Abstract
In epithelial cells, the tight junction divides the plasma membrane into distinct apical and basolateral domains. Polarization is essential for epithelial cell function, and apico-basal cell polarity is lost during the epithelial to mesenchymal transition (EMT), a program of events characterized not only by loss of cell polarity, but also by enhanced cell motility and increased cell invasion. Among several apically localized protein complexes, the Crumbs and Par protein complexes have pivotal roles in control of epithelial polarity and apical membrane formation. Here, we demonstrate that the Snail transcriptional repressor antagonizes expression of the Crumbs polarity complex. We show that Snail abolishes localization of the Crumbs and Par complexes to the tight junction, decreases Crumbs complex protein levels and suppresses Crumbs3 transcription. Evidence that Snail acts directly to antagonize Crumbs3 promoter activity is presented. Strikingly, we note that reexpression of exogenous Crumbs3 in Snail-expressing Madin–Darby Canine Kidney cells partially restores cell–cell junctions. Moreover, we find that the EMT inducer transforming growth factor- β elicits transcriptional repression of Crumbs3 and results in a measurable loss of Crumbs3 protein. Our findings provide new insights into the links between the transcriptional repression function of Snail and its role in antagonizing key apico-basal polarity factors during EMT.
Keywords: Crumbs, Snail, apico-basal polarity, tight junction, epithelial to mesenchymal transition
Epithelial cells form highly organized sheets of tissue that line organ cavities and create barriers between body compartments. The tight junction forms seals between adjacent epithelial cells and also divides the plasma membrane into distinct apical and basolateral domains. Recently, mammalian orthologs of two novel, interacting tight junction-associated complexes crucial in establishing and maintaining apico-basal polarity were identified. These evolutionarily conserved protein complexes are known as the Crumbs complex (Crumbs3-PALS1 (protein associated with Lin-7)-PATJ (PALS1-associated tight junction protein)) and the Par complex (Par6-Par3-aPKC (atypical protein kinase C); reviewed by Shin et al., 2006). In mammalian cells, there is strong evidence that the Crumbs and Par complexes control epithelial polarity and apical membrane formation.
The epithelial to mesenchymal transition (EMT) is a program of events characterized by loss of cell polarity, enhanced cell motility and increased cell invasion (reviewed by Thiery and Sleeman, 2006). While this process is essential in the development of many tissues and organs, the inappropriate activation of EMT in adult tissues has been linked to cancer. A central regulator of this cellular program is the transcription factor Snail, which binds to consensus E-box sequences 5′-CA(G/C)(G/C)TG-3′ in regulatory elements of its target genes (reviewed by Nieto, 2002). Snail has a profound effect in dissolving cell–cell junctions—among its bona fide target genes are structural elements of adherens and tight junctions, including the tumor suppressor E-cadherin, occludin and some claudin genes. Here, we test the hypothesis that the Crumbs and Par apico-basal polarity complexes are targeted by Snail and disrupted during EMT.
In order to evaluate the effects of Snail expression and EMT on the Crumbs and Par polarity protein complexes, we created Madin–Darby Canine Kidney (MDCK) clonal epithelial cell lines expressing either Snail-FLAG or a control empty vector. As previously reported, we noted that epithelial cells expressing Snail exhibit morphologic features consistent with EMT and concomitant downregulation of specific junctional proteins (Batlle et al., 2000; Cano et al., 2000; Ohkubo and Ozawa, 2004; Supplementary Figures 1a–e, 2a–d).
Using this system, we investigated the mechanisms underlying the loss of apico-basal cell polarity during EMT. Specifically, we examined the localization of the conserved Par and Crumbs polarity complexes. We discovered that the Par complex is displaced from the cell–cell junctions upon Snail expression, as judged by immunofluorescent staining for PKCζ and Par3 (Figures 1a and b). Moreover, our experiments show that the Crumbs complex is also absent from cell–cell junctions in Snail cells, as demonstrated by immuno-fluorescent staining for Crumbs3, PALS1 and PATJ (Figures 1c–e). Thus, our data demonstrate that the novel apico-basal Par and Crumbs polarity complexes are displaced from the tight junctions in MDCK cells undergoing Snail-induced EMT. Next, we collected a z-series of confocal images to examine apico-basal polarity more fully. In control cells, a clear demarcation of both basolateral membranes (stained with E-cadherin) and apical membranes (bordered by ZO-1) was seen. In contrast, apico-basal polarity was absent in Snail-expressing cells (Figure 1f).
Figure 1.
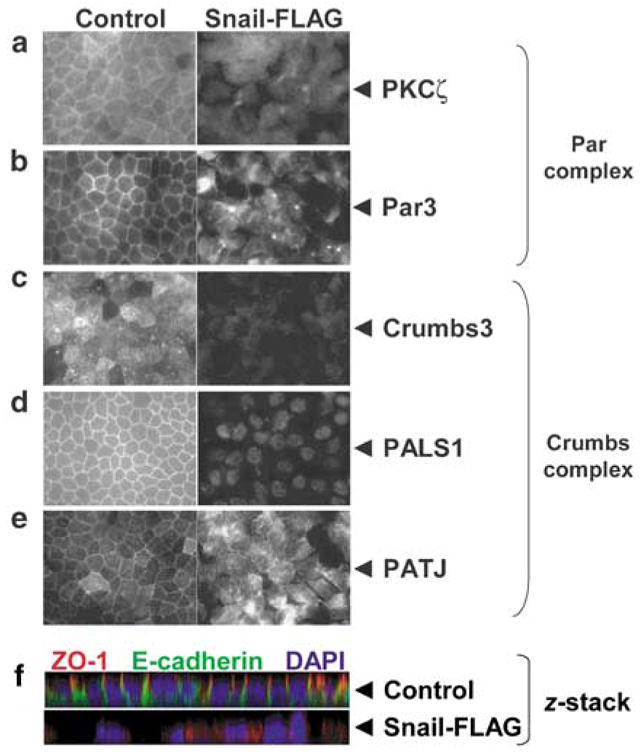
Snail expression disrupts junctional localization of the Crumbs and Par complexes and apico-basal polarity. A retroviral construct containing human Snail cDNA with a FLAG-epitope tag has been previously described (Hajra et al., 2002). Stable Madin–Darby Canine Kidney (MDCK) cell lines were created by retroviral mediated gene transfer and selected with 600 μg ml−1 G418 (Whiteman et al., 2003). Control and Snail-FLAG MDCK cells were grown on 24 mm Transwell filters (0.4 μm pore size; Corning Inc., Corning, NY, USA) for at least 72 h to achieve a tightly packed monolayer. The filters were immunostained for the indicated markers and images were captured as described (Straight et al., 2006). (a and b) Par complex: PKCζ and Par3 (tight junction), (c–e) Crumbs complex: Crumbs3, PALS1, PATJ (tight junction), (f) Confocal z-stack triple labeled for ZO-1 (tight junction), E-cadherin (adherens junction) and 4,6-diamidino-2-phenylindole (DAPI) (nuclei). The z-stack image was obtained using an Olympus FluoView 500 laser-scanning confocal microscope, and images were collected in 0.3 μm z-steps ( × 600). Antibodies used: polyclonal PKCζ (Millipore, Billerica, MA, USA); polyclonal Crumbs3, PALS1, PATJ and Par3 were previously described (Roh et al., 2002); monoclonal ZO-1, polyclonal E-cadherin, fluorochrome-conjugated secondary antibodies (Invitrogen, Carlsbad, CA, USA).
Because prior studies have shown that Snail is a transcriptional repressor, we questioned whether it might be affecting apico-basal cell polarity by reducing the levels of Par and Crumbs complex proteins. We found that Par3 and PKCζ proteins, components of the Par complex, were unaffected by Snail expression (Figures 2a–c). In contrast, we discovered that the Crumbs polarity complex was reduced by Snail expression. We noted measurable decreases in PATJ and PALS1 proteins and a striking loss of detectable Crumbs3 protein upon Snail expression (Figures 2d and e). We also assayed the effects of Snail expression on the lateral Scribble polarity complex by analysing Scribble and Dlg/SAP97 proteins by immunoblot. The levels of Scribble protein remain unchanged in the presence of Snail; however, we note a modest decrease in Dlg/SAP97 protein (Supplementary Figures 2e and f).
Figure 2.
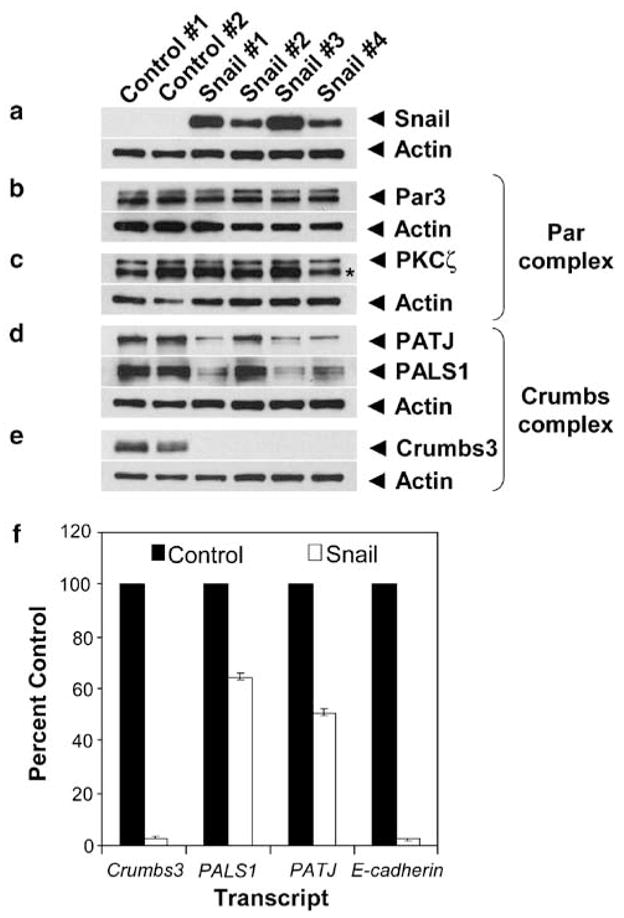
Snail expression downregulates components of the Crumbs complex and strongly represses Crumbs3 transcription. Two control and four Snail-FLAG clonal cell lines were cultured, harvested in denaturing buffer and 20 μg protein samples resolved by SDS–polyacrylamide gel electrophoresis (PAGE) and immuno-blotted for the Par and Crumbs complexes as described (Straight et al., 2006). (a) Blots probed for Snail expression, (b and c) Par complex (Par3, PKCζ asterisk denotes a cross-reactive band), (d and e) Crumbs complex (PATJ, PALS1, Crumbs3). Actin immunoblots are shown as loading controls. (f) Cytosolic RNA was isolated from two control and four Snail-FLAG clonal cell lines and subjected to quantitative reverse transcription–PCR (qRT–PCR) to detect levels of Crumbs complex transcripts. Bar graph depicts the mean transcript levels from three experiments (error bars indicate s.e.m.). Results were normalized to glycer-aldehyde-3-phosphate dehydrogenase (GAPDH). The qRT-PCR method was previously described (Straight et al., 2006). Additional antibodies used: polyclonal Snail E-18 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA); polyclonal actin (Sigma, St Louis, MO, USA); horseradish-peroxidase-conjugated secondary antibodies (GE Healthcare, Buckinghamshire, England).
The loss of Crumbs3 protein expression mirrored the marked reduction in the expression of other authentic targets of Snail transcriptional repression—namely occludin, E-cadherin and claudin-1 (Supplementary Figure 2). This observation prompted us to explore whether Snail might be transcriptionally repressing specific genes that encode components of the Crumbs complex. We performed quantitative reverse transcription–PCR and measured transcript levels for Crumbs3, PALS1, PATJ and E-cadherin (positive control) in control and Snail-expressing MDCK cells. We discovered that Snail expression resulted in a >95% decrease in Crumbs3 transcript and a modest 35–50% reduction in PALS1 and PATJ transcript levels (Figure 2f).
The dramatic reduction in Crumbs3 transcript levels led us to inspect the Crumbs3 promoter for consensus Snail binding sites (i.e., E-boxes). We discovered that the predicted promoter region of human Crumbs3 contains several E-boxes clustered near the transcription start site ‘TSS’ (Figure 3a). Of note, the first five E-box elements directly upstream of the TSS and the first E-box downstream of the TSS are conserved among the human, mouse and canine Crumbs3 promoters. For our initial analysis, we used the Genomatix software suite (Munich, Germany) to predict an ~1 kb human Crumbs3 promoter. Sequential 5′ deletions allowed us to refine the Crumbs3 promoter to a 556 bp fragment (−155/ + 401) that experiences a robust, approximately fivefold repression by Snail in luciferase reporter assays (Figures 3a and b). We then systematically altered each E-box (CANNTG → AANNTA) to create a series of mutant Crumbs3 promoters. Luciferase reporter assays show that Snail sensitivity is largely maintained among the single E-box mutant promoters and is likely conferred by multiple, redundant E-boxes (Figure 3c). Accordingly, we reasoned that Snail may be repressing Crumbs3 expression by directly binding to its promoter region. To address this possibility, we performed chromatin immunoprecipitation (ChIP) assays using anti-Snail antibodies. We discovered that Snail directly associates with the Crumbs3 promoter (Figure 3d). Thus, Crumbs3 appears to be a new target of transcriptional repression by Snail.
Figure 3.
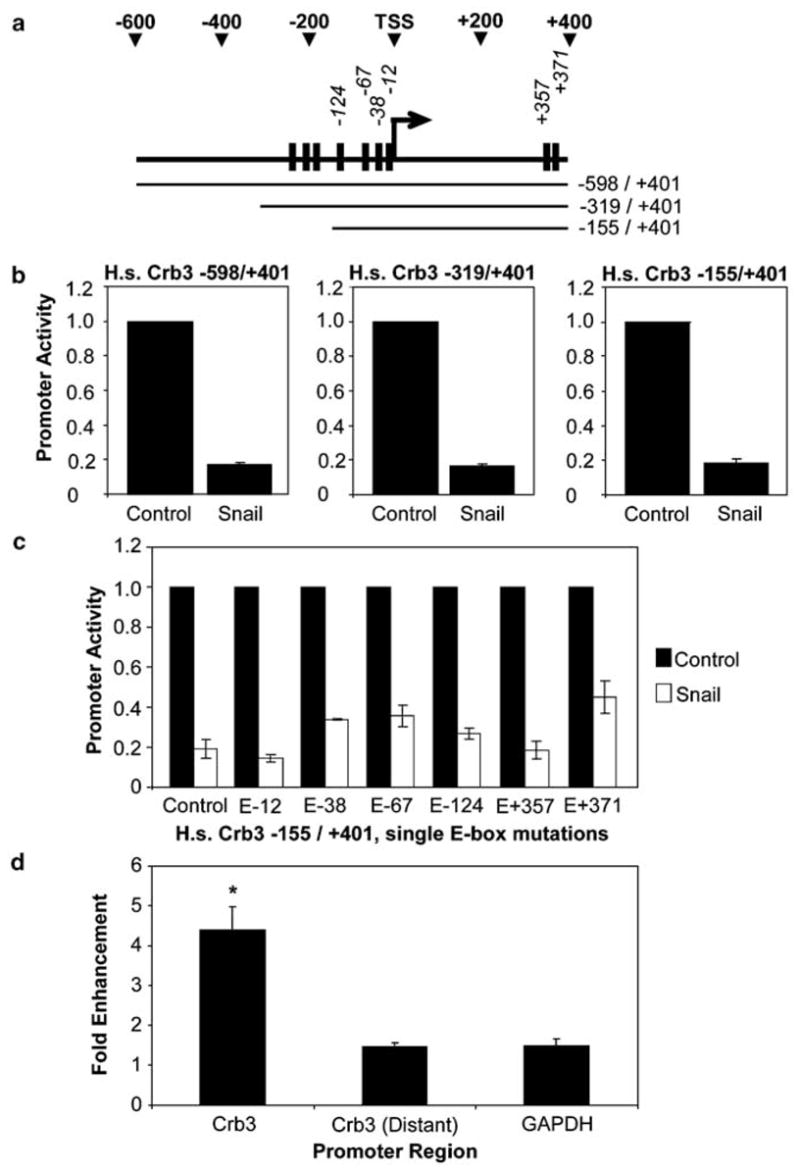
Snail represses Crumbs3 promoter activity and directly associates with the promoter. (a) Schematic of the human Crumbs3 proximal promoter region. The black boxes represent E-boxes. (b and c) Luciferase reporter assays were performed in 293 cells by transfecting equivalent amounts of the indicated human Crumbs3 promoter in pGL4.20 (firefly luciferase) and either pPGS-CMV-CITE-Neo-Snail-FLAG or empty vector. Co-transfection of pGL4.73 (SV40-Renilla luciferase) served as an internal control (Dual Luciferase Reporter kit; Promega, Madison, WI, USA). Point mutations CANNTG → AANNTA were created in the indicated E-boxes by Quikchange (Stratagene, La Jolla, CA, USA) and then assayed for luciferase reporter activity. (d) Chromatin immunoprecipitation (ChIP) assays were performed using polyclonal Snail H-130 antibodies (Santa Cruz) or control rabbit immunoglobulin G (IgG; Sigma). The purified DNA was amplified by quantitative reverse transcription–PCR to detect selected genomic regions, including the Crumbs3 proximal promoter, a distant region ~ 10 kb upstream of the Crumbs3 promoter (negative control) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (negative control). Asterisk (*) indicates Student’s t-test comparing Crb3 and Crb3 distant yields P<0.05. All results are the average of three experiments (error bars indicate s.e.m.).
We next tested the biological significance of Crumbs3 loss in epithelial cells experiencing EMT. First, we stably reexpressed exogenous myc-Crumbs3 in Snail MDCK cells (Supplementary Figures 3a and b) and found that it partially rescues the formation of cell–cell junctions in these cells (Figure 4a). The incomplete rescue of the junctions is presumably due to the ability of Snail to suppress multiple target genes. Conversely, we knocked down Crumbs3 in parental MDCK cells using shRNA and found that there are defects in junction formation (Supplementary Figures 4a and b). Interestingly, we found that high levels of Snail transcript do not always correlate with low level Crumbs3 message in a panel of breast cancer cell lines (Supplementary Figure 5). We speculate that there may be additional regulatory mechanisms that act dominantly on the Crumbs3 promoter.
Figure 4.
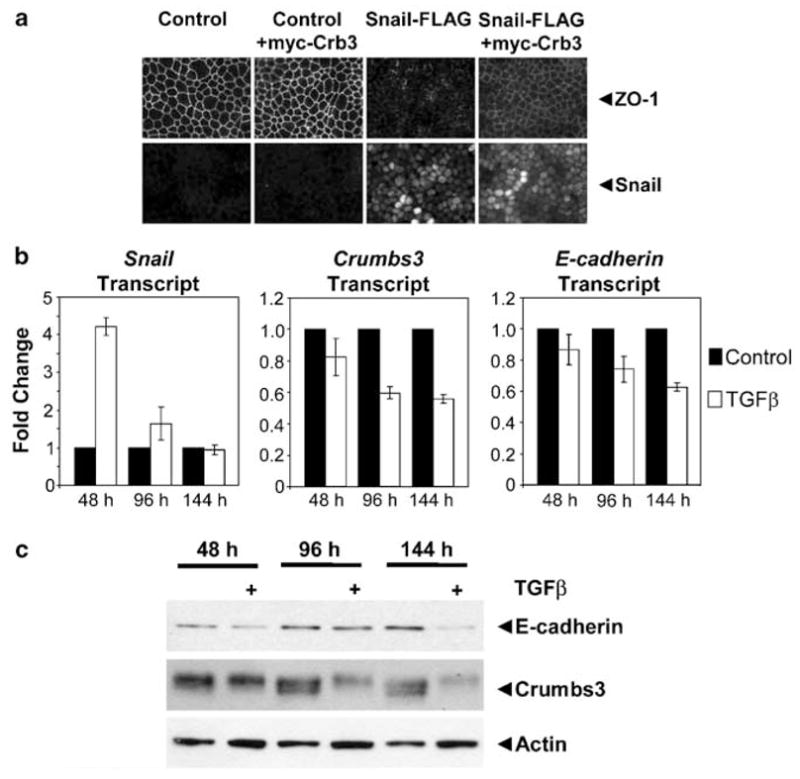
Crumbs3 may be an important physiologic target during EMT: Crumbs3 reexpression partially restores cell–cell junctions in Snail cells and Crumbs3 is downregulated in parental Madin–Darby Canine Kidney (MDCK) cells upon treatment with transforming growth factor-β (TGF-β). (a) Control and Snail-FLAG MDCK cells stably expressing empty vector or myc-Crumbs3 were grown on transwell filters and immunostained for ZO-1 or Snail. (b) MDCK cells were treated with 5 ng ml −1 TGF-β for the indicated time points, and cytosolic RNA was isolated and subjected to quantitative reverse transcription–PCR. Bar graph depicts the mean transcript levels from two experiments (error bars indicate standard deviation). Results were normalized to GAPDH. (c) MDCK cells were treated with TGF-β as above. Protein samples were subjected to immunoblot analysis for E-cadherin, Crumbs3 and actin (loading control). Additional antibodies used: polyclonal E-cadherin (BD Biosciences, San Jose, CA, USA).
Finally, we explored the physiologic relevance of Crumbs3 loss by examining the effects of transforming growth factor-β (TGF-β) on parental MDCK cells. TGF-β is a growth factor that is known to induce Snail expression and that is strongly associated with EMT in vitro and in vivo. We observed a marked, approximately fourfold upregulation of Snail transcript at earlier time points of TGF-β treatment (for example, 48 h) and a measurable, ~40% downregulation of Crumbs3 transcript that slightly overlaps Snail expression at later points (for example, 96–144 h; Figure 4b). In addition, we note a marked decrease in Crumbs3 protein levels upon TGF-β treatment for 96–144 h (Figure 4c). Similar reductions in E-cadherin transcript and protein levels were seen upon TGF-β addition.
In summary, our data demonstrate that Snail directly targets the Crumbs3 gene and its protein product, with resultant disruption of the apical polarity complexes. Historically, the altered epithelial morphology noted in Snail-induced EMT was attributed to the loss of junctional proteins, such as E-cadherin and the disruption of cellular adhesion. Here, we show that Snail also fundamentally alters cellular polarization by abolishing Crumbs complex and Par complex localization to the tight junction. In addition, the marked reduction in levels of Crumbs complex proteins and transcripts in response to Snail expression or exposure to TGF-β suggests that loss of this complex may be a pivotal event in cellular depolarization during EMT.
Our findings raise fundamental questions concerning how the apico-basal polarity complexes, and particularly Crumbs3, are regulated during EMT in normal development and disease. We speculate that while Snail may be an early mediator of Crumbs3 transcriptional downregulation, the sustained repression of Crumbs3 may be modulated by another transcription factor such as Slug (Snail2), E47, ZEB-1 or ZEB-2, which can also bind the consensus E-box sequence. Indeed, a recent study demonstrates that ZEB-1 can directly repress Crumbs3 transcription in MDA-MB-231 breast cancer cells (Aigner et al., 2007). Furthermore, evidence suggests that Slug, ZEB-1 and ZEB-2 may be induced downstream of Snail expression (reviewed by Peinado et al., 2007).
Finally, it will be important to define the biological implications of Crumbs3 loss during EMT. Absence of Crumbs3 may confer a simple loss of polarity that permits further phenotypic changes as EMT progresses. The downregulation of Crumbs3 and subsequent perturbation of tight junction function may expose lateral growth factor receptors to apical ligands leading to deregulated cell growth and proliferation. On the other hand, Crumbs3 depletion may alter cellular behaviors, such as motility, directional migration or invasion. Thus, further investigation into the role of Crumbs3 in normal development and tumor formation is warranted.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants DK058208 and DK069605 (to BM). ELW was supported by the Cancer Biology Training Program at the University of Michigan and an individual National Research Service Award (NIH Grants 5-T32-CA09676 and 5-F32-GM079906). This work utilized the Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center funded by NIH Grant 5-P60-DK20572. We thank Ron Koenig, Sanj Patel, Stephen Lentz, Toby Hurd, Shuling Fan, Jennifer Harder, Jay Pieczynski, Qian Wang and Kunyoo Shin for technical advice, reagents and critical discussions concerning this article.
References
- Aigner K, Dampier B, Descovich L, Mikula M, Sultan A, Schreiber M, et al. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, Del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Ozawa M. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J Cell Sci. 2004;117:1675–1685. doi: 10.1242/jcs.01004. [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Roh MH, Makarova O, Liu CJ, Shin K, Lee S, Laurinec S, et al. The Maguk protein, Pals1, functions as an adapter, linking mammalian homologues of Crumbs and discs lost. J Cell Biol. 2002;157:161–172. doi: 10.1083/jcb.200109010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Straight SW, Pieczynski JN, Whiteman EL, Liu CJ, Margolis B. Mammalian lin-7 stabilizes polarity protein complexes. J Biol Chem. 2006;281:37738–37747. doi: 10.1074/jbc.M607059200. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Whiteman EL, Chen JJ, Birnbaum MJ. Platelet-derived growth factor (PDGF) stimulates glucose transport in 3T3-L1 adipocytes overexpressing PDGF receptor by a pathway independent of insulin receptor substrates. Endocrinology. 2003;144:3811–3820. doi: 10.1210/en.2003-0480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


