Abstract
Once thought of as simply an oily barrier that maintains cellular integrity, lipids are now known to play an active role in a large variety of cellular processes. Phosphoinositides are of particular interest because of their remarkable ability to affect many signaling pathways. Ion channels and transporters are an important target of phosphoinositide signaling, but identification of the specific phosphoinositides involved has proven elusive. TRPV1 is a good example; although phosphatidylinositol (4,5)-bisphosphate (PI(4,5)P2) can potently regulate its activation, we show that phosphatidylinositol (4)-phosphate (PI(4)P) and phosphatidylinositol (3,4,5)-trisphosphate (PI(3,4,5)P3) can as well. To determine the identity of the endogenous phosphoinositide regulating TRPV1, we applied recombinant pleckstrin homology domains to inside-out excised patches. Although a PI(4,5)P2-specific pleckstrin homology domain inhibited TRPV1, a PI(3,4,5)P3-specific pleckstrin homology domain had no effect. Simultaneous confocal imaging and electrophysiological recording of whole cells expressing a rapamycin-inducible lipid phosphatase also demonstrates that depletion of PI(4,5)P2 inhibits capsaicin-activated TRPV1 current; the PI(4)P generated by the phosphatases was not sufficient to support TRPV1 function. We conclude that PI(4,5)P2, and not other phosphoinositides or other lipids, is the endogenous phosphoinositide regulating TRPV1 channels.
Although they make up only 1-5% of the total anionic lipids in a cell membrane (1-3), phosphoinositides have a prominent role in lipid signaling (4). Both phosphatidylinositol (4,5)-bisphosphate (PI(4,5)P2)3 and phosphatidylinositol (3,4,5)-trisphosphate (PI(3,4,5)P3) have been implicated in protein trafficking, cell motility, Ca2+ signaling, cell growth, endo- and exocytosis, and ion transporter and channel regulation (4, 5). Changes in the plasma membrane PI(4,5)P2 concentration that result from activation of cell surface receptors are believed to regulate the function of many ion channels and transporters, including K+ channels, voltage-gated Ca2+ channels, inositol trisphosphate receptors, and the family of transient receptor potential (TRP) channels (4).
Several different phosphoinositides have been implicated in modulation of TRP channels. The original TRP channel from Drosophila is thought to be gated by either diacyl glycerol or a polyunsaturated fatty acid (6). This is in contrast to original studies that implied an inhibitory role for PI(4,5)P2 on both TRP and TRPL. A recent study demonstrated phosphoinositide binding by TRPC6, a TRP channel involved in vasodilation, but did not determine whether PI(4,5)P2 or PI(3,4,5)P3 was the endogenous ligand (7). Finally, it has been shown that PI(4,5)P2 can alter the function of TRPM4, TRPM5, TRPM8, TRPM7, and TRPV5 (8-14), but whether PI(4,5)P2 is indeed the physiological regulator has not been addressed.
For the transient receptor potential vanilloid-type 1 (TRPV1) channel responsible for transduction of painful thermal and chemical stimuli, the role of phosphoinositides remains a topic of intense debate. In the context of excised membrane patches, both inhibition (15) and stimulation (16, 17) of TRPV1 by PI(4,5)P2 have been observed. Furthermore, in whole cells, PI(4,5)P2 has been reported either to be inhibitory (15) or to inhibit in response to weak or moderate TRPV1 stimuli but have no effect when cells are treated with strong TRPV1 stimuli (17). Synthesis of PI(4,5)P2 has also been proposed to play a role in recovery from Ca2+-mediated desensitization of TRPV1 (18), but whether changes in the plasma membrane levels of PI(4,5)P2 contribute to desensitization remains controversial.
Here, we combine biochemical, optical, and electrophysiological approaches to determine that: 1) PI(4,5)P2, and not other phosphoinositides, is bound to TRPV1 in both whole cells and excised patches; 2) sequestration of PI(4,5)P2 in excised patches inhibits TRPV1, whereas the addition of PI(4,5)P2 stimulates TRPV1; and 3) recruitment of inositol 5-phosphatase to the plasma membrane produces synchronous decreases in PI(4,5)P2 levels and TRPV1 current. Our work unites previous studies under a single paradigm that defines PI(4,5)P2 as an essential co-factor for activation of TRPV1 and yields important molecular constraints for the protein-phosphoinositide interactions.
EXPERIMENTAL PROCEDURES
Cell Culture—F-11 cells were cultured and transfected as described previously (16). Cells were transiently transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions and used for electrophysiology 1-4 days after transfection.
Human embryonic kidney tsA-201 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 0.1 mm nonessential amino acids, and 50 units/ml penicillin with 50 μg/ml streptomycin at 37 °C and 5% CO2. Cells were transiently transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After transfection, cells were passaged onto poly-l-lysine-coated glass coverslips and grown under normal culture conditions until experimentation 24-36 h later.
Molecular Biology—The PLCδ1-PH and GRP1-PH domain constructs were subcloned into pETGQ to incorporate a His6 tag for protein purification. PLCδ1-PHLLN (K30L, K32L, and W36N) was constructed from the wild type plasmid PLCδ1-PH.pETGQ using a two-step PCR method as described previously (16).
Biochemistry—All pleckstrin homology (PH) domain constructs were expressed and purified as described previously (19). 100 μl of PI(4,5)P2 or PI(3,4,5)P3 agarose beads (Echelon Biosciences), containing 10 μm of bound DiC6-PI(4,5)P2 or DiC6-PI(3,4,5)P3, were incubated with 10-20 μg of each purified protein for 1-2 h with gentle rocking at 4 °C. The beads were then centrifuged at 1000 rpm, and the supernatant was saved as the unbound fraction of the proteins. The beads were washed twice with 1 ml of washing buffer (10 mm HEPES, pH 7.4, 0.25% Nonidet P-40, 150 mm NaCl), and bound proteins were eluted with 100 μl of 2× Laemmli sample buffer by heating at 95 °C for 5 min. Proteins were separated by SDS-PAGE and subject to Coomassie Blue staining.
Phosphoinositides and Polylysine—All phosphoinositides were obtained from Avanti Polar Lipids. Natural PI(4,5)P2 solutions were prepared as described previously (16). Natural PI(4,5)P2 refers to lipids purified from porcine brain, with a fatty acid composition primarily composed of 18:0, 18:1, and 20:4 acyl chains. DiC8-PIPn solutions (Avanti Polar Lipids) were solubilized in water as a 2.5 mm stock, frozen at -20 °C, and used the same day they were diluted from the stock. Polylysine (70-150 kDa) was dissolved as a 2 mg/ml stock, aliquoted, and frozen at -20 °C. All chemicals were purchased from Sigma unless otherwise noted.
Electrophysiology and Confocal Imaging—For the excised patch experiments, coverslips were placed in a 12-mm open perfusion chamber. Solutions were changed using a“sewer pipe” solution changer controlled by RSC-200 (Biologic). Patch pipettes (2-4 megaohms) were filled with symmetrical recording solutions (in mm: 130 NaCl, 3 HEPES, and 0.2 EGTA). Currents were recorded with an Axopatch 200B amplifier (Axon Instruments) interfaced to a Dell computer controlled with Pulse (HEKA).
For simultaneous electrophysiology-imaging experiments, coverslips were placed on a custom-made open perfusion chamber that allowed for simultaneous imaging recording and perforated-patch voltage clamp. Perforated-patch recordings were performed using filamented borosilicate glass pipettes heat-polished with a microforge. Patch pipettes were filled with intracellular saline solution (in mm: 10 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 0.05 EGTA, and 10 HEPES, pH 7.2) and amphotericin B (1 mg/ml). Hank's buffered salt solution (in mm: 140 NaCl, 4 KCl, 1 MgCl2, 1.8 CaCl2, 5 glucose, 10 HEPES, pH 7.4) was continuously perfused until achieving perforation. After this, a nominally Ca2+-free solution (in mm: 140 NaCl, 4 KCl, 2 MgCl2, 5 glucose, 10 HEPES, pH 7.4) was perfused before the application of stimulus. Currents were recorded with a MultiClamp 700A amplifier (Axon Instruments, Inc.) interfaced to a Dell personal computer controlled with Clampex 8.2. Patches that showed unstable leak currents after perforation were discarded. Analysis of perforated-patch currents was performed using Clampfit 8.2 (Axon Instruments, Inc.).
Confocal images during simultaneous electrophysiology-imaging experiments were obtained using one of either two microscopes. For Fig. 5, images were obtained using a Radiance-2100 confocal system (Bio-Rad Laboratories Inc.) controlled with proprietary software (scanning rate of 500 lines/s). The confocal system was coupled to a Nikon TE300 inverted microscope using a Nikon ×60 oil immersion objective (numerical aperture = 1.4). For Fig. 6, images were obtained with a Nikon inverted microscope coupled to a swept field confocal system with a Photometrics EMCCD camera. Imaging was performed using a Nikon ×100 (1.49 NA) lens. PH-PLCδ1-YFP was excited using the 488 nm line of an argon laser, and emission from 500-525 nm was collected. Analysis of images was performed using Metamorph 7.0 (Universal Imaging, Inc.) and ImageJ (Scion Corp. and National Institutes of Health).
FIGURE 5.
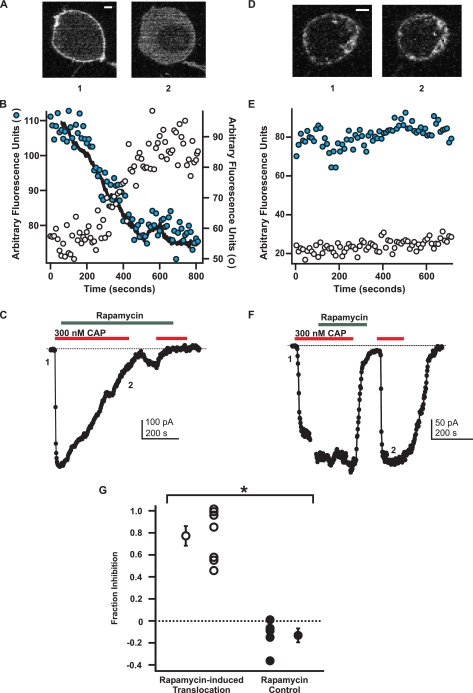
Depletion of PI(4,5)P2 inhibits TRPV1. A-C, experimental group for rapamycin-induced PI(4,5)P2 depletion. A, YFP-PLCδ1-PH signal in a tsA cell transfected with the Ins-5P system. Shown are confocal images for each numbered point in the current traces shown in C. The scale bar applies to both images and represents 1 μm. B, traces represent the mean fluorescence data from three different plasma membrane regions of interest (shaded circle) and from two cystosolic regions of interest (open circle). The overlaid black trace represents the inverse of the current shown in C. The ordinate on the left represents the scale for the plasma membrane regions, and the ordinate on the right represents the scale for the cytosolic regions of interest. C, current (black) from the cell depicted in A. The current and fluorescence traces are shown on the same time scale and are aligned such that the onset of the current recording matches the onset of the imaging trace. Currents (perforated-patch configuration, holding potential -60 mV) were elicited by capsaicin (CAP, 300 nm, red bar) in nominally Ca2+-free solution. Rapamycin (1 μm, green bar) was applied to cause translocation of the Ins-5P and PI(4,5)P2 depletion. The zero current level is shown as the dotted black line. D and E, negative control group for the rapamycin-induced system. D, YFP-PLCδ1-PH signal in a tsA cell transfected with the FKBP-only construct (no Ins-5P). Shown are confocal images for each numbered point in the current traces shown in F. The scale bar applies to both images and represents 1 μm. E, traces represent the mean fluorescence data from two different plasma membrane regions of interest (shaded circle) and from two cystolic regions of interest (open circle). F, as in C. G, summary plot showing the amount of current inhibition 1-(I/Imax) produced by rapamycin in cells that had probe translocation (white) and cells that did not have probe translocation (black). The separate single point in each group represents the mean of that group. Errors bars are S.E. * = p = 0.003.
FIGURE 6.
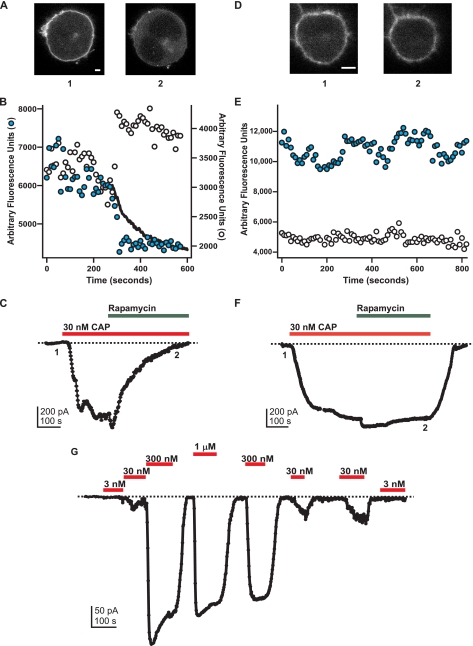
PI(4,5)P2 depletion inhibits TRPV1 even at a low concentration of capsaicin (CAP). A-C, experimental group for rapamycin-induced PI(4,5)P2 depletion. A, YFP-PLCδ1-PH signal in a tsA cell transfected with the Ins-5P system. Shown are confocal images for each numbered point in the current traces shown in C. The scale bar applies to both images and represents 4 μm. B, traces represent the fluorescence data from a plasma membrane region of interest (shaded circle) and from a cytosolic region of interest (open circle). The overlaid black trace represents the inverse of the current shown in C. The ordinate on the left represents the scale for the plasma membrane regions, and the ordinate on the right represents the scale for the cytosolic regions of interest. C, current (black) from the cell depicted in A. The current and fluorescence traces are shown on the same time scale and are aligned such that the onset of the current recording matches the onset of the imaging trace. Currents (perforated-patch configuration, holding potential -60 mV) were elicited by capsaicin (30 nm, red bar) in nominally Ca2+-free solution. Rapamycin (1 μm, green bar) was applied to cause translocation of the Ins-5P and PI(4,5)P2 depletion. The zero current level is shown as the dotted black line. D-E, in the absence of YFP-PLCδ1-PH PI(4,5)P2 probe translocation, rapamycin did not inhibit the capsaicin-activated current. D, YFP-PLCδ1-PH signal in a tsA cell transfected with the Ins-5P system. Shown are confocal images for each numbered point in the current traces shown in F. The scale bar applies to both images and represents 4 μm. E, traces represent the mean fluorescence data from three different plasma membrane regions of interest (shaded circle) and from three cytosolic regions of interest (open circle). F, as in C. G, responses to indicated capsaicin concentrations in a typical cell. The relaxation observed upon the application of the first saturating concentration (300 nm) was observed in almost all cases and was not studied further. In addition, a small increase over time in the response to low capsaicin concentrations was commonly observed, perhaps due to residual capsaicin partitioned into cell membranes. In all cases, rapamycin experiments were not performed until both the relaxation and the run-up had reached steady state.
Data Analysis—All electrophysiology data were analyzed with Igor Pro (Wavemetrics, Portland, OR). In box and whisker plots (Figs. 1C, 3C, and 4F), the boxes represent the range of data from the 25th to 75th percentiles, the whiskers represent the 10th and 90th percentiles, and the horizontal lines represent the median. Errors given in the text, and error bars shown in all figures represent the S.E. For electrophysiological measurements, all currents shown are difference currents in which the current in the absence of capsaicin was subtracted to yield the capsaicin-activated component of the current. Smooth curves shown in dose-response relations are fits of the data to the Hill equation: I = Imax ([ligand]n/(K½n + [ligand]n)), where n was constrained to 1. Statistical significance was assayed with the two-tailed Student's t test.
FIGURE 1.
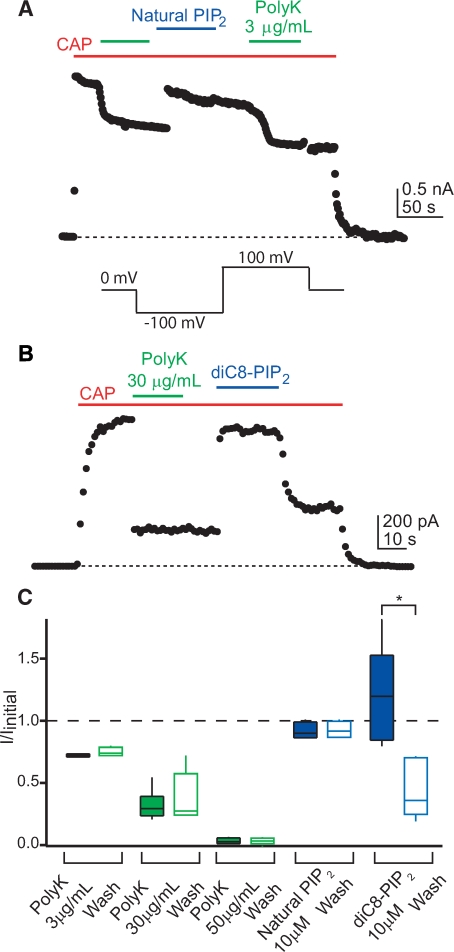
Natural and DiC8-PI(4,5)P2 rescue inhibition of TRPV1 by PolyK. A and B, time courses of TRPV1 activation in inside-out excised patches from F-11 cells. A voltage protocol (see inset) was used to drive current through the channels at the times indicated by the points (current at +100 mV shown), and capsaicin (CAP, 1 μm, red bars) was applied to the patch to elicit TRPV1 current. PolyK (A, 3 μg/ml; B, 30 μg/ml; green bars), natural PI(4,5)P2 (A, 10 μm, blue bar), or diC8-PI(4,5)P2 (B, 10 μm, blue bar) was applied as indicated. PIP2, phosphatidylinositol 4,5-bisphosphate. C, summary box plot of the fractional inhibition by PolyK and the restoration by PI(4,5)P2. Boxes encompass the 25th through 75th percentile of the data, the horizontal bar represents the median, and the whiskers extend to the 10th and 90th percentile of the data. Inhibition measurements during PolyK application are shown as filled green boxes, and after the removal of PolyK, they are shown as open green boxes (n = 4-6). The mean values for current remaining (I/Imax) after PolyK treatment are 0.72 ± 0.006 (3 μg/ml), 0.32 ± 0.05 (30 μg/ml), and 0.032 ± 0.013 (50 μg/ml) and 0.75 ± 0.02 (3 μg/ml), 0.38 ± 0.08 (30 μg/ml), and 0.033 ± 0.012 (50 μg/ml) after washing the PolyK from the bath. The restoration of the current by diC8-PI(4,5)P2 and natural PI(4,5)P2 is shown as filled blue boxes, and the currents after removing the PI(4,5)P2 are shown as open blue boxes (n = 4-6), with mean values of 1.21 ± 0.16 (diC8-PI(4,5)P2) and 0.92 ± 0.03 (natural PI(4,5)P2) in the presence of the phosphoinositide and 0.45 ± 0.11 (diC8-PI(4,5)P2) and 0.93 ± 0.03 (natural PI(4,5)P2) after washing the phosphoinositide from the bath. *, p = 0.005.
FIGURE 3.
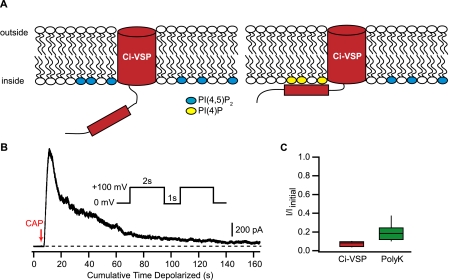
Activation of Ci-VSP inhibits TRPV1. A, schematic model of Ci-VSP mechanism. At hyperpolarized membrane potentials, the phosphatase domain of Ci-VSP is not close to the membrane. Upon depolarization of the membrane, the phosphatase domain moves closer to the membrane and dephosphorylates PI(4,5)P2 to produce PI(4)P. B, time course of TRPV1 inhibition by Ci-VSP activation in an excised inside-out patch. The patch was stepped repeatedly using the protocol shown in the inset (1-s intervals between each 2-s depolarization), and the current recorded at 100mV is shown. Capsaicin (100 nm) was applied at the point of the red arrow and remained present throughout the experiment. The dashed line shows the zero current level. C, summary box plot for the inhibition produced by Ci-VSP (red box) and PolyK (green box). Boxes are as in Fig. 1C.
FIGURE 4.
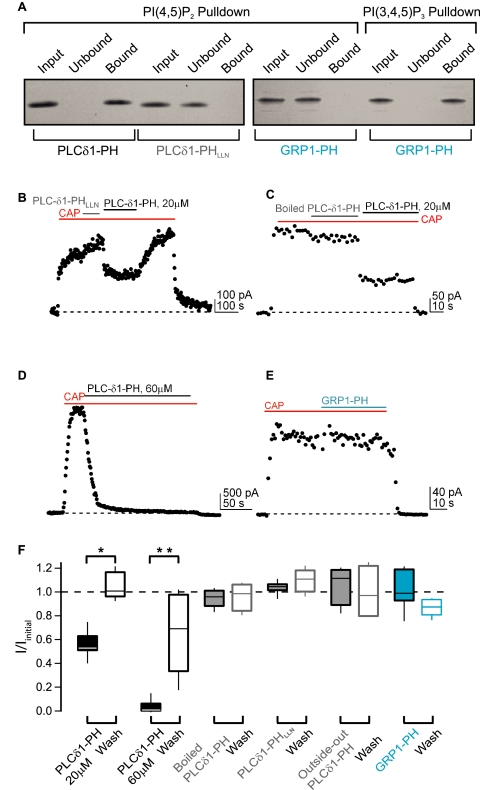
Only PI(4,5)P2-specific PH domain can modulate TRPV1. A, gels of PIPn pulldown assays with recombinant PH domain proteins. Equivalent amounts of each fraction were run to allow quantitative comparison between lanes. B-E, representative time courses of TRPV1 currents activated by capsaicin (300 nm, red bars) in the presence of different PH domain proteins (voltage protocol as in Fig. 2). B, PLCδ1-PH (20 μm, black bar) or PLCδ1-PHLLN (gray bar). C, boiled PLCδ1-PH (gray bar) or PLCδ1-PH (black bar). D, PLCδ1-PH (60 μm, black bar). E, GRP1-PH (cyan bar). F, summary box plot of the fraction current remaining (I/Imax) in the presence of each PH domain protein (black/gray/cyan bars) and the recovery of the current after PH domain protein washout (black/gray/cyan boxes). Boxes are as in Fig. 1C; * = p = 0.0005 and ** = 0.002. CAP, capsaicin.
RESULTS
Polylysine Inhibition of TRPV1—We hypothesized that PI(4,5)P2 is a required cofactor for activation of TRPV1 by agonists such as capsaicin. This hypothesis predicts that TRPV1 activated in excised patches is already bound to PI(4,5)P2 and that decreasing the concentration of free PI(4,5)P2 will prevent activation of the channels by capsaicin. One way to decrease the free PI(4,5)P2 concentration in the membrane is to use polycations, such as polylysine (PolyK), which have been proposed to sequester PI(4,5)P2 through electrostatic interactions with the negatively charged phosphoinositide headgroup (20-22). Decreases in activity of several K+ channels and TRP channels, including rundown, have been observed in response to PolyK, and these decreases are part of the evidence supporting PI(4,5)P2 regulation (14, 16, 23-25).
We applied PolyK to the intracellular surface of inside-out patches from F-11 cells transiently transfected with TRPV1. F-11 cells were derived from rat dorsal root ganglion neurons, the cells that express TRPV1, and are an excellent model system for studying TRPV1 function (26, 27). We found that PolyK reduced the capsaicin-activated current in a dose-dependent manner (Fig. 1, green bars/boxes), with a saturating concentration (50 μg/ml) producing complete inhibition (Fig. 1, green bars/boxes). The highest concentration of PolyK was not well tolerated by patches; lower concentrations were used in subsequent experiments.
Although inhibition of TRPV1 currents by PolyK is consistent with a decrease in the free PI(4,5)P2 concentration, it could also arise through other mechanisms such as block of the pore. One result that argues against this alternative mechanism can be seen upon removal of PolyK from the bath. As shown in Fig. 1C (compare filled green boxes with open green boxes), the capsaicin-activated current did not recover after removal of PolyK from the bath, even after minutes of washing with PolyK-free solution. This very slow off rate could be due to the multiple positive charges on PolyK (net charge of +1 per lysine at pH 7.2, molecular mass 70,000-150,000 Da) binding simultaneously to many PI(4,5)P2 molecules. Importantly, the addition of either natural PI(4,5)P2 (Fig. 1, A and C; blue bars/boxes) or the water-soluble diC8-PI(4,5)P2 (Fig. 1, B and C, blue bars/boxes) rescued the capsaicin-activated current from PolyK inhibition. As expected from the more favorable partitioning into the membrane of natural PI(4,5)P2 when compared with diC8-PI(4,5)P2, the rescue by natural PI(4,5)P2 persisted even after it was washed from the bath (Fig. 1, A and C), whereas the rescue by diC8-PI(4,5)P2 was rapidly lost when it was washed from the bath (Fig. 1, B and C). These data indicate that, at the very least, PI(4,5)P2 can substitute for the activating molecule sequestered by PolyK.
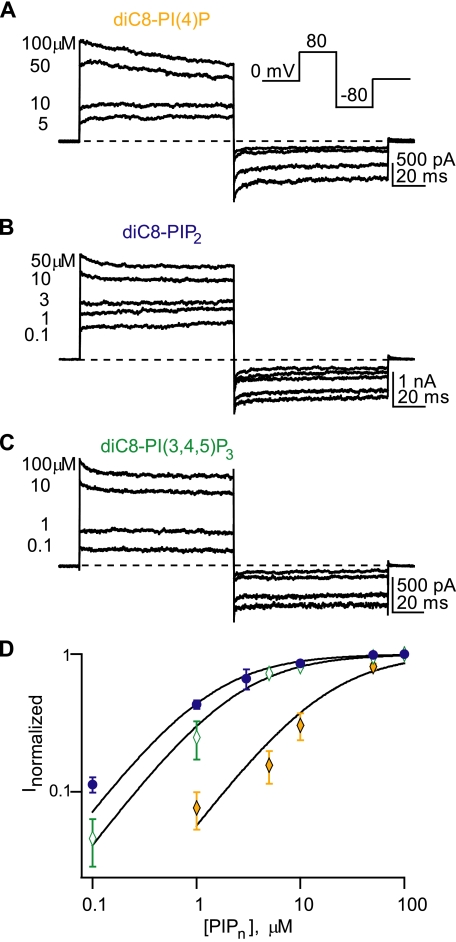
TRPV1 Can Be Modulated by Several Phosphoinositides—As discussed above, many ion channels that can be regulated by PI(4,5)P2 can be regulated by other phosphoinositides as well, making it difficult to establish which phosphoinositide is the endogenous regulator. PolyK can bind to all anionic species in the membrane and does not distinguish among different phosphoinositides. If PI(4,5)P2, and only PI(4,5)P2, could regulate TRPV1, then interpreting our PolyK data as meaning PI(4,5)P2 is bound to TRPV1 in patches would be reasonable. To address this question, we measured dose-response relations for three phosphoinositides, phosphatidylinositol (4)-phosphate (PI(4)P), PI(4,5)P2, and PI(3,4,5)P3. We used the water-soluble diC8 versions of PI(4)P, PI(4,5)P2, and PI(3,4,5)P3 to produce potentiation that reversed when the phosphoinositides were washed from the bath (as in Fig. 1B). All patches were pretreated with PolyK (see above) to control for cell-to-cell variability in endogenous phosphoinositide levels. A half-saturating concentration of capsaicin was used to activate the channels. As shown in Fig. 2, all three phosphoinositides potentiated activation of TRPV1. The channel exhibited the lowest apparent affinity for PI(4)P (Fig. 2, A and D;EC50 = 17.3 ± 3.2 μm, n = 4). The apparent affinities of the channel for PI(4,5)P2 (Fig. 2, B and D, EC50 = 1.5 ± 0.21 μm, n = 6) and PI(3,4,5)P3 (Fig. 2, C and D;EC50 = 2.5 ± 0.93 μm, n = 4), measured from fits to individual patches with the Hill equation, were not significantly different, and all of the phosphoinositides elicited the same maximal current in a given patch (data not shown). Others have reported similar results in comparing PI(4,5)P2 and PI(4)P modulation of TRPV1 (17).
FIGURE 2.
Candidate phosphoinositides modulate TRPV1. A-C, currents from representative excised inside-out patches of TRPV1 from transfected F-11 cells. Currents were activated by 300 nm capsaicin (a half-saturating concentration in this cell type) in response to the depicted voltage protocol (inset). The dashed lines represent the zero current level for each patch. The numbers represent the concentration of phosphoinositide, in micromolar, applied for the adjacent current trace. PIP2, phosphatidylinositol 4,5-bisphosphate. D, dose-response curves for diC8-PI(4)P (open diamond), diC8-PI(4,5)P2 (filled circle), and diC8-PI(3,4,5)P3 (shaded diamond). For each phosphoinositide concentration, the current was normalized to the maximal response with a saturating concentration of the same phosphoinositide. Each curve was fit with the Hill equation (see“Experimental Procedures”), yielding K½ values of 16.7, 1.3, and 2.4 μm for PI(4)P, PI(4,5)P2, and PIP3, respectively. Points represent mean data for each concentration (n = 4-9 patches).
Removal of PI(4,5)P2 by Ci-VSP Can Inhibit TRPV1—Determining which of the phosphoinositide species is the endogenous modulator is an important question because it bears on whether and how intracellular and extracellular signals may modulate the channels and thereby alter nociceptor excitability. Recently, a voltage-sensitive phosphatase from Ciona intestinalis has been described (Ci-VSP, (28, 29)). This protein contains two modules, a voltage-sensing domain and a cytoplasmic lipid phosphatase (Fig. 3A). The phosphatase module of Ci-VSP removes phosphate groups from both PI(3,4,5)P3 and PI(4,5)P2, resulting in the loss of both lipids from the membrane and the production of PI(4)P (29, 40). At hyperpolarizing potentials, the phosphatase is inactive. Depolarization brings the phosphatase domain closer to the membrane, resulting in robust activity (Fig. 3A). Activation of Ci-VSP has been previously shown to inhibit the current of several known PI(4,5)P2-sensitive ion channels (e.g. Kir, KCNQ2/3, (29)). We therefore asked whether Ci-VSP-mediated depletion of plasma membrane PI(4,5)P2 would inhibit TRPV1 channels.
We transiently transfected F-11 cells with both TRPV1 and a Ci-VSP-citrine fusion construct. When excised inside-out patches from these co-transfected cells were depolarized from a holding potential of 0 mV to 100 mV, we saw a slow decrease in the capsaicin-activated current (Fig. 3, B and C). The inhibition of the TRPV1 current produced by Ci-VSP (I/Iinitial = 0.073 ± 0.015, n = 3) was less variable than the inhibition produced by polyK (Fig. 3C). Furthermore, the specificity of Ci-VSP for PI(4,5)P2 and PI(3,4,5)P3 makes it unlikely that PI(4)P plays a role in TRPV1 regulation.
Specific Sequestration of PI(4,5)P2 Inhibits TRPV1—Our Ci-VSP experiments rule out PI(4)P as the endogenous regulator of TRPV1. However, they do not distinguish between PI(4,5)P2 and PI(3,4,5)P3. We turned to recombinant PH domains to provide the selectivity required for identifying the endogenous phosphoinositide regulating TRPV1. A number of PH domains appear to have remarkable selectivity for some phosphoinositides over others (30). In particular, the PH domain from PLC-δ1 binds the headgroup of PI(4,5)P2 almost 100-fold better than the headgroups of either PI(4)P or PI(3,4,5)P3 (31). In contrast, the PH domain of GRP1 binds PI(3,4,5)P3 650 times better than PI(4,5)P2 (32).
We wondered whether the application of the recombinant PLC-δ1 and GRP1-PH domains to excised patches could be used to selectively sequester PI(4,5)P2 or PI(3,4,5)P3, respectively. We examined whether bacterially expressed and purified PH domain proteins retained their selectivity using phosphoinositide pulldown experiments. Pulldown experiments of PLC-δ1-PH domain protein with diC6-PI(4,5)P2 immobilized on beads (PIPn concentration is 10 μm) show that all of the input protein bound to the PI(4,5)P2 beads, with no protein in the unbound fraction (Fig. 4A, first set of three lanes). A mutant PLC-δ1-PH domain (PLC-δ1-PHLLN) did not bind to the PI(4,5)P2 beads and instead was present only in the unbound fraction (Fig. 4A, second set of three lanes). These point mutations (K30L, K32L, W36N) are known to disrupt PI(4,5)P2 binding from structural studies of the PLCδ1-PH domain (33, 34). The GRP1-PH domain did not bind to the PI(4,5)P2 beads (Fig. 4A, third set of three lanes) but was pulled down by PI(3,4,5)P3 immobilized on beads (Fig. 4A, final set of three lanes). None of the proteins tested were pulled down by control agarose beads (data not shown). Our in vitro binding experiments validate the use of these recombinant PH domain proteins to target and sequester different phosphoinositides directly in excised membrane patches.
We next used the recombinant PH domains in electrophysiology experiments to test which phosphoinositide is the endogenous modulator of TRPV1. As shown in Fig. 4, B-D and F, when applied directly to inside-out patches, the PLC-δ1-PH domain inhibited the capsaicin-activated TRPV1 current (for 20 μm, I/Iinitial = 0.56 ± 0.03, n = 10, p = 0.0005, and for 60 μm, I/Iinitial = 0.035 ± 0.024, n = 6, p = 0.002; Fig. 4, B, D, and F). The application of boiled PLC-δ1-PH did not inhibit the current (I/Iinitial = 0.95 ± 0.02, n = 4, p > 0.05; Fig. 4, C and F), indicating that the effect of the PLC-δ1-PH domain requires folded protein. Furthermore, the mutant PLC-δ1-PHLLN domain protein did not inhibit the TRPV1 current (I/Iinitial = 1.04 ± 0.02, n = 6, p > 0.05; Fig. 4, B and F), indicating that the PI(4,5)P2 binding ability of the PLC-δ1-PH domain was required for inhibition. Importantly, the GRP1-PH domain did not produce inhibition of the TRPV1 current (I/Iinitial = 1.02 ± 0.06, n = 5, p > 0.05; Fig. 4, E and F), confirming that PI(3,4,5)P3 was not bound to and potentiating TRPV1. Finally PLC-δ1-PH applied to outside-out patches did not inhibit the TRPV1 current (I/Iinitial = 1.06 ± 0.08, n = 4, p > 0.05; Fig. 4F). Taken together, these results demonstrate that PI(4,5)P2 is the endogenous phosphoinositide bound to TRPV1 and modulating its current.
Manipulation of PI(4,5)P2 Levels Can Modulate TRPV1 in Intact Cells—The hydrolysis of PI(4,5)P2 has been reported to underlie both sensitization (15) and desensitization (18) of the TRPV1 response to noxious stimuli. However, a direct connection has not yet been established between changes in PI(4,5)P2 concentration and changes in TRPV1 excitability in an intact cell. We asked whether decreasing the PI(4,5)P2 concentration in intact cells could in fact produce changes in TRPV1 activity using a recently developed system for manipulating PI(4,5)P2 plasma membrane levels described by Suh et al. (35). In this system, rapamycin applied to intact cells recruits a FK506-binding protein (FKBP)-inositol-5-phosphatase fusion protein (normally cytosolic) to the plasma membrane by inducing the formation of heterodimers between it and a membrane-targeted FKBP-rapamycin-binding domain-Lyn protein. The recruitment of inositol-5-phosphatase (Ins-5P) to the plasma membrane then causes depletion of PI(4,5)P2 via its conversion to PI(4)P + Pi. This rapamycin-inducible system bypasses production of inositol 1,4,5-trisphosphate, diacylglycerol, and intracellular Ca2+ release. Rapamycin-induced changes in channel function can thus be attributed directly to changes in PI(4,5)P2 concentration, without concern over down-stream effects of phospholipases.
We transiently transfected tsA cells with the components of the rapamycin-inducible Ins-5P system, TRPV1, and YFP fused to the PLCδ1-PH domain (YFP-PLCδ1-PH) as a marker for PI(4,5)P2. YFP-PLCδ1-PH was localized to the plasma membrane in resting cells (supplemental Movies 1 and 2; Figs. 5, A and D, and 6, A and D) because of the relatively high concentration of PI(4,5)P2 in the plasma membrane (17, 35). We examined the effect of applying rapamycin to the bath in two groups of cells: an experimental group expressing all components of the rapamycin-inducible Ins-5P system and a negative control group expressing the same components but with a FKBP protein that lacked the Ins-5P fusion. Consistent with previous reports (35, 36), we found that the application of rapamycin caused translocation of YFP-PLCδ1-PH from the plasma membrane to the cytosol in the experimental cells (translocation observed in 13 out of 31 cells tested; supplemental Movies 1 and 2, and Fig. 5A, image 2) but not in the negative control cells (no translocation observed in 18 out of 18 cells tested; Fig. 5D, image 2).
The translocation of the YFP-PLCδ1-PH probe from the plasma membrane to the cytosol observed in the experimental group of cells was due to the rapamycin-induced recruitment of CFP-FKBP-Ins-5P from the cytosol to the plasma membrane. This was confirmed by measuring the YFP and CFP fluorescence from these proteins simultaneously within the same cells. As can be seen in supplemental Movie 2, the rapamycin-induced translocation of the CFP-FKBP-Ins-5P from the cytosol to the plasma membrane occurs with the same kinetics as the translocation of the YFP-PLCδ1-PH from the plasma membrane to the cytosol. These data indicate that, in the experimental group, rapamycin decreased the plasma membrane PI(4,5)P2 concentration by targeting the Ins-5P to the plasma membrane. It is important to note, however, that rapamycin did not produce a decrease in PI(4,5)P2 concentration in more than half of the cells tested (18 out of 31). Furthermore, many cells were not tested because the YFP-PLCδ1-PH probe was localized to the cytosol even at rest (typically 33% of the cells within a field), perhaps due to high expression of the FKBP-Ins-5P. We therefore concluded that this system could be used to study the PI(4,5)P2 dependence of TRPV1 only if the effect of rapamycin on PI(4,5)P2 levels could be measured at the same time as we measured TRPV1 function.
Using the rapamycin-inducible Ins-5P system, we tested the hypothesis that a decrease in the plasma membrane levels of PI(4,5)P2 would produce a concomitant decrease in TRPV1 activity. The incorporation of a confocal microscope into an electrophysiology work station allowed us to image the YFP-PLCδ1-PH probe in the same cells in which we recorded capsaicin-activated currents. Using perforated-patch recording to minimize disturbance of the normal intracellular milieu, we found that a saturating concentration of capsaicin (300 nm to 1 μm) activated large and stable inward currents (Fig. 5, C and F). Because these experiments were performed using a nominally Ca2+-free bath solution, Ca2+-dependent desensitization was not observed. In five out of seven cells from the FKBP-Ins-5P group, the addition of rapamycin to the bath produced a slow decrease in the capsaicin-activated current (Fig. 5C; supplemental Movie 1) together with a translocation of the YFP-PLCδ1-PH probe from the plasma membrane to the cytosol (Fig. 5, A and B, supplemental Movie 1). In the FKBP-negative control group, no change in TRPV1 activity (Fig. 5F) or YFP-PLCδ1-PH probe localization (Fig. 5, D and E) was induced by rapamycin.
In contrast to the data shown in Fig. 5, Lukacs et al. (17) reported, using a similar rapamycin-activated-Ins-5P system, that PI(4,5)P2 dephosphorylation at a saturating concentration of capsaicin did not alter TRPV1 currents. They also observed a potentiation of TRPV1 activity by rapamycin-induced Ins-5P at a subsaturating concentration of capsaicin. To test whether inhibition of TRPV1 by PI(4,5)P2 dephosphorylation was capsaicin-dependent in our system, we repeated the experiments of Fig. 5 with a concentration of capsaicin that activated less than half of the maximal current. Because co-expression of TRPV1 with the YFP-PLCδ1-PH probe and components of the rapamycin system can alter the apparent affinity of the channels for capsaicin, we first measured the concentration dependence of TRPV1 in cells expressing all these components. Based on dose-response measurements such as those shown in Fig. 6G, we chose 30 nm capsaicin, a concentration that activated 25 ± 9% (n = 4) of the maximal current. This capsaicin concentration produced a current large enough to measure reliably but small enough to be fairly called a“low” concentration.
As shown by the experiments in Fig. 6, rapamycin caused translocation of the YFP-PLCδ1-PH probe even when applied with the low (30 nm) concentration of capsaicin (Fig. 6, A and B). Like the rapamycin experiments with a saturating concentration of capsaicin, the application of rapamycin with 30 nm capsaicin caused a concomitant decrease in current for the experimental cells (Fig. 6C). As expected, when no translocation of YFP-PLCδ1-PH probe occurred, there was no inhibition of the capsaicin-activated current (Fig. 6, D-F). The values for fractional inhibition of the current upon the application of rapamycin with the low concentration of capsaicin are included in the aggregate data of Fig. 5G, with values not significantly different from those obtained with the high capsaicin protocol.
Remarkably, the kinetics of the rapamycin-induced decrease in TRPV1 activity tracked the kinetics of the rapamycin-induced translocation of the YFP-PLCδ1-PH PI(4,5)P2 probe (Figs. 5B and 6B, inverse of currents shown in C is shown as a superimposed black trace on the plasma membrane data points; supplemental Movie 1). Although this temporal correlation between PI(4,5)P2 degradation and TRPV1 inhibition does not rule out an indirect connection between the two events, the simplest interpretation of our data is that PI(4,5)P2 degradation caused TRPV1 inhibition. Together with the complete inhibition we observed in response to saturating concentrations of PolyK and the PLC-δ1-PH protein and the activation of Ci-VSP, these data suggest that PI(4,5)P2, and not PI(4)P or PI(3,4,5)P3, is the endogenous phosphoinositide that interacts with TRPV1 to regulate its activity.
DISCUSSION
Although regulation of some ion channels by PI(4,5)P2 appears to be selective (4), other channels can be regulated by a number of phosphoinositides (24, 37-39). The assumption that PI(4,5)P2 is the biologically relevant phosphoinositide involved in ion channel regulation is based on its abundance in the plasma membrane and its strong negative charge (1, 2). Technical challenges have made identifying the lipid bound to the channels in a natural system (e.g. intact cell or excised patch) difficult. Although PI(4,5)P2 and PI(3,4,5)P3 are equally effective at promoting activation of TRPV1 (Fig. 2, B and C) and PI(4)P has an EC50 10-fold higher than PI(4,5)P2 (Fig. 2A), we believe our data definitively establish that PI(4,5)P2 is the physiological regulator, both in intact cells and in excised patches. Conversion of plasma membrane PI(4,5)P2 to PI(4)P eliminated all capsaicin-activated current (Figs. 3, 5, and 6), and a PI(4,5)P2-selective PH domain also inhibited TRPV1 (Fig. 4), ruling out a role for PI(4)P. PI(3,4,5)P3 can be ruled out based on inhibition by the PI(4,5)P2-selective PH domain, the lack of inhibition by the PI(3,4,5)P3-selective PH domain (Fig. 4E), and more weakly, by the temporal correlation between PI(4,5)P2 degradation (i.e. translocation of the PLC-δ1-PH probe) and TRPV1 inhibition in the rapamycin experiments (Figs. 5 and 6). Taken together, our data indicate that PI(4,5)P2 is the physiological regulator of TRPV1.
The difficulty of studying lipid regulation of ion channels is well illustrated by the controversy surrounding whether PI(4,5)P2 stimulates or inhibits TRPV1. Although consensus is growing that PI(4,5)P2 is stimulatory in excised patches (16, 17), the story in whole cells may be more complex. It has recently been suggested, based in part on the same type of rapamycin-inducible system used here, that the polarity of PI(4,5)P2 regulation may depend on the concentration of capsaicin used (17). In particular, rapamycin-induced potentiation of TRPV1 was reported at low capsaicin concentrations or with moderate temperature elevations, suggesting an inhibitory role for PI(4,5)P2. We cannot explain the inconsistency between our results and the reported failure of rapamycin to alter TRPV1 responses to saturating capsaicin concentrations nor the potentiation reported with rapamycin at a low capsaicin concentration (17). Because the previous report did not examine whether PI(4,5)P2 was depleted in the cells in which currents were recorded, it is possible that, as we observed, the rapamycin system was not functional in all cells. Perhaps the different recording configurations (perforated patch versus whole-cell recording) or the different cell types used (tsA cells versus HEK 293 cells and Xenopus oocytes) generated differences in the properties of TRPV1 or of PI(4,5)P2 metabolism. A full understanding of the stimulatory and inhibitory roles of PI(4,5)P2 will clearly require additional studies.
We have shown that manipulating PI(4,5)P2 levels in the plasma membrane can alter the activity of TRPV1. However, the rapamycin-induced decrease in plasma membrane PI(4,5)P2 levels may not accurately reflect the amplitude of PI(4,5)P2 fluctuations under physiological conditions. Is PI(4,5)P2 a static cofactor that remains associated with TRPV1 channels throughout their lifetime in the plasma membrane? Otherwise, alternatively, do physiologically induced changes in the PI(4,5)P2 concentration constitute a system for actively tuning the excitability of TRPV1-expressing nociceptors? Deciphering PI(4,5)P2 regulation of TRPV1 is likely to reveal general themes that apply to other channels and membrane proteins as well.
Supplementary Material
Acknowledgments
We thank the following people for their generous gifts of materials: Dr. David Julius (University of California, San Francisco; TRPV1 cDNA). Dr. Mark Lemmon (University of Pennsylvania; GRP1 and PLCδ1-PH domain cDNAs); Drs. Bertil Hille (University of Washington (UW)) and Tobias Meyer (Stanford University; the rapamycin-inducible Ins-5P system); and Dr. Yasushi Okamura (Osaka University; Ci-VSP cDNA). We are indebted to Mika Munari for excellent technical assistance. We thank Drs. Luis Fernando Santana and Manuel Navedo for invaluable technical assistance in the combined imaging and electrophysiology experiments and Dr. Justin Taraska for fusing citrine to Ci-VSP. We also thank Dr. Greg Martin and the UW Keck Imaging Center for technical assistance and facilities for the confocal imaging. Finally, we thank Dr. Byung-Chang Suh for helpful discussions during the course of this study.
This work was supported, in whole or in part, by National Institutes of Health Grant R01EY017564 from the NEI (to S. E. G.). This work was also supported by the University of Washington Vision Training Grant 2T32EY007031-31 (to R. M. K.) and the University of Washington Molecular Neuroscience Training Grant 5T32NS007332-19 (to C. A. U.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked“advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains two supplemental movies.
Footnotes
The abbreviations used are: PI(4,5)P2, phosphatidylinositol (4,5)-bisphosphate; PI(4)P, phosphatidylinositol (4)-phosphate; PI(3,4,5)P3, phosphatidylinositol (3,4,5)-trisphosphate; IP3, inositol 1,4,5-trisphosphate; TRP, transient receptor potential; PH, pleckstrin homology; YFP, yellow fluorescent protein; CFP, cyan fluorescent protein; PLC, phospholipase C; polyK, polylysine; Ci-VSP, Ciona voltage-sensor containing phosphatase; FKBP, FK506-binding protein; Ins-5P, inositol-5-phosphatase.
References
- 1.Hilgemann, D. W., Feng, S., and Nasuhoglu, C. (2001) Science's STKE 2001 RE19. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin, S., and Murray, D. (2005) Nature 438 605-611 [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin, S., Wang, J., Gambhir, A., and Murray, D. (2002) Annu. Rev. Biophys. Biomol. Struct. 31 151-175 [DOI] [PubMed] [Google Scholar]
- 4.Suh, B. C., and Hille, B. (2005) Curr. Opin. Neurobiol. 15 370-378 [DOI] [PubMed] [Google Scholar]
- 5.Czech, M. P. (2000) Cell 100 603-606 [DOI] [PubMed] [Google Scholar]
- 6.Hardie, R. C. (2007) J. Physiol. (Lond.) 578 9-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon, Y., Hofmann, T., and Montell, C. (2007) Mol. Cell 25 491-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voets, T., and Nilius, B. (2007) J. Physiol. (Lond.) 582 939-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu, B., and Qin, F. (2005) J. Neurosci. 25 1674-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohacs, T., Lopes, C. M., Michailidis, I., and Logothetis, D. E. (2005) Nat. Neurosci. 8 626-634 [DOI] [PubMed] [Google Scholar]
- 11.Liu, D., and Liman, E. R. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 15160-15165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang, Z., Okawa, H., Wang, Y., and Liman, E. R. (2005) J. Biol. Chem. 280 39185-39192 [DOI] [PubMed] [Google Scholar]
- 13.Lee, J., Cha, S. K., Sun, T. J., and Huang, C. L. (2005) J. Gen. Physiol. 126 439-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozak, J. A., Matsushita, M., Nairn, A. C., and Cahalan, M. D. (2005) J. Gen. Physiol. 126 499-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuang, H. H., Prescott, E. D., Kong, H., Shields, S., Jordt, S. E., Basbaum, A. I., Chao, M. V., and Julius, D. (2001) Nature 411 957-962 [DOI] [PubMed] [Google Scholar]
- 16.Stein, A. T., Ufret-Vincenty, C. A., Hua, L., Santana, L. F., and Gordon, S. E. (2006) J. Gen. Physiol. 128 509-522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukacs, V., Thyagarajan, B., Varnai, P., Balla, A., Balla, T., and Rohacs, T. (2007) J. Neurosci. 27 7070-7080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, B., Zhang, C., and Qin, F. (2005) J. Neurosci. 25 4835-4843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenbaum, T., Gordon-Shaag, A., Munari, M., and Gordon, S. E. (2004) J. Gen. Physiol. 123 53-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toner, M., Vaio, G., McLaughlin, A., and McLaughlin, S. (1988) Biochemistry 27 7435-7443 [DOI] [PubMed] [Google Scholar]
- 21.Gabev, E., Kasianowicz, J., Abbott, T., and McLaughlin, S. (1989) Biochim. Biophys. Acta 979 105-112 [DOI] [PubMed] [Google Scholar]
- 22.Ben-Tal, N., Honig, B., Peitzsch, R. M., Denisov, G., and McLaughlin, S. (1996) Biophys. J. 71 561-575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohacs, T., Chen, J., Prestwich, G. D., and Logothetis, D. E. (1999) J. Biol. Chem. 274 36065-36072 [DOI] [PubMed] [Google Scholar]
- 24.Rohacs, T., Lopes, C. M., Jin, T., Ramdya, P. P., Molnar, Z., and Logothetis, D. E. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 745-750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulze, D., Rapedius, M., Krauter, T., and Baukrowitz, T. (2003) J. Physiol. (Lond.) 552 357-367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francel, P. C., Harris, K., Smith, M., Fishman, M. C., Dawson, G., and Miller, R. J. (1987) J. Neurochem. 48 1624-1631 [DOI] [PubMed] [Google Scholar]
- 27.Jahnel, R., Bender, O., Munter, L. M., Dreger, M., Gillen, C., and Hucho, F. (2003) Eur. J. Biochem. 270 4264-4271 [DOI] [PubMed] [Google Scholar]
- 28.Murata, Y., Iwasaki, H., Sasaki, M., Inaba, K., and Okamura, Y. (2005) Nature 435 1239-1243 [DOI] [PubMed] [Google Scholar]
- 29.Murata, Y., and Okamura, Y. (2007) J. Physiol. (Lond.) 583 875-889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemmon, M. A. (2003) Traffic 4 201-213 [DOI] [PubMed] [Google Scholar]
- 31.Lemmon, M. A., Ferguson, K. M., O'Brien, R., Sigler, P. B., and Schlessinger, J. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 10472-10476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klarlund, J. K., Tsiaras, W., Holik, J. J., Chawla, A., and Czech, M. P. (2000) J. Biol. Chem. 275 32816-32821 [DOI] [PubMed] [Google Scholar]
- 33.Ferguson, K. M., Kavran, J. M., Sankaran, V. G., Fournier, E., Isakoff, S. J., Skolnik, E. Y., and Lemmon, M. A. (2000) Mol. Cell 6 373-384 [DOI] [PubMed] [Google Scholar]
- 34.Harlan, J. E., Yoon, H. S., Hajduk, P. J., and Fesik, S. W. (1995) Biochemistry 34 9859-9864 [DOI] [PubMed] [Google Scholar]
- 35.Suh, B. C., Inoue, T., Meyer, T., and Hille, B. (2006) Science 314 1454-1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varnai, P., Thyagarajan, B., Rohacs, T., and Balla, T. (2006) J. Cell Biol. 175 377-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong, Q., Gamper, N., Medina, J. L., Shapiro, M. S., and Stockand, J. D. (2004) J. Biol. Chem. 279 22654-22663 [DOI] [PubMed] [Google Scholar]
- 38.Brown, D. A., Hughes, S. A., Marsh, S. J., and Tinker, A. (2007) J. Physiol. (Lond.) 582 917-925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gamper, N., and Shapiro, M. S. (2007) J. Physiol. (Lond.) 582 967-975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwasaki, H., Murata, Y., Kim, Y., Hossain, M.I., Worby, C.A., Dixon, J.E., McCormack, T., Sasaki, T., and Okamura, Y. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 7970-7975 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.