Abstract
In the cortex, NMDA receptors (NMDARs) play a critical role in the control of synaptic plasticity processes. We have previously shown in rat visual cortex that the application of a high frequency of stimulation (HFS) protocol used to induce long-term potentiation (LTP) in layer 2/3 leads to a parallel potentiation of excitatory and inhibitory inputs received by cortical layer 5 pyramidal neurons without changing the excitation/inhibition (E/I) balance of the pyramidal neuron, indicating a homeostatic control of this parameter.
We show here that the blockade of NMDARs of the neuronal network prevents the potentiation of excitatory and inhibitory inputs and this result opens to question the role of the NMDAR isoform involved in the induction of LTP, actually being strongly debated. In P18-P23 rat cortical slices, the blockade of synaptic NR2B-containing NMDARs prevents the induction of the potentiation induced by the HFS protocol, whereas the blockade of NR2A-containing NMDARs reduced the potentiation itself. In P29-P32 cortical slices, the specific activation of NR2A-containing receptors fully ensures the potentiation of excitatory and inhibitory inputs. These results constitute the first report of a functional shift in subunit composition of NMDARs during the critical period (P12-P36) which explains the relative contribution of both NR2B- and NR2A-containing NMDARs in synaptic plasticity processes. These effects of HFS protocol are mediated by the activation of synaptic NMDARs but our results also indicate that the homeostatic control of the E/I balance is independent of NMDARs activation and is due to specialized recurrent interactions between excitatory and inhibitory networks.
Keywords: 2-Amino-5-phosphonovalerate, pharmacology, Animals, Cerebral Cortex, cytology, growth & development, physiology, Electric Stimulation, Electrophysiology, Neuronal Plasticity, physiology, Patch-Clamp Techniques, Pyramidal Cells, drug effects, physiology, Rats, Receptors, N-Methyl-D-Aspartate, drug effects, physiology, Synapses, physiology, Visual Cortex, cytology, growth & development, physiology
Introduction
N-methyl D-aspartate receptors (NMDARs) are among the most important receptors in the central nervous system. Largely expressed in the brain, they play a major role in glutamatergic transmission due to their unique functional properties, which include a high Ca2+ permeability, slow activation and deactivation kinetics, and a voltage-sensitive block by external Mg2+ (Jahr & Stevens, 1990; Lester et al., 1990). As a result of these properties, NMDARs play key roles in numerous physiological and pathological brain processes such as synaptic plasticity (Choi & Rothman, 1990), schizophrenia (Coyle et al., 2002; Coyle et al., 2003) or epilepsy (Dingledine et al., 1990).
The role of NMDARs in the two major forms of synaptic plasticity that contribute to phenomena of learning and memory, long-term potentiation (LTP) and long-term depression (LTD) has been largely determined. Modest elevations of intracellular calcium due to NMDARs activation are thought to trigger depression, whereas large increases of intracellular calcium trigger potentiation (Bear & Malenka, 1994; Yang et al., 1999).
NMDARs are heterotetramers that consist of the NMD A receptor 1 (NR1) subunit which binds glycine or D-serine and one or more of the NR2 subunits: NR2A-NR2D which bind glutamate (Forrest et al., 1994; Hollmann & Heinemann, 1994). While, NR3A (Ciabarra et al., 1995; Sucher et al., 1995) and NR3B (Chatterton et al., 2002; Matsuda et al., 2002) have also been recently identified, it is reported that NR2A and NR2B subunits dominate in the forebrain (Monyer et al., 1994).
The functional properties of the receptor strongly depend on the type of NR2 subunit incorporated (Cull-Candy et al., 2001; Cull-Candy & Leszkiewicz, 2004) which changes with age. In the cortex, the NR2B subunit is predominantly expressed early in development (Babb et al., 2005) whereas expression of the NR2A subunit increases later. According to the NR2 composition, NMDARs will have different roles in synaptic plasticity, and it has been proposed that potentiation and depression are differentially mediated by NR2A and NR2B, respectively (Liu et al., 2004a; Massey et al., 2004). This differential effect could be related to the specific localization of NMDARs subtypes; the NR1/NR2A complex, characterized by rapid offset kinetics, would be present at the synapse, while the NR1/NR2B complex, characterized by slow kinetics, would mainly lie in extrasynaptic sites (Rumbaugh & Vicini, 1999; Tovar & Westbrook, 1999). However, recent studies suggest that NR2A and NR2B each contribute equally to the induction of potentiation and depression (Toyoda et al., 2005; Weitlauf et al., 2005; Zhao et al., 2005).
In the rat visual cortex it has been shown that the NR1 subunit is co-expressed with both the NR2A and NR2B subunits (Hawkins et al., 1999; Tongiorgi et al., 2003; Babb et al., 2005). The role of NMDARs in the induction of potentiation in the visual cortex is well established (Bear & Kirkwood, 1993; Bliss & Collingridge, 1993) but which NR2 subunit(s) might be involved in this plasticity process remains controversial. We have previously shown that application of a high frequency stimulation (HFS) protocol in layer 2/3 does not change the excitatory/inhibitory (E/I) ratio due to parallel potentiation of excitatory and inhibitory inputs received by pyramidal neurons (Le Roux et al., 2006). Here, we investigate the role of NMDARs in the induction of the potentiation of excitatory and inhibitory inputs of layer 5 pyramidal neurons after application of an HFS protocol in layer 2/3 in rat visual cortex (between P18 and P23 or between P29 and P32). We find that for young rats (between P18 and P32) the potentiation of excitatory and inhibitory inputs by an HFS protocol mainly depends on the activation of NR2B-containing receptors, whereas for P29-P32 old rats this plasticity becomes dependent on NR2A-containing receptors. We show that the prevention of potentiating effects on excitation and inhibition by blockade of NMDARs did not change the E/I ratio. Our results bring direct evidence of a functional shift in subunit composition of NMDARs during the critical period (P12-P36) which would explain the relative contribution of both NR2B- and NR2A-containing NMDARs in synaptic plasticity processes.
Materials and Methods
Slice preparation
Parasagittal slices containing primary visual cortex were obtained from P18-P23 or P29-P32 day-old Wistar rats. Briefly, in accordance with the guidelines of the American Neuroscience Association, a rat was decapitated, its brain quickly removed and placed in chilled (5°C) artificial cerebrospinal extracellular solution. Slices of 250 μm thickness were made using a vibratome from the primary visual cortex and then incubated for at least 1 hr at 36°C in a solution containing (in mM): 126 NaCl, 26 NaHCO3, 10 Glucose, 2 CaCl2, 1.5 KCl, 1.5 MgSO4 and 1.25 KH2PO4 (pH 7.5, 310–330 mOsm). This solution was bubbled continuously with a mixture of 95 % O2 and 5 % CO2.
Electrophysiological recordings and cell identification
Current-clamp and voltage-clamp recordings were performed using an Axopatch 1D (Axon Instruments, USA); filtered by a low-pass Bessel filter with a cut-off frequency set at 2 kHz, and digitally sampled at 4 kHz. Layer 5 pyramidal neurons, identified from the shape of their soma and primary dendrites and from their current-induced excitability pattern, were studied using the patch-clamp technique in whole-cell configuration. Somatic whole-cell recordings were performed at room temperature using borosilicate glass pipettes (of 3–5 MΩ resistance in the bath) filled with a solution containing (in mM): 140 K-gluconate, 10 HEPES, 4 ATP, 2 MgCl2, 0.4 GTP, 0.5 EGTA (pH 7.3 adjusted with KOH, 270–290 mOsm). The membrane potential was corrected off-line by −10 mV to account for the junction potential. Estimation of the access resistance (Rs) is critical to quantitatively evaluate the relative change of input conductance in response to synaptic activation. After capacitance neutralisation, bridge balancing was done on-line in current-clamp conditions, which provided us with an initial estimate of Rs. This value was checked and revised as necessary off-line by fitting the voltage response to a hyperpolarizing current pulse with the sum of two exponentials. Under voltage-clamp conditions, the holding potential was corrected off-line using this Rs value. Only cells with a resting membrane potential more negative than −55 mV and recordings with an access resistance lower than 25 MΩ were kept for further analysis. The firing behavior of neurons was determined in response to depolarizing current pulses ranging from −100 to 200 pA under current-clamp conditions.
The stimulating electrodes were positioned in layer 2–3. Electrical stimulations (1–10 μA, 0.2 ms duration) were delivered in these layers using 1 MΩ impedance bipolar tungsten electrodes (TST33A10KT, WPI). The intensity of the stimulation was adjusted in current-clamp conditions to induce a subthreshold postsynaptic response due to coactivation of excitatory and inhibitory circuits. Under voltage-clamp conditions, the frequency of stimulation was 0.05 Hz and five to eight trials were repeated for a given holding potential.
A control recording was made after 15 min of patch-clamp equilibration at 0.05 Hz, and then drugs were continuously superfused during 20 min, a “drug recording” was made in the same conditions. We next applied an HFS (high frequency of stimulation) protocol in order to induce long term modifications of synaptic strength in the recruited circuits. The HFS protocol was elicited with theta burst stimulation known to induce long term potentiation (LTP) at the synaptic level. It consists of 3 trains of 13 bursts applied at a frequency of 5 Hz, each burst containing 4 pulses at 100 Hz (Abraham & Huggett, 1997). Recordings were made at 0.05 Hz after 15, 30, 45 and 60 min application of HFS protocol. A LFS (low frequency of stimulation) protocol was also used, it consists of a 1 Hz stimulation applied during 15 min.
Drugs
D-AP5, Ro-256981 were purchased from Sigma (St-Louis, Missouri), NVP-AAM077 was a gift from Dr. Y.P. Auberson (Novartis Institutes for BioMedical Research, Basel Switzerland) and CP 101,606 was a gift from Pfizer (Groton, Connecticut, USA). Drugs were dissolved in the perfusate for at least 15 min before recordings.
Synaptic response analysis
Data were analyzed off-line with specialized software (Acquisl™ and Elphy™: written by Gérard Sadoc, Biologic UNIC–CNRS, France). The method is based on the continuous measurement of conductance dynamics during stimulus-evoked synaptic responses, as primarily described in vivo on cat cortex (Borg-Graham et al., 1998; Monier et al., 2003) and recently validated on rat visual cortex (Le Roux et al., 2006). This method has received further validation on other experimental models (Shu et al., 2003; Wehr & Zador, 2003; Higley & Contreras, 2006; Le Roux et al., 2006). Evoked synaptic currents were measured and averaged at a given holding potential. In I-V curves for each delay (t), the value of the holding potential (Vh) was corrected (Vhc) from the ohmic drop due to the leakage current through Rs, by the equation: Vhc(t) = Vh(t) − I(t) × Rs. An average estimate of the input conductance waveform of the cell was calculated from the best linear fit (mean least square criterion) of the I-V curve for each delay (t) following the stimulation onset. Only cells showing a Pearson correlation coefficient for the I-V linear regression higher than 0.95 between −90 and −40 mV were considered in order to calculate the conductance change of the recorded pyramidal neuron from the slope of the linear regression.
The evoked synaptic conductance term (gT(t)) was derived from the input conductance by subtracting the resting conductance (grest) estimated 90 ms before the electrical stimulation. The apparent reversal potential of the synaptic current at the soma (Esyn(t)) was taken as the voltage abscissa of the intersection point between the I-V curve obtained at a given time (t) and that determined at rest. Assuming that the evoked somatic conductance change reflects the composite synaptic input reaching the soma, Esyn(t) characterizes the stimulation-locked dynamics of the balance between excitation and inhibition.
Decomposition of the synaptic conductance
To decompose the global evoked synaptic conductance (gT(t)) into excitatory and inhibitory components (gE(t) and gI(t)), we used the following simplifications: Isyn(t) = gE(t) (Esyn(t) − Eexc) + gI(t) (Esyn(t) − Einh) and gT(t) = gE(t) + gI(t) where Isyn(t) is the total synaptic current, Esyn(t) is the apparent reversal potential at the soma (see the previous paragraph), gE(t) and gI(t) are excitatory and inhibitory conductances respectively and Eexc and Einh are the reversal potentials for excitation and inhibition. Values of these reversal potentials were equal to 0 mV for excitation (Eexc) and to −80 mV for inhibition (Einh). The value of −80 mV used in the decomposition method is the reversal potential of GABAA (and not an intermediate value between GABAA and GABAB) because in the presence of QX314 (which blocks K+ efflux), no variation of the synaptic response was observed.
We showed that the I-V curve in the presence of excitatory synaptic transmission blockers (CNQX, D-AP5) is linear between −80 to +10 mV with a reversal potential equal to −80 mV (Le Roux et al., 2006; supplementary material). In the presence of bicuculline, which blocks the inhibitory inputs on the layer 5 pyramidal neuron, the I/V curve for excitation is linear between −80 to +10 mV with a reversal potential equal to 0 mV (data not shown) as already shown by other studies (Wehr & Zador, 2003; Higley & Contreras, 2006). Under our experimental conditions of stimulation of cortical layers leading to subthreshold postsynaptic responses, Esyn(t) which was extrapolated from I-V curves took any intermediate values between −80 mV and −40 mV (Le Roux et al., 2006; supplementary material) i.e. within the limits of our voltage excursion (−90 to −40 mV) corresponding to the linearity of I-V curves and between the respective values of Einh and Eexc in such a way that the mathematical conditions of the oversimplification used to calculate gI(t) and gE(t) were fulfilled.
Like all somatic recordings, our recordings cannot make rigorous estimates of synaptic events in the distal dendrites and the conductance estimates are ratios of the overall excitatory and inhibitory drive contained in the local stimulated network (Haider et al., 2006). However, our measurements give relative changes in conductance magnitude which reflect the cumulative contributions of excitation and inhibition arriving at proximal portions of the neuron. These relative conductance changes at the somatic level is precisely the result of synaptic dendritic integration that we wish to record at the somatic level because it determines the output signal from the neuron (Wehr & Zador, 2003; Higley & Contreras, 2006).
To quantify the synaptic conductance changes we calculate the integral (int) over a time window of 200 ms. The contribution of each component was expressed by the ratio of its integral value (intgE or intgI) to that of total conductance change (intgT).
Statistical analysis
Data are expressed as the mean ± the standard deviation of the mean (SD) of n cells. Paired samples for IntgT, IntgE, IntgI, %E or %I between the control condition (before HFS) and a given time after HFS (15, 30, 45 or 60 min) were analysed using the parametrical t-test. Data were considered statistically significant for p ≤ 0.05 (*), p ≤ 0.01 (**) and p ≤ 0.001 (***).
Results
Involvement of NMDARs in the potentiation of excitatory and inhibitory inputs on cortical layer 5 pyramidal neurons induced by HFS protocol application in layer 2/3
Figure 1A–C presents representative current recordings in a layer 5 voltage-clamped pyramidal neuron to electrical stimulation (0.05 Hz) in layer 2/3. Current responses show a strong inward current followed by an outward component (Fig. 1A). Decomposition of these current responses into distinct conductance changes showed that, as already reported (Le Roux et al., 2006), inhibition (gI) represents roughly 80 % (light grey trace) and excitation (gE) 20 % (dark grey trace) of the global conductance change (black trace, Fig. 1A1). Application of 50 μM D-AP5, a specific antagonist of NMD A receptors (Yoshimura et al., 2003; Huemmeke et al., 2004), for 20 min did not induce significant differences in the global, excitatory and inhibitory conductance changes (Fig. 1A2). These results clearly show that the excitatory and inhibitory components recorded in a layer 5 pyramidal neuron in response to a low frequency of stimulation (0.05 Hz) of layer 2/3 does not depend on activation of NMDA receptors but corresponds to the activation of AMPA and GABAA receptors mainly located on the apical dendrite of the layer 5 pyramidal neuron.
Figure 1. Effects of NMDA receptor blockade on the potentiation of layer 5 pyramidal neurons inputs.
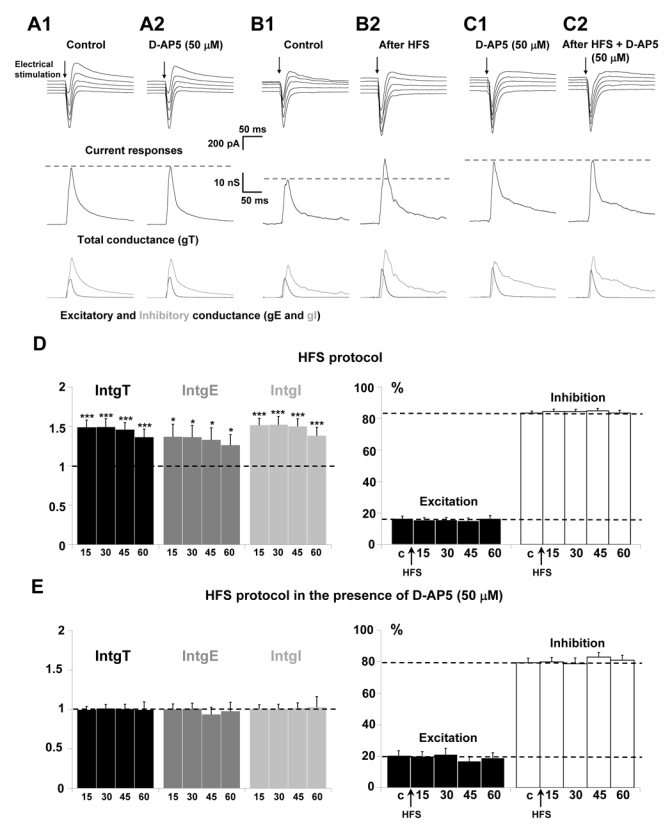
(A–C) Upper traces: current responses of a layer 5 pyramidal neuron to electrical stimulation (black arrow) applied in layer 2/3: (A1) under control conditions and (A2) 20 min after perfusion of 50 μM D-AP5; holding potentials scaled from − 75 (bottom trace) to − 55 mV (top trace, steps equal to 5 mV). (B1) Under control conditions and (B2) 15 min after HFS protocol; holding potentials scaled from − 70 (bottom trace) to − 50 mV (top trace, steps equal to 5 mV). (C1) 20 min after application of 50 μM D-AP5 and (C2) 15 min after HFS protocol in the presence of D-APS; holding potentials scaled from − 75 (bottom trace) to − 55 mV (top trace, steps equal to 5 mV). Medium traces: decomposition of the responses in total conductance change (gT). Lower traces: decomposition of gT in excitatory (gE, dark grey) and inhibitory (gI, light grey) conductance changes. Note that, for stimulation in layer 2/3 in the presence of D-AP5, no significant change of the response was observed. HFS protocol induced an increase of total, excitatory and inhibitory conductance changes which was prevented in the presence of D-AP5.
(D) Left part: relative changes (compared to control) of intgT (black), intgE (dark grey) and intgI (light grey), after application of HFS protocol in layer 2/3 under control conditions (n = 26) (*** p < 0.001, ** p < 0.01 and * p < 0.05, t-test). Right part: relative contribution of excitatory and inhibitory conductances to the total conductance change, after HFS (c: control before HFS protocol).
(E) Left part: relative changes (compared to control) of intgT (black), intgE (dark grey) and intgI (light grey), after HFS in layer 2/3 (n = 15) in the presence of 50 μM D-AP5. Right part: relative contribution of excitatory and inhibitory conductances to the total conductance change, after HFS in the presence of 50 μM D-AP5 (c: control before HFS protocol).
In the brain, information is encoded as action potential trains of higher frequency than that used in our protocol (0.05 Hz). We applied an HFS protocol with a higher frequency (see methods), usually used to induce LTP in numerous preparations. Application of this HFS protocol in layer 2/3 induced a long term potentiation of both excitatory and inhibitory inputs on layer 5 pyramidal neurons. Figure 1B shows representative current recordings in a voltage clamp pyramidal neuron, under control condition (Fig. 1B1) and 15 minutes after application of an HFS protocol (Fig. 1B2). HFS application elicits enhanced current responses and increases conductance changes. The statistical analysis of the global population (n = 24) is presented on figure 1D and shows that 15, 30, 45 and 60 min after the HFS protocol, the total, excitatory and inhibitory conductance changes are potentiated by about 45, 35 and 48 %, respectively. However, no significant change in excitation (intgE) and inhibition (intgI) conductance integrals expressed as percent of intgT was measured (Fig 1D, right part) in such a way that the E/I balance was unchanged.
It is worth noting that the detected potentiation is likely related to HFS protocol since in absence of such protocol, control experiments show no significant change of IntgT, IntgE or IntgI for one hour (n = 17, data not shown).
In order to examine the involvement of NMDARs activation in these potentiating effects of HFS on excitatory and inhibitory inputs, HFS protocol was applied in the presence of 50 μM D-AP5. No significant increase of current responses and conductance changes was measured (Fig. 1C1 and 1C2). No variation of the total conductance change and of inhibitory or excitatory conductance changes was observed through the statistical analysis of the total neuronal population (n = 15; Fig. 1E). Under this condition, the HFS protocol failed to induce potentiation of excitatory and inhibitory inputs. No significant changes in excitation (intgE) and inhibition (intgI) conductance integrals expressed as percent of intgT was measured (Fig. 1E) and the E/I ratio did not change. These results indicate that NMDARs are recruited by high frequency of stimulation applied in layer 2/3.
Involvement of NR2A and/or NR2B-containing receptors in the potentiation of excitatory and inhibitory inputs of cortical layer 5 pyramidal neurons induced by HFS protocol application in layer 2/3
The involvement of NR2A and/or NR2B-containing receptors in synaptic plasticity is presently strongly debated (Liu et al., 2004b; Massey et al., 2004; Berberich et al., 2005; Weitlauf et al., 2005). To further characterise whether NMDA currents in layer 2/3 pyramidal neurons were due to the activation of NR2A- or NR2B-containing receptors, we then used antagonists for each of these receptors.
Role of NR2B-containing receptors in P18-P23 old rats
Ro 25-6981 (Yang et al., 2006) or CP101,606 (Traxoprodil, (Chenard et al., 1995; Menniti et al., 1997) were used to specifically block NR2B-containing receptors. Representative recordings in the presence of 1 μM of Ro 25-6981 before and after application of HFS protocol showed that the HFS effect was blocked by the NR2B antagonist (Fig. 2A). The statistical analysis of the global population (n = 12; Fig. 2B) confirms that no significant variations of the global, excitatory and inhibitory conductance changes were observed after application of HFS protocol in layer 2/3 in the presence of Ro 25-6981. Consequently, the E/I ratio was unchanged (Fig. 2C). Identical results were obtained after application of HFS protocol in the presence of CP101,606 (n = 18; Fig. 2D, 2E, 2F). These results reproduced those observed in the presence of D-AP5 and provide evidence for an involvement of NR2B-containing receptors in the HFS-induced potentiation of both excitatory and inhibitory inputs on layer 5 pyramidal neurons.
Figure 2. Effects of the blockade of NR2B-containing receptors on the potentiation of layer 5 pyramidal neurons inputs for P18–P23 old rats.
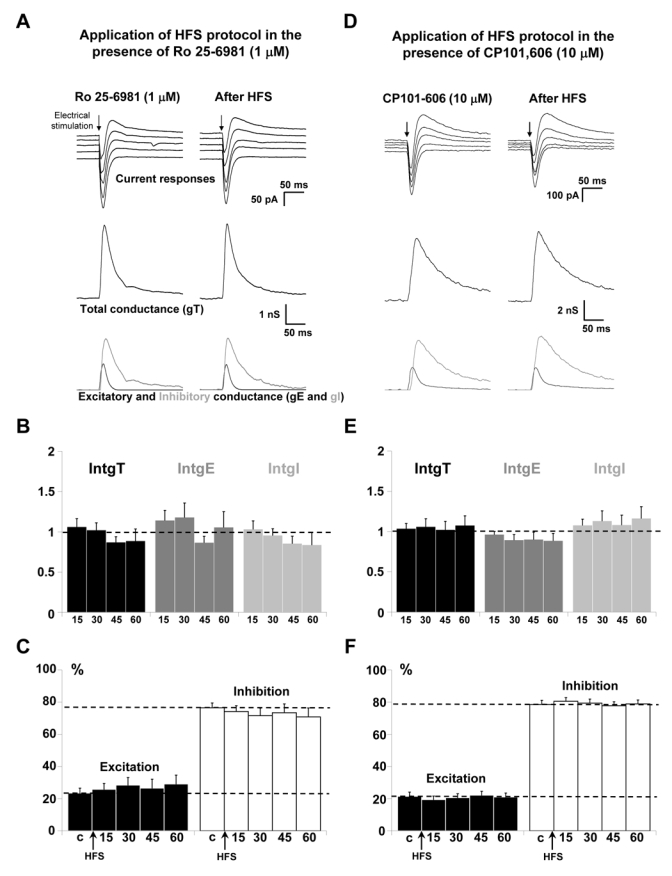
(A) The left column shows representative recordings before HFS application and the right column 60 min after application of HFS in layer 2/3 in the presence of 1 μM Ro 25-6981. Upper traces: current responses of a layer 5 pyramidal neuron to electrical stimulation. Imposed membrane potential ranged for −55 to −75 mV. The amplitude of current responses was unchanged after HFS application in the presence of Ro 25-6981. Medium traces: decomposition of the responses in total conductance change (gT). Lower traces: decomposition of gT in excitatory and inhibitory conductance changes (gE, dark grey and gI, light grey).
(B) Relative changes (compared to control) of intgT (black bars), intgE (dark grey bars) and intgI (light grey bars), after HFS in layer 2/3 (n = 12) in the presence of 1 μM Ro 25-6981.
(C) Relative contribution of excitation and inhibition conductance changes to the total conductance change, after HFS in the presence of 1 μM Ro 25-6981 (c:control before HFS protocol).
(D-E-F) Application of HFS protocol in the presence of 10 μM CP101,606 (n = 18). Legends are identical to those in A-B-C. No variations of total, excitatory and inhibitory conductance changes were observed after HFS (D-E) and the E/I ratio was unchanged (F).
Role of NR2A-containing receptors
NVP-AAM077 (Izumi et al., 2006) or Zn2+ (Paoletti & Neyton, 2006) were used to block NR2A-containing receptors. Representative recordings in the presence of 0.1 μM of NVP-AAM077, before and after application of HFS protocol showed an increase in the amplitude of current recordings (Fig. 3A, upper traces). Decomposition in conductance showed an increase of total, excitatory and inhibitory conductance changes (Fig. 3A, middle and lower traces). The statistical analysis of the global population (n = 13; Fig 3B) indicated that application of HFS protocol in the presence of NVP-AAM077 still induced a significant potentiation of the global and inhibitory conductance changes for 45 min. IntgT was enhanced by 26 % (p < 0.01), 21 % (p < 0.05) and 34 % (p < 0.05), 15, 30 and 45 min respectively after HFS protocol. IntgI was significantly enhanced in the same proportions but IntgE was not significantly increased (p > 0.05). In any case, no potentiating effect remains 60 min after HFS protocol. Consequently, the E/I ratio was decreased (p < 0.01, Fig. 3C) 15 min after HFS protocol but recovered its control value 30, 45 and 60 min after HFS protocol.
Figure 3. Effects of the blockade of NR2A-containing receptors on the potentiation of layer 5 pyramidal neurons inputs for P18-P23 old rats.
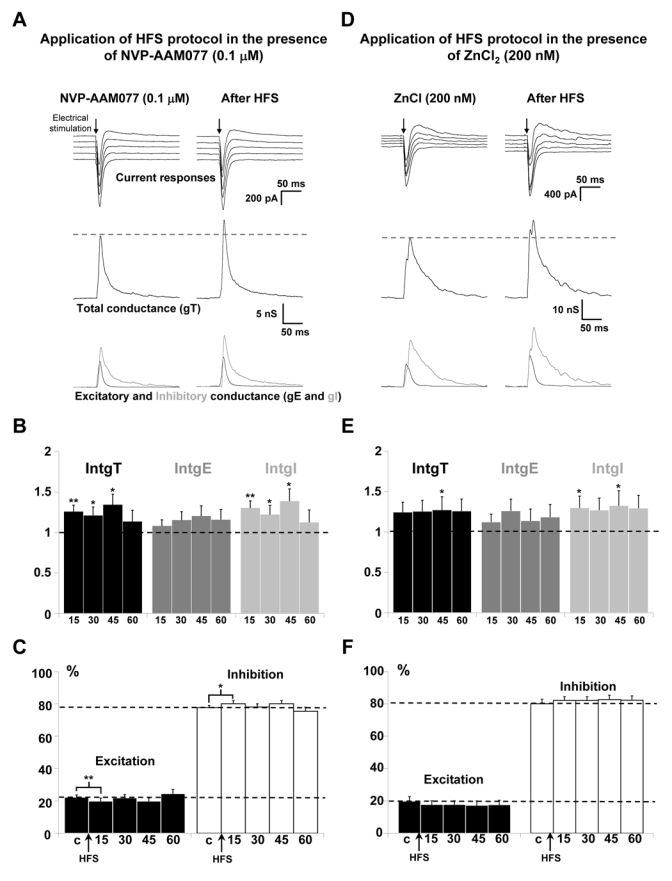
(A) The left column shows representative recordings before HFS application and the right column 60 min after application of HFS in layer 2/3 in the presence of 0.1 μM NVP-AAM077. Upper traces: current responses of a layer 5 pyramidal neuron to electrical stimulation. Imposed membrane potential ranged for −55 to −75 mV. The amplitudes of current responses were slightly increased after HFS application. Medium traces: decomposition of the responses in total conductance change (gT). Lower traces: decomposition of gT in excitatory and inhibitory conductance changes (gE, dark grey and gI, light grey). Total, excitatory and inhibitory conductance changes were all increased.
(B) Relative changes (compared to control) of intgT (black bars), intgE (dark grey bars) and intgI (light grey bars), after HFS in layer 2/3 (n = 13) in the presence of 0.1 μM NVP-AAM077. (*** p < 0.001, ** p < 0.01 and * p < 0.05, t-test).
(C) Relative contribution of excitation and inhibition conductance changes to the total conductance change, after HFS in the presence of 0.1 μM NVP-AAM077 (c:control before HFS protocol).
(D-E-F) Application of HFS protocol in the presence of 200 nM ZnCl2 (n = 13). Legends are identical to those in A-B-C. (D) Imposed membrane potential ranged for −60 to −80 mV. (E-F) Total, excitatory and inhibitory conductance changes were increased after HFS.
ZnCl2 was also used to block selectively NR2A-containing receptors because low concentrations of Zn2+ block NR2A receptors more than NR2B receptors (Amar et al., 2001; Kohr, 2006; Neyton & Paoletti, 2006). As observed for NVP-AAM077, similar results were obtained in the presence of 200 nM of ZnCl2, with excitatory and inhibitory conductance changes all increased (Fig. 3D). This result was confirmed by the statistical analysis of the global population (n = 13; Fig. 3E), which showed that HFS application in the presence of Zn2+ leads to a mean increase by 26 %, 18 % and 30 % for the total, excitatory and inhibitory conductance changes, respectively. In this case, a slight but not significant (p > 0.05) decrease of the E/I ratio was observed (Fig. 3F). In the presence of NR2A-containing receptors blockers, the potentiating effects of the HFS protocol on layer 5 pyramidal neurons still occurred but were reduced.
Involvement of extrasynaptic NR2B-containing receptors in potentiation of excitatory and inhibitory inputs on layer 5 pyramidal neurons
NR2B receptors are known to be present in both synaptic and extrasynaptic domains at glutamatergic synapses (Cull-Candy et al., 2001). To check the involvement of synaptic and/or extrasynaptic NR2B-containing receptors in HFS induced-potentiation of excitatory and inhibitory inputs on layer 5 pyramidal neurons, synaptic NMDARs were blocked according to the protocol described by Massey et al. (2004) using MK-801, an antagonist of NMDARs that acts only on open channels. MK-801 (10 μM) was bath-applied and a low frequency stimulation protocol (LFS) was delivered in order to activate synaptic NMDARs which can then be blocked whereas extrasynaptic receptors are not activated by this protocol of stimulation. MK-801 was then washed out for 30 min and HFS protocol of stimulation was applied in layer 2/3.
To start, a control condition (omitting MK-801) was used in order to check that application of LFS protocol does not prevent the potentiation induced by the HFS protocol. Significant potentiations were observed for IntgT (by 39 %, p < 0.05), for IntgI (by 42 %, p < 0.05) and for IntgE (by 22 %, p < 0.05) (Fig 4A for representative recordings and Fig. 4B for the statistical analysis of the global population, n = 14) and the E/I ratio slightly decreases (Fig. 4C). However, the increased percentage of intgE was lower than that obtained after a direct HFS protocol. This discrepancy might be due to the prolonged depressive effects (beyond 30 min) of LFS protocols (mainly on intgE) that we have shown (Le Roux et al., 2006). Nevertheless, it was possible to obtain a potentiating effect with an HFS protocol, and we could thus test the HFS protocol in the presence of MK801. In the presence of MK-801, no potentiation of excitatory and inhibitory inputs was obtained, but a slight decrease of IntgT, IntgE and IntgI was observed (Fig. 4D for representative recordings and Fig. 4E for statistical analysis of the global population, n = 13) and the E/I ratio remains stable (Fig. 4F), indicating that synaptic NMDARs can account for the potentiation of excitatory and inhibitory inputs of layer 5 pyramidal neurons.
Figure 4. Effect of synaptic NMDARs blockade on the potentiation of layer 5 pyramidal neurons inputs.
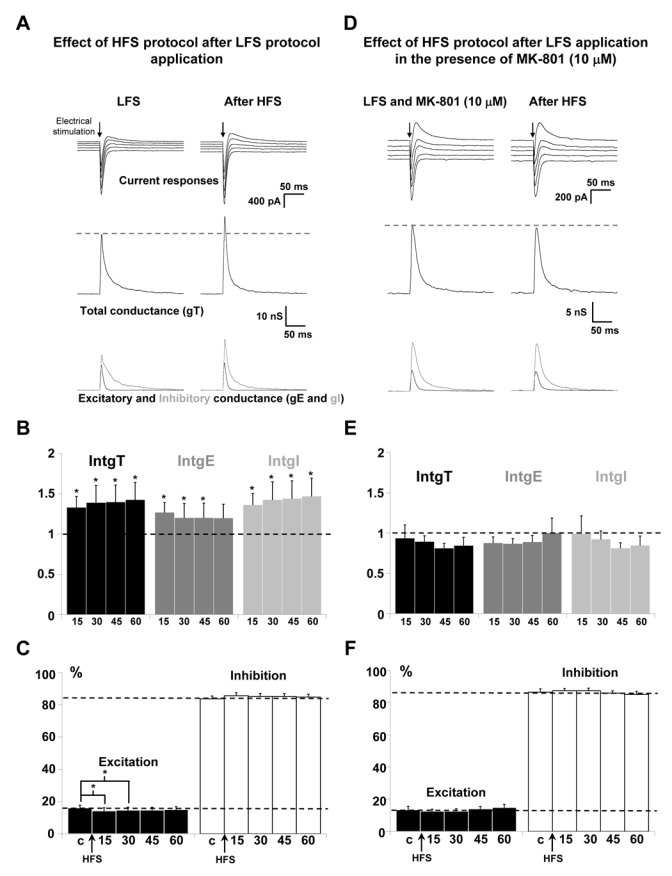
(A) Left column: representative recordings after application of LFS protocol in layer 2/3. Right column: the LFS protocol was followed by an HFS protocol and recordings were done 60 min after the HFS protocol. Upper traces: current responses of a layer 5 pyramidal neuron to electrical stimulation. Imposed membrane potential ranged for −60 to −80 mV. The amplitudes of current responses were increased after HFS application. Medium traces: decomposition of the responses in total conductance change (gT). Lower traces: decomposition of gT in excitatory and inhibitory conductance changes (gE, dark grey and gI, light grey). Total, excitatory and inhibitory conductance changes were all increased.
(B) Relative changes (compared to control) of intgT (black bars), intgE (dark grey bars) and intgI (light grey bars), after HFS in layer 2/3 (n = 14) after application of LFS protocol. (*** p < 0.001, ** p < 0.01 and * p < 0.05, t-test).
(C) Relative contribution of excitation and inhibition conductance changes to the total conductance change, after application of LFS protocol, without MK 801. (c:control before HFS protocol).
(D-E-F) Application of HFS after application of a LFS protocol in the presence of 10 μM MK-801 (n = 13). Legends are identical to those in A-B-C. No significant variations of total, excitatory and inhibitory conductance changes were observed after HFS.
Role of NR2A and/or NR2B-containing receptors in the potentiation of excitatory and inhibitory inputs in P29-P32 old rats
Some studies suggest that the activation of NR2A but not of NR2B induces long-term potentiation (Liu et al., 2004b; Massey et al., 2004) while other studies proposed that both types of NMDARs can contribute to the induction of LTP (Berberich et al., 2005; Weitlauf et al., 2005; Zhao et al., 2005). These discrepancies may be related to temporal regulation of NR2 subunits (Monyer et al., 1994; Liu et al., 2004b). The NR2A/NR2B ratio increases with maturation (Yoshimura et al., 2003; Liu et al., 2004c). Our results indicate that for rats between P18 and P23, synaptic NR2B-containing receptors are functional while NR2A-containing receptors may not yet be fully functional. To test this hypothesis, a new series of experiments was done on P29-P32 old rats.
Role of NR2B-containing receptors in P29-P32 rats
Representative recordings in the presence of 1 μM Ro 25-6981, before and after application of HFS protocol showed an increase of the amplitude of current recordings (Fig. 5A, upper traces). Decomposition in conductance changes showed an increase of total, excitatory and inhibitory conductance changes (Fig. 5A, middle and lower traces). The statistical analysis of the global population (n = 12; Fig 5B) indicated that application of HFS protocol in the presence of Ro 25-6981 still induced a significant potentiation of the global and inhibitory conductance changes for 45 min. IntgT was enhanced by 42%, intgE by 40% and intgI by 51% after HFS protocol application. Due to parallel potentiation of intgE and intgI, no significant change of the E/I ratio was observed (Fig. 5C).
Figure 5. Effects of the blockade of NR2B or NR2A-containing receptors on the potentiation of layer 5 pyramidal neurons inputs for P29-P32 old rats.
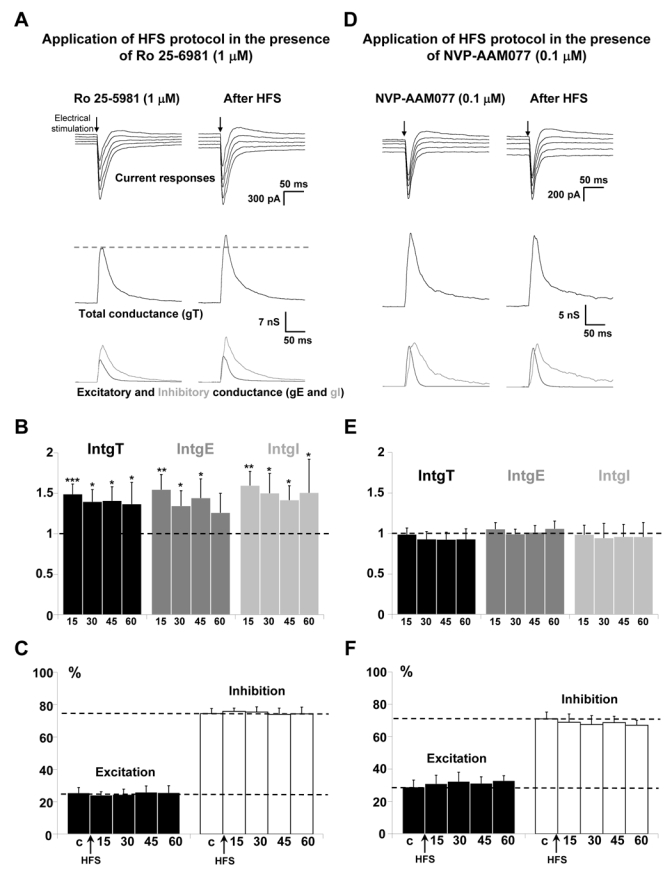
(A) The left column shows representative recordings from the statistical analysis of n = 12 experiments before HFS application and the right column 60 min after application of HFS in layer 2/3 in the presence of 1 μM Ro 25-6981. Upper traces: current responses of a layer 5 pyramidal neuron to electrical stimulation. Imposed membrane potential ranged for −55 to −75 mV. The amplitude of current responses was increased after HFS application. Medium traces: decomposition of the responses in total conductance change (gT). Lower traces: decomposition of gT in excitatory and inhibitory conductance changes (gE, dark grey and gI, light grey). Total, excitatory and inhibitory conductance changes were all increased.
(B) Relative changes (compared to control) of intgT (black bars), intgE (dark grey bars) and intgI (light grey bars), after HFS in layer 2/3 (n = 12) in the presence of 1 μM Ro 25-6981.
(C) Relative contribution of excitation and inhibition conductance changes to the total conductance change, after HFS in the presence of 1 μM Ro 25-6981 (c:control before HFS protocol).
(D-E-F) Application of HFS protocol in the presence of 0.1 μM NVP-AAM077 (n = 13). Legends are identical to those in A-B-C. No variations of total, excitatory and inhibitory conductance changes were observed after HFS (D-E) and the E/I ratio was unchanged (F).
Role of NR2A-containing receptors in P29-P32 rats
Representative recordings in the presence of 0.1 μM NVP-AAM077 before and after application of HFS protocol showed no significant variation (Fig. 5D). The statistical analysis of the global population (n = 13; Fig. 5E) confirms the block of the effects of the HFS protocol in the presence of the NR2A-containing receptors blocker for P29-P32 old rats. Consequently, the E/I ratio was unchanged (Fig. 5F).
Discussion
NMDAR activation is required for the potentiation of excitatory and inhibitory inputs on layer 5 pyramidal neurons after application of an HFS protocol in layer 2/3. Our results provide evidence for a major recruitment of synaptic NR2B-containing receptors in P18-P23 old rats, relative to the involvement of NR2A-receptors. As well as their possible differential role in physiological processes such as learning and memory (Collingridge et al., 2004), NR2A and NR2B-containing receptors are thought to be differentially involved in certain central disorders such as chronic pain or dementia (Kemp & McKernan, 2002; Chazot, 2004; Paoletti & Neyton, 2006). It therefore becomes important to determine the contribution and the function of distinct NMDAR subtypes. However, this physiological classification is highly dependent on the selectivity of antagonists that can be used. Ro 25-6981 (ifenprodil) and CP, 101–606 (traxoprodil) which bind the N-terminal lysine/isoleucine/valine-binding protein (LIVBP)-like domain of NR2B are now considered as selective antagonists of NR2B-containing receptors (Fischer et al., 1997; Berberich et al., 2005; Kohr, 2006). On the contrary, the specific blockade of NR2A-containing receptors is strongly debated (Feng & Macdonald, 2004; Paoletti & Neyton, 2006). First, Zn2+ binds the (LIVBP)-like domain of NR2A and displays a better selectivity for NR2A than for NR2B with a maximal selectivity of 70% (Amar et al., 2001; Kohr, 2006; Neyton & Paoletti, 2006). Second, NVP-AAM077 was originally described as a highly selective antagonist of NR2A-containing receptors (Auberson et al., 2002; Liu et al., 2004b; Massey et al., 2004) but later studies on rodents indicate that this compound lacks selectivity for NR2A vs NR2B receptors (Berberich et al., 2005; Weitlauf et al., 2005; Neyton & Paoletti, 2006). Other studies reported that NVP-AAM077 presents some selectivity at concentrations lower than 1 μM (Bartlett et al., 2006; Izumi et al., 2006) and concluded that it was not possible to find a concentration of NVP-AAM077 that fully block the NR2A receptors while sparing NR2B. According to the work by Bartlett et al. (2006), we used 0.1 μM NVP-AAM077 to check the specific blockade of NR2A receptors. Indeed, whatever the NR2A antagonist used, the potentiation of excitatory and inhibitory inputs of layer 5 pyramidal neurons occurred but was reduced by about 40 % in P18-P23 old rats. This result could be due to the blockade of NR2A, but also to an unspecific blockade of NR2B subunits.
Recent studies suggest that the activation of NR2A but not NR2B induces long-term potentiation (Liu et al., 2004b; Massey et al., 2004) although other studies proposed that both types of NMDARs can contribute to the induction of LTP (Berberich et al., 2005; Weitlauf et al., 2005; Zhao et al., 2005). These discrepancies could be related to the differential distribution of NMDARs subtypes. For example, an important role for extrasynaptic receptors exclusively containing the NR2B subunit (Cull-Candy et al., 2001) is reported in synaptic plasticity (Massey et al., 2004) or in neuronal cell death (Hardingham et al., 2002; Hardingham, 2006). However, both NR2A- or NR2B-NMDAR subtypes can be located either on synaptic or extrasynaptic sites (Thomas et al., 2006). Here, we show that predominantly synaptic NMDARs are implicated in the observed potentiating effects.
Another possibility to explain these discrepancies is to consider changes in the spatial distribution of NMDA receptors during the developmental stages. While NR1 subunit is ubiquitously expressed in the CNS, NR2 subunit expression is temporally regulated (Monyer et al., 1994; Liu et al., 2004b) with the NR2A/NR2B ratio increasing during maturation (Yoshimura et al., 2003; Liu et al., 2004c). In the rat visual cortex, morphological studies showed that NR1 and NR2B subunits are already expressed at birth and reach their highest level of expression during the second and third postnatal weeks (Kew et al., 1998; Tongiorgi et al., 2003; Babb et al., 2005). In contrast, NR2A subunits are poorly expressed at birth but progressively increase as development proceeds (Kew et al., 1998) and the insertion of NR2A receptors displace NR2B receptors at extrasynaptic sites (Liu et al., 2004c). Our results clearly show that the potentiation initially depends on NR2B-containing receptors in P18-P23 old rats, but becomes NR2A dependent for P29-P32 old rats. Thus in order to understand the particular role of NR2A or NR2B in the potentiation process, it is crucial to consider the temporal and spatial NMDARs expression. Hence, rather than a differential role for NR2A- or NR2B-containing receptors in the induction of LTP supposed by recent works (Berberich et al., 2005, Weitlauf et al., 2005, Zhao et al., 2005), we proposed that both types can be involved in the potentiation but their respective involvement depends on the developmental stage of the cortex.
We show that without under estimating the problem of selectivity of the NR2A or NR2B-containing receptors antagonists, it is necessary to consider the developmental stages of the animal. In addition, we cannot exclude the possibility that, for P18-P23 old rats, NR2A-containing receptors were present but not yet fully functional (not antagonist sensitive), whereas for P29-P32 old rats specific blockers were rather efficient.
The blockade of NMDA receptors prevents the potentiation of excitatory and inhibitory inputs on layer 5 pyramidal neurons to HFS in layer 2/3 resulting in an unchanged E/I ratio. These results suggest that the NMDARs activation is not required for the induction of the homeostatic control of the E/I balance but is necessary for the homeostatic plasticity expression. Only a few studies report a similar observation in which the blockade of NMDARs prevents the potentiation of inhibitory synapses (IPSCs recorded in cerebellar stellate cells (Liu & Lachamp, 2006)). It is obvious that we cannot localize facilitated synapses in the stimulated networks by HFS protocol and we observed that the stimulation of inhibitory interneurons is mainly disynaptic, involving a synapse between a glutamatergic terminal and the interneuron (Le Roux et al., 2006; Supplementary materials). Thus the potentiation of inhibitory inputs following HFS protocol in layer 2/3 is likely indirect, but in any case parallel increases of the strength of excitatory and inhibitory inputs on the layer 5 pyramidal neuron were observed resulting in a close control of the E/I ratio. Indeed, normal brain function requires a delicate balance between excitation and inhibition in neuronal networks, an imbalance of excitation and inhibition underlies various neurological diseases such as epilepsy (Cossart et al., 2005), Parkinson’s disease (Llinas et al., 1999) or schizophrenia (Wassef et al., 2003). A correlation between excitatory and inhibitory neuronal responses to sensory stimuli has been found in many cortical neurons in adult mammalian sensory cortices. For example, in cat primary visual cortex, excitation and inhibition exhibit similar tuning preference and tuning width in orientation-selective neurons (Anderson et al., 2000; Martinez et al., 2002; Monier et al., 2003; Hirsch et al., 2003; Marino et al., 2005). In addition, a proportionality of recurrent excitation and inhibition in cortical networks has been demonstrated to be associated with the persistent activity and is thought to underlie the working memory (Shu et al., 2003). So, neuronal networks adapt to environmental changes by learning and memory, in which the strength of excitatory or inhibitory synapses are changed. We show using a protocol of stimulation classically used in long-term plasticity, that the E/I ratio can remain unchanged while the strength of excitatory and inhibitory inputs altered. The present results provide evidence for the activation of the homeostatic control of the E/I balance previously reported by Le Roux et al. (2006). This control is independent of the activation of NMDARs but appears linked to recurrent interactions between excitatory and inhibitory circuits through layer 2/3 and layer 5 pyramidal neurons. This cortical organization can underlie the HFS-induced potentiation of some excitatory and inhibitory synapses (and the imprinting of some information) and may control the main output pathway of the cortex, layer 5 pyramidal neurons, through a homeostatic mechanism avoiding hyper- or hypo-excitability.
Acknowledgments
The authors are grateful to Dr. Y.P. Auberson (Novartis Institutes for BioMedical Research, Basel) for the kind gift of NVP-AAM077. We are grateful to Dr. D.W. Owens (Pfizer global research and development) for the kind gift of CP101,606. The authors thank Dr. S. O’Regan and Dr. R. Lewis for critical reading of the manuscript.
Abbreviations
- E
excitation
- GABA
gamma amino butyric acid
- HFS
high frequency of stimulation
- I
inhibition
- intgE
excitatory conductance integral
- intgI
inhibitory conductance integral
- intgT
total conductance integral
- LTP
long-term potentiation
- NMDAR
N-methyl D-aspartate receptor
References
- Abraham WC, Huggett A. Induction and reversal of long-term potentiation by repeated high-frequency stimulation in rat hippocampal slices. Hippocampus. 1997;7:137–145. doi: 10.1002/(SICI)1098-1063(1997)7:2<137::AID-HIPO3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Amar M, Perin-Dureau F, Neyton J. High-affinity Zn block in recombinant N-methyl-D-aspartate receptors with cysteine substitutions at the Q/R/N site. Biophys J. 2001;81:107–116. doi: 10.1016/S0006-3495(01)75684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J Neurophysiol. 2000;84:909–926. doi: 10.1152/jn.2000.84.2.909. [DOI] [PubMed] [Google Scholar]
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Babb TL, Mikuni N, Najm I, Wylie C, Olive M, Dollar C, MacLennan H. Pre- and postnatal expressions of NMDA receptors 1 and 2B subunit proteins in the normal rat cortex. Epilepsy Res. 2005;64:23–30. doi: 10.1016/j.eplepsyres.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2006 doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Bear MF, Kirkwood A. Neocortical long-term potentiation. Curr Opin Neurobiol. 1993;3:197–202. doi: 10.1016/0959-4388(93)90210-p. [DOI] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PM, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Borg-Graham LJ, Monier C, Fregnac Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature. 1998;393:369–373. doi: 10.1038/30735. [DOI] [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Chazot PL. The NMDA receptor NR2B subunit: a valid therapeutic target for multiple CNS pathologies. Curr Med Chem. 2004;11:389–396. doi: 10.2174/0929867043456061. [DOI] [PubMed] [Google Scholar]
- Chenard BL, Bordner J, Butler TW, Chambers LK, Collins MA, De Costa DL, Ducat MF, Dumont ML, Fox CB, Mena EE, et al. (1S,2S)-1-(4-hydroxyphenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-propanol: a potent new neuroprotectant which blocks N-methyl-D-aspartate responses. J Med Chem. 1995;38:3138–3145. doi: 10.1021/jm00016a017. [DOI] [PubMed] [Google Scholar]
- Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Ciabarra AM, Sullivan JM, Gahn LG, Pecht G, Heinemann S, Sevarino KA. Cloning and characterization of chi-1: a developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J Neurosci. 1995;15:6498–6508. doi: 10.1523/JNEUROSCI.15-10-06498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Cossart R, Bernard C, Ben-Ari Y. Multiple facets of GABAergic neurons and synapses: multiple fates of GABA signalling in epilepsies. Trends Neurosci. 2005;28:108–115. doi: 10.1016/j.tins.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann N Y Acad Sci. 2003;1003:318–327. doi: 10.1196/annals.1300.020. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G, Goff DC. Ionotropic glutamate receptors as therapeutic targets in schizophrenia. Curr Drug Targets CNS Neurol Disord. 2002;1:183–189. doi: 10.2174/1568007024606212. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004:re 16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Dingledine R, McBain CJ, McNamara JO. Excitatory amino acid receptors in epilepsy. Trends Pharmacol Sci. 1990;11:334–338. doi: 10.1016/0165-6147(90)90238-4. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Macdonald RL. Proton modulation of alpha 1 beta 3 delta GABAA receptor channel gating and desensitization. J Neurophysiol. 2004;92:1577–1585. doi: 10.1152/jn.00285.2004. [DOI] [PubMed] [Google Scholar]
- Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, Heitz MP, Kemp JA. Ro 25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther. 1997;283:1285–1292. [PubMed] [Google Scholar]
- Forrest D, Yuzaki M, Soares HD, Ng L, Luk DC, Sheng M, Stewart CL, Morgan JI, Connor JA, Curran T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE. 2B synaptic or extrasynaptic determines signalling from the NMDA receptor. J Physiol. 2006 doi: 10.1113/jphysiol.2006.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hawkins LM, Chazot PL, Stephenson FA. Biochemical evidence for the co-association of three N-methyl-D-aspartate (NMDA) R2 subunits in recombinant NMDA receptors. J Biol Chem. 1999;274:27211–27218. doi: 10.1074/jbc.274.38.27211. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Contreras D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J Neurosci. 2006;26:448–457. doi: 10.1523/JNEUROSCI.3506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Martinez LM, Pillai C, Alonso JM, Wang Q, Sommer FT. Functionally distinct inhibitory neurons at the first stage of visual cortical processing. Nat Neurosci. 2003;6:1300–1308. doi: 10.1038/nn1152. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Huemmeke M, Eysel UT, Mittmann T. Lesion-induced enhancement of LTP in rat visual cortex is mediated by NMDA receptors containing the NR2B subunit. J Physiol. 2004;559:875–882. doi: 10.1113/jphysiol.2004.069534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Auberson YP, Zorumski CF. Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. J Neurosci. 2006;26:7181–7188. doi: 10.1523/JNEUROSCI.1258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J Neurosci. 1990;10:3178–3182. doi: 10.1523/JNEUROSCI.10-09-03178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JA, McKernan RM. NMDA receptor pathways as drug targets. Nat Neurosci. 2002;5(Suppl):1039–1042. doi: 10.1038/nn936. [DOI] [PubMed] [Google Scholar]
- Kew JN, Richards JG, Mutel V, Kemp JA. Developmental changes in NMDA receptor glycine affinity and ifenprodil sensitivity reveal three distinct populations of NMDA receptors in individual rat cortical neurons. J Neurosci. 1998;18:1935–1943. doi: 10.1523/JNEUROSCI.18-06-01935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G. NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res. 2006;326:439–446. doi: 10.1007/s00441-006-0273-6. [DOI] [PubMed] [Google Scholar]
- Le Roux N, Amar M, Baux G, Fossier P. Homeostatic control of the excitation-inhibition balance in cortical layer 5 pyramidal neurons. Eur J Neurosci. 2006;24:3507–3518. doi: 10.1111/j.1460-9568.2006.05203.x. [DOI] [PubMed] [Google Scholar]
- Lester RA, Clements JD, Westbrook GL, Jahr CE. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990;346:565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Liu HN, Kurotani T, Ren M, Yamada K, Yoshimura Y, Komatsu Y. Presynaptic activity and Ca2+ entry are required for the maintenance of NMDA receptor-independent LTP at visual cortical excitatory synapses. J Neurophysiol. 2004a;92:1077–1087. doi: 10.1152/jn.00602.2003. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004b;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci. 2004c;24:8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Lachamp P. The activation of excitatory glutamate receptors evokes a long-lasting increase in the release of GABA from cerebellar stellate cells. J Neurosci. 2006;26:9332–9339. doi: 10.1523/JNEUROSCI.2929-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino J, Schummers J, Lyon DC, Schwabe L, Beck O, Wiesing P, Obermayer K, Sur M. Invariant computations in local cortical networks with balanced excitation and inhibition. Nat Neurosci. 2005;8:194–201. doi: 10.1038/nn1391. [DOI] [PubMed] [Google Scholar]
- Martinez LM, Alonso JM, Reid RC, Hirsch JA. Laminar processing of stimulus orientation in cat visual cortex. J Physiol. 2002;540:321–333. doi: 10.1113/jphysiol.2001.012776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Kamiya Y, Matsuda S, Yuzaki M. Cloning and characterization of a novel NMDA receptor subunit NR3B: a dominant subunit that reduces calcium permeability. Brain Res Mol Brain Res. 2002;100:43–52. doi: 10.1016/s0169-328x(02)00173-0. [DOI] [PubMed] [Google Scholar]
- Menniti F, Chenard B, Collins M, Ducat M, Shalaby I, White F. CP-101,606, a potent neuroprotectant selective for forebrain neurons. Eur J Pharmacol. 1997;331:117–126. doi: 10.1016/s0014-2999(97)10092-9. [DOI] [PubMed] [Google Scholar]
- Monier C, Chavane F, Baudot P, Graham LJ, Fregnac Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron. 2003;37:663–680. doi: 10.1016/s0896-6273(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PM. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nagy J, Horvath C, Farkas S, Kolok S, Szombathelyi Z. NR2B subunit selective NMDA antagonists inhibit neurotoxic effect of alcohol-withdrawal in primary cultures of rat cortical neurones. Neurochem Int. 2004;44:17–23. doi: 10.1016/s0197-0186(03)00100-1. [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2006 doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Vicini S. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J Neurosci. 1999;19:10603–10610. doi: 10.1523/JNEUROSCI.19-24-10603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA. Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J Neurosci. 1995;15:6509–6520. doi: 10.1523/JNEUROSCI.15-10-06509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Tongiorgi E, Ferrero F, Cattaneo A, Domenici L. Dark-rearing decreases NR2A N-methyl-D-aspartate receptor subunit in all visual cortical layers. Neuroscience. 2003;119:1013–1022. doi: 10.1016/s0306-4522(03)00196-9. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H, Zhao MG, Zhuo M. Roles of NMDA receptor NR2A and NR2B subtypes for long-term depression in the anterior cingulate cortex. Eur J Neurosci. 2005;22:485–494. doi: 10.1111/j.1460-9568.2005.04236.x. [DOI] [PubMed] [Google Scholar]
- Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol. 2003;23:601–640. doi: 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Woodhall GL, Jones RS. Tonic facilitation of glutamate release by presynaptic NR2B-containing NMDA receptors is increased in the entorhinal cortex of chronically epileptic rats. J Neurosci. 2006;26:406–410. doi: 10.1523/JNEUROSCI.4413-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN, Tang YG, Zucker RS. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J Neurophysiol. 1999;81:781–787. doi: 10.1152/jn.1999.81.2.781. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Ohmura T, Komatsu Y. Two forms of synaptic plasticity with distinct dependence on age, experience, and NMDA receptor subtype in rat visual cortex. J Neurosci. 2003;23:6557–6566. doi: 10.1523/JNEUROSCI.23-16-06557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]


