Abstract
Background
Emerging evidence implicates metabotropic glutamate receptor (mGluR) function in the neurobiological effects of ethanol. The recent development of subtype specific mGluR antagonists has made it possible to examine the roles of specific mGluRs in biochemical and behavioral responses to ethanol. The purpose of the present study was to determine if mGluRs modulate the acute sedative-hypnotic properties of ethanol in mice.
Methods
C57BL / 6J mice were tested for locomotor activity (sedation) and duration of loss of the righting reflex (hypnosis) following acute systemic administration of ethanol alone or in combination with the mGluR5-selective antagonist, 2-methyl-6-(phenylethynyl)pyridine (MPEP), the mGluR1-selective antagonist, 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt), or the mGluR2 / 3-selective antagonist (2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495)).
Results
MPEP (10 and 30 mg / kg) significantly enhanced both the sedative and hypnotic effects of ethanol, while LY341495 (10 and 30 mg / kg) significantly reduced the sedative-hypnotic effects of ethanol. CPCCOEt had no effect at any concentration tested. Further loss of righting reflex experiments revealed that LY341495 (30 mg / kg) significantly reduced hypnosis induced by the gamma-aminobutyric acid type A (GABAA) positive modulators, pentobarbital (50 mg / kg) and midazolam (60 mg / kg), and the N-methyl-D-aspartate (NMDA) receptor antagonist, ketamine (150 mg / kg), while MPEP (30 mg / kg) only significantly enhanced the hypnotic properties of ketamine (150 mg / kg).
Conclusions
These findings suggest that specific subtypes of the metabotropic glutamate receptor differentially modulate the sedative-hypnotic properties of ethanol through separate mechanisms of action, potentially involving GABAA and NMDA receptors.
Keywords: Ethanol, Metabotropic Glutamate Receptors, mGluR5, MPEP, Sedation
The amino acid glutamate is the primary excitatory neurotransmitter in the mammalian central nervous system. Glutamate receptors are divided into 2 categories: iono-tropic receptors (iGluRs), glutamate-gated cation channels including NMDA, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid, and kainate responsive receptors, and metabotropic receptors (mGluRs), a heterogeneous family of 7-transmembrane G-protein coupled receptors. Eight mGluR subtypes have been cloned. These receptors are divided into 3 broad groups based on amino acid sequence similarity, agonist pharmacology, and second messenger coupling. Group I mGlu receptors (mGluR1 and mGluR5) stimulate inositol phosphate metabolism and mobilization of intracellular Ca2+, whereas group II (mGluR2 and mGluR3) and group III (mGluR4, -R6, -R7, and -R8) inhibit adenylyl cyclase and reduce synaptic transmission (Gereau and Conn, 1995; Kew and Kemp, 2005; Pin and Duvoisin, 1995). In contrast to the iGluRs, which are responsible for the fast, excitatory responses to glutamate, mGluRs mediate the slower, modulatory responses to glutamate. In this capacity, mGluRs can modulate neurotransmission at both glutamatergic and nonglutamatergic synapses (Benquet et al., 2002; Diaz-Cabiale et al., 2002).
The availability of selective pharmacological agents has begun to reveal basic functional roles for the group I and II mGluR subtypes (Kew and Kemp, 2005). Group I selective agonists have been shown to increase glutamate and GABA levels in vivo (de Novellis et al., 2003) and enhance glutamate-evoked depolarization (Doherty et al., 1997; Pisani et al., 2001) and GABA-gated Cl− currents (Hoffpauir and Gleason, 2002). Group II receptors are predominantly presynaptic autoreceptors (Cartmell and Schoepp, 2000) and selective activation of these receptors has been shown to reduce glutamate-evoked excitatory postsynaptic currents through inhibition of glutamate release (Shen and Johnson, 2003). mGluR selective ligands have also demonstrated behavioral activity in rodent models of anxiety and epilepsy. Group I antagonists and group II agonists have been shown to have anxiolytic (Klodzinska et al., 1999; Spooren et al., 2000) and anticonvulsant properties (Chapman et al., 2000; Moldrich et al., 2001).
Emerging evidence implicates mGluR function in ethanol’s neurobiological and behavioral effects. Ethanol alters neuronal firing rates (Netzeband et al., 1997) and Ca2+ levels (Gruol et al. 1997) mediated by mGluRs in vitro. Chronic exposure to ethanol reduces mGluR1 mRNA levels in cerebellar Purkinje neurons of mice (Simonyi et al., 1996), and early withdrawal from ethanol leads to alterations in mGluR-evoked Ca2+ signaling in cerebellar neurons (Netzeband et al., 2002). In rats, chronic exposure to an ethanol containing liquid diet decreased mRNA levels for mGluR3 and mGluR5 in the dentate gyrus, whereas mGluR1, mGluR5, and mGluR7 mRNA was decreased in the CA3 regions of the hippocampus (Simonyi et al., 2004). Neurobehaviorally, mGluR5 selective antagonists reduce ethanol self-administration in mice and rats (Cowen et al., 2005, 2007; Hodge et al., 2006; Lominac et al., 2006), decrease relapse to ethanol self-administration in rats (Backstrom et al., 2004; Schroeder et al., 2005), and block the discriminative stimulus effects of ethanol (Besheer and Hodge, 2005; Besheer et al., 2006).
The purpose of this study was to investigate the role of specific mGluRs in the acute sedative hypnotic effects of ethanol in mice. The 3 selective, systemically active mGluR antagonists 2-methyl-6-(phenylethynyl)pyridine (MPEP; mGluR5), 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt; mGluR1), and LY341495 (mGluR2/ 3) were examined for their ability to alter ethanol-induced inhibition of spontaneous locomotor activity (sedation) and ethanol-induced loss of righting reflex (LORR) (hypnosis). To explore potential mechanisms by which mGluRs might modulate responses to ethanol, these antagonists were also tested with other sedative-hypnotics: the GABAA positive modulators pentobarbital and midazolam, and the NMDA antagonist ketamine. Blood-ethanol clearance studies were conducted to ensure that the observed effects were not due to alterations in ethanol clearance.
MATERIAL AND METHODS
Animals
Male adult C57BL/ 6J mice (Jackson Labs, Bar Harbor, ME; 10 to 24 weeks; 22 to 35 g) were housed 4 per cage and maintained on a 12-hour light/ dark cycle with food and water available ad libitum. Animal care and handling procedures were performed in accordance with approved institutional protocols and the National Institutes of Health Guide for Care and Use of Laboratory Animals (1996).
Drugs
Ethanol (95%w/ v) was diluted in physiological saline (0.9%) to a concentration of 20% (v/ v) and administered in various volumes to obtain the appropriate doses. The mGluR1 selective antagonist CPCCOEt, mGluR5 selective antagonist MPEP, and the group II selective (mGluR2 and mGluR3) antagonist LY 341495 were purchased from Tocris (Ellisville, MO, USA). The mGluR5 selective antagonist 3-[(2-methyl-1,3-thiazol-4-yl)-ethynyl]-pyridine (MTEP) was purchased from Ascent (Weston-super-Mare, UK). MPEP and MTEP were dissolved in physiological saline (0.9%). CPCCOEt and LY 341495 were suspended in (2-Hydroxypropyl)-β-cyclodextrin (45% w/ v, Sigma, St Louis, MO) in distilled water. Pentobarbital, midazolam, and ketamine were purchased from Sigma-Aldritch and were dissolved in physiological saline (0.9%). Drug and vehicle solutions were administered to mice in a volume of 0.1 ml/ 10 g body weight, and dose selections were made based on pilot experiments and published studies (Naveilhan et al., 2001; Quinlan et al., 1998).
LORR Studies
Animals were treated with vehicle, MPEP (10 to 30 mg/ kg), CPCCOEt (10 to 30 mg/ kg), or LY341495 (10 to 30 mg/ kg) 10 minutes prior to ethanol treatment (4.0 g/ kg, IP) and the onset and duration of LORR (sleep time) were measured. Onset was calculated as the time between ethanol injection and LORR. LORR was defined as the inability of an animal to right itself within 30 seconds. Upon LORR, mice were placed in V-shaped troughs (~90° angle) and the time to regain righting reflex was measured. Return of the righting reflex was defined as the ability of an animal to right itself 3 times in 30 seconds. Duration of LORR was calculated as the difference between loss and return of righting reflex.
The active antagonists (MPEP and LY341495) were then further tested with a series of ethanol doses (2.5 to 3.5 g/ kg) to examine ethanol dose-dependence. MTEP (10 mg/ kg) and MPEP (10 mg/ kg) were also tested with ethanol (3 g/ kg) to further confirm mGluR5 involvement. To examine possible mechanisms for the antagonist effects on ethanol-induced LORR, pentobarbital (50 mg/ kg)-, midazolam (60 mg/ kg)-, and ketamine (200 mg/ kg)-induced LORR were also examined following pretreatment with the active mGluR antagonists.
Locomotor Activity Studies
Spontaneous locomotor activity was measured in 8 ENV 250 activity chambers (Med. Associates, St Albans, VT). Infrared light sources and photodetectors were used to measure horizontal distance traveled during test sessions. All sessions were 60 minutes. Sessions were recorded in 5-minute time bins at 100 ms resolution on a computer interfaced with the chambers. Following each session, chambers were cleaned with glacial acetic acid and rinsed with water.
All animals were chamber naive prior to testing. Vehicle, MPEP (30 mg/kg), CPCCOEt (30 mg / kg), or LY341495 (30 mg/ kg) was administered by intraperitoneal injection (IP) 10 minutes prior to ethanol treatment (0 and 2.0 g/ kg, IP). The animals were placed in the activity chambers immediately after ethanol injection.
Blood Ethanol Clearance
Vehicle, MPEP (30 mg/ kg) or LY341495 (30 mg/ kg) was administered by IP injection 10 minutes prior to ethanol treatment (4.0 g/ kg). Blood (~30 μl) was taken from the tail vein at various time points (10, 30, 60, 120, 180 minutes) after ethanol administration. Plasma from the samples was analyzed using an alcohol analyzer (Analox Instruments, Lunenburg, MA).
Analysis of Drug Effects
One-way ANOVA was used to determine the effects of the antagonists on single doses of sedative-hypnotic compounds. Two-way ANOVA was used to analyze the effects of the antagonists on total spontaneous locomotor activity and ethanol dose-dependent LORR. Three-way ANOVA with repeated measures was used to analyze the time course of antagonist effects on spontaneous locomotor activity. Two-way ANOVA with repeated measures and area under the curve (AUC) calculations were used to evaluate the blood-ethanol data. Upon identification of statistical significance, post hoc comparisons were made with a Tukey’s test where appropriate. In all cases, a value of p < 0.05 was considered significant.
RESULTS
Ethanol-Induced LORR
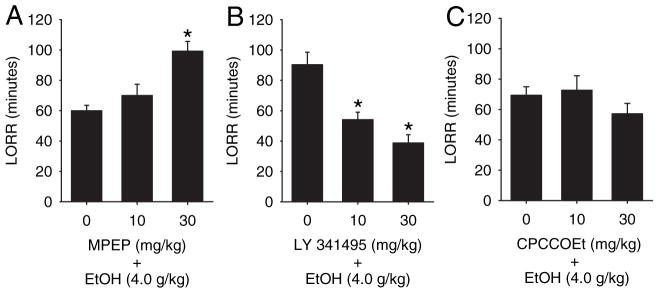
Systemic administration of the selective mGluR5 antagonist MPEP (0 or 30 mg/kg) or the mGluR2/ 3 antagonist LY 341495 (0 or 30 mg/ kg) produced differential effects on the time required for animals to regain their righting reflex following a high dose of ethanol (Fig. 1). Pretreatment with the highest dose of MPEP (30 mg/ kg) increased the duration of LORR induced by ethanol (4 g/ kg) by 65% (Fig. 1A; F[2,25] = 13; p < 0.001). Follow-up analysis shows that 30 mg/kg MPEP was significantly different from saline and 10 mg/kg MPEP (Tukey; p < 0.05), indicating a dose-dependent effect of MPEP. In contrast, pretreatment with the mGluR2/ 3 antagonist, LY341495, decreased the duration of ethanol-induced LORR (Fig. 1B). LY341495 significantly reduced the duration of LORR produced by ethanol (4 g/ kg) (F[2,31] = 11; p < 0.001), although responses to the 10 and 30 mg/kg doses (54.2 ± 5.0 minutes and 38.8 ± 5.3 minutes, respectively) were not significantly different from each other. Neither dose of the mGluR1 antagonist CPCCOEt tested (10 and 30 mg/ kg) altered the duration of ethanol-induced LORR (F[2,29] = 0.9; p = 0.415) (Fig. 1C).
Fig. 1.
Effects of mGluR antagonists on loss of righting reflex (LORR). Bars represent the mean (±SEM) duration of ethanol-induced LORR in minutes (n = 6 to 8) following pretreatment with MPEP (A), CPCCOEt (B), or LY341495 (C). *Significantly different from 4 g / kg ethanol alone (p < 0.05, Tukey’s test).
Ethanol-Induced Locomotor Inhibition
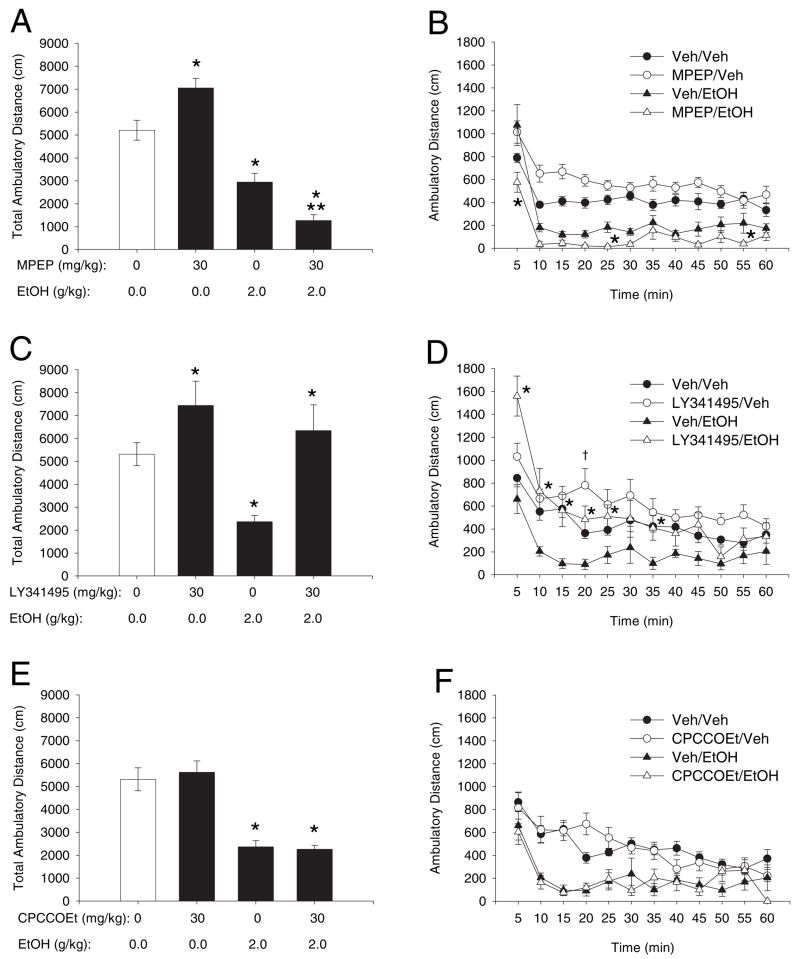
mGluR5 and mGluR2/ 3 antagonists differentially modulated the ability of a subhypnotic dose of ethanol (2.0 g/ kg) to reduce spontaneous locomotor activity as measured by distance traveled in a novel environment. Two-way ANOVA revealed a main effect of ethanol on total ambulatory distance (Fig. 2A; F[1,27] = 112; p < 0.001). When administered prior to ethanol, MPEP (30 mg/ kg) further reduced exploratory locomotor activity compared with ethanol control. Although the analysis shows no main effect of MPEP, there was a significant MPEP × ethanol interaction (F[1, 27] = 22;p < 0.001), indicating that the effect of MPEP on total motor activity depended on the dose of ethanol (Fig. 2A). Analysis of the time course of MPEP’s effects on ethanol-induced sedation by 3-way RM ANOVA showed a significant interaction among MPEP, ethanol, and time (Fig. 2B, F[11,297] = 2; p < 0.01) and a main effect of time (F[11,297] = 43; p < 0.001), in addition to confirming the main effect of ethanol. Follow-up analysis of these data showed that MPEP pretreatment significantly enhanced ethanol-induced motor impairment during the first 5 minutes (Tukey; p < 0.001), as well as 25 and 55 minutes post injection (Tukey; p < 0.05). Pretreatment with LY341495 (30 mg/ kg) reversed ethanol-induced locomotor inhibition, producing a main effect of LY341495 (Fig. 2C; F[1,24] = 16; p < 0.001) but no LY341495 × ethanol interaction. However, time course analysis by 3-way RM ANOVA showed an interaction among LY341495, ethanol, and time (Fig. 2D; F[11,220] = 2; p < 0.01) and a main effect of time (F[11,220] = 2; p < 0.001), while also confirming the main effect of LY341495. LY341495 pretreatment significantly diminished ethanol-induced motor impairment at 5, 10, 15, 20, 25, and 35 minutes after ethanol treatment (Tukey; p < 0.05). Treatment with CPCCOEt (30 mg/ kg) had no effect on total locomotor activity when administered alone or prior to ethanol treatment (Fig. 2E; F[1,24] = 0.05; p = 0.83) and temporal analysis showed no interaction among CPCCOEt, ethanol, and time (Fig. 2F).
Fig. 2.
Effects of mGluR antagonists on total locomotor activity alone and in the presence of a sedative dose of ethanol (A, C, and E). Bars represent the mean (±SEM) horizontal distance traveled in 60 minutes (n = 6 to 8) following pretreatment with vehicle, MPEP (30 mg / kg) (A), LY341495 (30 mg / kg) (C), or CPCCOEt (30 mg / kg) (E) with and without ethanol (2.0 g / kg). *Significantly different from vehicle / vehicle (p < 0.05, Tukey’s test). **Significantly different from vehicle / ethanol (p < 0.05, Tukey’s test). Temporal analysis of mean (±SEM) horizontal distance traveled in 5 minute time intervals (n = 6 to 8) following treatment with vehicle, MPEP (30 mg / kg) (B), LY341495 (30 mg / kg) (D), or CPCCOEt (30 mg / kg) (F) with and without ethanol (2.0 g / kg). *mGluR antagonist / ethanol treatment significantly different from vehicle / ethanol treatment at given time point (p < 0.05, Tukey’s test). *mGluR antagonist / vehicle treatment significantly different from vehicle / vehicle at given time point (p < 0.05, Tukey’s test).
Ethanol-Induced LORR: Ethanol Dose-Dependence
To further characterize the involvement of mGluR5 and mGluR2/ 3 receptors in ethanol-induced hypnosis, the highest effective dose of each antagonist was tested in combination with a range of ethanol doses. As shown in Fig. 3A, the duration of LORR was dose-dependently increased by ethanol (F[3, 53] = 103; p < 0.001). At a dose of 2.5 g/ kg, ethanol did not induce LORR. Doses of 3.0, 3.5, and 4.0 g/ kg of ethanol induced increasing durations of LORR. This dose-dependent effect of ethanol was enhanced by MPEP pretreatment. Two-way ANOVA showed a main effect of MPEP (30 mg/ kg) (F[1, 53] = 80; p < 0.001). After MPEP pretreatment, the time to regain the righting reflex was significantly increased compared with the corresponding saline pretreated controls at all doses of ethanol tested (Tukey; p-values ≤ 0.002). MPEP pretreatment also altered the sedative-hypnotic effects of the lowest dose of ethanol, resulting in a LORR when combined with 2.5 g/kg ethanol. However, there was no MPEP × ethanol interaction.
Fig. 3.
Ethanol-induced loss of righting reflex (LORR) plotted as a function of ethanol dosage, symbols represent the mean (±SEM) duration of LORR in minutes (n = 8) following ethanol with saline pretreatment (open symbols) or in combination with MPEP (A) or LY341495 (B) pretreatment (closed symbols). *Significantly different from vehicle at corresponding dose of ethanol (p < 0.05, Tukey’s test).
Figure 3B shows the ethanol dose–response curve following vehicle and LY341495 pretreatment. Two-way ANOVA showed that both ethanol and LY341495 produced main effects (EtOH: F[2, 41] = 27; p < 0.001); LY341495: (F[1, 41] = 12; p = 0.001). Analysis also showed a significant LY341495 × ethanol interaction (F[2, 41] = 5; p = 0.015), indicating that LY341495 effects are dependent on ethanol dose. LY341495 (30 mg/ kg) significantly reducfed the duration of ethanol-induced LORR for a 4.0 g/ kg ethanol dose (Tukey; p < 0.001), but not for the 3.0 or 3.5 g/ kg doses.
Examination of the time to onset of LORR also demonstrated differences in the actions of MPEP and LY341495. As shown in Table 1, MPEP pretreatment resulted in a more rapid onset of LORR for the 3.0 and 3.5 g/kg doses of ethanol (F[2, 39] = 20; p = 0.001), while LY341495 pretreatment had no effect on onset time.
Table 1.
Effects of mGluR Antagonists on Onset to Ethanol-Induced Loss of Righting Reflex
| MPEP dose(mg / kg)
|
LY341495 dose(mg/ kg)
|
|||
|---|---|---|---|---|
| Ethanol (g / kg) | 0 | 30 | 0 | 30 |
| 3.0 | 2.4 ± 0.1 | 1.9 ± 0.1* | 3.1 ± 0.2 | 3.2 ± 0.2 |
| 3.5 | 1.8 ± 0.2 | 1.2 ± 0.2* | 2.6 ± 0.2 | 2.5 ± 0.2 |
| 4.0 | 1.5 ± 0.1 | 1.2 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.2 |
Data expressed as mean ± SEM minutes (n = 8 to 10).
Significantly different from ethanol alone (p < 0.001, Tukey’s test).
Ethanol-Induced LORR: mGluR5 Specificity
To further confirm the mGluR5 involvement, a low dose of MPEP and the highly potent and selective mGluR5 antagonist MTEP were tested in parallel with a lower hypnotic dose of ethanol (3 g/ kg). As shown in Fig. 4, both MPEP (10 mg/ kg) and MTEP (10 mg/kg) significantly increased the duration of LORR induced by ethanol (3 g/ kg). The duration of ethanol-induced LORR was increased by 86% by MPEP and 133% by MTEP (F[2,21] = 13; p < 0.001). There was not a significant difference between the effects of MPEP and MTEP on ethanol-induced LORR suggesting that the effects observed following MPEP (30 mg/ kg) are attributable to its actions on mGluR5.
Fig. 4.
Confirmation of mGluR5 specificity in loss of righting reflex (LOSS). Bars represent mean (±SEM) duration of ethanol-induced LORR in minutes (n = 8) following pretreatment with saline, MPEP, or MTEP. *Significantly different from saline pretreatment (p < 0.05, Tukey’s test).
Pentobarbital and Midazolam-Induced LORR
The GABAAR positive modulator pentobarbital (50 mg/ kg) induced an average duration of LORR comparable to that produced by a 4 g/ kg dose of ethanol. Pretreatment with MPEP (30 mg/ kg) did not alter the onset time or duration of LORR in mice treated with pentobarbital (Table 2). In contrast, LY341495 (30 mg/ kg) significantly increased onset time (F[1,13] = 14; p = 0.002) and shortened the duration of pentobarbital-induced LORR by 67% (F[1,13] = 39; p < 0.001). The GABAAR benzodiazepine positive modulator midazolam (60 mg/ kg) also produced an average duration of LORR similar to the highest dose of ethanol. Midazolam-induced LORR was unaffected by MPEP and completely reversed by LY 341495 (Table 2; Onset: F[1,6] = 12; p = 0.013; duration: F[1,6] = 23; p = 0.003).
Table 2.
Effects of mGluR Antagonists on Loss of Righting Reflex Induced by GABAergic and Glutamatergic Sedative-Hypnotics
| MPEP dose (mg / kg)
|
LY341495 dose (mg / kg)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 0 | 30 | |||||
| Onset | Duration | Onset | Duration | Onset | Duration | Onset | Duration | |
| Pentobarbital (50 mg / kg) | 3.6 ± 0.4 | 63.9 ± 3.5 | 3.6 ± 0.5 | 56.3 ± 2.1 | 8.1 ± 0.7 | 46.2 ± 11.5 | 12.3 ± 0.9* | 15.1 ± 7.6* |
| Midazolam (60 mg / kg) | 10.6 ± 2.5 | 47.3 ± 3.2 | 6.2 ± 1.2 | 47.5 ± 3.6 | 25.5 ± 7.3 | 39.6 ± 8.2 | 0.0 ± 0.0* | 0.0 ± 0.0* |
| Ketamine (200 mg / kg) | 1.4 ± 0.1 | 41.0 ± 1.3 | 1.4 ± 0.1 | 56.8 ± 1.1* | 2.7 ± 0.6 | 40.2 ± 1.9 | 2.1 ± 0.1 | 32.3 ± 1.3* |
Data expressed as mean ± SEM minutes (n = 8 to 10).
Significantly different from pentobarbital, midazolam, or ketamine alone (p < 0.05, Tukey’s test).
Ketamine-Induced LORR
Like ethanol, the NMDA receptor antagonist ketamine (200 mg/ kg)-induced hypnosis was differentially affected by pretreatment with MPEP and LY341495 (Table 2). MPEP (30 mg/ kg) increased the duration of LORR in mice treated with ketamine by 39% (F[1,13] = 85; p < 0.001), while LY341495 (30 mg/ kg) reduced ketamine-induced LORR by 20% (F[1,13] = 12; p = 0.004). Onset time was unaffected by either MPEP or LY341495 treatment.
Blood Ethanol Determination
To address the possibility that MPEP and LY341495 are eliciting their effects by altering ethanol metabolism, blood-ethanol concentrations were measured following a 4 g/kg dose of ethanol (Table 3). In vehicle treated animals, blood-ethanol concentrations decreased significantly over time (saline: F[4,79] = 79; p < 0.001; cyclodextrin: F[4,88] = 42; p < 0.001). Neither MPEP (30 mg/ kg; F[1,22] = 0.987; p = 0.331) nor LY341495 (30 mg/ kg; F[1,14] = 1.3; p = 0.274) pretreatment altered the blood-ethanol clearance time course. However, comparisons of the blood ethanol concentrations of saline and cyclodextrin treated animals revealed significant differences between the 2 vehicle groups (F[1,100] = 10; p = 0.003; AUC: F[1,22] = 11; p = 0.003), specifically at the 120 and 180 minute time points (p < 0.05).
Table 3.
Effects of mGluR Antagonists on Blood-Ethanol Concentration
| Time interval (min)
|
||||||
|---|---|---|---|---|---|---|
| 10 | 30 | 60 | 120 | 180 | AUC | |
| Saline | 387.1 ± 18.6 | 381.6 ± 8.4 | 369.4 ± 6.9 | 314.7 ± 7.9 | 255.6 ± 8.8 | 57197.8 ± 1568.9 |
| MPEP | 391.2 ± 22.4 | 399.5 ± 14.6 | 387.1 ± 11.0 | 330.8 ± 11.3 | 275.5 ± 12.7 | 60347.3 ± 2627.5 |
| Cyclodextrin | 390.7 ± 10.9 | 406.2 ± 5.6 | 385.4 ± 8.4 | 353.0 ± 5.9* | 314.6 ± 6.1* | 62026.1 ± 680.4* |
| LY341495 | 399.9 ± 17.3 | 381.5 ± 11.6 | 379.3 ± 14.3 | 333.7 ± 16.0 | 305.8 ± 10.9 | 59802.8 ± 2008.8 |
AUC, mean area under the curve ± SEM.
Data expressed as mean ± SEM mg / dl (n = 7 to 16).
Significantly different from saline (Tukey; p < 0.05).
DISCUSSION
The purpose of this study was to investigate the role of specific mGluRs in the acute sedative-hypnotic effects of ethanol in mice by examining the effects of mGluR selective antagonists on ethanol-induced sedation and hypnosis. One of the primary findings of the present study is the mGluR5 antagonist MPEP increased ethanol-induced locomotor inhibition and LORR. These findings indicate that the inhibition of mGluR5 activity enhances the acute sedative-hypnotic effects of ethanol. Moreover, the more selective mGluR5 antagonist MTEP produced a comparable effect on ethanol-induced LORR, further supporting the conclusion that mGluR5 activity influences the sedative-hypnotic properties of ethanol. Although the exact role of mGluR5 in ethanol’s pharmacological effects has not been identified, these results suggest that ethanol produces some of its acute inhibitory effects through negative modulation of mGluR5 activity. This hypothesis is supported by evidence showing that pharmacologically relevant concentrations of ethanol inhibit glutamate-induced Ca2+-dependent Cl− currents in Xenopus oocytes expressing mGluR5 (Minami et al., 1998). Furthermore, this inhibition of mGluR5 is PKC dependent. Because acute ethanol activates PKCγ in vivo (Wilkie et al., 2007), which desensitizes mGluR5 through phosphorylation (Gereau and Heinemann, 1998), it is possible that ethanol inhibits mGluR5 activity through PKC-dependent desensitization.
The other group I antagonist tested, the mGluR1 selective CPCCOEt, had no effect on ethanol-induced sedation or hypnosis. The absence of an effect for CPCCOEt is consistent with evidence suggesting that group I mGluR mediation of ethanol effects is specific to mGluR5. Acute ethanol treatment does not affect Cl− currents in mGluR1-expressing oocytes (Minami et al., 1998), and our laboratory has shown no effect of CPCCOEt on ethanol consumption in either C57BL/ 6J mice or alcohol-preferring (P) rats (Hodge et al., 2006; Schroeder et al., 2005). However, our current results are inconsistent with a similar study (Lominac et al., 2006), which shows that pretreatment with CPCCOEt, but not MPEP, facilitates ethanol-induced motor impairment. While it is not entirely clear why these discrepancies exist, it may be due to differences in experimental procedure. We chose to use a higher dose of MPEP (30 mg/ kg) based on our initial LORR study that showed no effect of a 10 mg/kg when paired with a 4 g/ kg dose of ethanol. Furthermore, we employ a shorter pretreatment time. The onset to LORR and temporal distribution analysis of locomotor behavior both suggest that MPEP’s behavioral effects are rapidly induced, implying that a shorter pretreatment time is necessary to see MPEP effects. Furthermore, our data showing that both a lower dose of MPEP and another mGluR5 selective antagonist, MTEP, increase the duration of LORR produced by a lower dose of ethanol (3 g/ kg) support the conclusion that selective blockade of mGluR5 enhances ethanol’s sedative-hypnotic effects. As for the differences in CPCCOEt effects, the longer pretreatment time and longer testing time used by Lominac et al. (2006) may be necessary for the expression of CPCCOEt enhancement of ethanol-induced motor impairment.
This study also shows that the mGluR2/ 3 antagonist, LY341495, decreased both the sedative and hypnotic effect of ethanol. Both doses of LY341495 (10 and 30 mg/ kg) reduced ethanol-induced LORR. LY341495 (30 mg/ kg) also reversed the sedative effects of ethanol, as measured by spontaneous locomotor activity. These results were not unexpected given that LY341495 has been shown to have locomotor activating properties (David and Abraini, 2001). A growing body of evidence indicates that group II mGluRs are presynaptic autoreceptors and blockade of these receptors with LY341495 increases glutamate release (Xi et al. 2002). Increases in presynaptic glutamate release are known to promote increased locomotor activity (Vezina and Kim, 1999), suggesting that reductions in ethanol-induced sedation and hypnosis by LY341495 are due to increased glutamatergic activity.
The ethanol dose–response data highlight other differences between the mGluR5 and mGluR2/ 3 effects. The mGluR5 antagonist MPEP produced a significant increase in the duration of LORR regardless of the dose of ethanol administered. Furthermore, the increases produced by MPEP were the same for each dose of ethanol. Although MPEP alone produced no sedative-hypnotic effects, combination with a sub-hypnotic dose of ethanol (2.5 g/ kg) produced full hypnosis and a duration of LORR similar to the increases seen when MPEP was combined with fully hypnotic doses of ethanol. In contrast, the mGluR2/ 3 antagonist LY341495 produced a significant decrease in the duration of LORR only when administered with the highest dose of ethanol. These results indicate that the effects of LY341495 are dependent on the dose of ethanol administered. The onset to LORR data also present a distinction between the actions of MPEP and LY341495. Pretreatment with MPEP resulted in a more rapid onset of LORR for the 2 lower doses of ethanol (3.0 and 3.5 g/ kg), providing further evidence that MPEP enhances the hypnotic properties of ethanol and does so rapidly. Pretreatment with LY341495 did not alter the onset of LORR at any of the ethanol doses tested.
Ethanol-induced hypnosis has been attributed largely to ethanol’s ability to enhance inhibitory GABAergic responses and impair excitatory NMDA receptor activity (Beleslin et al., 1997). For an understanding of the mechanisms by which MPEP and LY341495 alter ethanol-induced hypnosis and a clarification as to whether these compounds selectively alter ethanol effects, MPEP and LY341495 were also tested ith the GABAA receptor positive modulators, pentobarbital and midazolam, and an NMDA receptor antagonist, ketamine, that exhibit hypnotic properties. MPEP pretreatment increased the duration of LORR for ketamine but had no effect on the hypnotic properties of pentobarbital or midazolam. This finding is consistent with reports that MPEP reduces NMDA-evoked responses (Attucci et al., 2001) and antagonism of mGluR5 and NMDA receptors have additive detrimental effects on learning and memory (Homayoun et al., 2004). Our results for pentobarbital and midazolam appear to be at odds with reports that mGluR5 activation positively modulates GABAA receptor function in vitro (Hoffpauir and Gleason, 2002), although this may be specific to benzodiazepine-sensitive GABAA receptors as MPEP inhibits the ethanol-like stimulus properties of diazepam, but not pentobarbital (Besheer and Hodge, 2005). However, it has also been reported that the anxiolytic effects of MPEP do not involve benzodiazepine-sensitive GABAA receptors (Wieronska et al., 2004), indicating that further research must be done to determine the extent to which mGluR5 interacts with GABAA receptors in vivo. Overall, these results suggest that blockade of mGluR5 increases the hypnotic properties of ethanol by enhancing ethanol-induced inhibition of NMDA receptors without affecting ethanol’s actions at GABAA receptors.
LY341495 reduced the duration of LORR for ketamine and pentobarbital and fully blocked induction of midazolam-induced LORR. These data are consistent with evidence showing that LY341495 has general stimulant effects (David and Abraini, 2001). While LY341495 reversed the hypnotic effects of all 3 drugs, it did so to varying degrees. LY341495 reduced ketamine-induced LORR by 20%, pentobarbital-induced LORR by 67%, and midazolam-induced LORR by 100%. Interestingly, LY341495 appears to be least effective at reversing hypnosis induced by NMDA receptor inhibition. Based on our hypothesis that LY341495 reverses hypnosis by increasing glutamate release, these data suggest that the inhibition of mGluR2/3 results in enough glutamate release to counteract enhanced GABAAR activity, but not enough to prevent decreased glutamatergic activity due to NMDAR inhibition.
One limitation of the present study that merits discussion is the potential off target effects of the pharmacological compounds tested. High concentrations of MPEP (20 μM and above) have been associated with NMDA receptor inhibition (O’Leary et al., 2000) and positive allosteric modulation of mGlu4 receptors (Mathiesen et al., 2003). However, it has been reported that systemic administration of a 3 mg/ kg dose of MPEP produces submicromolar concentrations in the brain (0.83 μM; (Cosford et al., 2003), making it unlikely that a 30 mg/kg dose of MPEP would result in brain concentrations high enough to significantly affect NMDA or mGlu4 receptors. It has also been reported that MPEP can inhibit the norepinephrine transporter at nanomolar concentrations (Heidbreder et al., 2003), which may be related to the effects we report, given that the norepinephrine transporter has been implicated in the differential ethanol sensitivities of the long-sleep and short-sleep mice (Haughey et al., 2005). However, given that a low dose of MPEP (10 mg/ kg) and the more selective mGluR5 antagonist MTEP (Varty et al., 2005) both enhanced the hypnotic effects of a low dose of ethanol, off target effects of MPEP do not appear to be a concern here.
Although there is currently no evidence that LY341495 has any off target effects, another mGluR2/ 3 antagonist has been shown to increase extracellular dopamine levels (Karasawa et al., 2006). Thus, additional research using gene knockout mice, RNA inhibition, or other more selective approaches are warranted to examine the selectivity of these compounds. The dose of ketamine used to induce LORR may also be having off target effects, namely at dopaminergic or nicotinic acetylcholine systems. As ketamine has been shown to increase extracellular dopamine release (Aalto et al., 2005), it is unlikely that interactions with dopaminergic systems are contributing to ketamine’s sedative-hypnotic profile. Ketamine has also been shown to inhibit α7-containing nicotinic acetylcholine receptors (nAChR) (Coates and Flood, 2001). This interaction may play a role in ketamine-induced hypnosis, as α7-subunit null mutant mice are more sensitive to the sedative-hypnotic properties of ethanol (Bowers et al., 2005), and in the MPEP and LY341495 modulation of induced sedation, as recent evidence suggests a link between mGluR function and α7-nAChR (Welsby et al., 2006). Again, further research is necessary to determine the roles of each of these systems.
Finally, neither MPEP nor LY341495 altered blood-ethanol clearance. However, cyclodextrin, the vehicle used for LY341495 and CPCCOEt, appears to slow the time course of ethanol elimination as compared with saline. While these differences do not invalidate results from individual experiments, it limits the comparisons that can be made between saline vehicle and cyclodextrin vehicle experiments. These differences may contribute to the variability seen for the 3 vehicle groups in the locomotor activity experiments.
In summary, the present data suggest that specific subtypes of metabotropic glutamate receptors differentially modulate ethanol-induced sedation and hypnosis without altering the pharmacokinetics of ethanol elimination. Inhibition of mGluR5 enhances the sedative-hypnotic effects of ethanol, whereas inhibition of mGluR2/ 3 reverses these effects of ethanol. Our results also suggest that mGluR5 and mGluR2/ 3 elicit these changes through differential modulation of GABAA and NMDA receptors.
Acknowledgments
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism to CWH (AA014983 and AA011605).
References
- Aalto S, Ihalainen J, Hirvonen J, Kajander J, Scheinin H, Tanila H, Nagren K, Vilkman H, Gustafsson LL, Syvalahti E, Hietala J. Cortical glutamate-dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacology (Berl) 2005;182:375–383. doi: 10.1007/s00213-005-0092-6. [DOI] [PubMed] [Google Scholar]
- Attucci S, Carla V, Mannaioni G, Moroni F. Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. Br J Pharmacol. 2001;132:799–806. doi: 10.1038/sj.bjp.0703904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Beleslin DB, Djokanovic N, Jovanovic Micic D, Samardzic R. Opposite effects of GABAA and NMDA receptor antagonists on ethanol-induced behavioral sleep in rats. Alcohol. 1997;14:167–173. doi: 10.1016/s0741-8329(96)00140-1. [DOI] [PubMed] [Google Scholar]
- Benquet P, Gee CE, Gerber U. Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J Neurosci. 2002;22:9679–9686. doi: 10.1523/JNEUROSCI.22-22-09679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Hodge CW. Pharmacological and anatomical evidence for an interaction between mGluR5- and GABA(A) alpha1-containing receptors in the discriminative stimulus effects of ethanol. Neuropsychopharmacology. 2005;30:747–757. doi: 10.1038/sj.npp.1300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Stevenson RA, Hodge CW. mGlu5 receptors are involved in the discriminative stimulus effects of self-administered ethanol in rats. Eur J Pharmacol. 2006;551:71–75. doi: 10.1016/j.ejphar.2006.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers BJ, McClure-Begley TD, Keller JJ, Paylor R, Collins AC, Wehner JM. Deletion of the alpha7 nicotinic receptor subunit gene results in increased sensitivity to several behavioral effects produced by alcohol. Alcohol Clin Exp Res. 2005;29:295–302. doi: 10.1097/01.alc.0000156116.40817.a2. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chapman AG, Nanan K, Williams M, Meldrum BS. Anticonvulsant activity of two metabotropic glutamate group I antagonists selective for the mGlu5 receptor: 2-methyl-6-(phenylethynyl)-pyridine (MPEP), and (E)-6-methyl-2-styryl-pyridine (SIB 1893) Neuropharmacology. 2000;39:1567–1574. doi: 10.1016/s0028-3908(99)00242-7. [DOI] [PubMed] [Google Scholar]
- Coates KM, Flood P. Ketamine and its preservative, benzethonium chloride, both inhibit human recombinant alpha7 and alpha4 beta2 neuronal nicotinic acetylcholine receptors in Xenopus oocytes. Br J Pharmacol. 2001;134:871–879. doi: 10.1038/sj.bjp.0704315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosford ND, Tehrani L, Roppe J, Schweiger E, Smith ND, Anderson J, Bristow L, Brodkin J, Jiang X, McDonald I, Rao S, Washburn M, Varney MA. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J Med Chem. 2003;46:204–206. doi: 10.1021/jm025570j. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Djouma E, Lawrence AJ. The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther. 2005;315:590–600. doi: 10.1124/jpet.105.090449. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Krstew E, Lawrence AJ. Assessing appetitive and consummatory phases of ethanol self-administration in C57BL / 6J mice under operant conditions: regulation by mGlu5 receptor antagonism. Psychopharmacology (Berl) 2007;190:21–29. doi: 10.1007/s00213-006-0583-0. [DOI] [PubMed] [Google Scholar]
- David HN, Abraini JH. Differential modulation of the D1-like- and D2-like dopamine receptor-induced locomotor responses by group II metabotropic glutamate receptors in the rat nucleus accumbens. Neuropharmacology. 2001;41:454–463. doi: 10.1016/s0028-3908(01)00082-x. [DOI] [PubMed] [Google Scholar]
- Diaz-Cabiale Z, Vivo M, Del Arco A, O’Connor WT, Harte MK, Muller CE, Martinez E, Popoli P, Fuxe K, Ferre S. Metabotropic glutamate mGlu5 receptor-mediated modulation of the ventral striopallidal GABA pathway in rats. Interactions with adenosine A(2A) and dopamine D(2) receptors. Neurosci Lett. 2002;324:154–158. doi: 10.1016/s0304-3940(02)00179-9. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Henley JM, Collingridge GL, Jane DE. (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5, but no mGlu1, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology. 1997;36:265–267. doi: 10.1016/s0028-3908(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Gereau RW, Conn PJ. Multiple presynaptic metabotropic glutamate receptors modulate excitatory and inhibitory synaptic transmission in hippocampal area CA1. J Neurosci. 1995;15:6879–6889. doi: 10.1523/JNEUROSCI.15-10-06879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereau RWt, Heinemann SF. Role of protein kinase C phosphorylation in rapid desensitization of metabotropic glutamate receptor 5. Neuron. 1998;20:143–151. doi: 10.1016/s0896-6273(00)80442-0. [DOI] [PubMed] [Google Scholar]
- Gruol DL, Parsons KL, DiJulio N. Acute ethanol alters calcium signals elicited by glutamate receptor agonists and K+ depolarization in cultured cerebellar Purkinje neurons. Brain Res. 1997;773:82–89. doi: 10.1016/s0006-8993(97)00912-8. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Kaiser AL, Johnson TE, Bennett B, Sikela JM, Zahniser NR. Norepinephrine transporter: a candidate gene for initial ethanol sensitivity in inbred long-sleep and short-sleep mice. Alcohol Clin Exp Res. 2005;29:1759–1768. doi: 10.1097/01.alc.0000183009.57805.a6. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Bianchi M, Lacroix LP, Faedo S, Perdona E, Remelli R, Cavanni P, Crespi F. Evidence that the metabotropic glutamate receptor 5 antagonist MPEP may act as an inhibitor of the norepinephrine transporter in vitro and in vivo. Synapse. 2003;50:269–276. doi: 10.1002/syn.10261. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL / 6J mice. Psychopharmacology (Berl) 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffpauir BK, Gleason EL. Activation of mGluR5 modulates GABA(A) receptor function in retinal amacrine cells. J Neurophysiol. 2002;88:1766–1776. doi: 10.1152/jn.2002.88.4.1766. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional interaction between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Karasawa J, Yoshimizu T, Chaki S. A metabotropic glutamate 2 / 3 receptor antagonist, MGS0039, increases extracellular dopamine levels in the nucleus accumbens shell. Neurosci Lett. 2006;393:127–130. doi: 10.1016/j.neulet.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Klodzinska A, Chojnacka-Wojcik E, Palucha A, Branski P, Popik P, Pilc A. Potential anti-anxiety, anti-addictive effects of LY 354740, a selective group II glutamate metabotropic receptors agonist in animal models. Neuropharmacology. 1999;38:1831–1839. doi: 10.1016/s0028-3908(99)00066-0. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Dep. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Mathiesen JM, Svendsen N, Brauner-Osborne H, Thomsen C, Ramirez MT. Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP. Br J Pharmacol. 2003;138:1026–1030. doi: 10.1038/sj.bjp.0705159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami K, Gereau RWt, Minami M, Heinemann SF, Harris RA. Effects of ethanol and anesthetics on type 1 and 5 metabotropic glutamate receptors expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;53:148–156. doi: 10.1124/mol.53.1.148. [DOI] [PubMed] [Google Scholar]
- Moldrich RX, Jeffrey M, Talebi A, Beart PM, Chapman AG, Meldrum BS. Anti-epileptic activity of group II metabotropic glutamate receptor agonists (–)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate ( LY379268) and (–)-2-thia-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate ( LY389795) Neuropharmacology. 2001;41:8–18. doi: 10.1016/s0028-3908(01)00044-2. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Canals JM, Valjakka A, Vartiainen J, Arenas E, Ernfors P. Neuropeptide Y alters sedation through a hypothalamic Y1-mediated mechanism. Eur J Neurosci. 2001;13:2241–2246. doi: 10.1046/j.0953-816x.2001.01601.x. [DOI] [PubMed] [Google Scholar]
- Netzeband JG, Parsons KL, Sweeney DD, Gruol DL. Metabotropic glutamate receptor agonists alter neuronal excitability and Ca2+ levels via the phospholipase C transduction pathway in cultured Purkinje neurons. J Neurophysiol. 1997;78:63–75. doi: 10.1152/jn.1997.78.1.63. [DOI] [PubMed] [Google Scholar]
- Netzeband JG, Schneeloch JR, Trotter C, Caguioa-Aquino JN, Gruol DL. Chronic ethanol treatment and withdrawal alter ACPD-evoked calcium signals in developing Purkinje neurons. Alcohol Clin Exp Res. 2002;26:386–393. [PubMed] [Google Scholar]
- de Novellis V, Marabese I, Palazzo E, Rossi F, Berrino L, Rodella L, Bianchi R, Rossi F, Maione S. Group I metabotropic glutamate receptors modulate glutamate and gamma-aminobutyric acid release in the periaqueductal grey of rats. Eur J Pharmacol. 2003;462:73–81. doi: 10.1016/s0014-2999(03)01342-6. [DOI] [PubMed] [Google Scholar]
- O’Leary DM, Movsesyan V, Vicini S, Faden AI. Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br J Pharmacol. 2000;131:1429–1437. doi: 10.1038/sj.bjp.0703715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Quinlan JJ, Homanics GE, Firestone LL. Anesthesia sensitivity in mice that lack the beta3 subunit of the gamma-aminobutyric acid type A receptor. Anesthesiology. 1998;88:775–780. doi: 10.1097/00000542-199803000-00030. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. Group II metabotropic glutamate receptor modulation of excitatory transmission in rat subthalamic nucleus. J Physiol. 2003;553:489–496. doi: 10.1113/jphysiol.2003.052209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyi A, Christian MR, Sun AY, Sun GY. Chronic ethanol-induced subtype- and subregion-specific decrease in the mRNA expression of metabotropic glutamate receptors in rat hippocampus. Alcohol Clin Exp Res. 2004;28:1419–1423. doi: 10.1097/01.alc.0000139825.35438.a4. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Zhang JP, Sun AY, Sun GY. Chronic ethanol on mRNA levels of IP3R1, IP3 3-kinase and mGluR1 in mouse Purkinje neurons. Neuroreport. 1996;7:2115–2118. doi: 10.1097/00001756-199609020-00010. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Vassout A, Neijt HC, Kuhn R, Gasparini F, Roux S, Porsolt RD, Gentsch C. Anxiolytic-like effects of the prototypical metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine in rodents. J Pharmacol Exp Ther. 2000;295:1267–1275. [PubMed] [Google Scholar]
- Varty GB, Grilli M, Forlani A, Fredduzzi S, Grzelak ME, Guthrie DH, Hodgson RA, Lu SX, Nicolussi E, Pond AJ, Parker EM, Hunter JC, Higgins GA, Reggiani A, Bertorelli R. The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology (Berl) 2005;179:207–217. doi: 10.1007/s00213-005-2143-4. [DOI] [PubMed] [Google Scholar]
- Vezina P, Kim JH. Metabotropic glutamate receptors and the generation of locomotor activity: interactions with midbrain dopamine. Neurosci Biobehav Rev. 1999;23:577–589. doi: 10.1016/s0149-7634(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Welsby P, Rowan M, Anwyl R. Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus. Eur J Neurosci. 2006;24:3109–3118. doi: 10.1111/j.1460-9568.2006.05187.x. [DOI] [PubMed] [Google Scholar]
- Wieronska JM, Smialowska M, Branski P, Gasparini F, Klodzinska A, Szewczyk B, Palucha A, Chojnacka-Wojcik E, Pilc A. In the amygdala anxiolytic action of mGlu5 receptors antagonist MPEP involves neuropeptide Y but not GABAA signaling. Neuropsychopharmacology. 2004;29:514–521. doi: 10.1038/sj.npp.1300322. [DOI] [PubMed] [Google Scholar]
- Wilkie MB, Besheer J, Kelley SP, Kumar S, O’Buckley TK, Morrow AL, Hodge CW. Acute ethanol administration rapidly increases phosphorylation of conventional protein kinase C in specific mammalian brain regions in vivo. Alcohol Clin Exp Res. 2007;31:1259–1267. doi: 10.1111/j.1530-0277.2007.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]