Abstract
Estrogen metabolism is suggested to play an important role in estrogen-induced breast carcinogenesis. Epidemiologic studies suggest that diets rich in phytoestrogens are associated with a reduced risk of breast cancer. Phytoestrogens are biologically-active plant compounds that structurally mimic 17β-estradiol (E2). We hypothesize that phytoestrogens, may provide protection against breast carcinogenesis by altering the expression of estrogen-metabolizing enzymes cytochrome P450 1A1 (Cyp1A1) and 1B1 (Cyp1B1). Cyp1A1 and Cyp1B1 are responsible for the metabolism of E2 to generate 2-hydroxyestradiol (2-OHE2) and 4-hydroxyestradiol (4-OHE2), respectively. Studies suggest that 2-OHE2 and 2-methoxyestradiol may protect against breast carcinogenesis, while 4-OHE2 is carcinogenic in rodent models. Thus, agents that increase the metabolism of E2 by Cyp1A1 to produce 2-OHE2 may have chemoprotective properties. The human immortalized non-neoplastic breast cell line MCF10F was treated with quercetin at 10 and 50 µM concentrations for time-points ranging from 3 to 48 hours. Total RNA and protein were isolated. Real-time PCR was used to measure the expression of Cyp1A1 and Cyp1B1 mRNA. Quercetin treatment produced differential regulation of Cyp1A1 and Cyp1B1 mRNA expression in a time-and dose-dependent manner. Treatment with 10 and 50 µM doses of quercetin produced 6-and 11-times greater inductions of Cyp1A1 mRNA over Cyp1B1 mRNA, respectively. Furthermore, quercetin dramatically increased Cyp1A1 protein levels and only slightly increased Cyp1B1 protein levels in MCF10F cells. Thus, our data suggest that phytoestrogens may provide protection against breast cancer by modulating expression of estrogen-metabolizing genes such that production of the highly carcinogenic estrogen metabolite 4-OHE2 by Cyp1B1 is reduced and the production of the less genotoxic 2-OHE2 by Cyp1A1 is increased.
Keywords: Quercetin, phytoestrogens, estrogen, cytochrome P450, catechol estrogens, estrogen metabolism
Introduction
In the United States breast cancer represents the most common neoplasm and the second most frequent cause of cancer death in women [1, 2]. The importance of endogenous estrogens in the etiology of breast cancer is widely recognized [3–6]. In 2001 the US government added steroidal estrogens to the list of known human carcinogens [7–9]. Estrogens have been implicated in the initiation and promotion stages of breast cancer, and lifetime estrogen exposure is a major risk factor for breast cancer [8, 10].
Estrogens exert their carcinogenic effects by both estrogen receptor (ER)-dependent and independent mechanisms [3, 7, 8, 11]. The ER-dependent mechanism underlying mammary carcinogenesis involves the activation of the ER by estrogens, leading to the expression of estrogen-responsive genes and stimulation of cell proliferation [3, 4, 7, 8, 11]. The ER-independent pathway entails the generation of toxic estrogen metabolites that are highly reactive and readily damage DNA, protein and lipids [5, 7, 8, 12–14]. E2, the main steroidal estrogen present in women, is metabolized to catechol estrogens 2-OHE2 and 4-OHE2 by Cyp1A1 and Cyp1B1, respectively [5, 6]. While, 2-OHE2 and 2-methoxyestradiol (2-MeOE2) have putative protective effects, 4-OHE2 is genotoxic and has potent estrogenic activity [15, 16]. 2-MeOE2 has antiproliferative and antiangiogenic activities which have been demonstrated both in vitro and in vivo [17, 18]. In contrast, 4-OHE2 induces DNA single strand breaks and oxidative damage [19, 20]. Tumorigenic estrogen metabolites such as 4-OHE2 undergo redox cycling to form electrophilic quinones which readily react with DNA to produce depurinating adducts [6, 11, 12, 21]. Furthermore, redox cycling results in the formation of free radicals and reactive oxygen species [20, 22]. The oxidative stress generated by estrogens is suspected to act in concert with the ER mediated signaling pathways to promote DNA damage and altered expression of genes responsible for controlling cell cycle and proliferation [3, 5, 6, 20, 22]. An understanding of the ways in which endogenous and exogenous compounds alter estrogen metabolism is a key step in the development of new breast cancer prevention strategies. Dietary or therapeutic agents that can interfere with receptor-mediated pathways or reduce the production of genotoxic estrogen metabolites may be effective in preventing estrogen-induced breast carcinogenesis. For example, a compound that increases the production of 2-OHE2 relative to 4-OHE2 would reduce the opportunity for genotoxic damage, and may result in a subsequent decrease in breast cancer risk.
Phytoestrogens are biologically active, plant-derived, phenolic compounds that structurally mimic the mammalian steroid hormone E2 [23–26]. These biologically-active plant compounds exhibit a wide array of pharmacological properties, and in recent years, interest in their potential benefits has increased dramatically. The most highly investigated properties of phytoestrogens are their possible chemoprotective characteristics, especially those relevant to breast cancer [1, 25, 27–29]. Epidemiological evidence suggests that diet and nutrition can influence cancer development, and Asian women report fewer post-menopausal symptoms and experience fewer breast cancers than women in Western countries [30–32]. More specifically, Asian women have a three-fold lower breast cancer risk than women in the United States, independent of body weight [33]. However, second and third generation descendants of women who migrated from Asia to Western countries have breast cancer risks similar to those of women in the host country, suggesting that lifestyle and dietary habits, and not genetic factors explain the low breast cancer risk of women in Asia [30, 34, 35]. Furthermore, epidemiologic studies have detected an association between phytoestrogen consumption, phytoestrogen levels in plasma and urine, and reduced risk of breast cancer [28, 36]. Studies using animal models of breast cancer have shown that a number of phytoestrogens, including quercetin, reduce the incidence and inhibit the development of N-methyl-N-nitrosourea (NMU) and DMBA-induced mammary carcinogenesis in rats [37–43]. Conversely, quercetin was shown to increase the severity of E2-induced kidney tumorigenesis in male Syrian hamsters [44].
While there have been a number of investigations regarding the effects of phytoestrogens on estrogen-metabolizing enzymes, very few of these studies have been carried out in breast cell lines and their relevance to human breast cancer prevention is unclear [45–50]. Previous studies have reported that quercetin treatment results in time- and dose-dependent increases in both Cyp1A1 mRNA levels and enzyme activity in MCF7 cells [49]. The ability of quercetin to modulate Cyp1A1 expression is thought to be mediated by its AhR-binding activity [49, 51]. Moreover, data from primate and human epidemiologic studies suggest that phytoestrogens may alter estrogen metabolism and metabolite patterns in vivo [52–55]. Thus, in the current study we have examined the effects of the widespread phytoestrogen quercetin on the RNA and protein expression of the estrogen-metabolizing enzymes Cyp1A1 and Cyp1B1 using the non-tumorigenic human breast epithelial cell line MCF10F.
Materials and Methods
Chemicals
Quercetin was purchased from Sigma-Aldrich (St. Louis, MO). Quercetin was dissolved in ethanol prior to treatments. The concentration of ethanol in control experiments or in experimental samples was always 1/1000th (vol/vol) of the final medium volume.
Cell Culture
MCF10F cells were cultured in DMEM/F12 (50:50) media (Mediatech, Herndon VA) as described previously [3, 12]. Twenty-four hours before treatment with quercetin or vehicle, cells were washed one time with 5 ml PBS and then grown in phenol red-free DMEM/F12 (50:50) supplemented with 5% charcoal dextran stripped horse serum (Cocalico Biologicals, Reamstown PA). Quercetin was used at 10 and 50 µM doses. Cells were cultured for either 12 hours, 24 hours, 48 hours or 72 hours. All treatments were done in quadruplicate. Experiments were performed in passages 2 to 10 of cells sub-cultured from a frozen stock of MCF10F cells.
Reverse Transcription and real-time PCR
Real-time PCR was used to quantify the expression of Cyp1A1 and Cyp1B1. After quercetin treatment, total RNA from cultured MCF10F cells was isolated by using RNAbee (Tel-Test Inc, Friendstown TX) according to the supplier’s protocols and as reported previously [56]. Five µg total RNA was reverse transcribed using the superscript II reverse transcription system and an oligo-dT18 primer (Invitrogen, Carlsbad CA). After reverse transcription, RNase H (2 units/µl) was added to all samples to ensure degradation of the remaining RNA. Real time PCR was performed in duplicate 25 µl reactions by using the Applied Biosystems (Foster City, CA) 7500 Real Time PCR System as described previously [57]. Data were analyzed using Applied Biosystems 7500 SDS version 1.7 software. The expression of cyclophilin, a housekeeping gene, was used for quantification and standardization purposes. Cychophilin is a housekeeping gene whose mRNA expression is not altered by estrogen or phytoestrogen treatment as shown by our unpublished data and previous research [58]. The expression of Cyp1A1 and Cyp1B1 relative to cyclophilin was determined by dividing the number of cDNA molecules for the gene of interest by the number of cyclophilin cDNA molecules. Standards were created for each gene and a standard curve was run on each plate to allow for accurate quantification of cDNA, as reported previously [56, 57].
Western blot analysis
Twenty micrograms total protein isolated from quadrupicates of control or quercetin-treated MCF10F cells was size fractionated on a 15% SDS-polyacrylamide gel, and transferred onto PVDF membranes under standard conditions. Membranes were blocked in 5% dry non-fat milk/PBS/0.5% Tween-20 at room temperature for 30 minutes. Affinity purified goat polyclonal antibodies against Cyp1A1 (Santa Cruz, sc-9828) and Cyp1B1 (Santa Cruz, sc-31667) were diluted 1:1000 in PBS/0.5% Tween-20 and used for immunodetection. After incubation overnight at room temperature with the primary antibody, membranes were washed four times for 8 minutes per wash using PBS/0.5% Tween-20. Horse radish peroxidase-conjugated bovine anti-goat IgG was diluted 1:2000 in PBS/0.5% Tween-20 and used as a secondary antibody (Santa Cruz, sc-2350). After incubation for 2 hours at room temperature, the membrance was washed again as described above. Chemiluminescent detection was performed using the BM Chemiluminescence Detection kit (Roche, Indianapolis, IN) and the Kodak Image Station 2000mm (Eastman Kodak Company, NY), with Kodak molecular imagaing software (Eastman Kodak Company, NY). Membranes probed for Cyp1A1 and Cyp1B1 were washed in PBS/0.5% Tween-20 and re-incubated overnight at room temperature with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal mouse antibody (RDI Fitzgerald, MA; TRK5G4-6C5) diluted 1:2000 in PBS/0.5% Tween-20. Horse radish peroxidase-conjugated anti-mouse IgG antibody (Amersham, NJ; NA931V) was diluted 1:2500 in PBS/0.5% Tween-20 and used as a secondary antibody for GAPDH detection. Secondary antibody was incubated with the membrane for 2 hours at room temperature prior to chemiluminescent detection using the method described above.
Statistical analysis
Statistical analyses were performed by using Sigma Plot 8.0 software (Systat Software Incorporated, CA). All cell culture treatments were done in quadruplicate and the student’s t test was used to compare the Cyp1A1 or Cyp1B1 mRNA expression changes in quercetin-treated MCF10F cells to Cyp1A1 or Cyp1B1 expression in MC10F cells. A p value of 0.05 was considered signficant in our experiments.
Results
MCF10F cells were treated with graded doses of quercetin for time points ranging from 3 to 48 hours. The amount of Cyp1A1 and Cyp1B1 mRNA in the control and quercetin-treated MCF10F cells was quantified by using real time PCR. The raw amounts of Cyp1A1 and Cyp1B1 mRNA were normalized to the amount of cyclophilin mRNA in each corresponding sample. Fold increases in Cyp1A1 and Cyp1B1 expression were determined by comparing mRNA levels of Cyp1A1 and Cyp1B1 in quercetin-treated MCF10F cells to mRNA levels of each respective gene in control MCF10F cells. Fold changes were used to compare the degree of increase or decrease in Cyp1A1 and Cyp1B1 expression at each time point and dose of quercetin.
Quercetin treatment differentially regulates the mRNA expression of the estrogen-metabolizing genes Cyp1A1 and Cyp1B1 in MCF10F cells
Treatment of MCF10F cells with graded doses of quercetin resulted in a time-and dose-dependent increase in Cyp1A1 mRNA expression that was significantly different from control at all time points tested (Figure 1A and B). MCF10F cells treated with 10 µM quercetin displayed a 5-fold increase in Cyp1A1 mRNA expression compared to vehicle-treated cells after 3 hours treatment (Figure 1A). Maximal induction of Cyp1A1 by 10 µM quercetin occurred after 12 hours treatment and was equal to a 15-fold increase in Cyp1A1 expression compared to vehicle-treated cells (Figure 1A). Similarly, 50 µM doses of quercetin increased expression of Cyp1A1 mRNA in MCF10F cells (Figure 1B). A 4-fold increase in Cyp1A1 expression compared to vehicle-treated control cells was observed following 6 hours treatment (Figure 1B). The maximal induction of Cyp1A1 mRNA expression produced by 50 µM quercetin was reached after 24 hours treatment and was equal to a 12-fold increase over control cells (Figure 1B).
Figure 1. Regulation of Cyp1A1 mRNA expression by quercetin.
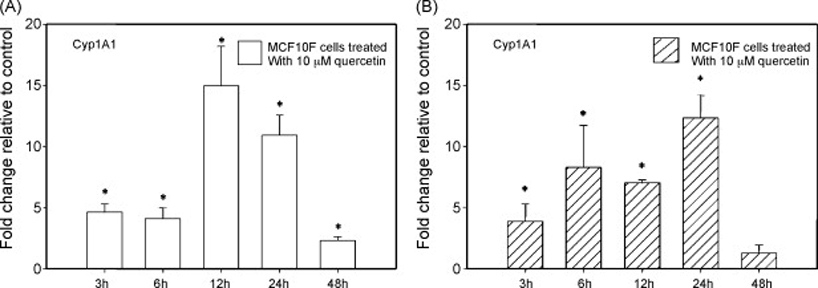
MCF10F human breast epithelial cells were treated with either vehicle (ethanol) or 10 and 50 µM concentrations of quercetin for 3 to 48 hours. Total RNA was isolated and reverse transcribed to generate cDNA. Real-time quantitative PCR was performed to analyze the expression of cyclophilin and Cyp1A1 mRNA. Expression of Cyp1A1 was determined by dividing the number of cDNA molecules of Cyp1A1 by the number of cDNA molecules of cyclophilin, a housekeeping gene. Fold change was determined by dividing the expression of Cyp1A1 in the quercetin-treated MCF10F cells by the expression of Cyp1A1 in the vehicle-treated cells. Each experiment was performed in quadruplicate and the data are expressed as fold change +/− standard deviation relative to control. A * indicates that Cyp1A1 expression is significantly different than Cyp1A1 expression in vehicle-treated cells, with a p value of less than 0.05.
MCF10F cells treated with 10 µM quercetin displayed an increase in Cyp1B1 mRNA expression (Figure 2A). The change in Cyp1B1 mRNA expression was significantly increased compared to vehicle-treated controls at the 3, 6, 12 and 24 hour time points but not the 48 hour time point (Figure 2A). After 3 hours treatment with 10 µM quercetin, Cyp1B1 mRNA expression was increased approximately 4-fold over vehicle-treated control cells (Figure 2A). The maximal induction of Cyp1B1 expression produced by 10 µM quercetin was equal to a 7-fold increase over control cells and occurred after 12 hours treatment (Figure 2A). The effect of 50 µM doses of quercetin on Cyp1B1 mRNA expression in MCF10F cells followed a different pattern (Figure 2B). Significant elevations in Cyp1B1 mRNA expression were observed at the 3, 6, 12 and 24 hour time points (Figure 2B). After 3 hours treatment with 50 µM quercetin, the expression of Cyp1B1 mRNA was increased slightly over control (Figure 2B). The maximal increase in Cyp1B1 expression in response to treatment with 50 µM quercetin was observed at the 12 hour time-point and was equal to a 4-fold increase over vehicle-treated cells (Figure 2B). However, unlike the 10 µM dose of quercetin, 48 hours treatment with 50 µM quercetin resulted in a significant decrease in Cyp1B1 mRNA expression (Figure 2B). Specifically, Cyp1B1 mRNA expression was approximately 8-fold lower in MCF10F cells treated with 50 µM doses of quercetin compared to vehicle-treated control cells (Figure 2B).
Figure 2. Regulation of Cyp1B1 mRNA expression by quercetin.
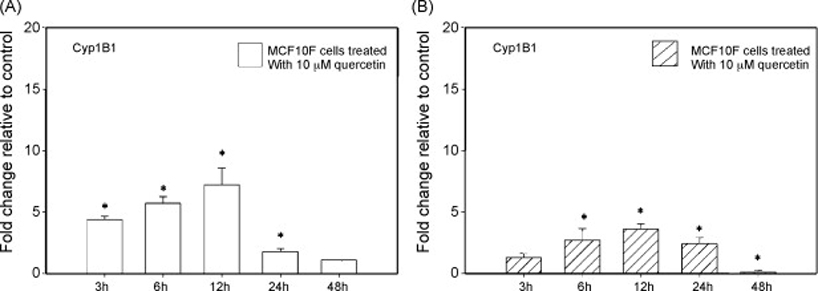
MCF10F human breast epithelial cells were treated with either vehicle or 10 and 50 µM concentrations of quercetin for 3 to 48 hours. Total RNA was isolated and reverse transcribed to generate cDNA. Real-time quantitative PCR was performed to analyze the expression of cyclophilin and Cyp1B1 mRNA. Expression of Cyp1B1 was determined by dividing the number of cDNA molecules of Cyp1B1 by the number of cDNA molecules of cyclophilin, a housekeeping gene. Fold change was determined by dividing the expression of Cyp1B1 in the quercetin-treated MCF10F cells by the expression of Cyp1B1 in the vehicle-treated cells. Each experiment was performed in quadruplicate and the data are expressed as fold change +/− standard deviation relative to control. A * indicates that Cyp1B1 expression is significantly different than Cyp1B1 expression in vehicle-treated cells, with a p value of less than 0.05.
Quercetin treatment elevates the expression of Cyp1A1 mRNA to significantly higher levels than Cyp1B1 mRNA in MCF10F cells
In order to compare the relationship between Cyp1A1 and Cyp1B1 mRNA expression in response to quercetin exposure in MCF10F cells, the ratios of Cyp1A1 to Cyp1B1 fold changes (Cyp1A1 fold change/Cyp1B1 fold change) were calculated for each dose and time-point, ranging from 3 to 48 hours. Ratios greater than one indicate that the expression of Cyp1A1 was induced to a higher level than the expression of Cyp1B1. At all time points tested, other than the 6 hour time-point, 10 µM doses of quercetin produced a ratio of Cyp1A1 to Cyp1B1 greater than one (Table 1). Likewise, 50 µM doses of quercetin resulted in ratios of Cyp1A1 to Cyp1B1 greater than one at all time-points tested. The highest ratios of Cyp1A1 to Cyp1B1 caused by 10 and 50 µM quercetin were observed after 24 and 48 hours and were equal to 6 and 11, respectively (Table 1).
Table 1.
Ratio of Cyp1A1 to Cyp1B1 mRNA expression in quercetin-treated MCF10F cells
| Dose (µ M) | Time (hours) | Cyp1A1 Fold Change | Cyp1B1 Fold Change | Ratio (Cyp1A1/Cry1B1) |
|---|---|---|---|---|
| 10 | 3 | 4.6 | 4.3 | 1.1 |
| 50 | 3 | 3.9 | 1.3 | 2.9 |
| 10 | 6 | 4.1 | 5.7 | 0.7 |
| 50 | 6 | 1.1 | 2.7 | 3.1 |
| 10 | 12 | 15.0 | 7.2 | 2.1 |
| 50 | 12 | 7.0 | 3.6 | 1.9 |
| 10 | 24 | 10.9 | 1.8 | 6.1 |
| 50 | 24 | 12.3 | 2.4 | 5.1 |
| 10 | 48 | 2.3 | 1.1 | 2.1 |
| 50 | 48 | 1.3 | 0.1 | 10.7 |
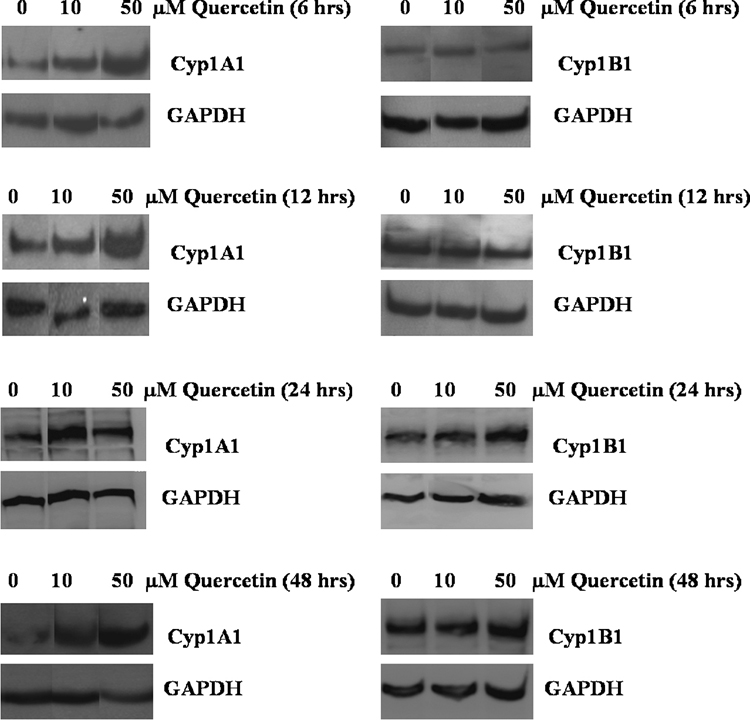
Quercetin strikingly increases Cyp1A1 but not Cyp1B1 protein levels
Western blotting was performed in order to determine whether the changes observed in Cyp1A1 and Cyp1B1 mRNA levels in quercetin-treated MCF10F cells resulted in altered Cyp1A1 and Cyp1B1 protein levels. MCF10F cells displayed dramatic increases in Cyp1A1 protein levels following 6, 12, 24 and 48 hour exposures to either 10 or 50 µM doses of quercetin. However, 10 and 50 µM concentrations of quercetin produced only slight elevations in Cyp1B1 protein levels in MCF10F cells. These data are in agreement with the greater increase in Cyp1A1 compared to Cyp1B1 expression at the RNA level quantified by real-time PCR.
Discussion
In light of the chemoprotective properties attributed to quercetin and other phytoestrogens by recent epidemiological and animal studies, we set out to elucidate the possible mechanisms by which quercetin may exert its anti-cancer effects. One such mechanism may involve quercetin’s ability to interfere with estrogen metabolism and the production of estrogen metabolites by altering the mRNA and protein levels of the key estrogen metabolizing enzymes Cyp1A1 and Cyp1B1 [5, 6]. Cyp1A1 metabolizes E2 to generate 2-OHE2, a relatively non-genotoxic metabolite, while Cyp1B1 metabolizes E2 to produce 4-OHE2, a highly genotoxic and carcinogenic metabolite [3, 5, 6, 11, 12, 15, 16, 19–22]. Although quercetin increases the expression of both Cyp1A1 and Cyp1B1, it increases Cyp1A1 to a higher level compared to Cyp1B1. Thus, quercetin, may shift estrogen metabolism toward Cyp1A1 and the production of 2-OHE2 and away from Cyp1B1 and the production of 4-OHE2. As a result, quercetin exposure may decrease levels of 4-OHE2, thereby reducing the opportunity for genotoxic insult by this highly reactive estrogen metabolite.
Evidence from primate studies suggests that exposure to phytoestrogens alters the pathways of estrogen metabolism by Cyp1A1 and Cyp1B1 in vivo, so that it is shifted toward the production of fewer genotoxic metabolites [52–54]. Recent epidemiological studies provide evidence that ingestion of phytoestrogens can alter endogenous estrogen metabolism [54, 55]. The consumption of soy isoflavones decreased urinary 4-OHE2 and 16-α-OHE1 in pre-menopausal women [54, 55]. Women eating phytoestrogen-rich diets had an increased ratio of 2-OHE2 to 4-OHE2 and a decreased ratio of genotoxic estrogens to total estrogens, suggesting a pattern of estrogen metabolism that is shifted away from the formation of genotoxic estrogen metabolites [54, 55]. In addition, several studies suggest that phytoestrogens may enhance the clearance of endogenous estrogens and may modestly lower serum estrogen concentrations [52, 59–62].
In the current study we have shown that quercetin differentially affects the expression of Cyp1A1 and Cyp1B1, such that the estrogen metabolic pathway is shifted toward Cyp1A1 and favors the production of fewer genotoxic estrogen metabolites. This could be one possible mechanism by which this phytoestrogen exerts protective effects against estrogen-induced breast carcinogenesis.
Figure 3. Western blot shows quercetin increases protein levels of Cyp1A1 more than Cyp1B1.
MCF10F human breast epithelial cells were treated with either vehicle or 10 and 50 µM concentrations of quercetin for 3 to 48 hours. Protein was isolated and subjected to Western blotting analysis. PVDF membranes were probed using antibodies to Cyp1A1, Cyp1B1 and GAPDH. Equal amounts of protein were loaded into each lane.
Acknowledgements
This work was supported by the National Institutes of Health Grants 5P30ES009089 and R01 CA 109551 (HKB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galati G, O'Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37(3):287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 2.Ju YH, Allred KF, Allred CD, Helferich WG. Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis. 2006;27(6):1292–1299. doi: 10.1093/carcin/bgi370. [DOI] [PubMed] [Google Scholar]
- 3.Bhat HK, Calaf G, Hei TK, Loya T, Vadgama JV. Critical role of oxidative stress in estrogen-induced carcinogenesis. Proc Natl Acad Sci U S A. 2003;100(7):3913–3918. doi: 10.1073/pnas.0437929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, Muti P, Rogan E, Russo J, Santen R, Sutter T. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006;1766(1):63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Cavalieri EL, Rogan EG. A unifying mechanism in the initiation of cancer and other diseases by catechol quinones. Annals of the New York Academy of Sciences. 2004;1028:247–257. doi: 10.1196/annals.1322.029. [DOI] [PubMed] [Google Scholar]
- 6.Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, Johansson SL, Patil KD, Gross ML, Gooden JK, Ramanathan R, Cerny RL, Rogan EG. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc Natl Acad Sci U S A. 1997;94(20):10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 8.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344(4):276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 9.Nelson R. Steroidal oestrogens added to list of known human carcinogens. Lancet. 2002;360(9350):2053. doi: 10.1016/S0140-6736(02)12045-9. [DOI] [PubMed] [Google Scholar]
- 10.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 11.Liehr JG, Han X, Bhat HK. 32P-postlabelling in studies of hormonal carcinogenesis. IARC Sci Publ. 1993;124:149–155. [PubMed] [Google Scholar]
- 12.Patel MM, Bhat HK. Differential oxidant potential of carcinogenic and weakly carcinogenic estrogens: Involvement of metabolic activation and cytochrome P450. J Biochem Mol Toxicol. 2004;18(1):37–42. doi: 10.1002/jbt.20005. [DOI] [PubMed] [Google Scholar]
- 13.Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annual review of pharmacology and toxicology. 1996;36:203–232. doi: 10.1146/annurev.pa.36.040196.001223. [DOI] [PubMed] [Google Scholar]
- 14.Cavalieri EL, Rogan EG, Chakravarti D. Initiation of cancer and other diseases by catechol ortho-quinones: a unifying mechanism. Cell Mol Life Sci. 2002;59(4):665–681. doi: 10.1007/s00018-002-8456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badawi AF, Cavalieri EL, Rogan EG. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16alpha-hydroxylation of 17beta-estradiol. Metabolism. 2001;50(9):1001–1003. doi: 10.1053/meta.2001.25592. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler RG, Rossi SC, Fears TR, Bradlow HL, Adlercreutz H, Sepkovic D, Kiuru P, Wahala K, Vaught JB, Donaldson JL, Falk RT, Fillmore CM, Siiteri PK, Hoover RN, Gail MH. Quantifying estrogen metabolism: an evaluation of the reproducibility and validity of enzyme immunoassays for 2-hydroxyestrone and 16alpha-hydroxyestrone in urine. Environ Health Perspect. 1997;105(Suppl 3):607–614. doi: 10.1289/ehp.97105s3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaVallee TM, Zhan XH, Herbstritt CJ, Kough EC, Green SJ, Pribluda VS. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res. 2002;62(13):3691–3697. [PubMed] [Google Scholar]
- 18.Brueggemeier RW, Bhat AS, Lovely CJ, Coughenour HD, Joomprabutra S, Weitzel DH, Vandre DD, Yusuf F, Burak WE., Jr 2-Methoxymethylestradiol: a new 2-methoxy estrogen analog that exhibits antiproliferative activity and alters tubulin dynamics. J Steroid Biochem Mol Biol. 2001;78(2):145–156. doi: 10.1016/s0960-0760(01)00090-5. [DOI] [PubMed] [Google Scholar]
- 19.Li JJ, Li SA. Estrogen carcinogenesis in Syrian hamster tissues: role of metabolism. Fed Proc. 1987;46(5):1858–1863. [PubMed] [Google Scholar]
- 20.Liehr JG, Fang WF, Sirbasku DA, Ari-Ulubelen A. Carcinogenicity of catechol estrogens in Syrian hamsters. J Steroid Biochem. 1986;24(1):353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 21.Cao K, Stack DE, Ramanathan R, Gross ML, Rogan EG, Cavalieri EL. Synthesis and structure elucidation of estrogen quinones conjugated with cysteine, N-acetylcysteine, and glutathione. Chem Res Toxicol. 1998;11(8):909–916. doi: 10.1021/tx9702291. [DOI] [PubMed] [Google Scholar]
- 22.Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr. 2000;27:67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- 23.Hodek P, Trefil P, Stiborova M. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem Biol Interact. 2002;139(1):1–21. doi: 10.1016/s0009-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 24.Stopper H, Schmitt E, Kobras K. Genotoxicity of phytoestrogens. Mutat Res. 2005;574(1–2):139–155. doi: 10.1016/j.mrfmmm.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Sirtori CR, Arnoldi A, Johnson SK. Phytoestrogens: end of a tale? Ann Med. 2005;37(6):423–438. doi: 10.1080/07853890510044586. [DOI] [PubMed] [Google Scholar]
- 26.Limer JL, Parkes AT, Speirs V. Differential response to phytoestrogens in endocrine sensitive and resistant breast cancer cells in vitro. Int J Cancer. 2006;119(3):515–521. doi: 10.1002/ijc.21863. [DOI] [PubMed] [Google Scholar]
- 27.Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;98(18):1275–1284. doi: 10.1093/jnci/djj356. [DOI] [PubMed] [Google Scholar]
- 28.Peeters PH, Keinan-Boker L, van der Schouw YT, Grobbee DE. Phytoestrogens and breast cancer risk. Review of the epidemiological evidence. Breast Cancer Res Treat. 2003;77(2):171–183. doi: 10.1023/a:1021381101632. [DOI] [PubMed] [Google Scholar]
- 29.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 30.Usui T. Pharmaceutical prospects of phytoestrogens. Endocr J. 2006;53(1):7–20. doi: 10.1507/endocrj.53.7. [DOI] [PubMed] [Google Scholar]
- 31.Le Corre L, Chalabi N, Delort L, Bignon YJ, Bernard-Gallon DJ. Resveratrol and breast cancer chemoprevention: molecular mechanisms. Mol Nutr Food Res. 2005;49(5):462–471. doi: 10.1002/mnfr.200400094. [DOI] [PubMed] [Google Scholar]
- 32.Nichenametla SN, Taruscio TG, Barney DL, Exon JH. A review of the effects and mechanisms of polyphenolics in cancer. Crit Rev Food Sci Nutr. 2006;46(2):161–183. doi: 10.1080/10408390591000541. [DOI] [PubMed] [Google Scholar]
- 33.Ursin G, Bernstein L, Pike MC. Breast cancer. Cancer Surv. 1994;19–20:241–264. [PubMed] [Google Scholar]
- 34.Pike MC, Kolonel LN, Henderson BE, Wilkens LR, Hankin JH, Feigelson HS, Wan PC, Stram DO, Nomura AM. Breast cancer in a multiethnic cohort in Hawaii and Los Angeles: risk factor-adjusted incidence in Japanese equals and in Hawaiians exceeds that in whites. Cancer Epidemiol Biomarkers Prev. 2002;11(9):795–800. [PubMed] [Google Scholar]
- 35.Probst-Hensch NM, Pike MC, McKean-Cowdin R, Stanczyk FZ, Kolonel LN, Henderson BE. Ethnic differences in post-menopausal plasma oestrogen levels: high oestrone levels in Japanese-American women despite low weight. Br J Cancer. 2000;82(11):1867–1870. doi: 10.1054/bjoc.1999.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brooks JD, Thompson LU. Mammalian lignans and genistein decrease the activities of aromatase and 17beta-hydroxysteroid dehydrogenase in MCF-7 cells. J Steroid Biochem Mol Biol. 2005;94(5):461–467. doi: 10.1016/j.jsbmb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Verma AK, Johnson JA, Gould MN, Tanner MA. Inhibition of 7,12-dimethylbenz(a)anthracene- and N-nitrosomethylurea-induced rat mammary cancer by dietary flavonol quercetin. Cancer Res. 1988;48(20):5754–5758. [PubMed] [Google Scholar]
- 38.Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12- dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62(17):4945–4954. [PubMed] [Google Scholar]
- 39.Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61(20):7456–7463. [PubMed] [Google Scholar]
- 40.Fritz WA, Coward L, Wang J, Lamartiniere CA. Dietary genistein: perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat. Carcinogenesis. 1998;19(12):2151–2158. doi: 10.1093/carcin/19.12.2151. [DOI] [PubMed] [Google Scholar]
- 41.Lamartiniere CA, Zhang JX, Cotroneo MS. Genistein studies in rats: potential for breast cancer prevention and reproductive and developmental toxicity. Am J Clin Nutr. 1998;68(6 Suppl):1400S–1405S. doi: 10.1093/ajcn/68.6.1400S. [DOI] [PubMed] [Google Scholar]
- 42.Murrill WB, Brown NM, Zhang JX, Manzolillo PA, Barnes S, Lamartiniere CA. Prepubertal genistein exposure suppresses mammary cancer and enhances gland differentiation in rats. Carcinogenesis. 1996;17(7):1451–1457. doi: 10.1093/carcin/17.7.1451. [DOI] [PubMed] [Google Scholar]
- 43.Hilakivi-Clarke L, Onojafe I, Raygada M, Cho E, Skaar T, Russo I, Clarke R. Prepubertal exposure to zearalenone or genistein reduces mammary tumorigenesis. Br J Cancer. 1999;80(11):1682–1688. doi: 10.1038/sj.bjc.6690584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu BT, Liehr JG. Quercetin increases the severity of estradiol-induced tumorigenesis in hamster kidney. Toxicol Appl Pharmacol. 1994;125(1):149–158. doi: 10.1006/taap.1994.1059. [DOI] [PubMed] [Google Scholar]
- 45.Chang TK, Chen J, Lee WB. Differential inhibition and inactivation of human CYP1 enzymes by trans-resveratrol: evidence for mechanism-based inactivation of CYP1A2. J Pharmacol Exp Ther. 2001;299(3):874–882. [PubMed] [Google Scholar]
- 46.Chun YJ, Kim MY, Guengerich FP. Resveratrol is a selective human cytochrome P450 1A1 inhibitor. Biochem Biophys Res Commun. 1999;262(1):20–24. doi: 10.1006/bbrc.1999.1152. [DOI] [PubMed] [Google Scholar]
- 47.Berge G, Ovrebo S, Botnen IV, Hewer A, Phillips DH, Haugen A, Mollerup S. Resveratrol inhibits benzo[a]pyrene-DNA adduct formation in human bronchial epithelial cells. Br J Cancer. 2004;91(2):333–338. doi: 10.1038/sj.bjc.6601898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Revel A, Raanani H, Younglai E, Xu J, Rogers I, Han R, Savouret JF, Casper RF. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J Appl Toxicol. 2003;23(4):255–261. doi: 10.1002/jat.916. [DOI] [PubMed] [Google Scholar]
- 49.Ciolino HP, Yeh GC. Inhibition of aryl hydrocarbon-induced cytochrome P-450 1A1 enzyme activity and CYP1A1 expression by resveratrol. Mol Pharmacol. 1999;56(4):760–767. [PubMed] [Google Scholar]
- 50.Chen ZH, Hurh YJ, Na HK, Kim JH, Chun YJ, Kim DH, Kang KS, Cho MH, Surh YJ. Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis. 2004;25(10):2005–2013. doi: 10.1093/carcin/bgh183. [DOI] [PubMed] [Google Scholar]
- 51.Ramadass P, Meerarani P, Toborek M, Robertson LW, Hennig B. Dietary flavonoids modulate PCB-induced oxidative stress, CYP1A1 induction, and AhR-DNA binding activity in vascular endothelial cells. Toxicol Sci. 2003;76(1):212–219. doi: 10.1093/toxsci/kfg227. [DOI] [PubMed] [Google Scholar]
- 52.Lu LJ, Cree M, Josyula S, Nagamani M, Grady JJ, Anderson KE. Increased urinary excretion of 2-hydroxyestrone but not 16alpha-hydroxyestrone in premenopausal women during a soya diet containing isoflavones. Cancer Res. 2000;60(5):1299–1305. [PubMed] [Google Scholar]
- 53.Wood CE, Register TC, Cline JM. Soy isoflavonoid effects on endogenous estrogen metabolism in postmenopausal female monkeys. Carcinogenesis. 2006 doi: 10.1093/carcin/bgl163. [DOI] [PubMed] [Google Scholar]
- 54.Xu X, Duncan AM, Wangen KE, Kurzer MS. Soy consumption alters endogenous estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2000;9(8):781–786. [PubMed] [Google Scholar]
- 55.Xu X, Duncan AM, Merz BE, Kurzer MS. Effects of soy isoflavones on estrogen and phytoestrogen metabolism in premenopausal women. Cancer Epidemiol Biomarkers Prev. 1998;7(12):1101–1108. [PubMed] [Google Scholar]
- 56.Bhat HK, Epelboym I. Suppression of calbindin D28K in estrogen-induced hamster renal tumors. J Steroid Biochem Mol Biol. 2004;92(5):391–398. doi: 10.1016/j.jsbmb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 57.Bhat HK, Epelboym I. Quantitative analysis of total mitochondrial DNA: competitive polymerase chain reaction versus real-time polymerase chain reaction. J Biochem Mol Toxicol. 2004;18(4):180–186. doi: 10.1002/jbt.20024. [DOI] [PubMed] [Google Scholar]
- 58.Bhat H, Brad TE, Liehr JG. Differential estrogen regulation of c-fos in hamster kidney and kidney tumor cells: Receptor mediation verses metabolic activation. International Journal of Oncology. 1995;7:527–534. doi: 10.3892/ijo.7.3.527. [DOI] [PubMed] [Google Scholar]
- 59.Wood CE, Register TC, Franke AA, Anthony MS, Cline JM. Dietary soy isoflavones inhibit estrogen effects in the postmenopausal breast. Cancer Res. 2006;66(2):1241–1249. doi: 10.1158/0008-5472.CAN-05-2067. [DOI] [PubMed] [Google Scholar]
- 60.Duncan AM, Underhill KE, Xu X, Lavalleur J, Phipps WR, Kurzer MS. Modest hormonal effects of soy isoflavones in postmenopausal women. J Clin Endocrinol Metab. 1999;84(10):3479–3484. doi: 10.1210/jcem.84.10.6067. [DOI] [PubMed] [Google Scholar]
- 61.Lu LJ, Anderson KE, Grady JJ, Kohen F, Nagamani M. Decreased ovarian hormones during a soya diet: implications for breast cancer prevention. Cancer Res. 2000;60(15):4112–4121. [PubMed] [Google Scholar]
- 62.Wu AH, Stanczyk FZ, Seow A, Lee HP, Yu MC. Soy intake and other lifestyle determinants of serum estrogen levels among postmenopausal Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev. 2002;11(9):844–851. [PubMed] [Google Scholar]