Abstract
We present a concept for reducing formation of fibrotic deposits by inhibiting self-assembly of collagen molecules into fibrils, a main component of fibrotic lesions. Employing monoclonal antibodies that bind to the telopeptide region of a collagen molecule, we found that blocking telopeptide-mediated collagen/collagen interactions reduces the amount of collagen fibrils accumulated in vitro and in keloid-like organotypic constructs. We conclude that inhibiting extracellular steps of the fibrotic process provides a novel approach to limit fibrosis in a number of tissues and organs.
Collagen I is the most abundant structural protein of connective tissues such as skin, bone, and tendon. This protein is first synthesized as a precursor molecule, procollagen, that is characterized by the presence of a rod-like central triple-helical domain flanked by short linear telopeptides and globular N-terminal and C-terminal propeptides (1). Single procollagen molecules are the building blocks for the biologically and mechanically relevant collagen fibrils. Formation of collagen fibrils is initiated by enzymatic cleavage of the N-terminal and the C-terminal propeptides. The N-terminal propeptides are cleaved by a group of enzymes that includes a disintegrin and metalloprotease with thrombospondin motifs (ADAMTS)-2, -3, and -14, whereas the C-terminal propeptides are cleaved by the metalloprotease bone morphogenetic protein 1 (BMP-1)2 and by the other members of a closely related family of mammalian tolloid-like metalloproteases (2–4). Such a removal of procollagen propeptides exposes telopeptides, which by engaging in site-specific intermolecular interactions drive collagen self-assembly.
In native tissues a precise balance between the processes of biosynthesis and degradation maintains the physiological homeostasis of tissue collagens. At the same time, accelerated biosynthesis is required for proper wound healing, whereas excessive accumulation of collagen is the hallmark of a number of localized fibrotic diseases, such as keloids and hypertrophic scars, and systemic fibrosis, such as systemic scleroderma.
Localized fibrotic reactions are quite common and frequently develop as a consequence of surgical procedures. For instance, after surgery of the abdomen, the formation of excessive scar tissue around abdominal organs, such as the intestines, can interfere with the functionality of such organs and may cause severe pain and even death. Another situation where excessive scar formation presents a major complication is in the eye after glaucoma surgery performed to create a pressure-maintenance valve. Frequently, however, excessive scar formation closes this pressure-reducing valve, thereby forcing the intraocular pressure to rise (5). Moreover, excessive scarring of the vocal folds may severely alter their ability to vibrate, thereby causing a number of voice disorders (6).
At present, several biological processes critical for development of fibrotic lesions are considered potential targets for inhibitors of fibrosis. These inhibitors aim at (i) reducing inflammatory processes associated with fibrosis, (ii) inhibiting biological functions of cytokines and growth factors that promote fibrosis, (iii) reducing cell proliferation, and (iv) decreasing biosynthesis and processing of procollagens. Because most of those potential targets are involved not only in pathological fibrosis but also in a number of physiological processes, their inhibition is frequently associated with significant adverse effects (7–11).
Here, we tested a new approach to reduce excessive scarring by specifically targeting the extracellular process of formation of collagen fibrils, a main component of fibrotic scars. By employing custom-designed antibodies that specifically bind to the C-terminal telopeptide of the α2-chain of collagen I, we demonstrated that blocking telopeptide-mediated collagen/collagen interaction limits accumulation of collagen fibrils in vitro and in organotypic constructs formed by keloid-derived fibroblasts. Because excessive deposition of collagen fibrils is characteristic of all fibrotic processes, we predict that the basic design for the inhibitors of collagen fibril formation we tested in a skin-based keloid model will be applicable for reducing a number of localized and systemic fibrotic changes in other tissues and organs as well.
EXPERIMENTAL PROCEDURES
Purification of Human Procollagen I—Procollagen I was purified from cell culture media of normal human dermal fibroblasts, as described (12, 13).
Monoclonal Antibodies—Hybridomas were prepared by a commercial company (Abgent, Inc. San Diego, CA). Inhibitory antibodies were raised against a synthetic peptide (GGGYDFGYDGDFYRA) whose sequence corresponds to that of the C-terminal telopeptide of the α2 chain (α2Ct). Initial screening of these antibodies produced by various hybridoma clones was done with synthetic α2Ct. Finally, the specificity of selected antibodies to bind a native epitope was tested against intact human procollagen I and against enzymatically processed procollagen I. In brief, purified procollagen I was processed by BMP-1 (R&D Systems), lysyl endopeptidase (Lys-C; Roche Applied Science) or pepsin (Sigma-Aldrich), as described (14, 15). Note that enzymatic cleavage of procollagen I with BMP-1 or Lys-C leaves telopeptide regions intact, whereas digestion of procollagen with pepsin degrades the telopeptides. At the same time, digestion with all enzymes employed here preserves the triple-helical region of collagen I. Intact procollagen I and products of its enzymatic cleavage were electrophoresed in polyacrylamide gels in denaturing conditions. Subsequently, protein bands were transferred onto nitrocellulose membranes and detected with primary anti-α2Ct antibody and secondary antibodies conjugated with horseradish peroxidase (Sigma-Aldrich). Finally, the protein bands were visualized by chemiluminescence.
The ability of the anti-α2Ct antibody to interact with a native antigen was also tested by applying unprocessed procollagen I and products of its enzymatic digestion directly onto nitrocellulose membranes and detecting these proteins as described above. Moreover, to ensure the lack of any cross-reactivity of analyzed antibodies with employed enzymes, BMP-1, Lys-C, and pepsin alone were also loaded onto nitrocellulose membranes.
Monoclonal antibody, described by Fisher et al. (16) as LF-41, was raised against a peptide (PLDVGAPDQEFGFDVGPVCFL) that corresponds to the C-terminal propeptide of the pro-α1 chain of procollagen I (α1Cp). Specificity of the anti-α1Cp antibody to recognize the native epitopes was tested in a way similar to that described for the anti-α2Ct antibody.
Purification of Monoclonal Antibodies—Isotyping of antibodies was performed by Rockland Immunochemicals, Inc., Gilbertsville, PA. The anti-α2Ct monoclonal antibody (an IgA isoform with kappa light chains) produced by a selected hybridoma clone was purified from cell culture media. In brief, cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum with reduced IgG content (Ultra-Low IgG fetal bovine serum, Invitrogen). About 10 liters of cell culture medium was collected and filtered through glass fiber filters (Millipore, Inc.) to remove cell debris. Subsequently, a protein fraction that included immunoglobulins was precipitated with a 50% saturation of ammonium sulfate. A protein pellet was collected by centrifugation and then resuspended in a buffer consisting of 0.1 m Tris-HCl, pH 7.8, 120 mm NaCl, and 0.02% NaN3. The anti-α2Ct monoclonal antibody was finally purified by affinity chromatography on a Protein-L column (Pierce). Column-bound IgA was eluted with 0.1 m glycine, pH 3.0. Collected fractions were immediately neutralized with a buffer consisting of 1.5 m Tris-HCl, pH 8.0, 120 mm NaCl, and 25 mm EDTA. The purity of the antibody was determined by electrophoresis in polyacrylamide gels.
The anti-α1Cp antibody (an IgG type) was prepared in a similar way, but instead of Protein-L, Protein-G was employed for purification (Pierce). Purified antibodies were dialyzed against phosphate-buffered saline (PBS) and stored at –80 °C.
Cleavage of Procollagen I by BMP-1 in the Presence of Anti-α2Ct Antibody—Because the anti-α2Ct antibody was designed to bind the C-terminal α2 telopeptide, we tested whether its binding would interfere with the enzymatic activity of BMP-1, an enzyme that specifically cleaves procollagen I at the C telopeptide/C propeptide junction (17). Samples containing 50 μg/ml procollagen I and 1.2 or 0.2 μg/ml purified anti-α2Ct antibody solubilized in a buffer supplemented with 5 mm CaCl2 were incubated for 0.5 h at room temperature. Subsequently, recombinant BMP-1 at a concentration of 25 μg/ml was added to the procollagen I-antibody mixtures, and the samples were incubated at 37 °C. At defined time points aliquots were withdrawn and boiled with a protein-loading buffer. After collecting all samples, the products of cleavage of procollagen I by BMP-1 were separated in 7.5% polyacrylamide gels. Proteins were visualized by staining with Coomassie Blue (BioSafe Coomassie, Bio-Rad). Subsequently, employing a computer program, the pixel intensities of bands corresponding to substrates and products of enzymatic digestion were determined (ImageQuant, GE Healthcare). Data from three independent measurements were used to derive rates of cleavage of procollagen I. In addition, samples that contained the anti-α1Cp antibody and those with no antibodies were prepared and analyzed in a similar way.
To exclude the possibility of the presence of any nonspecific proteolytic activities derived from enzymes that could co-purify with procollagen itself or with antibodies, in one set of experiments we employed EDTA, an inhibitor of BMP-1. In brief, samples containing procollagen I, BMP-1, and antibodies were prepared as described above. EDTA was added to these samples to a final concentration of 10 mm. Subsequently, the samples were incubated for 4 h at 37°C and then they were analyzed by gel electrophoresis as described above.
Fibril Formation Assays in Vitro—De novo collagen fibril formation assays were employed to analyze the inhibitory effect of the anti-α2Ct antibody on collagen fibril formation in vitro, as described (15, 18–20). In these assays self-assembly of collagen monomers into fibrils is initiated by enzymatic digestion of procollagen propeptides. In brief, procollagen I at a concentration 230 μg/ml was digested with Lys-C (1 μg/ml) for 0.5 h at 25 °C, experimental conditions in which formation of collagen fibrils does not occur (21). After initial incubation, a possible enzymatic digestion of Lys-C-sensitive antibodies was inhibited by Nα-p-tosyl-l-lysine chloromethyl ketone (TPCK, Sigma-Aldrich) added to a final concentration of 1 mm (19, 22). Subsequently, the anti-α2Ct antibody was added to the Lys-C-processed collagen samples at concentrations of 180 or 10 μg/ml to achieve collagen/antibody molar ratios of about 1:2 or 10:1, respectively. Samples were incubated for 0.5 h at room temperature followed by incubation at 37 °C. To determine the kinetics of fibril formation, samples were analyzed at defined time points, as described (14). In brief, fibril incorporated collagen was separated from the soluble collagen monomers by centrifugation. Supernatant was transferred to a fresh tube, and a fibril-containing pellet was resuspended in 20 μl of a buffer containing 0.1 m Tris-HCl, pH 7.4, 0.4 m NaCl, 25 mm EDTA, and 0.02% NaN3. After boiling all samples with a protein loading buffer, pellet and supernatant collagen fractions were electrophoresed in 7.5% polyacrylamide gels. Subsequently, protein bands were visualized by staining with Coomassie Blue, and the pixel intensities of bands corresponding to the collagen α-chains present in a pellet or in a supernatant fraction were determined by densitometry. Data from independent studies were plotted to determine rates of fibril formation, as described (23). Fibril formation studies in the presence of the anti-α1Cp antibody and those with no antibodies were carried out in a similar way.
Microscopic Analyses of Morphology of Individual Collagen Fibrils—For ultrastructural analysis by transmission electron microscopy, the collagen fibrils were processed as described (18). In brief, 10-μl samples containing fibrils formed for 24 h were transferred onto 200-mesh Formvar-supported and carbon-coated copper grids (Ted Pella, Inc.). Collagen fibrils deposited on the grids were stained negatively with 1% phosphotungstate (pH 7.0) and visualized by a transmission electron microscope operating at 75 kV (model H-7000, Hitashi Ltd., Japan).
Organotypic Cultures of Keloid-derived Fibroblasts—To test the ability of the anti-α2Ct antibody to limit collagen fibril formation in vivo, we employed a system that utilizes keloid-derived fibroblasts cultured in a three-dimensional environment. Keloid fibroblasts from patients undergoing elective surgery were obtained from the Department of Dermatology and Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, South Korea. The cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mm l-glutamine, a mixture of penicillin and streptomycin, and l-ascorbic acid phosphate magnesium salt n-hydrate at a concentration of 40 μg/ml (WAKO Inc., Japan). The organotypic constructs were prepared by employing three-dimensional sponge-like scaffolds fabricated from polylactic acid (OPLA, BD Biosciences). Cells were seeded dynamically into scaffolds by a method adopted from Wang et al. (24). In brief, the scaffolds were placed into cell culture chambers of a bioreactor (Sythecon, Inc.) filled with cell suspension containing 2 × 105 cells/ml, and the cells were allowed to populate scaffolds for 7 days with the culture chambers rotating at 20 rpm. For fibril formation assay, in vitro individual scaffolds were transferred into wells of low-cell adhesion culture plates (Corning, Inc.) and placed into a cell culture incubator onto a platform rotating at 80 rpm. Each day cell culture media supplemented with the tested antibody at a concentration of 70 μg/ml was exchanged in each well. After 2 weeks of culture, samples were collected and prepared for analysis.
Inhibition of collagen deposition was also tested in organotypic constructs implanted subcutaneously into nude mice (Nude-Foxn1nu, HARLAN). After 7 days of incubation in cell culture chambers, four constructs seeded with keloid fibroblasts were implanted subcutaneously into an athymic nude mouse. Subsequently, two of those constructs received a 100-μl dose of an inhibitory antibody present at a concentration of 1.5 mg/ml, injected every 3 days directly into the scaffold and the surrounding area. Two remaining constructs received injections of equal amounts of PBS. After 1 month, the mice were sacrificed, and the scaffolds were prepared for biochemical and morphological analyses.
In the first set of experiments, in which the organotypic cultures were grown in cell culture conditions, one control group received the anti-α1Cp antibody treatment, and the second one was treated with PBS only. Because of the limits in supply of the anti-α1Cp antibody in studies with the subcutaneous implants, the only control group was the one receiving PBS injections.
Analysis of the Total Collagen Content in Keloid-like Constructs—Scaffolds were rinsed with PBS, cut into two portions, and then lyophilized. Subsequently, the dry mass of lyophilized samples was measured. Next, one portion of a scaffold was hydrolyzed in 6 n HCl, and the hydroxyproline concentration was analyzed to determine collagen content, as described (25). The remaining portion of a scaffold was utilized to determine the total protein content.
Microscopic Analyses of Organotypic Keloid-like Constructs—Keloid-like constructs were harvested and then processed for histology and electron microscopy analyses. The samples were stained for collagen deposits with Sirius red. In addition, the human origin of cells present in the constructs grown subcutaneously in mice was confirmed by immunostaining with anti-human vimentin-specific antibody (Santa Cruz Biotechnology, Inc.). The stained samples were observed under a microscope equipped with a digital camera (model Eclipse E600, Nikon, Japan). For electron microscopy, the samples were fixed in 2% glutaraldehyde and 1% tannic acid in 0.1 m phosphate buffer (Electron Microscopy Sciences, Inc.). Subsequently, the samples were post-fixed in 2% osmium tetraoxide in 0.1 m phosphate buffer, rinsed in distilled water, and then stained with 1% uranyl acetate (Electron Microscopy Sciences). After that, the samples were embedded in 3% agarose followed by dehydration in graded concentrations of acetone. Next, the samples were embedded in Spurr's resin (Electron Microscopy Sciences). After polymerization, blocks of samples were sectioned to 70-nm sections with a diamond knife (Diatome, AG, Switzerland) mounted on a Leica Ultracut UCT microtome (Leica Microsystems, Germany). Finally, the sections were post-stained with 2.5% uranyl acetate in 50% ethanol and sodium bismuth (Electron Microscopy Sciences) and then observed with a transmission electron microscope (model H-7000, Hitashi Ltd., Japan).
Statistics—Collected data were analyzed, and results are presented as the mean ± S.E. for each sample. Comparisons between control groups and other groups were performed by an unpaired, two-tailed t test. In experiments with keloid-like constructs grown subcutaneously in mice, a paired t test was employed. In all tests the α level was set to 0.05. Statistical analyses were performed with GraphPad Prism Version 5.0 (GraphPad Software Inc.).
RESULTS
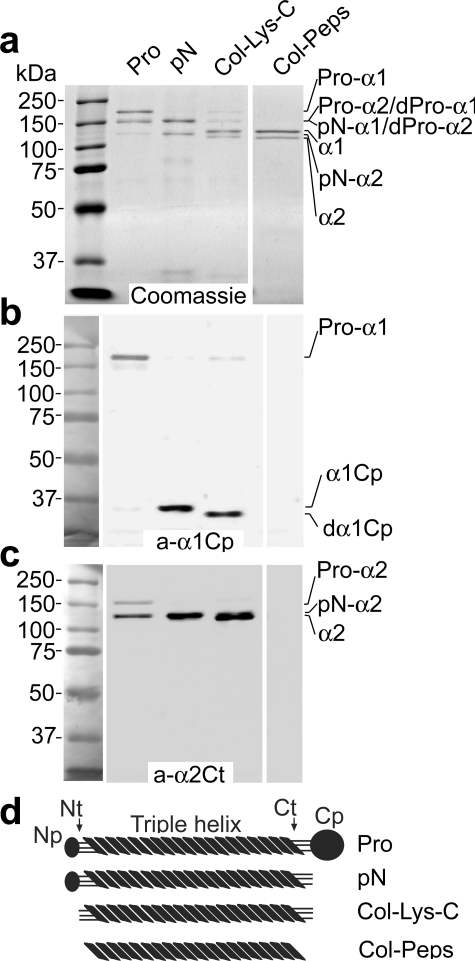
Epitope Specificity of Monoclonal Antibodies—Monoclonal anti-α1Cp and anti-α2Ct antibodies were purified from cell culture media of selected hybridomas. The monoclonal anti-α2Ct antibodies were an IgA type with the kappa light chains, whereas the anti-α1Cp antibody belonged to the IgG class. As determined by Western blot assays, monoclonal antibodies produced by selected hybridoma clones specifically recognized their respective epitopes (Figs. 1 and 2). In particular, the anti-α2Ct antibody-specific signal was apparent only in collagen variants in which the telopeptides were intact. Specifically, a group of collagen variants interacting with the anti-α2Ct antibody included intact procollagen I and procollagen processed with BMP-1 or with Lys-C (Fig. 1c). Moreover, we observed that the α2Ct-specific signal was stronger for a partially processed pro-α2 chain than the signal for the intact pro-α2 chain, suggesting more unrestricted access of the tested antibody to the target telopeptide in the absence of the C-terminal propeptide (Fig. 1c). There was no apparent signal from the pepsin-digested collagen I in which telopeptides were enzymatically degraded (Fig. 1c).
FIGURE 1.
Analysis of specificity of epitope recognition by the anti-α1Cp and anti-α2Ct antibodies. Panel a depicts intact procollagen I and products of its digestion by BMP-1, Lys-C, or pepsin separated in polyacrylamide gels and stained with Coomassie Blue. Specific epitopes present in proteins represented in panel a were analyzed by Western blot with the anti-α1Cp antibody (panel b) or with the anti-α2Ct antibody (panel c). In panel d schematics of domains in intact procollagen I or in products of its digestion by BMP-1, Lys-C, or pepsin are presented. In addition, molecular mass markers are presented. Pro, intact procollagen I; pN, procollagen variant in which the C-terminal propeptide have been cleaved by BMP-1; Col-Lys-C, collagen I variant, includes telopeptides, generated by enzymatic cleavage of procollagen with Lys-C; Col-Peps, collagen I variant, with telopeptides absent, generated by enzymatic cleavage of procollagen I with pepsin; Pro-α1, pro-α1 chain of procollagen I; Pro-α2, pro-α2 chain of procollagen I; dPro-α1 and dPro-α2, partially processed pro-α chains of procollagen I; pN-α1 and pN-α2, procollagen α chains in which the C-terminal propeptides were cleaved by BMP-1; α1 and α2, collagen I α chains; a-α1Cp and a-α2Ct, antibodies against the C-terminal propeptide of the α1 chain and the C-terminal telopeptide of the α2 chain of procollagen I, respectively; α1Cp and dα1Cp, C propeptide derived by cleavage of the procollagen α1 chain with BMP-1 or Lys-C, respectively; Np, Nt, Ct, and Cp, N-terminal propeptides, N-terminal telopeptides, C-terminal telopeptides, and C-terminal propeptides of procollagen I, respectively.
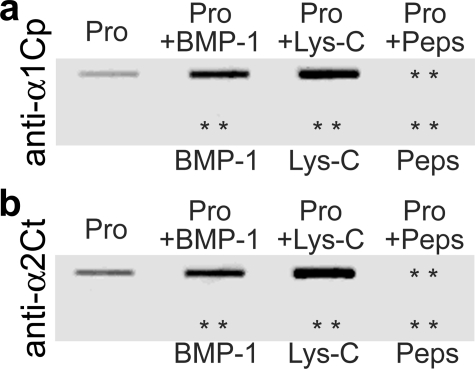
FIGURE 2.
Slot blot analysis of the specificity of recognition of native epitopes by the anti-α1Cp and anti-α2Ct antibodies. Panel a shows results of assays of binding of the anti-α1Cp antibody to native procollagen I and to the products of its cleavage with BMP-1, Lys-C, or pepsin. Panel b depicts results of analyses of binding of the anti-α2Ct antibody to the same proteins. In addition, both panels show the lack of binding of analyzed antibodies to BMP-1, Lys-C, and pepsin. Pro, procollagen I; + indicates samples digested with BMP-1, Lys-C, or pepsin; asterisks indicate positions of proteins present on nitrocellulose membranes but not detected by analyzed antibodies.
Similarly, the anti-α1Cp antibody specifically recognized the C propeptide of the α1 chain of procollagen I. As indicated in Fig. 1b, the α1 C propeptides present in the intact procollagen as well as C propeptides cleaved by BMP-1 or Lys-C were recognized by the anti-α1Cp antibody. There was an apparent difference in molecular masses of products generated by digestion of the C propeptides with Lys-C and BMP-1, most likely due to the differences in location of the specific cleavage sites recognized by these enzymes (Fig. 1b). The lack of binding of the anti-α1Cp antibody to the pepsin-treated procollagen in which the C propeptides were degraded provides even more evidence for its epitope-specific binding (Fig. 1b).
As determined by slot-blot assays, both the anti-α1Cp and anti-α2Ct antibodies were able to interact with native epitopes (Fig. 2). Specifically, the antibodies interacted with the intact procollagen I and with products generated by cleavage of procollagen I with BMP-1 or Lys-C. In contrast, the product generated by digestion with pepsin was not recognized by analyzed antibodies. As demonstrated in Fig. 2, the enzymes themselves did not react with tested antibodies.
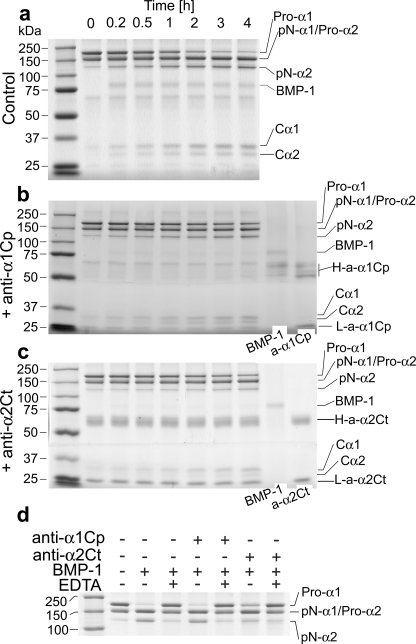
Processing of the C Propeptide by BMP-1 in Vitro in the Presence of the Anti-α2Ct Antibody—Because the anti-α2Ct antibody binds to the C-terminal telopeptide, we tested whether its binding could hinder BMP-1 cleavage of the Ala-Asp bond present at the C telopeptide/C propeptide junction (Fig. 3). As demonstrated in Table 1, the presence of the anti-α2Ct antibody decreased the rate of cleavage of procollagen chains in a concentration-dependent fashion (Fig. 3 and Table 1). Under the same conditions, there was no statistically significant difference between the rates of cleavage of procollagen α chains by BMP-1 in control samples containing the anti-α1Cp antibody and those to which no antibody was added (Fig. 3 and Table 1).
FIGURE 3.
Kinetics of cleavage of procollagen I by BMP-1 in the presence of the anti-α1Cp or the anti-α2Ct antibody. Panel a illustrates kinetics of cleavage of procollagen I by BMP-1 in the absence of antibodies, whereas panels b and c show kinetics of cleavage of procollagen I in the presence of 1.2 μg/ml anti-α1Cp or the anti-α2Ct antibody, respectively. Time points at which samples were analyzed are indicated. In panels b and c, protein bands corresponding to BMP-1 and to antibodies employed in this study are also indicated. In addition, molecular mass markers are presented in the left lanes of all panels. Panel d presents analysis of the specificity of cleavage of procollagen I with BMP-1. Note that in the presence of EDTA there was no cleavage of a procollagen substrate by BMP-1, thereby indicating the absence of other active enzymes. Pro-α1, pro-α1 chain of procollagen I; Pro-α2, pro-α2 chain of procollagen I; pN-α1 and pN-α2, procollagen α chains in which the C-terminal propeptides were cleaved by BMP-1; Cα1 and Cα2, C propeptides cleaved off respective procollagen chains by BMP-1; a-α1Cp and a-α2Ct, antibodies against the C-terminal propeptide of the α1 chain and the C-terminal telopeptide of the α2 chain of procollagen I, respectively; H-a-α1Cp and L-a-α1Cp, heavy and light chains of an anti-α1Cp immunoglobulin, respectively; H-a-α2Ct and L-a-α2Ct, heavy and light chains of an anti-α2Ct immunoglobulin, respectively; + and – indicate the presence or the absence of selected compounds.
TABLE 1.
Summary of results on inhibitory effects of the anti-α2Ct antibody on BMP-1 cleavage, kinetics of fibril formation, and accumulation of collagen in keloid-like constructs
NA, not applicable.
| Control | (+) Anti-α2Ct | (+) Anti-α1Cp | |
|---|---|---|---|
| Mean ± S.E. (% of control) | Mean ± S.E. (% of control) | Mean ± S.E. (% of control) | |
| Rate of cleavage of procollagen I with BMP-1 in vitro (μg/min/ml) | 0.22 ± 0.01; n = 3 (100%) | a0.04 ± 0.02; n = 3; p = 0.001 (18%) | b0.18 ± 0.009; n = 3; p = 0.07 (82%) |
| b0.03 ± 0.003; n = 3; p = 0.0001 (14%) | |||
| Rate of fibril formation in vitro (μg/min/ml) | 0.27 ± 0.046; n = 4 (100%) | c0.053 ± 0.02; n = 4; p = 0.005 (20%) | d0.25 ± 0.054; n = 4; p = 0.8 (93%) |
| d0.132 ± 0.003; n = 4; p = 0.02 (49%) | |||
| Total accumulated fibrils in vitro (μg/ml) | 72.08 ± 8.3; n = 4 (100%) | c21.87 ± 7.04; n = 3; p = 0.007 (30%) | d73.38 ± 10.01; n = 4; p = 0.9 (100%) |
| d40.77 ± 6.11; n = 4; p = 0.02 (57%) | |||
| Accumulation of collagen in keloid-like constructs grown in cell culture conditions (collagen (mg)/total protein (mg)) | 0.104 ± 0.016; n = 3 (100%) | 0.026 ± 0.004; n = 5; p = 0.0008 (25%) | 0.117 ± 0.01; n = 2; p = 0.6 (113%) |
| Accumulation of collagen in keloid-like constructs grown subcutaneously in nude mice (collagen (mg)/total protein (mg)) | 0.495 ± 0.07; n = 7 (100%) | 0.209 ± 0.03; n = 8; p = 0.001 (42%) | NA |
0.2 μg/ml anti-α2Ct was used.
1.2 μg/ml anti-α2Ct or the anti-α1Cp was used.
The collagen/antibody molar ratio was 1:2.
The collagen/antibody molar ratio was 10:1; NA, not assayed.
Employing EDTA, we determined that there was no proteolytic activity other than that derived from BMP-1. In particular, in the presence of EDTA there was not any detectable cleavage of procollagen I (Fig. 3d).
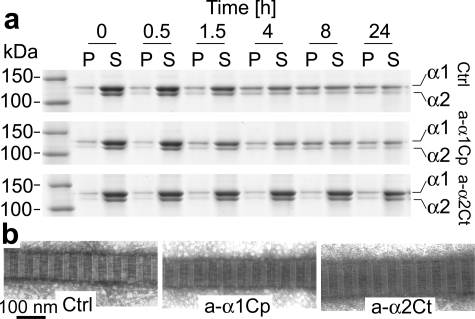
Kinetics of Self-assembly of Collagen and Morphology of Fibrils Formed in Vitro in the Presence of the Anti-α2Ct Antibody—In a number of in vitro assays it has been established that de novo fibril formation, i.e. where individual collagen molecules derived from enzyme-processed procollagen self-assemble into fibrils, is characterized by distinct phases of nucleation, propagation, and equilibrium (21). Here, in vitro fibril formation assays demonstrated that binding of the anti-α2Ct antibody to the C-terminal α2 telopeptide decreased both the rate of fibril formation and the amount of accumulated collagen fibrils (Fig. 4a, Table 1). By employing various concentrations of inhibitory antibodies, we demonstrated that the decrease in the rates of fibril formation and in the amounts of accumulated fibrils at equilibrium was concentration-dependent (Table 1). In contrast, the presence of the anti-α1Cp antibody did not have any significant effects on the kinetics of fibril assembly or on the total amount of accumulated fibrils (Fig. 4a, Table 1).
FIGURE 4.
Kinetics of de novo formation of collagen fibrils. Panel a represents analysis of collagen present in fibril and monomer fractions at indicated time points. The upper row depicts control samples, whereas the middle and lower rows show kinetics of fibril formation in the presence of 180 μg/ml anti-α1Cp and anti-α2Ct antibodies, respectively. In the left lane molecular mass markers are indicated. Panel b demonstrates morphology of individual fibrils formed in the presence or absence of tested antibodies. P, pellet fractions that represents collagen fibrils; S, supernatant fractions that represents collagen monomers; α1 and α2, collagen I α chains; Ctrl, fibril formation in the absence of antibodies; a-α1Cp and a-α2Ct, fibril formation in the presence of antibodies against the C-terminal propeptide of the α1 chain or the C-terminal telopeptide of the α2 chain of procollagen I, respectively.
In addition to electrophoretic assays of the kinetics of formation of fibrils and densitometric quantitation of their amounts, electron microscopy was employed to observe the morphology of individual collagen fibrils. These assays demonstrated that regardless of quantitative differences in the amounts of accumulated fibrils, the morphology of individual fibrils formed in the presence of tested antibodies was similar to that of a nontreated control. On average, fibrils formed in the control group and those assembled in the presence of antibodies were 100 nm in diameter. In addition, as evident by the uniform banding pattern, all analyzed fibrils were characterized by correct packing of collagen molecules (Fig. 4b).
Collagen Content and Morphology of Collagenous Matrices Formed in Organotypic Cultures in the Presence of the Anti-α2Ct Antibody—Keloid-derived fibroblasts were cultured in the presence of the anti-α2Ct or the anti-α1Cp antibodies in scaffolds in cell culture conditions and under the skin of nude mice. Subsequently, the total collagen content and the morphology of those organotypic constructs were analyzed. As indicated in Table 1, there was a statistically significant reduction of accumulated collagen in keloid constructs grown under cell culture conditions in the presence of the anti-α2Ct antibody. In contrast, the collagen content in constructs grown in the presence of the anti-α1Cp antibody was similar to that of the control group (Table 1). Similarly, in keloid-like constructs grown subcutaneously in mice, the total amount of collagen decreased in samples treated with the anti-α2Ct antibody in comparison to nontreated controls (Table 1).
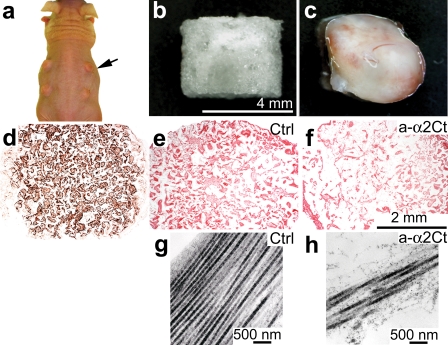
General morphological analysis of keloid-like constructs formed subcutaneously in mice showed that they were well organized into tissue-like structures (Fig. 5). As evident by the positive staining with antibody, which detects human vimentin and does not cross-react with mouse vimentin, the cells present in the subcutaneous constructs were fibroblasts derived from human keloid (Fig. 5d). Collagen-specific staining of samples demonstrated the presence of collagenous matrices deposited in the scaffold (Fig. 5, e and f). In samples treated with the anti-α2Ct antibody, however, the apparent density of collagenous deposits was relatively lower than that seen in control (Fig. 5, e and f). Similarly, in the presence of the anti-α2Ct antibody, the apparent density of collagen fibrils in the keloid-like constructs as seen under an electron microscope was relatively lower than in control samples (Fig. 5, g and h).
FIGURE 5.
Analysis of morphology of keloid-like constructs formed subcutaneously in nude mice. Panel a shows keloid-like constructs (arrow) implanted under skin of a mouse. Panel b presents a sponge-like scaffold that was employed to host human keloid-derived fibroblasts. Panel c represents overall morphology of a keloid-like construct harvested after 1 month of culture under skin of a nude mouse. Panel d demonstrates immunostaining for human-specific vimentin to confirm human origin of cells cultured in subcutaneous scaffolds, whereas panels e and f present Sirius red staining of collagen deposits. Panels g and h show electron microscopy images of collagen fibrils present in sections of keloid-like constructs. Individual panels show morphology of collagenous matrices formed in the absence (e and g) or presence (f and h) of inhibitory antibodies. Ctrl, keloid-like constructs cultured in the absence of antibodies; a-α2Ct, keloid-like constructs cultured in the presence of inhibitory antibodies against the C-terminal telopeptide of the α2 chain of procollagen I. Scale bars are included for specific sets of panels.
DISCUSSION
Fibrotic scarring is a reactive process that is modulated by the local proliferation of cells responsible for fibrosis and by altered regulation of various metabolic reactions governing biosynthesis and degradation of the connective tissue components. In addition, fibrotic processes are influenced by cytokines and growth factors, a group of diverse molecules derived from blood cells, such as platelets, or elaborated locally by mesenchymal and epithelial cells (26).
A number of processes and factors critical for the progression of fibrosis have been identified as targets to limit fibrotic scarring. Because most of these potential targets have broad biological functions, many of which are not related to fibrosis, their inhibition frequently leads to significant adverse effects.
Unlike prior approaches, which have attempted to limit excessive scarring by targeting upstream intracellular events, our strategy focuses on downstream extracellular events of collagen fibril formation, a process that directly contributes to formation of fibrotic deposits. The rationale for such an approach is that collagen fibril formation depends on site-specific interactions between telopeptides of one collagen molecule and a specific region located within a triple-helix of an interacting partner molecule (27). Free collagen molecules that do not incorporate into fibrils are then readily accessible for degradation by a number of enzymes present in the extracellular space (28, 29). The telopeptide/triple-helix interaction is particularly critical at the initial stages of fibril assembly, and our earlier studies demonstrated that in vitro fibril formation is completely blocked by peptides that compete for the telopeptide binding sites (28).
Although our method of blocking the collagen/collagen interaction represents a novel approach to limit fibrotic scar formation, conceptually similar approaches were tested in other pathologies. For instance, inhibition of protein aggregation has been tested as a method to prevent formation of insoluble amyloid plaques, a main feature of Alzheimer disease, that are formed in a process of fibrillation from an amyloid precursor protein (30). Moreover, inhibitors of fibrillation of sickle-cell hemoglobin have been tested in clinical trials, and inhibitors of systemic amyloidosis have been shown to be effective in animal models (31, 32).
Collagen fibril formation is not the only process that depends on telopeptide/triple-helix interaction. Site-specific binding of telopeptides positions them in a way that enables formation of intermolecular cross-links, critical for stabilization of collagen fibrils, between specific hydroxylysine residues (33). Thus, by blocking telopeptide/triple-helix binding, our approach not only prevents formation of new fibrils, but it may also prevent formation of cross-links in existing fibrils. Because poorly cross-linked collagen molecules are not fully functional and they are readily degraded by proteolytic enzymes, blocking telopeptide-mediated interactions may further reduce formation of excessive collagenous deposits in fibrotic scars.
Although collagen fibrils in fibrotic scars are heterotypic and consist mainly of collagen I, collagen III, and collagen V, we postulate that specific blocking collagen I/collagen I interaction will most likely prevent not only formation of homotypic collagen I fibrils but also formation of their heterotypic assemblies. This notion is based on the observation that incorporation of collagen III and collagen V into fibrils requires the presence of preassembled collagen I and that these proteins alone do not form stable fibrils (13, 34, 35).
Because the steric hindrance imposed by the presence of a bulky C propeptide prevents tight packing of collagen molecules required for the correct structure of a fibril, processing of this propeptide by BMP-1 is critical for fibril formation (21). Here, we demonstrated that a telopeptide binding antibody reduces the rate of cleavage of the C-terminal propeptides by BMP-1. Based on this observation, we postulate that such a reduction could offer an additional mechanism for reducing formation of fibrillar deposits. The idea of blocking BMP-1 to limit fibrosis was suggested by others, and a number of inhibitors of BMP-1 have been developed and tested as potential inhibitors of fibrosis (36). At the same time, however, it has been demonstrated that, in addition to procollagen I, BMP-1 cleaves other substrates and that its activity is critical for a number of processes not related to fibrosis (37–39). Moreover, it has been shown that in mice with targeted ablation of the gene for BMP-1, procollagen is still processed, thereby indicating an involvement of other members of the family of tolloid-related metalloproteases (40, 41). These observations significantly reduce the usefulness of BMP-1 to serve as a target to limit fibrosis. In contrast, our approach, in which a substrate rather than an enzyme is a target for inhibition, makes altering cleavage of the C-propeptide of procollagen I both specific and feasible.
We demonstrated that blocking α2-Ct telopeptides limits fibril formation both in vitro and in organotypic keloid-like constructs. As an in vitro system provides a more controllable experimental environment suitable for measuring rates of enzymatic processing of procollagen by BMP-1 and rates of fibril formation, the keloid-like system we employed provides a more biologically relevant model in which active anabolic and catabolic processes resemble, to a certain extent, those occurring in native tissues. With this model we demonstrated that anti-α2Ct antibody significantly reduced the total amount of deposited collagen but at the same time did not alter overall morphology of individual fibrils formed in its presence (Fig. 4b). As indicated in Table 1, the extent of inhibition of collagen accumulation was greater in keloid-like constructs cultured in cell culture conditions than in those grown subcutaneously in mice. We postulate that this difference was mainly due to more efficient diffusion of antibodies to specific epitopes and perhaps slower rates of their degradation in cell culture conditions.
Overall, our research presents experimental support for a new concept that excessive deposition of fibrotic tissue can be limited by inhibition of formation of collagen fibrils. In the future, humanized antibodies or small compounds with specific and high affinities for binding collagen telopeptides may advance the effectiveness of the presented approach to inhibit formation of localized fibrotic changes and also make it applicable for limiting systemic fibrotic diseases.
This work was supported, in whole or in part, by National Institutes of Health Grants R01AR048544 and R01AR049537 (to A. F.) and R01AR41439 (to J. U.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: BMP-1, bone morphogenetic protein 1; PBS, phosphate-buffered saline.
References
- 1.Prockop, D. J., and Kivirikko, K. I. (1995) Annu. Rev. Biochem. 64 403–434 [DOI] [PubMed] [Google Scholar]
- 2.Colige, A., Vandenberghe, I., Thiry, M., Lambert, C. A., Van Beeumen, J., Li, S. W., Prockop, D. J., Lapiere, C. M., and Nusgens, B. V. (2002) J. Biol. Chem. 277 5756–5766 [DOI] [PubMed] [Google Scholar]
- 3.Kessler, E., Takahara, K., Biniaminov, L., Brusel, M., and Greenspan, D. S. (1996) Science 271 360–362 [DOI] [PubMed] [Google Scholar]
- 4.Hopkins, D. R., Keles, S., and Greenspan, D. S. (2007) Matrix Biol. 26 508–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Addicks, E. M., Quigley, H. A., Green, W. R., and Robin, A. L. (1983) Arch. Ophthalmol. 101 795–798 [DOI] [PubMed] [Google Scholar]
- 6.Lim, X., Tateya, I., Tateya, T., Munoz-Del-Rio, A., and Bless, D. M. (2006. Ann. Otol. Rhinol. Laryngol. 115 921–929 [DOI] [PubMed] [Google Scholar]
- 7.Roseborough, I. E., Grevious, M. A., and Lee, R. C. (2004) J. Natl. Med. Assoc. 96 108–116 [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders, K. W., Gage-White, L., and Stucker, F. J. (2005) Arch. Facial Plast. Surg. 7 172–175 [DOI] [PubMed] [Google Scholar]
- 9.Shah, M., Foreman, D. M., and Ferguson, M. W. (1995) J. Cell Sci. 108 985–1002 [DOI] [PubMed] [Google Scholar]
- 10.Ovens, A., Joule, J. A., and Kadler, K. E. (2000) J. Pept. Sci. 6 489–495 [DOI] [PubMed] [Google Scholar]
- 11.Riley, D. J., Berg, R. A., Edelman, N. H., and Prockop, D. J. (1980) J. Clin. Investig. 65 643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiedler-Nagy, C., Bruckner, P., Hayashi, T., and Prockop, D. J. (1981) Arch. Biochem. Biophys. 212 668–677 [DOI] [PubMed] [Google Scholar]
- 13.Romanic, A. M., Adachi, E., Kadler, K. E., Hojima, Y., and Prockop, D. J. (1991) J. Biol. Chem. 266 12703–12709 [PubMed] [Google Scholar]
- 14.Fertala, A., Sieron, A. L., Hojima, Y., Ganguly, A., and Prockop, D. J. (1994) J. Biol. Chem. 269 11584–11589 [PubMed] [Google Scholar]
- 15.Steplewski, A., Ito, H., Rucker, E., Brittingham, R. J., Alabyeva, T., Gandhi, M., Ko, F. K., Birk, D. E., Jimenez, S. A., and Fertala, A. (2004) J. Struct. Biol. 148 326–337 [DOI] [PubMed] [Google Scholar]
- 16.Fisher, L. W., Stubbs, J. T., III, and Young, M. F. (1995) Acta Orthop. Scand. 66 (Suppl. 266), 61–65 [PubMed] [Google Scholar]
- 17.Hojima, Y., van der Rest, M., and Prockop, D. J. (1985) J. Biol. Chem. 260 15996–16003 [PubMed] [Google Scholar]
- 18.Fertala, A., Holmes, D. F., Kadler, K. E., Sieron, A. L., and Prockop, D. J. (1996) J. Biol. Chem. 271 14864–14869 [DOI] [PubMed] [Google Scholar]
- 19.Ito, H., Rucker, E., Steplewski, A., McAdams, E., Brittingham, R. J., Alabyeva, T., and Fertala, A. (2005) J. Mol. Biol. 352 382–395 [DOI] [PubMed] [Google Scholar]
- 20.Cabral, W. A., Makareeva, E., Letocha, A. D., Scribanu, N., Fertala, A., Steplewski, A., Keene, D. R., Persikov, A. V., Leikin, S., and Marini, J. C. (2007) Hum. Mutat. 28 396–405 [DOI] [PubMed] [Google Scholar]
- 21.Kadler, K. E., Hojima, Y., and Prockop, D. J. (1987) J. Biol. Chem. 262 15696–15701 [PubMed] [Google Scholar]
- 22.Elliott, B. W., Jr., and Cohen, C. (1986) J. Biol. Chem. 261 11259–11265 [PubMed] [Google Scholar]
- 23.Gelman, R. A., Williams, B. R., and Piez, K. A. (1979) J. Biol. Chem. 254 180–186 [PubMed] [Google Scholar]
- 24.Wang, H. J., Bertrand-de Haas, M., van Blitterswijk, C. A., and Lamme, E. N. (2003) Tissue Eng. 9 909–917 [DOI] [PubMed] [Google Scholar]
- 25.Woessner, J. F. (1961) Archiv. Biochem. Biophys. 93 440–447 [DOI] [PubMed] [Google Scholar]
- 26.Butler, P. D., Longaker, M. T., and Yang, G. P. (2008) J. Am. Coll. Surg. 206 731–741 [DOI] [PubMed] [Google Scholar]
- 27.Prockop, D. J., and Fertala, A. (1998) J. Struct. Biol. 122 111–118 [DOI] [PubMed] [Google Scholar]
- 28.Prockop, D. J., and Fertala, A. (1998) J. Biol. Chem. 273 15598–15604 [DOI] [PubMed] [Google Scholar]
- 29.Chang, K., Uitto, J., Rowold, E. A., Grant, G. A., Kilo, C., and Williamson, J. R. (1980) Diabetes 29 778–781 [DOI] [PubMed] [Google Scholar]
- 30.Lansbury, P. T., Jr. (1997) Curr. Opin. Chem. Biol. 1 260–267 [DOI] [PubMed] [Google Scholar]
- 31.Kisilevsky, R., Lemieux, L. J., Fraser, P. E., Kong, X., Hultin, P. G., and Szarek, W. A. (1995) Nat. Med. 1 143–148 [DOI] [PubMed] [Google Scholar]
- 32.Merlini, G., Ascari, E., Amboldi, N., Bellotti, V., Arbustini, E., Perfetti, V., Ferrari, M., Zorzoli, I., Marinone, M. G., Garini, P., et al. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 2959–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eyre, D. R., Paz, M. A., and Gallop, P. M. (1984) Annu. Rev. Biochem. 53 717–748 [DOI] [PubMed] [Google Scholar]
- 34.Fleischmajer, R., Timpl, R., Tuderman, L., Raisher, L., Wiestner, M., Perlish, J. S., and Graves, P. N. (1981) Proc. Natl. Acad. Sci. U. S. A. 78 7360–7364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleischmajer, R., Olsen, B. R., Timpl, R., Perlish, J. S., and Lovelace, O. (1983) Proc. Natl. Acad. Sci. U. S. A. 80 3354–3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fish, P. V., Allan, G. A., Bailey, S., Blagg, J., Butt, R., Collis, M. G., Greiling, D., James, K., Kendall, J., McElroy, A., McCleverty, D., Reed, C., Webster, R., and Whitlock, G. A. (2007) J. Med. Chem. 50 3442–3456 [DOI] [PubMed] [Google Scholar]
- 37.Imamura, Y., Steiglitz, B. M., and Greenspan, D. S. (1998) J. Biol. Chem. 273 27511–27517 [DOI] [PubMed] [Google Scholar]
- 38.Scott, I. C., Imamura, Y., Pappano, W. N., Troedel, J. M., Recklies, A. D., Roughley, P. J., and Greenspan, D. S. (2000) J. Biol. Chem. 275 30504–30511 [DOI] [PubMed] [Google Scholar]
- 39.Amano, S., Scott, I. C., Takahara, K., Koch, M., Champliaud, M. F., Gerecke, D. R., Keene, D. R., Hudson, D. L., Nishiyama, T., Lee, S., Greenspan, D. S., and Burgeson, R. E. (2000) J. Biol. Chem. 275 22728–22735 [DOI] [PubMed] [Google Scholar]
- 40.Scott, I. C., Blitz, I. L., Pappano, W. N., Imamura, Y., Clark, T. G., Steiglitz, B. M., Thomas, C. L., Maas, S. A., Takahara, K., Cho, K. W., and Greenspan, D. S. (1999) Dev. Biol. 213 283–300 [DOI] [PubMed] [Google Scholar]
- 41.Suzuki, N., Labosky, P. A., Furuta, Y., Hargett, L., Dunn, R., Fogo, A. B., Takahara, K., Peters, D. M., Greenspan, D. S., and Hogan, B. L. (1996) Development 122 3587–3595 [DOI] [PubMed] [Google Scholar]