Abstract
Serotonin (5-HT) receptors are classified into seven groups (5-HT1–7), comprising at least 14 structurally and pharmacologically distinct receptor subtypes. Pharma-cological antagonism of ionotropic 5-HT3 receptors has been shown to modulate both behavioral and neuro-chemical aspects of the induction of sensitization to cocaine. It is not known, however, if specific molecular subunits of the 5-HT3 receptor influence the development of cocaine sensitization. To address this question, we studied the effects of acute and chronic intermittent cocaine administration in mice with a targeted deletion of the gene for the 5-HT3A-receptor subunit (5-HT3A −/−). 5-HT3A (−/−) mice showed blunted induction of cocaine-induced locomotor sensitization as compared with wild-type littermate controls. 5-HT3A (−/−) mice did not differ from wild-type littermate controls on measures of basal motor activity or response to acute cocaine treatment. Enhanced locomotor response to saline injection following cocaine sensitization was observed equally in 5-HT3A (−/−) and wild-type mice suggesting similar conditioned effects associated with chronic cocaine treatment. These data show a role for the 5-HT3A-receptor subunit in the induction of behavioral sensitization to cocaine and suggest that the 5-HT3A molecular subunit modulates neurobehavioral adaptations to cocaine, which may underlie aspects of addiction.
Keywords: 5-HT3, 5-HT3A-receptor subunit, cocaine, conditioning, environment, locomotion, sensitization
Behavioral sensitization to psychostimulants refers to a progressive augmentation of responding associated following repeated treatment with amphetamine, cocaine or related drugs (Robinson et al. 1998). The process of sensitization is thought to produce enduring adaptive changes in brain and behavioral function that may underlie components of drug addiction (Kalivas et al. 1998; Robinson & Berridge 2000). Thus, behavioral sensitization is in vivo model in which progressive alterations in drug-induced behavior can be linked to changes in specific neurobiological systems (Carlezon & Nestler 2002).
In general, psychostimulant sensitization is mediated by a set of integrated brain structures termed the ‘motive circuit’ (Pierce & Kalivas 1997). This limbic circuitry, which includes the mesocorticolimbic pathways, is believed to gate and modulate appropriate behavioral responses to environmental stimuli. Although cocaine sensitization has been linked to a number of neurotransmitter systems within this pathway such as dopamine, γ-aminobutyric acid (GABA) and glutamate [recently reviewed by (Steketee 2005)], the precise mechanisms of drug sensitization remain to be fully characterized.
Evidence suggests that serotonin systems may play a role in behavioral sensitization to cocaine (Cunningham et al. 1992). Cocaine-induced motor activity was enhanced by pretreatment with the serotonin uptake inhibitor fluoxetine (Herges & Taylor 1998). Other studies have shown that 5-HT3 receptor antagonists reduce cocaine-induced locomotion in the absence of effects on basal locomotion (Costall et al. 1990; King et al. 1997, 1998; Reith 1990; Svingos & Hitzemann 1992), and to attenuate hyperlocomotion induced by direct infusion of dopamine into the nucleus accumbens (NAcb) (Costall et al. 1987). Of particular interest to the present study, reports have indicated that 5-HT3 antagonists block the development, or induction, of behavioral sensitization to cocaine (King et al. 1997) but have no effect on expression of sensitization tested after a period of withdrawal (Szumlinski et al. 2003). Accordingly, 5-HT3 immunoreactivity is down-regulated in the shell of the NAcb during the induction of cocaine sensitization but not 2 weeks after withdrawal of cocaine (Ricci et al. 2004). These data suggest that 5-HT3 receptors may mediate the induction but not expression of cocaine sensitization.
The 5-HT3 receptor is a ligand-gated ion channel comprising a pentameric cylindrical-shaped structure surrounding a centrally gated ion channel (Barnes & Sharp 1999; Fletcher & Barnes 1998; Yan et al. 1999). 5-HT3 receptors are known to be expressed in limbic brain regions, with dense binding found in frontal cortical layers II–III, amygdala, hippocampus and moderately dense binding found in the NAcb and striatum (Kilpatrick et al. 1996; Morales et al. 1998). To date, two channel subunits are known, the 5-HT3A subunit (Belelli et al. 1995; Maricq et al. 1991), shown to be present in the hippocampus, amygdala, cortex and NAcb (Tecott et al. 1993); and the 5-HT3B subunit (Davies et al. 1999), known to be co-expressed with the 5-HT3A subunit in the amygdala, caudate and hippocampus in humans. Heteromeric assemblies of both subunits display physiological properties closely resembling those of neuronal 5-HT3 channels (Davies et al. 1999). In rats, however, 5-HT3B subunit transcripts are restricted to peripheral neurons (Morales & Wang 2002), which suggest that rodent neural 5-HT3 receptors might be 5-HT3A homomeric receptors or heteromeric receptors containing 5-HT3A subunits combined with subunits other than the 5-HT3B subunit. Indeed, comparison of electrophysiological properties of rodent neural 5-HT3 receptors has shown the presence of receptors with both high and low conductances (Hussy et al. 1994; Jones & Surprenant 1994; Yang et al. 1992), which suggests the presence of at least two different 5-HT3 receptor channels (Fletcher & Barnes 1998).
The role that specific molecular subunits of 5-HT3 receptors play in the temporal development of cocaine sensitization has not been studied. The following experiments were designed to evaluate the effects of total absence of the 5-HT3A-receptor subunit on induction of locomotor sensitization to cocaine.
Materials and methods
Mice
5-HT3A receptor (−/−) mice were derived using homologous recombination as described (Zeitz et al. 2002). F1 hybrid C57BL/6J × 129 heterozygous progeny were backcrossed with C57BL/6J mice to produce F9 generation congenics. Heterozygotes from the F9 generation were bred to generate wild-type and 5-HT3A (−/−) mutants used in the present study. Only male mice were used in the present study. Genotyping was conducted in Dr David Julius’ laboratory at the University of California, San Francisco as previously described (Hodge et al. 2004; Kelley et al. 2003; Zeitz et al. 2002). The experimenter conducting behavioral studies was blind to genotype. 5-HT3A (−/−) and wild-type C57BL/6 littermate control male mice (age, 15–20 weeks; weight, 28–35 g) were used as test subjects for all behavioral studies. All animals were drug naïve prior to testing, and separate batches of mice were used for each experimental set. The mice were housed in plastic cages lined with Cell Sorb bedding and provided with food (Harlan, Indianapolis, IN, USA) and water ad libitum. The vivarium was maintained on a 12 h light/dark cycle (lights on at 06:00) at a temperature of 22°C. All procedures were carried out in accordance with the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines.
Locomotor apparatus
Locomotor activity was measured in Plexiglass chambers (43 cm2) located in sound-attenuating cubicles equipped with exhaust fans that masked external noise (Med Associates, Lafayette, IN, USA). Two sets of 16 pulse-modulated infrared photo beams were placed on opposite walls at 1-inch centers to record X–Y ambulatory movements. Activity chambers were computer-interfaced (Med Associates) for data sampling at 100-millisecond resolution. Horizontal distance traveled (cm) was recorded for all sessions.
Locomotor ability and effects of acute cocaine
5-HT3A (−/−) mice (n = 13) and wild-type controls (N = 10) were handled and weighed daily for 1 week prior to activity testing. Mice were transported from the adjacent vivarium to the test room and allowed to acclimatize for a period of 30 min prior to all testing sessions. Spontaneous locomotor activity and habituation to a novel environment was tested during 1-h sessions on three consecutive days. Subsequently, the effects of acute cocaine [0, 10.0 and 20.0 mg/kg intraperitoneal (i.p.)] were tested in all mice once per week. Cocaine doses were administered immediately prior to 15-min locomotor test sessions according to a Latin square randomized dosing design. Sessions were set at 15 min to limit exposure to the test chambers because other work has shown that 5-HT3 receptors regulate contextual conditioning (Harrell & Allan 2003), which may influence sensitization (Robinson et al. 1998).
Locomotor effects of chronic intermittent cocaine
The effects of chronic intermittent cocaine were studied in wild-type (n = 7) and 5-HT3A (−/−) mice (n = 9) mice. All mice initially received four consecutive days of saline treatment and exposure to the test chambers for 1-h locomotor trials before exposure to cocaine. Cocaine (10.0 mg/kg i.p.) was administered every other day (e.g. at 48-h intervals) for a total of six injections and locomotor trials were run immediately following dosing. All cocaine injections were administered immediately prior to the start of each locomotor session, in the familiar context of the testing room in close proximity to the locomotor chambers.
Locomotor responses to saline injections pre- and post-cocaine sensitization
Locomotor response to saline injection was tested 2 days following the final cocaine challenge trial in the wild-type (n = 7) and 5-HT3A (−/−) mice (n = 9) that received repeated intermittent exposure to cocaine. The data were compared with the fourth saline control injection that preceded sensitization testing.
Drugs
Cocaine hydrochloride (Sigma-Aldrich, St Louis, MO, USA) was dissolved in 0.9% saline and administered intraperitoneally in a volume of 0.01 ml/g body weight. Drug solutions were prepared immediately prior to each administration.
Data presentation and analysis
Data are presented from locomotor trials that tested: (1) spontaneous locomotor behavior and habituation in a novel environment, (2) acute cocaine treatment, (3) repeated intermittent (every other day) injections of cocaine to induce sensitization and (4) response to saline injection pre- and post-development of cocaine sensitization to characterize conditioning effects of drug-environment pairings. Horizontal distance traveled (cm) was recorded during 60-min trials for baseline locomotor testing and 15-min trials after drug administration. Data were analyzed by two-way repeated measures ANOVA with cocaine (dose or treatment day) and genotype as factors. Planned post hoc comparisons were conducted using the Tukey test, P < 0.05. All statistical analyses were conducted with SigmaStat (Systat Software, San Jose, CA, USA).
Results
Locomotor ability and effects of acute cocaine
Mice lacking the 5-HT3A-receptor subunit showed normal spontaneous locomotor activity and habituation to the open field over 3 days of testing as compared with wild-type littermate controls (Fig. 1a). A trend toward reduced habituation by the 5-HT3A (−/−) mice was observed during day 1 of testing but did not result in statistically significant differences between genotypes. Acute treatment with cocaine (10–20 mg/kg i.p.) after habituation produced a dose-dependent increase in locomotor activity in all mice (F2,68 = 38.423, P < 0.001) (Fig. 1b). Wild-type and 5-HT3A (−/−) mice displayed similar levels of locomotor response to acute cocaine treatment. No effects of genotype (F1,68 = 0.0647, P > 0.05) or genotype × dose interaction (F2,68 = 0.321, P > 0.05) were found, showing absence of a differential genotypic sensitivity to the locomotor-stimulating effects of acute cocaine treatment.
Figure 1. Deletion of the 5-HT3A receptor subunit does not affect motor ability or acute locomotor response to cocaine.
(a) Spontaneous locomotor activity and habituation to a novel open field environment measured over three consecutive days. (b) Locomotor response to acute cocaine (0–20.0 mg/kg i.p.) measured during 15-min sessions. Data are plotted as mean horizontal distance traveled (cm) (±SEM), for wild-type (filled circles, n = 10), and 5-HT3A (−/−) mice (open circles, n = 13).
*Significantly different from saline control; †significantly different from cocaine (10.0 mg/kg), Tukey test (P < 0.05).
Locomotor effects of repeated intermittent cocaine
Figure 2 shows that repeated intermittent treatment with cocaine (10 mg/kg i.p.) resulted in significantly heightened locomotion over successive days in both wild-type and 5-HT3A (−/−) mice (F9,126 = 48.8, P < 0.001). A significant genotype × day interaction was observed (F9,126 = 2.9, P = 0.004) indicating that genotypic differences in locomotor activity depended on treatment day. Follow-up multiple comparison procedures (Tukey Test) showed that 5-HT3A (−/−) mice did not differ in response to saline or the first cocaine treatment but showed diminished cocaine-induced locomotor response to repeated cocaine exposure (Fig. 2; cocaine trial 2, 4, 5 and 6).
Figure 2. Targeted deletion of the 5-HT3A receptor subunit blunted the development of cocaine locomotor sensitization in response to chronic intermittent administration of cocaine (10 mg/kg i.p.).
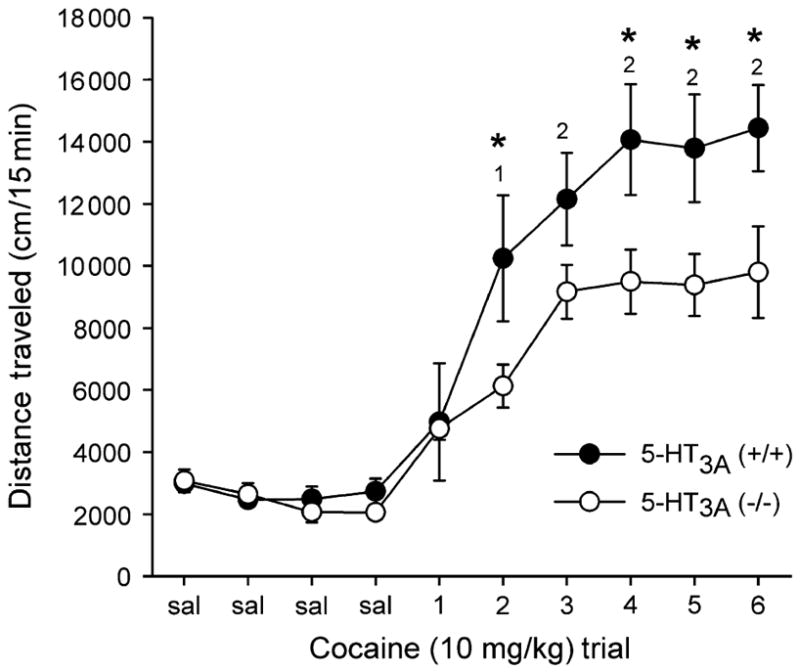
Data are presented as mean distance traveled (cm) (±SEM), for 15-min locomotor trials in wild-type (filled circles, n = 7), and 5-HT3A (−/−) mice (open circles, n = 9) across four initial saline trials (S1–S4) and six intermittent cocaine exposures.
*Significant difference among genotypes at corresponding x-axis point. 1, wild type significantly different from acute cocaine (trial 1); 2, both genotypes significantly different from acute cocaine, Tukey test (P < 0.05).
Effects of drug-associated conditioned cues
Figure 3 shows the locomotor response to saline injection during baseline conditions before cocaine exposure and 2 days following cocaine sensitization for both wild-type and 5-HT3A (−/−) mice. Basal activity in response to saline injection did not significantly differ between the groups either when measured pre- or post-cocaine sensitization [group (F1,31 = 2.709, P > 0.05)]. The locomotor response to saline was significantly heightened when measured following the final period of cocaine sensitization [day (F1,31 = 26.133, P < 0.001)] as compared with the pre-cocaine baseline. This may suggest an anticipatory or conditioned effect to chronic cocaine exposure, produced by repeated pairings of cocaine with the specific environmental cues associated with the location in which the drug was administered. No group × day interaction was observed (F1,31 = 0.513, P > 0.05), indicating that both wild-type and 5-HT3A (−/−) mice displayed similar degrees of hyperlocomotion in response to saline when tested post-cocaine sensitization.
Figure 3. No genotypic difference when mice were exposed to the drug-paired environment.

The locomotor response to saline injection (0.01 ml/g) at baseline, prior to cocaine exposure (left panel) and 2 days post-cocaine sensitization (right panel) in wild-type (filled bars, n = 7) and 5-HT3A (−/−) mice (open bars, n = 9). Data are presented as mean distance traveled (cm) (±SEM), for 15-min locomotor trials. Two-way ANOVA showed an effect of treatment (P < 0.05) but not genotype, with both genotypes showing significantly heightened activity post-cocaine.* Significantly different from pre-cocaine within genotype, Tukey test (P < 0.05).
Discussion
Behavioral sensitization to psychostimulant drugs is characterized by a progressive increase in locomotor behavior following repeated intermittent exposure. This drug effect has been studied extensively in animals as a model of behavioral and molecular adaptations that underlie aspects of drug addiction (Carlezon & Nestler 2002; Robinson & Berridge 1993, 2000; Thomas et al. 2001; Wolf 1998). Sensitization is thought to consist of components of induction and expression, which are believed to be temporally distinct and regulated by different neurochemical and neuroanatomical systems (Pierce & Kalivas 1997; Vanderschuren & Kalivas 2000). Induction of sensitization refers to the adaptive changes that take place as a result of first drug exposures. These initial cellular events precede the enduring changes thought to underlie the expression of sensitization, which occurs after a period of drug withdrawal.
Results of the present study add to growing evidence that implicates serotonin receptor systems in the process of behavioral sensitization (Cunningham et al. 1992; Parsons & Justice 1993). In particular, 5-HT3 receptor antagonists have been reported to attenuate locomotor stimulatory effects of both systemically administered cocaine (Costall et al. 1990; Reith 1990; Svingos & Hitzemann 1992) and direct infusion of dopamine into the NAcb (Costall et al. 1987). Other evidence indicates that coadministration of the 5-HT3 antagonist on-dansetron with cocaine blocks the induction of cocaine sensitization (King et al. 1997). Similarly, 5-HT3 immunoreactivity is downregulated in the shell of the NAcb during the induction of cocaine sensitization (Ricci et al. 2004), suggesting that initial adaptations to cocaine may involve activation of 5-HT3 receptors. In the present study, targeted deletion of the 5-HT3A-receptor subunit gene resulted in diminished locomotor response to repeated intermittent cocaine exposure in C57BL/6J mice. This effect appeared to be specifically related to blunted induction of cocaine sensitization in the 5-HT3A (−/−) mice because these mutants displayed similar basal activity and locomotor response to acute cocaine treatment when compared with wild-type mice. These results suggest that full induction of cocaine sensitization requires the 5-HT3A- receptor subunit.
It will be of interest in future studies to examine involvement of 5-HT3A receptors in the expression of cocaine sensitization to determine if this system also regulates the progressive adaptations in brain and behavioral function after cocaine exposure. Evidence suggests that 5-HT3-receptor antagonists can alter expression of cocaine sensitization but the effect may depend on when the antagonist compounds are administered in relation to cocaine treatment. For example, injections of the 5-HT3 antagonist, ondansetron on the first 5 days of withdrawal from intermittent cocaine significantly blocked the expression of sensitization when tested after 7 days of withdrawal in rats (King et al. 1998). Alternatively, in another study, the 5-HT3 antagonists MDL 72222, Y-25130 or ondansetron were administered before each repeated cocaine treatment (Szumlinski et al. 2003). Consistent with the results of our study in 5-HT3A (−/−) mice, 5-HT3 antagonists inhibited the progressive locomotor effects of cocaine during treatment. However, there was no effect of the antagonist treatment when expression of cocaine sensitization was tested 3 weeks later (Szumlinski et al. 2003). Together these studies suggest that 5-HT3-mediated neuro-adaptations that occur during withdrawal are important for expression of behavioral sensitization to cocaine. Whether 5-HT3A receptors mediate the withdrawal-associated changes remains to be determined.
In general, induction of behavioral sensitization to cocaine is thought to be mediated by cellular events in the ventral tegmental area (VTA), NAcb, and prefrontal cortex [reviewed by (Vanderschuren & Kalivas 2000)]. However, as the 5-HT3A-receptor subunit has been found to predominate in the hippocampus, amygdala, cortex and NAcb (Tecott et al. 1993), loss of ligand-gated ion channel function in any or all of these limbic structures may have impacted development of cocaine sensitization in the 5-HT3A (−/−) mice. Further research is needed to determine if 5-HT3A receptors regulate the induction cocaine sensitization in a brain region-dependent manner.
One possible mechanism of action to explain the blunted development of cocaine sensitization following loss of the 5-HT3A-receptor subtype is that 5-HT3 receptors may modulate midbrain dopamine systems. In vivo microdialysis studies have confirmed that 5-HT3 receptors regulate extracellular dopamine levels within the NAcb (Imperato & Angelucci 1989), and that agonists at the 5-HT3 receptor increase (Chen et al. 1991), while antagonists inhibit accumbal dopamine release (Carboni et al. 1989). Chronic cocaine treatment increases both dopamine and serotonin in the NAcb via interactions with and blockade of their transporters (Ritz et al. 1990), increasing the likelihood that these transmitters interact to modulate behavioral sensitization. Electrophysio-logical studies have shown that increased synaptic 5-HT inhibits firing of neurons in the NAcb (Henry et al. 1989). Also, treatment with LY-278584, a drug which displays high affinity and selectivity for rat and human brain 5-HT3 receptors (Abi-Dargham et al. 1993; Wong et al. 1989), decreases the activity of dopaminergic neurons in the VTA (Minabe et al. 1991). Thus, interaction with dopaminergic systems is one potential mechanism of 5-HT3 receptor modulation of the induction of cocaine sensitization.
Another potential mechanism by which deletion of the 5-HT3A receptor may have blunted induction of cocaine sensitization is through modulation of glutamate transmission. A variety of studies implicate ionotropic N-methyl-D-aspartate (NMDA) receptors in the induction of cocaine sensitization (Druhan & Wilent 1999; Ida et al. 1995; Karler et al. 1989; Li et al. 1999; Pudiak & Bozarth 1993). Recent evidence indicates that repeated cocaine administration results in a down-regulation of NMDA NR1 and NR2B receptor subunits in striatum, NAcb and cortical brain regions (Yamaguchi et al. 2002), which is consistent with cocaine-induced increases in extracellular glutamate in brain regions that regulate sensitization (Kalivas & Duffy 1998; Smith et al. 1995; Williams & Steketee 2004). Other evidence indicates that 5-HT3-receptor activation increases neural glutamate release (Ashworth-Preece et al. 1995; Funahashi et al. 2004). Thus, it is plausible that absence of 5-HT3–mediated glutamate release may have resulted in blunted cocaine-induced sensitization in the 5-HT3A (−/−) mice in this study. It would be of interest to determine if blockade of 5-HT3-receptor function, or 5-HT3A gene deletion, can prevent cocaine-induced increases in glutamate as seen in behavioral sensitization.
Another factor to consider when interpreting changes in behavioral sensitization is the role of conditioning that occurs when repeated drug effects are tested in a specific environment. Many studies have found evidence to suggest that environmental factors associated with drug exposure can influence behavioral sensitization (Browman et al. 1998; Partridge & Schenk 1999; Robinson et al. 1998). Specifically, groups of animals in which drug treatments are paired with the test environment express sensitization at a higher level than groups of animals that receive drug in an environment, which is novel and unassociated with the test conditions (Robinson et al. 1998). This issue is important to consider in the present study because 5-HT3 over-expressing mice exhibit enhanced contextual conditioning (Harrell & Allan 2003), which suggests that 5-HT3A (−/−) mice may have shown blunted sensitization because of impaired conditioning. This did not appear to be the case, however, because the 5-HT3A (−/−) mice showed normal habituation to a novel environment and similar levels of drug-conditioned effects when tested with saline injection post-cocaine sensitization. Moreover, response to acute cocaine appeared to be equally attenuated in both genotypes after repeated saline injections (compare the acute response to cocaine in Fig. 1 to that seen in Fig. 2), which is consistent with evidence showing that locomotor response to cocaine is attenuated as a function of increasing exposure to the test environment after repeated saline injections (Todtenkopf & Carlezon 2006). These observations support the hypothesis that 5-HT3A receptor–related neuroadaptations and not psychological factors, such as conditioning or stress responding to the drug or environment, formed the basis for the blunted development of cocaine sensitization in the 5-HT3A (−/−) mouse. This is of particular relevance as our group has previously reported differences in anxiety-related behaviors in this mouse (Kelley et al. 2003).
Results of this study suggest that the 5-HT3A-receptor subunit is required for the full induction of cocaine sensitization. This conclusion is based on the observation that mice carrying a null mutation for the 5-HT3A-receptor gene were less responsive to the progressive increase in locomotor activity seen following repeated intermittent cocaine treatment. Although targeted gene deletion has been used to create numerous null mutant mice that have been used to identify a variety of gene-behavior relations (Heisler et al. 1998; Hodge et al. 1999; Jones et al. 1998; Kelley et al. 2003; Saarelainen et al. 2003), use of the traditional gene knockout strategy raises several interpretive concerns including the potential role of developmental compensation and influence of mouse genetic background (Gerlai 2001). For example, null mice that lack the α1 subunit of ionotropic GABAA receptors show developmental compensation in other molecular subunits of the receptor including decreased expression of β2/3 and γ2 subunits and increased expression of α2 and α3 subunits (Kralic et al. 2002). At present, there is no evidence available regarding potential compensatory changes in other 5-HT3 molecular subunits in 5-HT3A (−/−) mice. However, it appears that deletion of the 5-HT3A subunit prevents the formation of functional 5-HT3 receptors because the 5-HT3 agonist m-Chlorophenylbiguanide (mCPBG) has no effect on the firing rate of peripheral nociceptors or cultured dorsal root ganglion neurons obtained from the 5-HT3A (−/−) mice (Zeitz et al. 2002). Whether compensatory changes or genetic background contributed to the blunted sensitization seen in the 5-HT3A (−/−) mice in this study remains to be determined; however, converging evidence from this study and reports cited above (i.e. King et al. 1997; Ricci et al. 2004) are consistent with the conclusion that 5-HT3 receptors regulate neurobehavioral adaptations to repeated cocaine.
In summary, 5-HT3A (−/−) mice showed blunted cocaine-induced locomotor sensitization in the absence of differential motor ability, acute response to cocaine or response to the environment paired with cocaine. These data suggest that the 5-HT3A-receptor subunit modulates adaptive changes in behavior that occur following repeated cocaine exposure. The precise mechanism(s) underlying blunted development of cocaine sensitization found in the 5-HT3A (−/−) mice requires further study. However, it is apparent that alterations in neural functioning and not conditioning effects related to repeated cocaine exposure underlie these differences. The current data support a role for the 5-HT3A-receptor subunit in the induction of cocaine sensitization and suggest that agents with selectivity for the 5-HT3A-receptor subunit may hold therapeutic potential in the treatment of psychostimulant abuse.
Acknowledgments
This work was supported by NIH grants AA011605 and AA014983 and by funds provided by the State of California for medical research on alcohol and substance abuse through the University of California at San Francisco to C.W.H. We are grateful to David Julius, PhD for initial contribution of the mice, and Kimberly Iller for technical assistance with behavioral studies.
References
- Abi-Dargham A, Laruelle M, Wong DT, Robertson DW, Weinberger DR, Kleinman JE. Pharmacological and regional characterization of [3H] LY278584 binding sites In human brain. J Neurochem. 1993;60:730–737. doi: 10.1111/j.1471-4159.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Ashworth-Preece MA, Jarrott B, Lawrence AJ. 5-Hydroxytryptamine3 receptor modulation of excitatory amino acid release in the rat nucleus tractus solitarius. Neurosci Lett. 1995;191:75–78. doi: 10.1016/0304-3940(95)11564-5. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Belelli D, Balcarek JM, Hope AG, Peters JA, Lambert JJ, Blackburn TP. Cloning and functional expression of a human 5-hydroxytryptamine type 3AS receptor subunit. Mol Pharmacol. 1995;48:1054–1062. [PubMed] [Google Scholar]
- Browman KE, Badiani A, Robinson TE. The influence of environment on the induction of sensitization to the psychomotor activating effects of intravenous cocaine in rats is dose-dependent. Psychopharmacol. 1998;137:90–98. doi: 10.1007/s002130050597. [DOI] [PubMed] [Google Scholar]
- Carboni E, Acquas E, Frau R, Di Chiara G. Differential inhibitory effects of a 5-HT3 antagonist on drug-induced stimulation of dopamine release. Eur J Pharmacol. 1989;164:515–519. doi: 10.1016/0014-2999(89)90259-8. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Nestler EJ. Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends Neurosci. 2002;25:610–615. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- Chen JP, van Praag HM, Gardner EJ. Activation of 5-HT3 receptor by 1-phenylbiguanide increases dopamine release in the rat nucleus accumbens. Brain Res. 1991;543:354–357. doi: 10.1016/0006-8993(91)90050-6. [DOI] [PubMed] [Google Scholar]
- Costall B, Domeney AM, Naylor RJ, Tyers MB. Effects of the 5-HT3 receptor antagonist, GR38032F, on raised dopaminergic activity in the mesolimbic system of the rat and marmoset brain. Br J Pharmacol. 1987;92:881–894. doi: 10.1111/j.1476-5381.1987.tb11394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Naylor RJ, Tyers MB. The psychopharmacology of 5-HT3 receptors. Pharmacol Ther. 1990;47:181–202. doi: 10.1016/0163-7258(90)90086-h. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Paris JM, Goeders NE. Serotonin neurotransmission in cocaine sensitization. Ann N Y Acad Sci. 1992;654:117–127. doi: 10.1111/j.1749-6632.1992.tb25960.x. [DOI] [PubMed] [Google Scholar]
- Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature. 1999;397:359–363. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- Druhan JP, Wilent WB. Effects of the competitive N-methyl-D-aspartate receptor antagonist, CPP, on the development and expression of conditioned hyperactivity and sensitization induced by cocaine. Behav Brain Res. 1999;102:195–210. doi: 10.1016/s0166-4328(99)00017-0. [DOI] [PubMed] [Google Scholar]
- Fletcher S, Barnes NM. Desperately seeking subunits: are native 5-HT3 receptors really homomeric complexes? Trends Pharmacol Sci. 1998;19:212–215. doi: 10.1016/s0165-6147(98)01210-3. [DOI] [PubMed] [Google Scholar]
- Funahashi M, Mitoh Y, Matsuo R. Activation of presynaptic 5-HT3 receptors facilitates glutamatergic synaptic inputs to area postrema neurons in rat brain slices. Methods Find Exp Clin Pharmacol. 2004;26:615–622. doi: 10.1358/mf.2004.26.8.863726. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Gene targeting: technical confounds and potential solutions in behavioral brain research. Behav Brain Res. 2001;125:13–21. doi: 10.1016/s0166-4328(01)00282-0. [DOI] [PubMed] [Google Scholar]
- Harrell AV, Allan AM. Improvements in hippocampal-dependent learning and decremental attention in 5-HT(3) receptor overexpressing mice. Learn Mem. 2003;10:410–419. doi: 10.1101/lm.56103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DJ, Greene MA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. J Pharmacol Exp Ther. 1989;251:833–839. [PubMed] [Google Scholar]
- Herges S, Taylor DA. Involvement of serotonin in the modulation of cocaine-induced locomotor activity in the rat. Pharacol Biochem Behav. 1998;59:595–611. doi: 10.1016/s0091-3057(97)00473-5. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Kelley SP, Bratt AM, Iller K, Schroeder JP, Besheer J. 5-HT(3A) receptor subunit is required for 5-HT3 antagonist-induced reductions in alcohol drinking. Neuropsychopharmacology. 2004;29:1807–1813. doi: 10.1038/sj.npp.1300498. [DOI] [PubMed] [Google Scholar]
- Hussy N, Lukas W, Jones KA. Functional properties of a cloned 5-hydroxytryptamine ionotropic receptor subunit: comparison with native mouse receptors. J Physiol. 1994;481:311–323. doi: 10.1113/jphysiol.1994.sp020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida I, Asami T, Kuribara H. Inhibition of cocaine sensitization by MK-801, a noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist: evaluation by ambulatory activity in mice. Jpn J Pharmacol. 1995;69:83–90. doi: 10.1254/jjp.69.83. [DOI] [PubMed] [Google Scholar]
- lmperato A, Angelucci L. 5-HT3 receptors control dopamine release in the nucleus accumbens of freely moving rats. Neurosci Lett. 1989;101:214–217. doi: 10.1016/0304-3940(89)90533-8. [DOI] [PubMed] [Google Scholar]
- Jones KA, Surprenant A. Single channel properties of the 5-HT3 subtype of serotonin receptor in primary cultures of rodent hippocampus. Neurosci Lett. 1994;174:133–136. doi: 10.1016/0304-3940(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem. 1998;70:1497–1502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Pierce RC, Cornish J, Sorg BA. A role for sensitization in craving and relapse in cocaine addiction. J Psychopharmacol. 1998;12:49–53. doi: 10.1177/026988119801200107. [DOI] [PubMed] [Google Scholar]
- Karler R, Calder LD, Chaudhry IA, Turkanis SA. Blockade of ‘‘reverse tolerance’’ to cocaine and amphetamine by MK-801. Life Sci. 1989;45:599–606. doi: 10.1016/0024-3205(89)90045-3. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Bratt AM, Hodge CW. Targeted gene deletion of the 5-HT3A receptor subunit produces an anxiolytic phenotype in mice. Eur J Pharmacol. 2003;461:19–25. doi: 10.1016/s0014-2999(02)02960-6. [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, Hagan RM, Gale JD. 5-HT3 and 5-HT4 receptors in terminal regions of the mesolimbic system. Behav Brain Res. 1996;73:11–13. doi: 10.1016/0166-4328(96)00063-0. [DOI] [PubMed] [Google Scholar]
- King GR, Xiong Z, Ellinwood EH., Jr Blockade of cocaine sensitization and tolerance by the co-administration of ondansetron, a 5-HT3 receptor antagonist, and cocaine. Psychopharmacology. 1997;130:159–165. doi: 10.1007/s002130050224. [DOI] [PubMed] [Google Scholar]
- King GR, Xiong Z, Ellinwood EH., Jr Blockade of the expression of sensitization and tolerance by ondansetron, a 5-HT3 receptor antagonist, administered during withdrawal from intermittent and continuous cocaine. Psychopharmacology. 1998;135:263–269. doi: 10.1007/s002130050508. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Korpi ER, O’Buckley TK, Homanics GE, Morrow AL. Molecular and pharmacological characterization of GABA(A) receptor alpha1 subunit knockout mice. J Pharmacol Exp Ther. 2002;302:1037–1045. doi: 10.1124/jpet.102.036665. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu XT, Berney TG, Vartanian AJ, Stine CD, Wolf ME, White FJ. Both glutamate receptor antagonists and prefrontal cortex lesions prevent induction of cocaine sensitization and associated neuroadaptations. Synapse. 1999;34:169–180. doi: 10.1002/(SICI)1098-2396(19991201)34:3<169::AID-SYN1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotoningated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Minabe Y, Ashby CR, Jr, Wang RY. The effect of acute and chronic LY277359, a selective 5-HT3 receptor antagonist, on number of spontaneously active midbrain dopamine neurons. Eur J Pharmacol. 1991;209:151–156. doi: 10.1016/0014-2999(91)90163-k. [DOI] [PubMed] [Google Scholar]
- Morales M, Wang SD. Differential composition of 5-hydroxytryptamine3 receptors synthesized in the rat CNS and peripheral nervous system. J Neurosci. 2002;22:6732–6741. doi: 10.1523/JNEUROSCI.22-15-06732.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Battenberg E, Bloom FE. Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol. 1998;402:385–401. [PubMed] [Google Scholar]
- Partridge B, Schenk S. Context-independent sensitization to the locomotor-activating effects of cocaine. Pharmacol Biochem Behav. 1999;63:543–548. doi: 10.1016/s0091-3057(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pudiak CM, Bozarth MA. L-NAME and MK-801 attenuate sensitization to the locomotor-stimulating effect of cocaine. Life Sci. 1993;53:1517–1524. doi: 10.1016/0024-3205(93)90559-l. [DOI] [PubMed] [Google Scholar]
- Reith ME. 5-HT3 receptor antagonists attenuate cocaine-induced locomotion in mice. Eur J Pharmacol. 1990;186:327–330. doi: 10.1016/0014-2999(90)90454-e. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Stellar JR, Todtenkopf MS. Subregion-specific down-regulation of 5-HT3 immunoreactivity in the nucleus accumbens shell during the induction of cocaine sensitization. Pharmacol Biochem Behav. 2004;77:415–422. doi: 10.1016/j.pbb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Cone EJ, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castren E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Mo Q, Guo H, Kunko PM, Robinson SE. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res. 1995;683:264–269. doi: 10.1016/0006-8993(95)00383-2. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Cortical mechanisms of cocaine sensitization. Crit Rev Neurobiol. 2005;17:69–86. doi: 10.1615/critrevneurobiol.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Hitzemann R. 5-HT3 receptor antagonists block cocaine-induced locomotion via a PCPA-sensitive mechanism. Pharmacol Biochem Behav. 1992;43:871–879. doi: 10.1016/0091-3057(92)90420-k. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Frys KA, Kalivas PW. Pretreatment with serotonin 5-HT(3) receptor antagonists produces no observable blockade of long-term motor sensitization to cocaine in rats. Psychopharmacology (Berl) 2003;165:329–336. doi: 10.1007/s00213-002-1274-0. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci U S A. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Carlezon WA., Jr Contribution of drug doses and conditioning periods to psychomotor stimulant sensitization. Psychopharmacology (Berl) 2006;185:451–458. doi: 10.1007/s00213-005-0259-1. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Williams JM, Steketee JD. Cocaine increases medial prefrontal cortical glutamate overflow in cocaine-sensitized rats: a time course study. Eur J Neurosci. 2004;20:1639–1646. doi: 10.1111/j.1460-9568.2004.03618.x. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wong DT, Robertson DW, Reid LR. Specific [3H] LY278584 binding to 5-HT3 recognition sites in rat cerebral cortex. Eur J Pharmacol. 1989;166:107–110. doi: 10.1016/0014-2999(89)90689-4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Abe S, Hori T, Kurita H, Asada T, Okado N, Arai H. Repeated cocaine administration differentially affects NMDA receptor subunit (NR1, NR2A-C) mRNAs in rat brain. Synapse. 2002;46:157–169. doi: 10.1002/syn.10132. [DOI] [PubMed] [Google Scholar]
- Yan D, Schulte MK, Bloom KE, White MM. Structural features of the ligand-binding domain of the serotonin 5HT3 receptor. J Biol Chem. 1999;274:5537–5541. doi: 10.1074/jbc.274.9.5537. [DOI] [PubMed] [Google Scholar]
- Yang J, Mathie A, Hille B. 5-HT3 receptor channels in dissociated rat superior cervical ganglion neurons. J Physiol. 1992;448:237–256. doi: 10.1113/jphysiol.1992.sp019039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]



