwestern American Indian Centers were supported directly by the NIDDK and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources supported data collection at many of the clinical centers. Funding for data collection and participant support was also provided by the Office of Research on Minority Health, the National Institute of Child Health and Human Development, the National Institute on Aging, the Centers for Disease Control and Prevention, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided medication. This research was also supported, in part, by the intramural research program of the NIDDK. A complete list of centers, investigators, and staff can be found in reference 1.
- DPP
Diabetes Prevention Program
- FPG
fasting plasma glucose
- HOMA
homeostasis model assessment
- IGR
insulinogenic index
- IGT
impaired glucose tolerance
- OGTT
oral glucose tolerance test
References
- 1.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Diabetes Prevention Program Research Group. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Diabetes Prevention Program Research Group. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Tech. Rep. Ser., no. 727. Geneva: World Health Org.; 1985. Diabetes Mellitus: Report of a WHO Study Group. [PubMed] [Google Scholar]
- 7.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, Porte D., Jr Quantification of the relationship between insulin sensitivity and β-cell function in human subjects: evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 8.Hanson RL, Pratley RE, Bogardus C, Narayan KM, Roumain JM, Imperatore G, Fagot-Campagna A, Pettitt DJ, Bennett PH, Knowler WC. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151:190–198. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- 9.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11:286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 10.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol. 1999;9:314–324. doi: 10.1016/s1047-2797(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 11.Mayer-Davis EJ, Sparks KC, Hirst K, Costacou T, Lovejoy JC, Regensteiner JG, Hoskin MA, Kriska AM, Bray GA. Dietary intake in the diabetes prevention program cohort: baseline and 1-year post randomization. Ann Epidemiol. 2004;14:763–772. doi: 10.1016/j.annepidem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Kriska AM, Caspersen CJ. A Collection of Physical Activity Questionnaires for Health-Related Research. Atlanta, GA: Centers For Disease Control and Prevention; 1997. Introduction to the collection of physical activity questionnaires. [Google Scholar]
- 13.Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg. 1996;23:104–115. doi: 10.1016/s0741-5214(05)80040-0. [DOI] [PubMed] [Google Scholar]
- 14.Snedecor GW, Cochran WG. Statistical Methods. 7. Ames, IA: Iowa State University Press; 1980. [Google Scholar]
- 15.Diggle PJ, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. New York: Oxford University Press; 1994. [Google Scholar]
- 16.Cox DR. Regression models and life-tables. JRSS (B) 1972;34:187–220. [Google Scholar]
- 17.Lachin JM. Biostatistical Methods: The Assessment of Relative Risks. New York: John Wiley and Sons; 2000. [Google Scholar]
- 18.Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:4059–4067. doi: 10.1210/jcem.81.11.8923861. [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- 20.Hundal RS, Krssak M, DuFour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care. 2003;26:977–980. doi: 10.2337/diacare.26.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halban PA. Structural domains and molecular lifestyles of insulin and its precursors in the pancreatic beta cell. Diabetologia. 1991;34:767–778. doi: 10.1007/BF00408349. [DOI] [PubMed] [Google Scholar]
- 23.Kahn SE, Halban PA. Release of incompletely processed proinsulin is the cause of the disproportionate proinsulinemia of NIDDM. Diabetes. 1997;46:1725–1732. doi: 10.2337/diab.46.11.1725. [DOI] [PubMed] [Google Scholar]
- 24.Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE American Diabetes Association GENNID Study Group. β-Cell function is the major determinant of oral glucose tolerance in four ethnic groups in the U.S. Diabetes. 2002;51:2170–2178. doi: 10.2337/diabetes.51.7.2170. [DOI] [PubMed] [Google Scholar]


