Abstract
Clostridium botulinum neurotoxins are the most potent toxins to humans. The recognition and cleavage of SNAREs are prime evente in exhibiting their toxicity. We report here the crystal structure of the catalytically active full-length botulinum serotype E catalytic domain (BoNT E) in complex with SNAP-25 (a SNARE protein) substrate peptide Arg180-Ile181-Met182-Glu183 (P1–P3′). It is remarkable that the peptide spanning the scissile bond binds to but bypasses cleavage by the enzyme and inhibits the catalysis fairly with Ki ∼69 μm. The inhibitory peptide occupies the active site of BoNT E and shows well defined electron density. The catalytic zinc and the conserved key residue Tyr350 of the enzyme facilitate the docking of Arg180 (P1) by interacting with its carbonyl oxygen that displaces the nucleophilic water. The general base Glu212 side chain interacts with the main chain amino group of P1 and P1′. Conserved Arg347 of BoNT E stabilizes the proper docking of the Ile181 (P1′) main chain, whereas the hydrophobic pockets stabilize the side chains of Ile181 (P1′) and Met182 (P2′), and the 250 loop stabilizes Glu183 (P3′). Structural and functional analysis revealed an important role for the P1′ residue and S1′ pocket in driving substrate recognition and docking at the active site. This study is the first of its kind and rationalizes the substrate cleavage strategy of BoNT E. Also, our complex structure opens up an excellent opportunity of structure-based drug design for this fast acting and extremely toxic high priority BoNT E.
Clostridium botulinum neurotoxins (BoNTs)2 are produced as an inactive single chain (∼150 kDa) and released as active dichains, heavy (∼100 kDa) and light chain (∼50 kDa) (1–4). BoNT serotype E is an exception, since it is released as a single chain (4). The C-terminal domain of the heavy chain mediates binding of the toxin to specific neuronal receptors, and the N-terminal domain enables the catalytically active light chain translocation to the cytosol (5). The light chain is a zinc-dependent endopeptidase, which recognizes and cleaves one of the three SNARE proteins (SNAP-25 (synaptosome-associated protein of 25,000 daltons), VAMP-2 (vesicle-associated membrane protein 2), or syntaxin) (5). SNARE proteins associate to form a low energy ternary complex required for fusion and docking of neurotransmitter carrying vesicles to target membranes of peripheral neurons enabling neurotransmitter release (6). Cleavage of any one of them prevents complex formation, resulting in flaccid paralysis. BoNTs A and E cleave SNAP-25, whereas BoNTs B, D, F, and G cleave VAMP-2. BoNT C is unique and cleaves both SNAP-25 and syntaxin (7).
BoNTs are listed as category A bioterrorism agents by the Centers for Disease Control and Prevention. Accordingly, developing inhibitors to counter their activity is a priority. BoNTs A, B, and E are more potent than the other serotypes. Although BoNTs E and A cleave the same substrate SNAP-25, E blocks neurotransmission faster (8). Crystal structures of catalytic domains of all serotypes are available (9–15). However, insight into the substrate recognition and binding in each serotype, crucial for developing common and/or serotype specific inhibitors, has yet to be established.
As of now, the structure of enzyme-substrate peptide (residues 146–206) complex has been reported only for BoNT A (Protein Data Bank code 1XTG) (16). However, to prevent cleavage during co-crystallization, an inactive double mutant enzyme was used. Although this structure maps the exosite interactions, the region flanking the scissile bond (about 6 or 7 residues) is disordered and does not make contact with the active site residues of the enzyme (16). Recently, crystal structures of BoNT A in complex with peptide inhibitors Arg-Arg-Gly-Cys (RRGC) and its variants at the Cys position and N-Ac-Cys-Arg-Ala-Thr-Lys-Met-Leu (N-Ac-CRATKML) have been reported (17–19). However, variation of substrate residues flanking the scissile bond and their subsites in BoNT E and other serotypes warrants determination of structures of substrate-enzyme complex. Structural information of substrate recognition and binding at the active site is crucial in better understanding the substrate specificity among various serotypes and for developing potent serotype-specific inhibitors.
To our knowledge, structural information for BoNT-substrate/analog peptide complexes is not available for any serotype except BoNT A. Here, we present for the first time the crystal structure of the full-length BoNT E catalytic domain with its substrate SNAP-25-(180–183) Arg-Ile-Met-Glu-amide (RIME; P1:Arg180, P1′:Ile181, P2′:Met182, P3′:Glu183) peptide spanning the scissile bond. The S1, S1′, S2′, and S3′ pockets of BoNT E and their crucial interactions with P1–P3′ residues are identified. We also modeled the P2:Asp179 residue of the substrate and identified its possible interactions in the S2 pocket. Interestingly, we found that the substrate peptide RIME itself acts as a moderate inhibitor of catalysis. This study rationalizes the substrate cleavage strategy of BoNT E, which could be extended to other serotypes too. The present complex structure is the first report of substrate binding to the active site of BoNT E and helps to better understand the mechanism of catalysis and substrate recognition and binding at and near the active site. This will serve as an important starting point for designing specific structure-based potent inhibitors/drugs for highly toxic BoNT E.
EXPERIMENTAL PROCEDURES
Cloning, Expression, and Purification of BoNT E Catalytic Domain—The clone for BoNT E catalytic domain in pET9c vector was used as described (20). Protein was expressed using an autoinduction protocol (21) and was purified (20). This yielded >20 mg protein/500 ml of culture, and the protein was concentrated to 10 mg/ml in Hepes buffer (20 mm, pH 7.8, 200 mm NaCl).
Kinetic Parameters and Ki Determination—Kinetic values km and kcat were determined for SNAP-25-(146–186) as substrate with BoNT E. Enzyme concentrations were adjusted (0.5 nm) to cleave <20% substrate at various concentrations of substrate ranging from 35 to 500 μm. The reactions were carried out in 20 μl of reaction buffer containing 20 mm Hepes, pH 7.4, 2 mm DTT, and 10 μm zinc acetate. The reaction mixtures were incubated at 37 °C for 15 min and stopped by adding 2 μl of 1% trifluoroacetic acid. The samples were run in HPLC (Beckman) with a Discovery Bio-Wide pore C18 column with acetonitrile and aqueous phase gradient (Buffer A: 0.1% trifluoroacetic acid in water; Buffer B: 0.1% trifluoroacetic acid, 90% acetonitrile). The amount of cleaved product was calculated using software provided by Beckman. Kinetic constants were calculated from Lineweaver-Burk plot using Enzyme Kinetic Module and Sigmaplot (Systat Software, Inc.). IC50 and Ki determination for RIME (P1–P3′) (50–950 μm concentrations) was performed in similar assay conditions, and data were analyzed using GraphPad software and the Cheng-Prusoff equation, respectively. Peptide 2.0 Inc. synthesized this tetrapeptide.
Crystallization and Structure Determination—The catalytic domain was cocrystallized with RIME in 0.5 m ammonium sulfate, 0.1 m sodium citrate buffer, pH 5.6, 2 m Li2SO4 in sitting drop in 24-well plates. Protein at 7 mg/ml and peptide at ∼10 mm concentration was used for cocrystallization. Lower concentrations of the peptide during cocrystallization led to poor occupancy of the substrate at the active site. Diamond-shaped crystals appeared in 2–7 days at 22 °C. Crystals were immersed in mother liquor containing 15% (v/v) glycerol and immediately flash frozen in liquid nitrogen. X-ray data were collected at beamline X29 of the National Synchotron Light Source, Brookhaven National Laboratory, at 1.08 Å wavelength, and crystals diffracted to better than 2.25 Å resolution. Crystals belong to space group P21212 with 2 molecules/asymmetric unit. A rigid body refinement was performed using a BoNT E catalytic domain model (Protein Data Bank code 1T3A), followed by simulated annealing in CNS (22). The refinement statistics are given in Table 1. Molecules A and B are complete for amino acids 1–411 and 1–409, respectively, but molecule A has weak electron density for amino acids 235–238. Residues 59–60 are not modeled in both molecules due to poorly defined electron density. The SNAP-25 substrate RIME (P1–P3′) bound to molecule B could be modeled unambiguously; however, the C-terminal residue P3′ and P1 side chain could not be modeled for peptide bound to molecule A because of weak electron density. P1:Arg180-P2′:Met182 (RIME) aligns well in both molecules of enzymes (root mean square deviation = 0.051Å) with minor variations in interacting distances of P1:Arg180 (peptide RIME) main chain amino group with the zinc and Glu212 of the enzyme. All of our discussion in this paper is based on the substrate peptide RIME bound to the B molecule.
TABLE 1.
Data collection and refinement statistics
| Parameter | Value |
|---|---|
| Data collection statistics | |
| Wavelength (Å) | 1.08 |
| Resolution (Å) | 50-2.25 |
| Space group | P21212 |
| Cell dimensions | a = 89.41 Å, b = 144.738 Å, c = 83.303 Å, α = β = γ = 90° |
| Molecules/asymmetric unit | 2 |
| Redundancy (overall/outermost shell) | 13.1 (8.5) |
| I/σ(I) | 11.1 (3) |
| Rmerge (overall/outmost shell) | 0.06 (0.22) |
| Completeness (%) (overall/outmost shell) | 94.5 (67.3) |
|
No. of Reflections
|
49224
|
| Refinement statistics | |
| Resolution range (Å) | 50-2.25 |
| No. of reflections | 47715 |
| Completeness (working+test) (%) | 91.6 |
| Rfactor | 0.23 |
| Rfree | 0.28 |
| No. of protein atoms | 6595 |
| No. of sulfate ions | 8 |
| No. of water molecules | 251 |
| B values | |
| From Wilson plot (Å2) | 21.2 |
| Mean B value (overall, Å2) | 37.6 |
| Root mean square deviation bonds (Å) | 0.008 |
| Root mean square deviation angles (degrees) | 1.4 |
| Ramachandran plot analysis: | |
| Most favored region (additionally allowed) (%) | 85.6 (13.2) |
| Generously allowed region (disallowed) (%) | 1.0 (0.3) |
RESULTS AND DISCUSSION
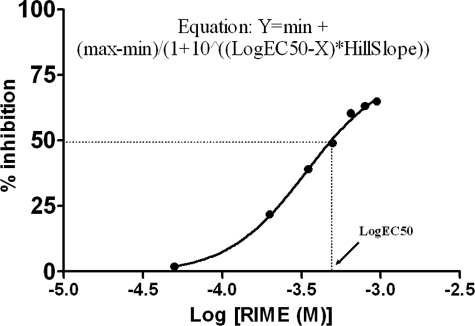
Arg180-Ile181-Met182-Glu183-Amide (RIME; P1–P3′) Catalytically Competes with SNAP-25-(146–186)—We assayed the catalytic activity of BoNT E on SNAP-25-(146–186) using HPLC and determined the kinetic values. It showed km of 8.57 × 10–5 m and a kcat of 2830 min–1. The tetrapeptide RIME of SNAP-25-(180–183) represents P1–P3′ residue for BoNT E and fairly competes with SNAP-25-(146–186) with Ki ∼69 μm, suggesting its binding to the active site and its role as a competitive substrate inhibitor (Fig. 1).
FIGURE 1.
BoNT E enzyme inhibition assay with SNAP-25-RIME (P1–P3′) using the 146–186-amino acid-long peptide substrate. The tetrapeptide showed an IC50 value of 340 μm (GraphPad software; logEC50 = IC50) and Ki of 69 μm using the Cheng-Prusoff equation, Ki = IC50/1 + ([S]/km). [S] and km are the substrate concentration and Michaelis-Menten constant, respectively.
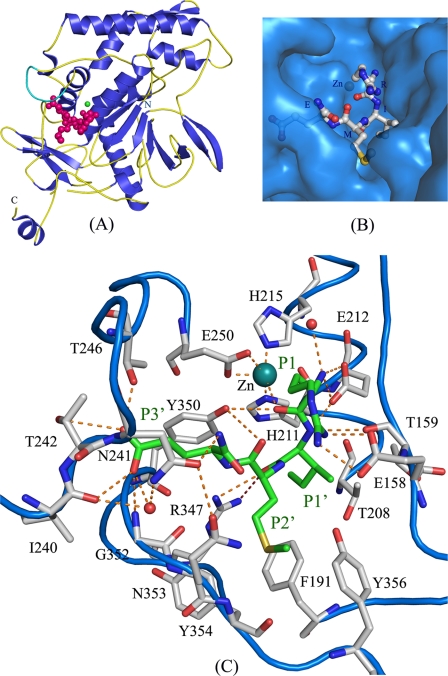
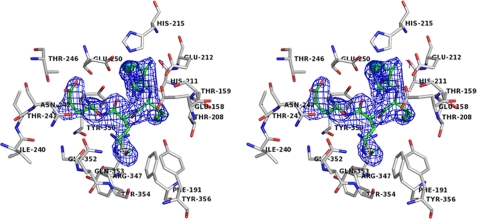
Overall Structure and Crucial Interactions of BoNT E with Inhibitory Peptide (RIME)—BoNT E recognizes and cleaves SNAP-25 substrate specifically between P1:Arg180-P1′:Ile181. In our complex structure, RIME binds to the active site cavity of BoNT E without being cleaved (Fig. 2, A and B), interacts with several residues (Fig. 2C), and shows well defined electron density in the omit map in molecule B (Fig. 3). The crucial interactions of various peptide residues (P1–P3′) and P2:Asp179 modeling results are discussed below.
FIGURE 2.
The SNAP-25 P1:Arg180-P1′:Ile181-P2′:Met182-P3′:Glu183-amide (RIME) binding and interactions at and near the active site of BoNT E. A, schematic diagram of the BoNT-E-RIME complex structure. The native substrate-free and complex structures vary with the addition of the 250 loop (cyan diagram). BoNT E is shown in a blue and yellow diagram, RIME in a magenta ball and stick model, and zinc in a green sphere model. Only molecule B is shown for clarity. B, BoNT E is shown in a blue surface model and RIME in a white ball and stick model to show that the substrate peptide binds in a cavity at and near the active site. C, the dashed line in orange shows the interactions of P1–P3′ residues of RIME with various residues of the S1–S3′ pocket of BoNT E. The RIME and enzyme residues are shown in green and white ball and stick models, respectively. BoNT E secondary structure is shown in a blue loop diagram. The RIME (P1–P3′) and BoNT E residues are labeled in green and black, respectively. Figs. 2, 3, 5, and 6 are made using the PYMOL program (38).
FIGURE 3.
The walleye stereo view of the substrate electron density omit map contoured at the 1σ level at RIME peptide (green ball and stick model, unlabeled for clarity). BoNT E residues interacting with RIME are shown in a white ball and stick model and labeled in black.
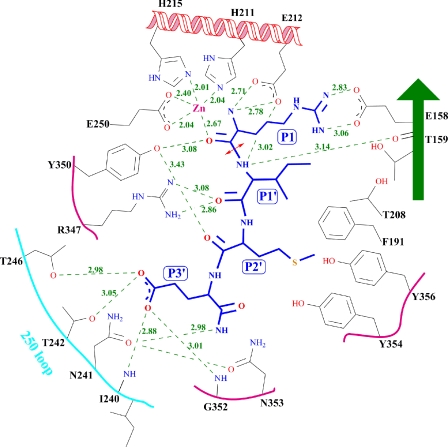
Docking of P1:Arg180 at the Active Site—P1:Arg180 makes interactions required for proper docking of P1 residue to the active site (Figs. 2C and 4). Its carbonyl oxygen interacts with both the catalytic zinc (2.69 Å) and Tyr350 (3.08 Å) and displaces the nucleophilic water (Figs. 2C and 4). In wild type protein structure (Protein Data Bank code 1T3A), the nucleophilic water is at 2.18 Å from zinc. The oxygen of P1 makes a tetrahedral arrangement similar to that of nucleophilic water. Interactions between zinc, Tyr350 (conserved in all BoNTs), and nucleophilic water are common to all catalytic domain structures. The Y350A mutant of BoNT E led to complete loss of catalytic inactivity (at ≤500 nm concentration) (23). The P1:Arg180 main chain amino group interacts with both Oe1 and Oe2 of Glu212, displacing a water molecule present in the native structure. However, it interacts with another water present in the current structure. Glu212, a general base, plays a crucial role in catalysis, and the E212Q mutation led BoNT E to be catalytically inefficient without alteration in protein conformation (23).
FIGURE 4.
The schematic representation of interactions and distances of the RIME peptide representing the P1-P1′-P2′-P3′ residues of SNAP-25 with BoNT E residues identifying the S1-S1′-S2′-S3′ pocket. The RIME peptide and BoNT E substrate subsite residues are in a blue and gray stick model. The distances (Å) are given in green numbers. The red double arrowhead points to the scissile bond of cleavage. The figure is made using the Chemdraw program (CambridgeSoft).
In the crystal structure, P1:Arg180 side chain NH1 and NH2 form a strong salt bridge with Glu158 and stabilize P1 at the active site (Fig. 2C). Glu158 is conserved in BoNTs E, F, and B and is Asp in C1, D, and G and Gln in A. The sequence analysis of SNAP-25 and VAMP2 showed that they have one of Q/R/K at the P1 position (except BoNT G), which can complement well with E/D/Q at the equivalent position of Glu158 (BoNT E) in all serotypes (24). A triple mutation E158A/T159A/N160A in BoNT E decreased the catalytic efficiency by ∼8-fold and decreased substrate affinity as compared with the wild type enzyme (23). Interestingly, the R180K (P1) variant in murine SNAP-23 still shows cleavage by BoNT E (25).
P1′:Ile181 Is Important for Specificity and Docking at the Active Site—P1′:Ile181 main chain N interacts with the side chain Oe1 and Oe2 of catalytic base Glu212 and also with Thr159 oxygen (Figs. 2C and 4). P1′ oxygen interacts with the side chain NH1 and NH2 groups of the conserved residue Arg347. The R347A mutation reduced the catalytic efficiency ∼1000-fold relative to the wild type (23). These interactions are presumably conserved among the other serotypes with their specific substrates and help to properly position and stabilize the substrate. However, the ones that are not conserved are side chain interactions of P1′ and the integrity of the S1′ pocket. Here, the hydrophobic P1′:Ile181 is stabilized in the S1′ pocket comprising Thr159, Phe191, and Thr208, confirming our earlier prediction (Figs. 2C and 4) (23). This seems to be typically different for various serotypes defining the specificity. Mutational studies on Thr159, Thr208, and Phe191 showed markedly lower kcat values by ∼20, ∼30, and ∼80-fold, respectively, for SNAP25 cleavage, relative to wild type (26). F191N mutation also showed a lower (∼100-fold) kcat. Mutagenesis of P1′:Ile181 to Cys in 141–206-amino acid-long SNAP-25 led it to be completely resistant to cleavage by BoNT E.3 However, change to Ala reduced the activity by ∼75-fold relative to wild type (27).
P1′ is the crucial residue where mutations are hardly tolerated and any mutation leads to either no or poor cleavage (25, 27). The S1′ pocket residues also play a prime role in delivering substrate specificity by allowing optimal docking of a P1′ residue of specific size and charge. It is tempting to speculate that probably an improper docking of the P1′ residue also affects the proper docking of the P1 residue, which abrogates the phenotype. We therefore conclude that the P1′ residue and its respective S1′ pocket together contribute primarily to the optimum docking of the substrate at the active site and to scissile bond specificity. Not surprisingly, most of the variations observed are at the P1′ position in various substrates as compared with the P1 position (24).
P2′:Met182 Interactions and the Dual Role of Phe191 of BoNT E—In the crystal structure, Tyr350 OH interacts with P2′: Met182 oxygen and helps in stabilizing its main chain (Figs. 2C and 4). However, the hydrophobic side chain resides in the hydrophobic pocket formed by Phe191, Tyr354, and Tyr356. Phe191 plays a dual role of stabilizing both P1′:Ile181 and P2′: Met182 side chains (Figs. 2C and 4). The above observation is supported by the fact that F191A mutation showed ∼75-fold lower kcat than the wild type enzyme (26). All of the above three residues are not conserved in various serotypes except for Phe191, corresponding to Phe194 in BoNT A, but the Phe194 side chain takes a different rotamer position in both native substrate-free (Protein Data Bank code 1E1H) and complex structure of BoNT A (Protein Data Bank code 1XTG), possibly due to the presence of different surrounding residues unlike BoNT E. Mutation of P2′:Met182 to Val reduced the catalytic activity of BoNT E on SNAP-25 by ∼25% relative to wild type (27).
The current structure confirms that Arg347 and Tyr350 play a crucial role in transition state stabilization by allowing proper docking of the main chain of P1, P1′, and P2′ residues at the active site.
P3′:Glu183 Interactions with the 250 Loop—In the native substrate-free protein crystal structure (Protein Data Bank code 1T3A), the electron density for the region 234–244 residues (250 loop) was disordered and thus was not modeled, but in the present structure, the electron density is well defined, allowing this region to be modeled unambiguously in molecule B (Fig. 2A). Ordering of the 250 loop is probably due to the interactions of P3′:Glu183 with Ile240, Thr241, Asn242, and Thr246 of BoNT E (Figs. 2C and 4). In the present structure, the carboxylate side chain of P3′: Glu183 takes two rotamer positions, both involved in multiple interactions with the 250 loop. Two additional water molecules and Gly352 are also contributing in stabilizing the P3′ (Fig. 2C). A functional importance assay on the P3′:E183A mutant showed the catalytic activity reduced by ∼25%, suggesting the requirement of Glu for optimal activity (27).
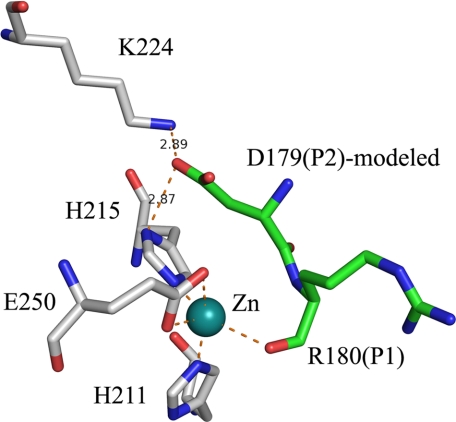
Modeling of P2:Asp179 in BoNT E Structure—P2:Asp179 is one of the crucial residues for better positioning of the scissile residue of the substrate. Mutation of P2:Asp179 to Val and Ala reduced the catalytic efficiency of BoNT E by ∼290- and 60-fold, respectively (27). Therefore, to trace the S2 pocket in BoNT E, the current and BoNT A-SNAP-25 peptide structures (Protein Data Bank code 1XTG) (16) are compared. P2:Asp179 of SNAP-25 is modeled similar to P2:Asn196 (in BoNT A), close to P1:Arg180 in BoNT E structure with some minor adjustments in rotation and translation. P2:Asn196 (in BoNT A) side chain interacts with His227 (BoNT A) ND1 in 1XTG, but here, P2:Asp179 side chain will be stabilized by interactions with Lys224 NZ and His215 ND1 of BoNT E (Fig. 5). These interactions are probably stronger than for Asn196 in BoNT A, since the K224A BoNT E mutant showed reduced (<80%) catalytic activity (26).
FIGURE 5.
Modeling of P2:Asp179 and identification of the S2 pocket residues. This modeling is based on P2:Asn196 of SNAP-25 as in a complex structure of SNAP-25-BoNT A (Protein Data Bank code 1XTG) with few adjustments. RIME and BoNT E are shown in green and white ball and stick models.
Interestingly, variation at the P2 position observed in human SNAP-23 and murine SNAP-23, which have 184IKRI187 and 183IQKI186 sequence in place of 178IDRI181 of SNAP-25 and show resistance and poor cleavage, respectively, by BoNT E (25). P2:Asp179 (SNAP-25) complements well with His215 and Lys224, whereas P2:Lys185 (human SNAP-23) in its place will not and may affect drastically the scissile bond alignment and thus the phenotype. However, P2:Gln184 (murine SNAP-23) still may form a hydrogen bond with Lys224 and may not affect the phenotype that severely.
S1′ Pockets in BoNTs Are Unique—The BoNTs E/C/D/G crystal structures (Protein Data Bank entries 1T3A, 2QN0, 2FPQ, and 1ZB7) are compared for the integrity of the S1 and S1′ pockets, since they have a positively charged side chain for P1 (except BoNT G) and a hydrophobic P1′ residue (Table 2). The S1′ pocket of E, C, and D are hydrophobic, but fine variations in S1′ in each serotype make them very specific for a particular residue. Pro168 in place of Thr159 and also relative narrowing of the S1′ pocket in BoNT C may not allow any bigger side chain residue at P1′. Although the S1′ pocket is unique in all serotypes, BoNT C and D share some similarity. The presence of Ile instead of Ala at position 226 and the flexibility of the S1′ pocket (Protein Data Bank code 2FPQ), might facilitate BoNT D to dock the bigger side chain of P1′. However, P1′ mutations to Ala did not completely abrogate but rather drastically reduced the activity of BoNTs, since the presence of Ala at P1′ may not allow a stable docking and optimal alignment at the active site required for catalysis. The lower catalytic efficiency of BoNT C (scissile bond Arg–Ala) could also be due to similar reasons.
TABLE 2.
The comparison of the S1 and S1′ side chain pocket residues of BoNT E with other serotypes (C/D/G) displaying similarity in P1 and P1′ residue by charge and hydrophobicity, respectively
The S1 pocket also includes zinc and Tyr (Tyr350 in BoNT E), common to all serotypes.
| BoNT E | BoNT C | BoNT D | BoNT G | |
|---|---|---|---|---|
| P1—P1′ | Arg-Ile | Arg/Lys-Ala | Lys-Leu | Ala-Ala |
| S1 pocket | Glu158 | Asp167 | Asp167 | Asp169 |
| S1′ pocket | Thr159 | Pro168 | Tyr168 | Asn170 |
| Phe191 | Leu200 | Leu200 | Asn201 | |
| Thr208 | Ile226 | Ala226 | Thr227 | |
| Met221 | Met221 | Tyr376 |
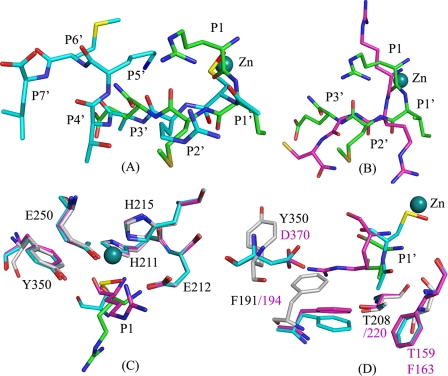
Substrate Binding Subsites Vary between BoNT E and A—In order to evaluate the variation/similarities among serotypes about substrate recognition/specificity at the active site, we structurally aligned BoNT E-SNAP-25-(180–183) with the existing structures of BoNT A with inhibitors Arg-Arg-Gly-Cys (Protein Data Bank code 3C88) and N-Ac-CRATKML (Protein Data Bank code 3BOO) (Fig. 6, A and B) (17–19). DALILITE structural alignment showed a root mean square deviation of 2.3 Å between RIME and RRGC structures for 386 Cα atoms. Although both BoNT E and A recognize SNAP-25, they cleave at different scissile bonds. BoNT E cleaves between P1:Arg180 and P1′:Ile181, whereas BoNT A cleaves between P1:Gln197 and P1′:Arg198. N-Ac-CRATKML has SNAP-25 sequence 198RATKML203 (P1′–P6′).
FIGURE 6.
The superposition of BoNT E-substrate peptide RIME (P1–P3′) with the BoNT A inhibitor peptides, N-Ac-CRATKML (Protein Data Bank code 3BOO) and RRGC (Protein Data Bank code 3C88). Here the P1, P1′, P2′, and P3′ positions in the RIME structure are Arg-Ile-Met-Glu; in RRGC they are Arg-Arg-Gly-Cys; and in N-Ac-CRATKML they are oxidized Cys-acetate-Arg-Ala. BoNT E, RIME, BoNT A-RRGC, and BoNT A-N-Ac-CRATKML structures are shown in white, green, magenta, and cyan ball and stick models, respectively. Zinc is in a deep cyan color for RIME (zinc of other structures is not shown for clarity). A, the position of oxidized cysteine (CSO), acetate (ACE), Arg, and Ala in the N-Ac-CRATKML structure aligns well with the P1:Arg-, P1′:Ile-, P2:Met-, and P3′:Glu- of the BoNT E-RIME structure but for BoNT A in actual RATKML represents P1′–P6′. B, the P1-P1′-P2′ of RRGC aligns with RIME with few differences. Cys of RRGC goes in a very different spatial position, which is different from the equivalent P3′:Glu for BoNT E and Ala in N-Ac-CRATKML. C, superposition of P1 residues and S1 pockets in all three structures. Only BoNT E residues are labeled. D, superposition of P1′ residues and S1′ pockets in all three structures. Black and magenta labels represent BoNT E and A, respectively.
A structural alignment with the above two structures showed that the position of the P1 main chain oxygen of the RIME structure aligns reasonably with the oxygen of Arg of RRGC and the sulfur of cysteine of N-Ac-CRATKML, but the spatial position of the amino group of the RIME structure is at variance with RRGC (Fig. 6C). Also, the side chain rotamer position of P1:Arg180 is different from the side chain of Arg of RRGC (the residue is underlined for clarity). In our structure, the P1:Arg180 side chain makes a strong salt bridge with the Glu158 side chain, whereas the side chain of Arg of RRGC interacts with a sulfate ion and Glu164 (Asn160 in BoNT E) in BoNT A. However, in the real substrate, P1 is Gln instead of Arg for BoNT A.
Comparison of P1′:Ile181 with Arg in RRGC reveals variation in spatial positioning as well as docking (Fig. 6D). Ile has a smaller side chain and goes deep inside the cavity and is stabilized by the Phe191 (Phe194 in BoNT A), Thr159 (Phe163 in BoNT A), and Thr208 (Thr220 in BoNT A). A residue corresponding to Phe191 exists in a similar position in BoNT A but has a different rotamer position in both native substrate-free and complex structures (Protein Data Bank codes 1E1H and 1XTG). Also, Thr159 is replaced with a relatively bigger residue, Phe163 (Fig. 6D). The long side chain of Arg of RRGC takes a different rotamer position, making a salt bridge interaction with the Asp370 (BoNT A). Asp370 (BoNT A) corresponds to Tyr354 in BoNT E, which has a different rotamer position and helps in stabilizing the P2′:Met182 side chain (Fig. 6D).
Interestingly, in the N-Ac-CRATKML structure, the P1′ position is occupied by the N-terminal acetate moiety. The methyl group of the acetate occupies the same position as CG1 of P1′:Ile181 in the RIME structure and is stabilized by Phe163 (Thr159) and Phe194 (Phe191). However, the carboxylate part of acetate interacts with the main chain amino and carbonyl groups of the enzyme, indicating a facilitated docking.
In BoNT E, P2′:Met182 is stabilized in a hydrophobic pocket formed by Phe191, Tyr354, and Tyr356. BoNT A lacks this pocket due to the presence of Asp370 (Fig. 6D). Gly in RRGC and Ala in N-Ac-CRATKML do not interact with protein, unlike P2′ in BoNT E. P3′:Glu183 interacts with 250 loop residues, whereas Cys (RRGC) takes a different turn (Fig. 6B) and is stabilized by the hydrophobic pocket of the 250 loop. But the actual P3′ residue is Thr, which might not bind in a similar manner as Cys. In BoNT A, the 250 loop is bigger and has a spatial position different from that of the BoNT E 250 loop. Thr in N-Ac-CRATKML was stabilized by a water molecule interacting with Asp370 in BoNT A. Interestingly, RATKML (P1′–P6′) of N-Ac-CRATKML represents P2′–P7′ and occupies the S2′–S7′ subsites instead of S1′–S6′ (Fig. 6A). This is because the oxidized cysteine side chain occupies the place of the P1 main chain, and its N-terminal acetate part goes in the S1′ pocket, and from then on the substrate residues slide by one. Surprisingly, this indicated the very interesting possibility that the docking at the S1 and S1′ pocket can decide the overall docking of other residues in BoNT A by challenging the substrate specificity. The P1′–P6′ residues dock in the S2′–S7′ pockets, although they do not match with the actual P2′–P6′ residues.
Overall analysis of structures suggested a variation in the S1′-S3′ pocket integrity between BoNT E and A. However, the presence of hydrophobic residues at the S1′ pocket in BoNT A, Phe194 (Phe191 in BoNT E) and Phe163 (Thr159 in BoNT E) might allow docking of hydrophobic side chains, but the hydrophobic interactions of both phenylalanines and a different rotamer position of Phe194, unlike Phe191 (BoNT E), cause a shallower S1′ pocket in BoNT A than in E. The Arg side chain at P1′ takes a suitable rotamer position and is stabilized differently than Ile in BoNT E. Also, in BoNT E, the Phe191 side chain adopts different rotamer positions, opening the S1′ pocket for residues like Ile of suitable size to fit in. The absence of a similar residue at the Asp370 (BoNT A) position will restrict the binding of residues like Arg or charged residue at P1′ and P2′ position in BoNT E.
Substrate Cleavage Strategy for BoNT E—Initially, it was a surprise in our crystal structure to have well defined electron density for the full tetrapeptide. However, combining and comparing the results of the enzyme inhibition and structural studies led us to better understand the molecular events of binding and cleavage. We assume that the reason the peptide did not get cleaved is due to the requirement of a minimal length as mentioned earlier. However, the sequence of 178IDRIME183 as an N-terminal GST fusion protein preceded by SNAP-25-(93–146) is also shown as cleavable length (27). The SNAP-25-(166–181) sequence that has C-terminal Arg-Ile showed inhibition (50%) of the catalytic activity of BoNT E at 400 μm concentration (27).
Also, our crystal structure clearly reveals that the mere presence of P1–P1′ and additional P2′ and P3′ are not sufficient for the cleavage of the substrate but are sufficient for recognition and binding at the active site. Thus, both P1 and P1′ with either N- or C-terminal extensions are not sufficient enough to bring the proper cleavage. Rather, they can serve as potential candidates for substrate inhibitors.
To understand BoNT E specificity for P1:Arg181-P1′:Ile181, we analyzed the SNAP-25 sequence (amino acids 1–206). It has at least three regions containing a similar scissile bond (RI), 57LERIEE62, 178IDRIME183, and 189KTRIDE194, but BoNT E selects only 178IDRIME183 for cleavage. This is probably due to the prime requirement of exosite interactions and/or cleavage region in larger peptides. Also, the size and type of P2 and P2′ may play significant roles.
Therefore, we assume that at least three residues on either side of the scissile bond are needed for precise alignment of the scissile bond required for the cleavage. However, all of them may not contribute to substrate affinity or specificity, although longer substrates serve better for high affinity binding and faster scissile bond cleavage. Also, simultaneous substrate recognition both at the exosites and the active site facilitates the high affinity binding and improved rate of cleavage in longer substrates. Together, the unique exosites and subsites at and near the active site determine the substrate selectivity and scissile bond specificity, which are unique in all BoNTs.
Implications in Inhibitor Design for BoNT E—The extreme toxicity and fast action of BoNT E in blocking neurotransmission requires advancement in developing countermeasures against it. Although several inhibitors are being developed for BoNT A and B and a few for BoNT F (18, 19, 28–36), a knowledge gap exists for BoNT E. The unavailability of structural information regarding substrate-enzyme interactions is the major reason for the existing gap. We for the first time report the substrate binding at the active site of full-length catalytically active enzyme on any BoNTs. Also, the identification of S1–S3′ subsites, hydrophobic nature of S1′ and S2′ pockets, and the positive charge nature of S2 can be exploited in designing of better specific inhibitors for BoNT E. Interestingly, the substrate RIME itself acts as an inhibitor and specifically binds at the active site. Overall, the BoNT E-substrate peptide complex crystal structure delivers the specific required information essential for structure-based design of potent inhibitors.
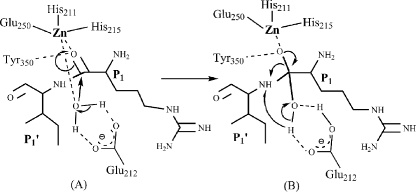
Catalytic Mechanism of BoNT Serotype E—Based on our complex structure analysis and previous mutational analysis of active site residues of BoNT E, we propose a mechanism of catalysis for botulinum neurotoxins (Fig. 7). Here in our structure, Arg181 carbonyl oxygen displaces the nucleophilic water, and the main chain nitrogen displaces the water coordinated to Glu212. Glu212, being a general base, activates a water molecule, which donates OH to the carbonyl oxygen of Arg180 and hydrogen to the leaving amino of Ile181. In our structure, we do not see any water close to Glu212. Perhaps in the presence of larger substrates with P2/P3 residues and/or the S4 SNARE motif (37), there is a possibility of subtle rearrangements at the active site to achieve the transition state. The transition state is often the result of strain or distortion of the reactants to form the particular electronic structure needed for the proper collision and product formation. However, due to smaller substrate length, a transition state is not achieved, which leads RIME to act as a substrate inhibitor.
FIGURE 7.
A mechanism of catalysis and transition state interactions adopted by BoNT E. The precise substrate docking at the active site (stage A) leads to a transition state (stage B), which facilitates cleavage of the scissile bond. The figure is made using Chemdraw program (CambridgeSoft).
Acknowledgments
We gratefully acknowledge data collection support from beamline X29 (National Synchotron Light Source).
This work was supported, in whole or in part, by National Institutes of Health Grant 1R56Al058175. This work was also supported by Grants DAMD17-02-2 and DTRA BO742081 under Department of Energy Prime Contract DEAC02-98CH10886 with the Brookhaven National Laboratory. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. The atomic coordinates and structure factors (code 3D3X) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
Footnotes
The abbreviations used are: BoNT, C. botulinum neurotoxin; SNARE, soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor; HPLC, high pressure liquid chromatography.
R. Agarwal and S. Swaminathan, unpublished results.
References
- 1.DasGupta, B. R., and Rasmussen, S. (1983) Toxicon 21 566–569 [DOI] [PubMed] [Google Scholar]
- 2.Dasgupta, B. R., and Rasmussen, S. (1983) Toxicon 21 535–545 [DOI] [PubMed] [Google Scholar]
- 3.Sathyamoorthy, V., and DasGupta, B. R. (1985) Biochem. Biophys. Res. Commun. 127 768–772 [DOI] [PubMed] [Google Scholar]
- 4.Sathyamurthy, V., and Dasgupta, B. R. (1985) J. Biol. Chem. 260 10461–10466 [PubMed] [Google Scholar]
- 5.Montecucco, C., and Schiavo, G. (1994) Mol. Microbiol. 13 1–9 [DOI] [PubMed] [Google Scholar]
- 6.Fiebig, K. M., Rice, L. M., Pollock, E., and Brunger, A. T. (1999) Nat. Struct. Biol. 6 117–123 [DOI] [PubMed] [Google Scholar]
- 7.Foran, P., Lawrence, G. W., Shone, C. C., Foster, K. A., and Dolly, J. O. (1996) Biochemistry 35 2630–2636 [DOI] [PubMed] [Google Scholar]
- 8.Wang, J., Meng, J., Lawrence, G. W., Zurawski, T. H., Sasse, A., Bodeker, M. O., Gilmore, M. A., Fernandez-Salas, E., Francis, J., Steward, L. E., Aoki, K. R., and Dolly, J. O. (2008) J. Biol. Chem. 283 16993–17002 [DOI] [PubMed] [Google Scholar]
- 9.Segelke, B., Knapp, M., Kadkhodayan, S., Balhorn, R., and Rupp, B. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 6888–6893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson, M. A., and Stevens, R. C. (2000) Nat. Struct. Biol. 7 687–692 [DOI] [PubMed] [Google Scholar]
- 11.Jin, R., Sikorra, S., Stegmann, C. M., Pich, A., Binz, T., and Brunger, A. T. (2007) Biochemistry 46 10685–10693 [DOI] [PubMed] [Google Scholar]
- 12.Arndt, J. W., Yu, W., Bi, F., and Stevens, R. C. (2005) Biochemistry 44 9574–9580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arndt, J. W., Chai, Q., Christian, T., and Stevens, R. C. (2006) Biochemistry 45 3255–3262 [DOI] [PubMed] [Google Scholar]
- 14.Agarwal, R., Eswaramoorthy, S., Kumaran, D., Binz, T., and Swaminathan, S. (2004) Biochemistry 43 6637–6644 [DOI] [PubMed] [Google Scholar]
- 15.Agarwal, R., Binz, T., and Swaminathan, S. (2005) Biochemistry 44 11758–11765 [DOI] [PubMed] [Google Scholar]
- 16.Breidenbach, M. A., and Brunger, A. (2004) Nature 432 925–929 [DOI] [PubMed] [Google Scholar]
- 17.Silvaggi, N. R., Wilson, D., Tzipori, S., and Allen, K. N. (2008) Biochemistry 27 5736–5745 [DOI] [PubMed] [Google Scholar]
- 18.Silvaggi, N. R., Boldt, G. E., Hixon, M. S., Kennedy, J. P., Tzipori, S., Janda, K. D., and Allen, K. N. (2007) Chem. Biol. 14 533–542 [DOI] [PubMed] [Google Scholar]
- 19.Kumaran, D., Rawat, R., Ludivico, M. L., Ahmed, S. A., and Swaminathan, S. (2008) J. Biol. Chem. 283 18883–18891 [DOI] [PubMed] [Google Scholar]
- 20.Agarwal, R., Eswaramoorthy, S., Kumaran, D., Dunn, J. J., and Swaminathan, S. (2004) Protein Expression Purif. 34 95–102 [DOI] [PubMed] [Google Scholar]
- 21.Studier, F. W. (2005) Protein Expression Purif. 41 207–234 [DOI] [PubMed] [Google Scholar]
- 22.Brunger, A. T., Adams, P. D., Clore, G. M., Delano, W. L., Gros, P., GrosseKunstleve, R. W., Jiang, J. S., Kuszwewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Somonsom, T., and Warren, G. L. (1998) Acta. Crystallogr. Sect. D 54 905–921 [DOI] [PubMed] [Google Scholar]
- 23.Agarwal, R., Binz, T., and Swaminathan, S. (2005) Biochemistry 44 8291–8302 [DOI] [PubMed] [Google Scholar]
- 24.Schiavo, G., Matteoli, M., and Montecucco, C. (2000) Physiol. Rev. 80 717–766 [DOI] [PubMed] [Google Scholar]
- 25.Vaidyanathan, V. V., Yoshino, K., Jahnz, M., Dorries, C., Bade, S., Nauenburg, S., Niemann, H., and Binz, T. (1999) J. Neurochem. 72 327–337 [DOI] [PubMed] [Google Scholar]
- 26.Chen, S., and Barbieri, J. T. (2007) J. Biol. Chem. 282 25540–25547 [DOI] [PubMed] [Google Scholar]
- 27.Chen, S., and Barbieri, J. T. (2006) J. Biol. Chem. 281 10906–10911 [DOI] [PubMed] [Google Scholar]
- 28.Adler, M., Nicholson, J. D., Cornille, F., and Hackley, B. E., Jr. (1998) FEBS Lett. 429 234–238 [DOI] [PubMed] [Google Scholar]
- 29.Anne, C., Turcaud, S., Quancard, J., Teffo, F., Meudal, H., Fournie-Zaluski, M. C., and Roques, B. P. (2003) J. Med. Chem. 46 4648–4656 [DOI] [PubMed] [Google Scholar]
- 30.Anne, C., Turcaud, S., Blommaert, A. G., Darchen, F., Johnson, E. A., and Roques, B. P. (2005) Chembiochem 6 1375–1380 [DOI] [PubMed] [Google Scholar]
- 31.Johnson, S. L., Chen, L. H., Harbach, R., Sabet, M., Savinov, A., Cotton, N. J., Strongin, A., Guiney, D., and Pellecchia, M. (2008) Chem. Biol. Drug. Des. 71 131–139 [DOI] [PubMed] [Google Scholar]
- 32.Burnett, J. C., Opsenica, D., Sriraghavan, K., Panchal, R. G., Ruthel, G., Hermone, A. R., Nguyen, T. L., Kenny, T. A., Lane, D. J., McGrath, C. F., Schmidt, J. J., Vennerstrom, J. L., Gussio, R., Solaja, B. A., and Bavari, S. (2007) J. Med. Chem. 50 2127–2136 [DOI] [PubMed] [Google Scholar]
- 33.Burnett, J. C., Ruthel, G., Stegmann, C. M., Panchal, R. G., Nguyen, T. L., Hermone, A. R., Stafford, R. G., Lane, D. J., Kenny, T. A., McGrath, C. F., Wipf, P., Stahl, A. M., Schmidt, J. J., Gussio, R., Brunger, A. T., and Bavari, S. (2007) J. Biol. Chem. 282 5004–5014 [DOI] [PubMed] [Google Scholar]
- 34.Burnett, J. C., Schmidt, J. J., McGrath, C. F., Nguyen, T. L., Hermone, A. R., Panchal, R. G., Vennerstrom, J. L., Kodukula, K., Zaharevitz, D. W., Gussio, R., and Bavari, S. (2005) Bioorg. Med. Chem. 13 333–341 [DOI] [PubMed] [Google Scholar]
- 35.Blommaert, A., Turcaud, S., Anne, C., and Roques, B. P. (2004) Bioorg. Med. Chem. 12 3055–3062 [DOI] [PubMed] [Google Scholar]
- 36.Schmidt, J. J., and Stafford, R. G. (2005) Biochemistry 44 4067–4073 [DOI] [PubMed] [Google Scholar]
- 37.Washbourne, P., Pellizzari, R., Baldini, G., Wilson, M. C., and Montecucco, C. (1997) FEBS. Lett. 418 1–5 [DOI] [PubMed] [Google Scholar]
- 38.DeLano, W. L. (2002) The PyMOL User's Manual, DeLano Scientific, San Carlos, CA