Abstract
The function of dopamine D3 receptors present in the striatum has remained elusive. In the present study evidence is provided for the existence of dopamine D1–D3 receptor heteromers and for an intramembrane D1–D3 receptor cross-talk in living cells and in the striatum. The formation of D1–D3 receptor heteromers was demonstrated by fluorescence resonance energy transfer and bioluminescence resonance energy transfer techniques in transfected mammalian cells. In membrane preparations from these cells, a synergistic D1–D3 intramembrane receptor-receptor interaction was observed, by which D3 receptor stimulation enhances D1 receptor agonist affinity, indicating that the D1–D3 intramembrane receptor-receptor interaction is a biochemical characteristic of the D1–D3 receptor heteromer. The same biochemical characteristic was also observed in membrane preparations from brain striatum, demonstrating the striatal co-localization and heteromerization of D1 and D3 receptors. According to the synergistic D1–D3 intramembrane receptor-receptor interaction, experiments in reserpinized mice showed that D3 receptor stimulation potentiates D1 receptor-mediated behavioral effects by a different mechanism than D2 receptor stimulation. The present study shows that a main functional significance of the D3 receptor is to obtain a stronger dopaminergic response in the striatal neurons that co-express the two receptors.
Dopamine receptors are grouped into two classes, D1-like receptors, which include D1 and D5 receptors, and D2-like receptors, which include D2 (with two isoforms, D2S and D2L), and D3 and D4 receptors (1, 2). The striatum receives the densest dopamine innervation and contains the highest density of dopamine receptors in the brain (3). The localization and function of the different subtypes of striatal dopamine receptors has been a matter of considerable debate, particularly that of the postsynaptic D1 and D2 receptors. It is widely accepted that D1 and D2 receptor subtypes are largely segregated in the two most populated types of striatal neurons, the γ-aminobutyric-acidergic (GABAergic)2 dynorphinergic neuron, which also expresses substance P (SP), and the GABAergic enkephalinergic neuron (4, 5). In fact, results obtained with in vivo techniques indicate that dopamine exerts differential effects on the two types of GABAergic efferent neurons, by acting on stimulatory D1 receptors localized in the GABAergic SP-dynorphinergic neurons and inhibitory D2 receptors localized in the GABAergic enkephalinergic neurons (6–8). However, functional D1-like and D2-like receptors, as well as significant levels of D1 and D2 receptor mRNA expression, were detected in acutely dissociated striatonigral neurons (9). A more detailed and extensive analysis of the mRNA expression of the different receptor subtypes indicated that there is a limited subset of striatal neurons (∼15% of all GABAergic efferent neurons) with a mixed phenotype of GABAergic SP-dynorphinergic and GABAergic enkephalinergic neurons, with D1 and D2 receptors (10). This co-expression of D1 and D2 receptors has been confirmed in neostriatal neurons at the confocal microscopy level (11, 12). George and coworkers (12, 13) have also found evidence for D1–D2 receptor heteromerization (by co-immunoprecipitation) and for the generation of a unique pharmacology of the D1–D2 receptor heteromer, with binding to selective ligands and with selective coupling to Gq/11 and phospholipase C-mediated calcium signaling.
In contrast to the few GABAergic neurons expressing D1 and D2 receptors, the study of Surmeier et al. (10) showed that at least half of the GABAergic SP-dynorphinergic neurons and very few GABAergic enkephalinergic neurons express D3 receptors, which have a very similar pharmacology to the D2 receptors. Therefore, the D3 receptors could in fact mediate most of the reported D1–D2-like receptor interactions demonstrated at the striatal neuronal level (reviewed in ref. 14). In the striatum, D3 receptors are more concentrated in the shell of the nucleus accumbens and islands of Calleja, where they are also predominantly localized in GABAergic SP-dynorphinergic neurons, and pharmacological studies suggest that D1–D3 receptor interactions could occur at the single cell level (15).
The evidence for striatal co-localization of D1 and D3 receptors gives a frame for the possible existence of direct D1–D3 receptor intermolecular interactions. In the present study we demonstrate that D1 and D3 receptors can, in fact, interact at the molecular level, with the ability to form D1–D3 receptor heteromers in transfected cells and in the striatum. These receptor heteromers show a synergistic D1–D3 intramembrane receptor-receptor interaction that is most probably involved in the ability of D3 receptor to potentiate the effects of D1 receptor stimulation at the neuronal and behavioral level.
EXPERIMENTAL PROCEDURES
Animals—Male Swiss Webster mice (Charles River Laboratories, Inc., Wilmington, MA) and homozygous D3 knockout (D3KO) mice and their wild-type (WT) littermates (kindly donated by J. Drago from the Department of Medicine, Monash University, Australia) (8–12 weeks old) were used. D3KO mice used in these experiments were 5th through 8th generations of congenic C57BL/6J mice and generated by a backcrossing strategy. Genotypes of the D3KO and WT mice were identified by PCR with two pairs of primers flanking either exon 3 of the WT D3 receptor or the PGK (phosphoglycerate kinase 1 gene promoter) cassette of the mutated gene (16). Animals were housed four per cage with food and water available ad libitum in temperature- and humidity-controlled rooms and were maintained on a 12-h light/dark cycle. They were experimentally naive at the start of the study and were used only once for locomotor activity experiments. Animals were maintained in accordance with guidelines of the Institutional Care and Use Committee of the Intramural Research Program, National Institute on Drug Abuse and the European Community Council Directive 86/609/EEC. The rationale, design, and methods of the experiments with D3KO mice and their WT littermates were approved by the Ethical Committee for Animal Research, University of Catania.
Cell Culture and Transfection—Human embryonic kidney (HEK)-293T cells were grown in Dulbecco's modified Eagle's medium supplemented with 2 mm l-glutamine, 100 units/ml penicillin/streptomycin, and 10% (v/v) fetal bovine serum at 37 °C and in an atmosphere of 5% CO2. HEK-293T cells were transiently transfected with different amounts of cDNA encoding the indicated proteins by calcium phosphate precipitation (17) except for confocal FRET acceptor photobleaching experiments in which the transfection reagent, FuGENE-6™ (Roche Molecular Biochemicals, Indianapolis, IN) was utilized following the protocol given by the manufacturer. In all cases, to maintain the total amount of DNA constant (10 μg) in co-transfections, the empty vector, pcDNA3.1, was used to equilibrate the amount of total DNA transfected. Cells were used 48 h after transfection. For confocal microscopy FRET experiments cells were fixed with a 3.5% paraformaldehyde in PBS for 15 min at room temperature, washed in PBS, and mounted onto slides using Mowiol coverslip mounting solution.
Expression Vectors—The human cDNA for the D3 receptor without its stop codon was amplified using sense and antisense primers harboring unique EcoRI and KpnI sites. The fragment was then subcloned to be in-frame with either Renilla luciferase (Rluc) or green fluorescence protein2 (GFP2) into the EcoRI and KpnI restriction site of a Rluc-expressing vector (pRluc-N1, PerkinElmer Life Sciences) or a variant of GFP (pGFYP2-N3, Clontech, Heidelberg, Germany), respectively, to give the two plasmids, pD3-Rluc and pD3-GFP2, that express D3 receptors fused to Rluc or GFP2 on the C-terminal ends of the receptor (D3-Rluc and D3-GFP2). The human D1 receptor was cloned in the enhanced yellow variant of GFP pEYFP-N1 (Clontech) vectors in a similar fashion and subcloned into the EcoRI and KpnI site to be in-frame with the GFP fluorescent protein variant enhanced yellow fluorescent protein (EYFP) (D1-YFP). The positive control vector used for the FRET experiments, pGFP2-EYFP (encoding for a fusion protein of GFP2 and EYFP linked by six amino acids), was a gift from R. Pepperkok (EMBL, Heidelberg, Germany).
cAMP Determination—The accumulation of cAMP was measured in transfected HEK-293T cells (2 × 106 cells/sample) stimulated with different concentrations of the D1 receptor agonist SKF 38393 (Tocris, Ellisville, MO) the D2–3 receptor agonist quinpirole (Sigma) and/or forskolin (Sigma) for 15 min prior to the determination of cAMP levels by a [3H]cAMP assay system (Amersham Biosciences) as described by the manufacturer.
FRET Experiments—For FRET-based acceptor photobleaching experiments analyzed by confocal microscopy, the protocol described elsewhere was used (18). Confocal laser scanning microscopy was performed using a Leica SP2 microscope (Leica Microsystems, Mannheim, Germany) equipped with an acousto-optical beamsplitter, a 100-milliwatt argon laser for excitation at 514 nm, and a 20-milliwatt blue diode laser for excitation at 405 nm, and images were acquired using the acceptor photobleaching element of the Leica software. For FRET experiments analyzed by fluorometry, 48 h after transfection, cells were rapidly washed twice in PBS, detached, and resuspended in the same buffer. To control the number of cells, the protein concentration of the samples was determined using a Bradford assay kit (Bio-Rad) using bovine serum albumin dilutions as standards. FRET experiments were performed as described previously (18). The contribution of the GFP variants, GFP2 and YFP proteins alone, to the two detection channels (spectral signature (19)) was measured in experiments with cells expressing only one of these proteins and normalized to the sum of the signal obtained in the two detection channels. The spectral signatures of the different receptors fused to either GFP2 or YFP did not significantly vary from the determined spectral signatures of the fluorescent proteins alone. FRET quantitation was performed as described previously (18).
BRET Experiments—Forty-eight hours after transfection, cells were rapidly washed twice in PBS, detached, and resuspended in the same buffer. To control the number of cells, sample protein concentration was determined using a Bradford assay kit (Bio-Rad, Munich, Germany) using bovine serum albumin dilutions as standards. To quantify D1-YFP expression, cells (20 μg of protein) were distributed in 96-well microplates (black plates with a transparent bottom), and fluorescence was read in a Fluostar Optima fluorometer (BMG Labtechnologies, Offenburg, Germany) using a 10 nm bandwidth excitation filter at 400 nm for D2R-GFP2 reading or 485 nm for YFP reading. Receptor-fluorescence expression was determined as fluorescence of the sample minus the fluorescence of cells expressing D3-Rluc alone. For BRET measurements, the protocol previously described was used (18). To quantify D3-Rluc expression luminescence readings were performed after 10 min of adding 5 μm coelentrazine H. The net BRET is defined as [(long-wavelength emission)/(short-wavelength emission)] - Cf, where Cf corresponds to [(long-wavelength emission)/(short-wavelength emission)] for the Rluc construct expressed alone in the same experiment.
Membrane Preparation—Transfected HEK cells were washed twice with ice-cold PBS and sonicated for three 30-s periods in 10 volumes of 20 mm Tris-HCl buffer containing 100 mm NaCl, 7 mm MgCl2, and 1 mm EDTA, pH 7.4. The homogenate was centrifuged at 1,500 × g for 10 min at 4 °C. The supernatant was centrifuged at 105,000 × g, 40 min, 4 °C. Membrane suspensions from striatal tissue were obtained by tissue disruption with a Polytron homogenizer (PTA 20 TS rotor, setting 3, Kinematica, Basel, Switzerland) for three 5-s periods in 10 volumes of 50 mm Tris-HCl buffer, pH 7.4, and the homogenates were processed as described above for cell membranes. Membranes were stored at -80 °C and were washed once more as described above and resuspended in 50 mm Tris-HCl buffer for immediate use. Protein was quantified by the bicinchoninic acid method (Pierce) using bovine serum albumin dilutions as standard.
Radioligand Binding Experiments—Membrane suspensions (0.5 mg of protein/ml) were incubated 1 h at 25 °C in 50 mm Tris-HCl buffer, pH 7.4, containing 10 mm MgCl2 with 2.5 nm of the D1 receptor antagonist [3H]R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine ([3H]SCH 23390, PerkinElmer Life Sciences), 1.7 nm of the D3 receptor agonist [3H]7-hydroxy-N,N-di-n-propyl-2-aminotetralin ([3H]R(+)-7-OH-DPAT, Amersham Biosciences), or 5.0 nm of the D2–3 receptor antagonist [3H]3,5-dichloro-N-[[(2S)-1-ethyl-2-pyrrolidinyl]methyl]-2-hydroxy-6-methoxybenzamide ([3H]raclopride, PerkinElmer Life Sciences) and increasing concentrations of the partial D1 receptor agonist (±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrobromide (SKF 38393, triplicates of 13 different competitor concentrations from 0.1 nm to 50 μm), the full D1 receptor agonist 6-chloro-2,3,4,5-tetrahydro-1-phenyl-1H-3-benzazepine hydrobromide (SKF 81297, triplicates of 12 different competitor concentrations from 0.001 nm to 10 μm, Tocris) or the D3 receptor agonist R(+)-7-OH-DPAT (triplicates of 13 different competitor concentrations from 0.01 nm to 10 μm; Sigma), in the absence or the presence of 10 nm of the D3 receptor agonist R(+)-7-OH-DPAT, 100 nm of the D1 agonist SKF 38393 or 100 nm of the D1 agonist SKF 81297. Nonspecific binding was determined in the presence of 10 μm SCH 23390 (Tocris) for the D1 receptor or 1 μm of the D3 receptor antagonist GR 103691 (Tocris) for the D3 receptor and confirmed that the value was the same as calculated by extrapolation of the displacement curves. In some experiments the D3 receptor antagonist (E)-N-(4-[1,2,3,4-tetrahydroisoquinolin-2-yl]butyl)-3-phenylacrylamide (ST 198, 1 μm, prepared and analyzed by two of us (H.S. and O.S.) at the Johann Wolfgang Goethe University, Germany) was added instead R(+)-7-OH-DPAT to show its lack of modulation of D1 receptor binding. In all cases, free and membrane-bound ligand were separated by rapid filtration of 500-μl aliquots in a cell harvester (Brandel, Gaithersburg, MD) through Whatman GF/C filters (Brandel) embedded in 0.3% polyethyleneimine, which were subsequently washed for 5 s with 5 ml of ice-cold Tris-HCl buffer. The filters were incubated with 10 ml of Ecoscint H scintillation mixture (National Diagnostics, Atlanta, GA) overnight at room temperature, and radioactivity counts were determined using a Tri-Carb 1600 scintillation counter (PerkinElmer Life Sciences) with an efficiency of 62% (20). Binding data from competition experiments were analyzed by nonlinear regression to the previously described equations (20, 21), using the commercial Grafit curve-fitting software (Erithacus Software, Surrey, UK). Goodness of fit was tested according to reduced χ2 value given by the nonlinear regression program and biphasic over monophasic competition curves were selected according to a p < 0.05 using an F test (22). The equilibrium dissociation constants for the high affinity (KDH) and low affinity (KDL) binding sites were determined from IC50 values using the Cheng-Prusoff relation (23). Differences in KDH and KDL in the presence and absence of R(+)-7-OH-DPAT were tested for significance (two-tailed, p < 0.05) using Student's t test for paired samples.
Locomotor Activity—Activity chambers with 25.40 × 25.40 × 40.64 (high) cm open fields (Coulbourn Instruments, Allentown, PA) were used for the experiments with Swiss Webster mice and 41 × 41 × 33 (high) cm activity cages (7433 cage, Ugo Basile Instruments, Italy) were used for the experiments with D3KO mice and their WT littermates. Reserpine was purchased from Sigma and the D2–3 receptor agonist quinpirole, the D3 receptor agonist (4aR,10bR)-3,4a,4,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano-[4,3-b]-1,4-oxazin-9-ol hydrochloride (PD 128907) and the D2 receptor antagonist 3-[4-(4-chlorophenyl)-4-hydroxyperidin-l-yl]methyl-1H-indole (L741626) were from Tocris. Reserpine was dissolved in a drop of glacial acetic acid, which was made up to volume with 5.5% glucose and was administered subcutaneously 20 h prior to the start of the locomotor activity recording. ST 198 was dissolved in sterile water and administered per os (due to the low pH of the solution). All the other drugs were dissolved in sterile saline and administered intraperitoneally (i.p.). The volume of administration was 10 ml/kg for all drugs. L741626 and ST 198 were administered 15 min prior to the locomotor activity recording and SKF 38393, quinpirole, and PD 128907 were administered immediately before the locomotor activity recording. Locomotor activity was recorded immediately after the animals were introduced in the open field. All values (in centimeters of ambulation) registered per 10 min were transformed (square root (24)), and the average of the results obtained during the 10-min periods of the first hour of recording were used for calculations. Differences between genotypes and differently treated groups of animals were analyzed by two-way and one-way ANOVA followed by post-hoc Newman-Keuls test.
RESULTS
Heteromerization of D1 and D3 Receptors in Living Cells—The formation of D1–D3 receptor heteromers was demonstrated by FRET and BRET techniques in cells transfected with fusion proteins consisting of each receptor and either a fluorescent protein (GFP2 and YFP) or Renilla luciferase (Rluc). Expression of fusion proteins was assessed by Western blot and immunocytochemistry (data not shown). The functionality of the receptor-Rluc, -GFP2, or -YFP constructs was assessed by the determination of cAMP levels produced in transfected cells in response to agonists. According to the positive coupling of D1 receptor to the adenylyl cyclase, the D1 receptor agonist SKF 38393 properly induced cAMP accumulation in cells transfected with D1-YFP similar to the wild-type untagged receptor. On the other hand, in agreement with the inhibitory role of D3 receptor on adenylyl cyclase activity, the D2–3 receptor agonist quinpirole was able to reduce forskolin-induced cAMP levels in cells transfected with either D3-Rluc or D3-GFP2 similar to the wild-type untagged receptor (data not shown). The functional receptor fusion proteins were co-expressed in cells and the degree of co-localization was assessed. In HEK-293 cells cotransfected with the vectors encoding for both D3-GFP2 and D1-YFP both receptors were expressed, and highly co-localized, at the membrane level (Fig. 1).
FIGURE 1.
Colocalization of D1 and D3 receptors on the plasma membrane of transiently transfected HEK-293 cells. HEK-293 cells transiently transfected with D3-GFP2 and D1-YFP were fixed and analyzed by confocal laser microscopy. A, D3 receptor immunoreactivity, in green. B, D1 receptor immunoreactivity in red. C, co-localization of both proteins is shown in yellow at the plasma membrane. D, overlapping ofgreen and redpixels is shown in white. Images are representative of four coverslips from two independent transfections.
By using the FRET approach with the D3-GFP2 and D1-YFP pair, and using the acceptor photobleaching technique and confocal microscopy analysis on cells expressing both receptors, it was possible to demonstrate the heteromerization between D1 and D3 receptors (Fig. 2). FRET was measured at the membrane level in different areas of the bottom section of a given cell and FRET efficiency was determined to be in the range of 14–23% (Fig. 2). To calculate the average of FRET efficiency in a co-transfected cell population, FRET was measured by fluorometric techniques determining the sensitized emission of YFP-tagged acceptor in live cells. The FRET efficiency was determined to be 34.8% (±7.8 S.D.) (Fig. 3). The low FRET efficiency found for a control constituted by mixing transfected D3-GFP2-expressing cells with D1-YFP-expressing cells proved the specificity in the energy transfer between D3-GFP2 and D1-YFP when co-expressed in the same cell (Fig. 3).
FIGURE 2.
Imaging FRET efficiency of the D3R-GFP2 and D1R-YFP pair by acceptor photobleaching. HEK-293T cells were transiently transfected with the plasmid DNA for the D3R-GFP2 and D1R-YFP constructs using a ratio of donor to acceptor DNA of 1:2 and fixed 48 h after transfection. Left panels are images of the D3-GFP2 donor before (Donor pre-bleach) and after (Donor post-bleach) photobleaching of the D1R-YFP acceptor obtained by spectral imaging and subsequent liner un-mixing (see “Experimental Procedures”) in several regions (ROI 1 to 5) of the lowest plane of the cell. The extent of the photobleaching is shown in the central panels as a lack of acceptor fluorescence in the selected region after photobleaching (Acceptor post-bleach) with respect to the image of the acceptor before photobleaching (Acceptor pre-bleach). The right panel represents donor un-quenching following acceptor photobleaching as donor post-bleach minus donor pre-bleach (subtraction) and a color representation of the FRET efficiency normalized to a scale from 0 to 1. The FRET efficiencies from different ROIs within the cell are given in the table below the images. As negative controls ROI 7 (out of the cell) and ROI 6 (cell section without photobleaching) were also analyzed. Images are representative of four coverslips from two independent transfections.
FIGURE 3.
FRET efficiency of the D3-GFP2 and D1-YFP pair by sensitized emission in living cells. HEK-293T cells were transiently transfected with the plasmid DNA corresponding to D3-GFP2 (donor) and D1-YFP (acceptor) proteins using a ratio of donor to acceptor DNA of 1:2 (same transfect), or with the positive control plasmid GFP2-YFP. Fluorescence readings were performed 48 h after transfection, and linear un-mixing of the emission signals was applied to the data (see “Experimental Procedures”). Results are shown as the sensitized emission of the acceptor when the cells were excited at 400 nm. Cells separately transfected with either the D3-GFP2 (donor) or D1-YFP (acceptor) proteins and subsequently mixed before FRET measurements (sep.transfect) were used as a negative control. Data are the mean ± S.D. of five independent experiments performed in duplicate. One-way ANOVA followed by Newman-Keuls test shows significant differences between GFP2-YFP and D3-GFP2 + D1-YFP (same transfect) and between D3-GFP2 + D1-YFP (same transfect) and D3-GFP2 + D1-YFP (sep. tranfect) (p < 0.001 in all cases).
In BRET experiments we studied D1–D3 receptor heteromerization by constructing a BRET saturation curve in living cells co-transfected with a constant amount of the D3-Rluc construct while increasing concentrations of the D1-YFP plasmid. A positive BRET signal for the transfer of energy between D3-Rluc and D1-YFP was obtained (Fig. 4). The BRET signal increased as a hyperbolic function of the concentration of the YFP-fusion construct added (assessed by the fluorescence emitted upon direct excitation at 480 nm) reaching an asymptote. From the saturation curve, a BRETmax of 0.021 ± 0.001 and a BRET50 of 2.2 ± 0.6 were calculated. The specificity of the interaction was demonstrated by including a negative control, in this case, D3-Rluc and CXCR4-YFP. Because the pair D3-Rluc and CXCR4-YFP led to a linear BRET signal (Fig. 4), the significant and hyperbolic BRET signal found for the D3-Rluc-D1-YFP indicates that the interaction between D3 and D1 is specific.
FIGURE 4.
BRET experiments in HEK-293 cells. BRET was measured in HEK-293T cells co-expressing D3-Rluc and D1-YFP (closed squares) or D3-Rluc and CXCR4-YFP (open squares) constructs. Co-transfections were performed with increasing amounts of plasmid DNA for the YFP construct (1 μg of DNA to 10 μg of DNA), whereas the DNA for the Rluc construct was maintained constant (3 μg). Both fluorescence and luminescence of each sample were measured prior to every experiment to confirm equal expression of Rluc while monitoring the increase of YFP expression. The relative amounts of BRET acceptor are expressed as the ratio between the fluorescence of the acceptor and the luciferase activity of the donor. “YFP0” corresponds to the fluorescence value in cells expressing the BRET donor alone. BRET data are expressed as means ± S.D. of three to nine different experiments grouped as a function of the amount of BRET acceptor.
D1–D3 Intramembrane Receptor Interaction—Intramembrane receptor-receptor interactions are a common biochemical characteristic of receptor heteromers (25, 26). Therefore, we investigated the possible existence of striatal D1–D3 intramembrane receptor interactions in transfected HEK cells. Competition experiments with the D1 receptor antagonist [3H]SCH 23390 and increasing concentrations of SKF 81297 as a displacer produced biphasic competition curves (significantly better fitting than monophasic; F test: p < 0.05) with KDH and KDL values for agonist binding to the D1 receptor of 1.06 ± 0.22 nm and 52.2 ± 5.4 nm, respectively (Fig. 5A). The addition of the D3-selective agonist R(+)-7-OH-DPAT (10 nm) produced a significant decrease in KDH and KDL values to 0.36 ± 0.19 nm and 39.5 ± 4.3 nm, respectively (Student's paired t test; p < 0.01 in both cases; five independent experiments); no significant differences were observed in the proportion of receptors in high and low affinity state (Fig. 5A). These results indicate that there is an increase in the affinity of both the high and low affinity states of the D1 receptor for agonists when the D3 receptor is activated. To investigate the possible existence of a D1 receptor modulation of agonist binding to the D3 receptor, competition experiments (five independent experiments) with the D2–3 receptor antagonist [3H]raclopride (which can only bind selectively to the D3 receptor in transfected cells) as radioligand and increasing concentrations of R(+)-7-OH-DPAT as displacer demonstrated monophasic competition curves, which were not modified by the addition of the D1 receptor agonist SKF 81297 (100 nm) (Fig. 5B). KD values for R(+)-7-OH-DPAT binding to the D3 receptor in the presence and absence of SKF 38393 were 4.3 ± 0.6 nm and 3.9 ± 0.6 nm, respectively. These results demonstrate that D3 receptor stimulation enhances the binding of the D1 receptor agonist SKF 81297, but that D1 receptor stimulation does not modify the binding characteristics of the D3 receptor agonist R(+)-7-OH-DPAT, indicating that the intramembrane interaction is not reciprocal. Because, in addition, no R(+)-7-OH-DPAT-mediated modulation of D1 receptor agonist binding was observed with [3H]SCH 23390/SKF 81297 competition experiments in cells only transfected with the D1 receptor (data not shown), the synergistic D1–D3 intramembrane receptor-receptor interaction can be considered as a biochemical characteristic of the D1–D3 receptor heteromers.
FIGURE 5.
D1–D3 intramembrane receptor cross-talk in transfected cells. A, D3 receptor agonist-mediated modulation of D1 receptor agonist binding. Competition experiments of the D1 receptor antagonist [3H]SCH 23390 (2.5 nm) versus increasing concentrations of the D1 receptor agonist SKF 81297 in transfected cells were performed in the presence (○) or in the absence (▪) of the D3 receptor agonist R(+)-7-OH-DPAT (10 nm). B, D1 receptor agonist-mediated modulation of D3 receptor agonist binding. Competition experiments of the D2–3 receptor antagonist [3H]raclopride (5 nm) versus increasing concentrations of R(+)-7-OH-DPAT in transfected cells were performed in the presence (○) or in the absence (▪) of the D1 receptor agonist SKF 81297 (100 nm). Values are expressed as percentage of specific binding of the sample without competing ligand (control). Data are means ± S.D. from a representative experiment performed in triplicate.
Using the synergistic D1–D3 intramembrane receptor-receptor interaction as a possible biochemical fingerprint to identify the D1–D3 receptor heteromer in the brain, we performed similar biochemical experiments in striatal membrane preparations. Competition experiments with the D1 receptor antagonist [3H]SCH 23390 as radioligand and increasing concentrations of the partial D1 receptor agonist SKF 38393 as displacer were carried out in the presence and in the absence of R(+)-7-OH-DPAT (10 nm). As shown in Fig. 6A, both competition curves were biphasic (significantly better fitting than monophasic; F test: p < 0.05) and, the same as with transfected cells, there was a significant decrease in KDH and KDL values for agonist binding to the D1 receptor in the presence of R(+)-7-OH-DPAT, from 21 ± 3 nm and 1.1 ± 0.1 μm to 10 ± 2 nm and 0.6 ± 0.1 μm, respectively (Student's paired t test; p < 0.05 in both cases; four independent experiments). There were no significant changes in the proportion of receptors in high and low affinity state. The D3 receptor antagonist ST 198 (1 μm) did not produce any change in the binding properties of the D1 receptor (data not shown), indicating that modulation is due to D3 receptor activation. Competition experiments with the D3 receptor agonist [3H]R(+)-7-OH-DPAT as radioligand and increasing concentrations of R(+)-7-OH-DPAT as displacer were carried out in the presence or the absence of the selective D1 receptor agonist SKF 38393 (100 nm). Although there are some concerns about the in vivo selectivity of R(+)-7-OH-DPAT, it is important to point out that [3H]R(+)-7-OH-DPAT has been previously shown to bind in vitro to the D3 receptors (27, 28). As shown in Fig. 6B, both competition curves were monophasic and the presence of the D1 receptor agonist did not significantly change the equilibrium binding parameters. From four independent experiments in the absence of D1 receptor agonist the KD values for R(+)-7-OH-DPAT binding to D3 receptor was 3.2 ± 0.8 nm, whereas in the presence of SKF 38393 the KD value was 2.9 ± 0.7 nm. These results demonstrate the existence of the same kind of D1–D3 intramembrane receptor-receptor interaction in the striatum and transfected cells, demonstrating the existence of D1–D3 receptor heteromers in the striatum.
FIGURE 6.
D1–D3 intramembrane receptor cross-talk in the striatum. A, D3 receptor agonist-mediated modulation of D1 receptor agonist binding. Competition experiments of the D1 receptor antagonist [3H]SCH 23390 (2.5 nm) versus increasing concentrations of the D1 receptor agonist SKF 38393 in calf striatal membranes were performed in the presence (○) or in the absence (▪) of the D3 receptor agonist R(+)-7-OH-DPAT (10 nm). B, D1 receptor agonist-mediated modulation of D3 receptor agonist binding. Competition experiments of the D3 receptor agonist [3H]R(+)-7-OH-DPAT (1.7 nm) versus increasing concentrations of R(+)-7-OH-DPAT in calf striatal membranes were performed in the presence (○) or in the absence (▪) of the D1 receptor agonist SKF 38393 (100 nm). Values are expressed as percentage of specific binding of the sample without competing ligand (control). Data are means ± S.D. from a representative experiment performed in triplicate.
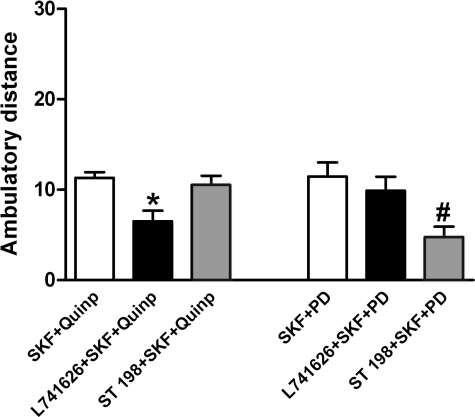
D1–D3 Behavioral Receptor Interaction in Reserpinized Mice—Administration of the selective D1 receptor agonist SKF 38393 (15 mg/kg, i.p.) or the putative non-selective D2–3 receptor agonist quinpirole (0.5 mg/kg, i.p.) (minimal doses producing significant effects; see Table 1) produced a similar degree of motor activation in Swiss Webster reserpinized mice (Table 1), and their co-administration produced a synergistic effect (Fig. 7). On the contrary, the administration of the putative selective D3 receptor agonist PD 128907 (29) did not produce any significant motor activation (up to 3 mg/kg, i.p.). Nevertheless, PD 128907 selectively and dose-dependently potentiated the locomotor activation induced by SKF 38393, but not when induced by quinpirole, a D2–3 receptor agonist in vitro (Fig. 7). In pilot studies, higher doses of the D1 receptor agonist were not potentiated by the co-administration of the D3 receptor agonist. In addition, locomotor activation induced by co-administration of SKF 38393 (15 mg/kg, i.p.) and quinpirole (0.5 mg/kg, i.p.) was also significantly potentiated by PD 128907 (0.3–3 mg/kg, i.p.) (Fig. 7). Interestingly, when co-administered, PD 128907 and quinpirole exerted additive effects on D1 receptor-mediated behavior, suggesting that simultaneous stimulation of D3 and D2 receptors induces a potentiation of D1 receptor effects through different mechanisms of action. In fact, the selective D2 receptor agonist L741626 (3 mg/kg, i.p. (30)) significantly counteracted the potentiating effect of quinpirole (0.5 mg/kg, i.p.), but not the potentiating effect of PD 128907 (3 mg/kg, i.p.) on the locomotor activation induced by SKF 38393 (15 mg/kg, i.p.) (Fig. 8). On the other hand, the selective D3 receptor antagonist ST 198 (10 mg/kg i.p. (28, 31)) significantly counteracted the potentiating effect of PD 128907 (3 mg/kg, i.p.) but not the potentiating effect of quinpirole (0.5 mg/kg, i.p.), on the locomotor activation induced by SKF 38393 (15 mg/kg, i.p.) (Fig. 8). Thus, these results strongly suggest that D3 receptor stimulation potentiates D1 receptor-mediated behavioral effects by a different mechanism than D2 receptor stimulation.
TABLE 1.
Effects of the D1 receptor agonist SKF 38393 (5 and 15 mg/kg, i.p.), the D2–3 receptor agonist quinpirole (0.15 and 0.5 mg/kg, i.p.), and the D3 receptor agonist PD 128907 (0.3–3 mg/kg, i.p.) on the locomotor activation in reserpinized Swiss Webster mice Results represent means ± S.E. of the average of the values obtained during the 10-min periods of the first hour of recording.
| Treatment | Locomotor activity |
|---|---|
| mg/kg | mean ± S.E. |
| Saline | 1.5 ± 0.2 |
| SKF 38393 (5) | 2.7 ± 0.7 |
| SKF 38393 (15) | 4.5 ± 0.8a |
| Quinpirole (0.15) | 2.5 ± 1.5 |
| Quinpirole (0.5) | 4.4 ± 1.2a |
| PD 128907 (0.3) | 1.1 ± 0.4 |
| PD 128907 (1) | 2.2 ± 0.6 |
| PD 128907 (3) | 2.3 ± 0.4 |
Significantly different compared to the control, saline-treated group (ANOVA: p < 0.05)
FIGURE 7.
D1–D3 behavioral receptor interactions in reserpinized Swiss Webster mice. Effects of the D3 receptor agonist PD 128907 (PD, 0.3–3 mg/kg, i.p.) on the locomotor activation induced by the D1 receptor agonist SKF 38393 (SKF, 15 mg/kg, i.p.) and/or the D2–3 receptor agonist quinpirole (Quinp, 0.5 mg/kg, i.p.) in reserpinized mice. Results represent means ± S.E. of the average of the values obtained during 10-min periods of the first hour of recording. * and **, significantly different compared with SKF (ANOVA: p < 0.05 and p < 0.01, respectively); ##, significantly different compared with SKF+Quinp without PD co-administration (ANOVA: p < 0.01).
FIGURE 8.
D1–D3 behavioral receptor interactions in reserpinized Swiss Webster mice. Differential effects of the D2 receptor antagonist L741626PD (3 mg/kg, i.p.) and the D3 receptor antagonist ST198 (10 mg/kg, per os) on the locomotor activation induced by the co-administration of the D1 receptor agonist SKF 38393 (SKF, 15 mg/kg, i.p.) plus the D2–3 receptor agonist quinpirole (Quinp, 0.5 mg/kg, i.p.) and the co-administration of SKF plus the D3 receptor agonist PD 128907 (PD, 3 mg/kg. i.p.) in reserpinized mice. Results represent means ± S.E. of the average of the values obtained during 10-min periods of the first hour of recording. *, significantly different compared with SKF+Quinp (ANOVA: p < 0.05); #, significantly different compared with SKF+PD (ANOVA: p < 0.05).
In view of the debated D3 receptor selectivity of PD 128907, its motor-activating effects were also analyzed in reserpinized D3KO mice and their WT littermates. Four different treated groups were studied in the two different genotypes: saline, PD 1218907 (3 mg/kg, i.p.), SKF 38393 (15 mg/kg, i.p.), and PD 1218907 plus SKF 38393. A two-way ANOVA demonstrated a significant genotype effect (F1,36 = 58.1, p < 0.0001) and a significant treatment effect (F3,36 = 71.9, p < 0.0001) with no significant interaction. The significant genotype effect was due to higher locomotor activity in reserpinized wild-type mice compared to with reserpinized D3KO mice. In fact, motor activity in reserpinized wild-type mice (C57BL/6J strain) was higher than in reserpinized Swiss Webster mice (compare Fig. 9 with Fig. 7). The results indicate a higher sensitivity to the motor-depressant effects of reserpine in D3KO mice. The D1 receptor agonist SKF 38393 significantly increased locomotor activity in both wild-type and D3KO mice, and the putative selective D3 receptor agonist PD 12807 was ineffective on its own, but significantly potentiated the locomotor activation induced by SKF 38393 in wild-type, but not in D3KO mice (Fig. 9). Finally, the effect of quinpirole was also analyzed in D3KO mice and their WT littermates. Quinpirole (5 mg/kg, i.p.) produced a similar locomotor activation to that of SKF 38393 (15 mg/kg, i.p.) in both D3KO and WT mice (in means ± S.E., 8.9 ± 0.5 and 12.2 ± 0.9, respectively; non-paired Student's t test: p < 0.01 compared with the respective saline-treated groups in both cases). In view of the lower locomotor activity of D3KO mice compared with their WT littermates, these results confirm that D3 receptors do not play a significant role in the locomotor-activating effects of quinpirole.
FIGURE 9.
D1–D3 behavioral receptor interactions in D3KO reserpinized mice and their WT littermates. Effects of the D3 receptor agonist PD 128907 (PD, 3 mg/kg, i.p.) on the locomotor activation induced by the D1 receptor agonist SKF 38393 (SKF, 15 mg/kg, i.p.) in reserpinized D3KO and WT mice. Results represent means ± S.E. of the average of the values obtained during 10-min periods of the first hour of recording. **, significantly different compared with respective saline (ANOVA: p < 0.01, respectively); #, significantly different compared with SKF alone, without PD co-administration (ANOVA: p < 0.05).
DISCUSSION
By using FRET and BRET techniques we demonstrate that D1 and D3 receptors can form heteromers if co-expressed in the same cell. This opens up the possibility of understanding the functional significance of the previously known co-localization of these receptors in the GABAergic SP-dynorphinergic striatal neuron (see the introduction). Receptor heteromerization does not only bring together two receptors to allow the interaction between their intracellular signaling cascades. Often those receptors show “intramembrane receptor-receptor interactions,” promoting changes in their ability to bind ligands (25, 26, 32). Intramembrane receptor-receptor interactions are shown in crude membrane preparations of brain tissue or cells in culture, therefore not requiring the involvement of receptor signaling mechanisms. This implies the existence of an intermolecular interaction between two adjacent receptors and is a strong evidence of receptor heteromerization. Furthermore, intramembrane receptor-receptor interactions observed with cell membranes in which heteromerization is demonstrated can be used as a “biochemical fingerprint.” Thus, the demonstration in the brain tissue of a specific intramembrane receptor-receptor interaction previously shown to depend on receptor heteromerization in an artificial cell system indicates the presence of the receptor heteromer in the brain (26).
In the present work we demonstrate the existence of the same kind of D1–D3 intramembrane receptor-receptor interaction in membrane preparations of both striatal tissue and co-transfected cells in which expression of D1–D3 receptor heteromers has been demonstrated, strongly suggesting the existence of D1–D3 receptor heteromers in the striatum. Because by far the main striatal localization of D1 receptors is postsynaptic to dopaminergic terminals, predominantly in the GABAergic dynorphinergic neurons (see the introduction), the D1–D3 intramembrane receptor-receptor interaction also indicates the existence of a postsynaptic striatal co-localization of both receptors. In this D1–D3 interaction, D3 receptor stimulation enhances the binding of D1 receptor agonists, indicating that D3 receptor stimulation could potentiate the effects of endogenous dopamine on D1 receptor. On the other hand, D1 receptor stimulation did not modify the binding of the D3 receptor agonist R(+)-7-OH-DPAT, indicating that the intramembrane modulatory interaction is not reciprocal within the D1–D3 receptor heteromer.
Previous studies about D1–D3 receptor interactions at the biochemical level have shown either facilitation or inhibition of the effects of D1 receptor stimulation upon D3 receptor activation, depending on the striatal compartment and on the signaling pathway (15, 33). On the one hand, stimulation of D3 receptor counteracted D1 receptor-mediated increase in c-fos expression in the islands of Calleja, but not in other striatal regions (15). This antagonistic interaction is in line with the differential coupling of D1 and D3 receptors to G proteins. D1 receptor is a Gs-olf-coupled receptor, the stimulation of which activates adenylyl cyclase (1, 2), whereas the D3 receptor, like any other D2-like receptor, couples mostly to Gi-o proteins, and its stimulation inhibits adenylate cyclase. On the other hand, βγ-subunit-mediated mechanisms of activation of other signaling pathways, such as mitogen-activated protein kinases, have been suggested to be involved in the ability of D3 receptor stimulation to potentiate an increase in SP expression in the nucleus accumbens induced by D1 receptor agonist (15). The present radioligand binding experiments provide an additional biochemical mechanism for a D3 receptor-mediated facilitation of D1 receptor function, which could be independent to, or be responsible for the D1–D3 synergistic interaction demonstrated at the signaling level.
Some studies have analyzed the existence of D1–D3 receptor interactions at the behavioral level, but without isolating pre- and post-synaptic mechanisms (29, 34). Thus, although it was initially a matter of debate, it is now quite well established that D3 receptors are expressed on dopaminergic neurons, where they act as autoreceptors (reviewed in Ref. 28). It is also generally accepted that low doses of D3 receptor agonists produce motor depression through the involvement of presynaptically located D3 receptors and that the motor activation induced by higher doses of D3 agonists are most probably related to loss of ligand selectivity and activation of postsynaptically located D2 receptors (29, 35, 36). Thus, the in vivo selectivity of putative selective D3 receptor ligands has been repeatedly questioned (28). Consequently, it has been suggested that the D3 receptor is not involved in the potentiating effects of putative D3 receptor agonists on D1 receptor agonist-induced motor activation (29, 34). In contrast, in the present study we found clear evidence for a selective D3 receptor-mediated potentiation of D1 receptor agonist-induced locomotor activation.
The evaluation of locomotor activity induced by dopamine receptor agonists in reserpinized mice is a very useful in vivo model to study the function of striatal postsynaptic D1-like and D2-like receptors localized in GABAergic dynorphinergic and GABAergic enkephalinergic neurons without the influence of endogenous dopamine (24, 37, 38). In the present study we found that the D3 receptor agonist PD 128907 potentiates the D1-mediated locomotor activation and that the effect of PD 128907 was counteracted by a specific D3 but not by a specific D2 receptor antagonist, indicating a selective involvement of the D3 receptor. The specific involvement of D3 receptors in the effects of PD 128907 was further demonstrated in experiments with D3KO mice. A rather surprising result was that quinpirole, a non-selective D2–3 receptor agonist in vitro, demonstrated a selective D2 receptor-mediated potentiation of D1 receptor agonist-induced locomotor activation. Thus, the effect of quinpirole was counteracted by a D2 but not by a D3 receptor antagonist. Therefore, our results indicate that the dose of quinpirole used in the present experiments did not produce a significant in vivo stimulation of striatal postsynaptic D3 receptors in mice. Accordingly, quinpirole produced the same degree of motor activation in D3KO mice and their WT littermates. Furthermore, co-administration of quinpirole or PD 128907 produced a very significant potentiation of the locomotor activation induced by the D1 receptor agonist SKF 38393, strongly suggesting the existence of independent D2- and D3-mediated potentiating mechanisms of D1 receptor action.
In addition to the intramembrane receptor-receptor interaction, D1–D3 receptor heteromerization can potentially modify D1 receptor signaling and internalization as it has been shown for other receptor heteromers (25, 26). The demonstration of tight intermolecular physical and functional interactions between both receptors gives a new framework for the understanding of dopamine receptor physiology and pharmacology. GABAergic enkephalinergic and GABAergic dynorphinergic neurons give rise to two striatal efferent systems, which connect the striatum with the output structures of the basal ganglia: the substantia nigra pars reticulata and the internal segment of the globus pallidus (entopeduncular nucleus in rodents) (3). These are called “direct” and “indirect” pathways. The direct pathway is made of GABAergic dynorphinergic neurons, which directly connect the striatum with the output structures. The indirect pathway consists mostly of GABAergic enkephalinergic neurons, which connect the striatum with the external segment of the globus pallidus (globus pallidus in rodents), GABAergic neurons, which connect the globus pallidus with the subthalamic nucleus, and glutamatergic neurons, which connect the subthalamic nucleus with the output structures. Stimulation of the direct pathway results in motor activation and stimulation of the indirect pathway produces motor inhibition (39). It is generally accepted that dopamine, or dopamine agonists, induces motor activation by activating the direct pathway (acting on stimulatory D1 receptors localized in GABAergic dynorphinergic neurons) and by depressing the indirect pathway (acting on inhibitory D2 receptors localized in GABAergic enkephalinergic neurons) (3, 6–8). An important locus of D1–D2 receptor agonist behavioral synergism is at the circuit level, due to the simultaneous inhibition (direct pathway) and release of stimulation (indirect pathway) of the tonic neuronal activity of the output structures (40, 41). However, the D1–D3 receptor agonist behavioral synergism most probably takes place at the cellular level, within the GABAergic SP-dynorphinergic neurons (direct pathway). The present results add strong support for this hypothesis, although we cannot discard a D1–D3 receptor interaction at the circuit level. However, because the binding properties of D1 receptors that are not co-localized with D3 receptors cannot be modulated by D3 receptor ligands (as we demonstrate in cells only transfected with D1 receptors), the results obtained with radioligand binding experiments in membranes preparations demonstrate that a significant amount of D1 receptors are co-localized with D3 receptors in the striatum. The two mechanisms, intercellular D1–D2 and intracellular D1–D3 receptor interactions, could explain the remarkable locomotor activation obtained with the simultaneous administration of D1, D2, and D3 receptor agonists reported herein and fit very well with the role of D3 receptors in behavioral sensitization to l-dopa in different animal models of Parkinson disease (31, 42). Chronic striatal dopamine depletion followed by l-dopa treatment leads to an increased sensitivity to l-dopa or dopamine D1 receptor agonists, which seems to be causally related to an up-regulation of D3 receptors in the GABAergic SP-dynorphinergic neurons (42). These experimental findings suggest that targeting D1–D3 receptor heteromers can have important implications for the treatment of basal ganglia disorders.
During the resubmission of this manuscript another research group also demonstrated D1–D3 receptor heteromerization in co-transfected cells with BRET techniques (43). Evidence was also obtained for a D3 receptor-mediated potentiation of D1 receptor signaling (cAMP accumulation) and modifications of agonist-mediated internalization with D1–D3 receptor heteromerization (43).
This work was supported, in whole or in part, by the National Institutes of Health Intramural Research Program, National Institute on Drug Abuse, Dept. of Health and Human Services. This work was also supported by the Ministerio de Ciencia y Tecnologia (Grants SAF2006-00170). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: GABAergic, γ-aminobutyric-acidergic; BRET, bioluminescence resonance energy transfer; FRET, fluorescence resonance energy transfer; GFP, green fluorescent protein; HEK, human embryonic kidney; PBS, phosphate-buffered saline; RLuc, Renilla luciferase; SP, substance P; i.p., intraperitoneally; SP, Substance P; D3KO, homozygous D3 knockout mice; WT, wild type; GFP2, green fluorescence protein2; YFP, yellow fluorescent protein; EYFP, enhanced YFP; R(+)-7-OH-DPAT, 7-hydroxy-N,N-di-n-propyl-2-aminotetralin; ANOVA, analysis of variance; ROI, regions of interest.
References
- 1.Missale, C., Nash, S. R., Robinson, S. W., Jaber, M., and Caron, M. G. (1998) Physiol. Rev. 78 189-225 [DOI] [PubMed] [Google Scholar]
- 2.Neve, K. A., Seamans, J. K., and Trantham-Davidson, H. (2004) J. Recept. Signal Transduct. Res. 24 165-205 [DOI] [PubMed] [Google Scholar]
- 3.Gerfen, C. R. (2004) The Rat Nervous System (Paxinos, G., ed) pp. 445-508, Elsevier Academic Press, Amsterdam
- 4.Gerfen, C. R., Engber, T. M., Mahan, L. C., Susel, Z., Chase, T. N., Monsma, F. J., Jr., and Sibley, D. R. (1990) Science 250 1429-1432 [DOI] [PubMed] [Google Scholar]
- 5.Le Moine, C., Normand, E., and Bloch, B. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 4205-4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerfen, C. R. (1992) Trends Neurosci. 15 133-139 [DOI] [PubMed] [Google Scholar]
- 7.Ferré, S., O'Connor, W. T., Fuxe, K., and Ungerstedt, U. (1993) J. Neurosci. 13 5402-5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferré, S., O'Connor, W. T., Svenningsson, P., Bjorklund, L., Lindberg, J., Tinner, B., Stromberg, I., Goldstein, M., Ogren, S. O., Ungerstedt, U., Fredholm, B. B., and Fuxe, K. (1996) Eur. J. Neurosci. 8 1545-1553 [DOI] [PubMed] [Google Scholar]
- 9.Surmeier, D. J., Eberwine, J., Wilson, C. J., Cao, Y., Stefani, A., and Kitai, S. T. (1992) Proc. Natl. Acad. Sci. U. S. A. 89 10178-10182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surmeier, D. J., Song, W. J., and Yan, Z. (1996) J. Neurosci. 16 6579-6591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aizman, O., Brismar, H., Uhlen, P., Zettergren, E., Levey, A. I., Forssberg, H., Greengard, P., and Aperia, A. (2000) Nat. Neurosci. 3 226-230 [DOI] [PubMed] [Google Scholar]
- 12.Lee, S. P., So, C. H., Rashid, A. J., Varghese, G., Cheng, R., Lanca, A. J., O'Dowd, B. F., and George, S. R. (2004) J. Biol. Chem. 279 35671-35678 [DOI] [PubMed] [Google Scholar]
- 13.Rashid, A. J., So, C. H., Kong, M. M., Furtak, T., El-Ghundi, M., Cheng, R., O'Dowd, B. F., and George, S. R. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 654-659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surmeier, D. J., Reiner, A., Levine, M. S., and Ariano, M. A. (1993) Trends Neurosci. 16 299-305 [DOI] [PubMed] [Google Scholar]
- 15.Ridray, S., Griffon, N., Mignon, V., Souil, E., Carboni, S., Diaz, J., Schwartz, J. C., and Sokoloff, P. (1998) Eur. J. Neurosci. 10 1676-1686 [DOI] [PubMed] [Google Scholar]
- 16.Accili, D., Fishburn, C. S., Drago, J., Steiner, H., Lachowicz, J. E., Park, B. H., Gauda, E. B., Lee, E. J., Cool, M. H., Sibley, D. R., Gerfen, C. R., Westphal, H., and Fuchs, S. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 1945-1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan, M., Schallhorn, A., and Wurml, F. M. (1996) Nucleic Acids Res. 24 596-601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canals, M., Marcellino, D., Fanelli, F., Ciruela, F., de Benedetti, P., Goldberg, S. R., Neve, K., Fuxe, K., Agnati, L. F., Woods, A. S., Ferré, S., Lluis, C., Bouvier, M., and Franco, R. (2003) J. Biol. Chem. 278 46741-46749 [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann, T., Rietdorf, J., Girod, A., Georget, V., and Pepperkok, R. (2002) FEBS Lett. 531 245-249 [DOI] [PubMed] [Google Scholar]
- 20.Sarrió, S., Casadó, V., Escriche, M., Ciruela, F., Mallol, J., Canela, E. I., Lluis, C., and Franco, R. (2000) Mol. Cell. Biol. 20 5164-5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casadó, V., Cantí, C., Mallol, J., Canela, E. I., Lluís, C., and Franco, R. (1990) J. Neurosci. Res. 26 461-473 [DOI] [PubMed] [Google Scholar]
- 22.Ciruela, F., Casado, V., Rodrigues, R. J., Lujan, R., Burgueno, J., Canals, M., Borycz, J., Rebola, N., Goldberg, S. R., Mallol, J., Cortes, A., Canela, E. I., Lopez-Gimenez, J. F., Milligan, G., Lluis, C., Cunha, R. A., Ferré, S., and Franco, R. (2006) J. Neurosci. 26 2080-2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng, Y., and Prusoff, W. H. (1973) Biochem. Pharmacol. 22 3099-3108 [DOI] [PubMed] [Google Scholar]
- 24.Ferré, S., Gimenez-Llort, L., Artigas, F., and Martinez, E. (1994) Eur. J. Pharmacol. 255 203-213 [DOI] [PubMed] [Google Scholar]
- 25.Agnati, L. F., Ferré, S., Lluis, C., Franco, R., and Fuxe, K. (2003) Pharmacol. Rev. 55 509-550 [DOI] [PubMed] [Google Scholar]
- 26.Ferré, S., Ciruela, F., Woods, A. S., Lluis, C., and Franco, R. (2007) Trends Neurosci. 30 440-446 [DOI] [PubMed] [Google Scholar]
- 27.Schoemaker, H. (1993) Eur. J. Pharmacol. 242 R1-R2 [DOI] [PubMed] [Google Scholar]
- 28.Sokoloff, P., Diaz, J., Le Foll, B., Guillin, O., Leriche, L., Bezard, E., and Gross, C. (2006) CNS Neurol. Disord. Drug Targets 5 25-43 [DOI] [PubMed] [Google Scholar]
- 29.Pugsley, T. A., Davis, M. D., Akunne, H. C., MacKenzie, R. G., Shih, Y. H., Damsma, G., Wikstrom, H., Whetzel, S. Z., Georgic, L. M., Cooke, L. W., Demattos, S. B., Corbin, A. E., Glase, S. A., Wise, L. D., Dijkstra, D., and Heffner, T. G. (1995) J. Pharmacol. Exp. Ther. 275 1355-1366 [PubMed] [Google Scholar]
- 30.Millan, M. J., Dekeyne, A., Rivet, J. M., Dubuffet, T., Lavielle, G., and Brocco, M. (2000) J. Pharmacol. Exp. Ther. 293 1063-1073 [PubMed] [Google Scholar]
- 31.Bezard, E., Ferry, S., Mach, U., Stark, H., Leriche, L., Boraud, T., Gross, C., and Sokoloff, P. (2003) Nat. Med. 9 762-767 [DOI] [PubMed] [Google Scholar]
- 32.Franco, R., Canals, M., Marcellino, D., Ferré, S., Agnati, L., Mallol, J., Casado, V., Ciruela, F., Fuxe, K., Lluis, C., and Canela, E. I. (2003) Trends Biochem. Sci. 28 238-243 [DOI] [PubMed] [Google Scholar]
- 33.Schwartz, J. C., Diaz, J., Bordet, R., Griffon, N., Perachon, S., Pilon, C., Ridray, S., and Sokoloff, P. (1998) Brain Res. Rev. 26 236-242 [DOI] [PubMed] [Google Scholar]
- 34.Mori, T., Murase, K., Tanaka, J., and Ichimaru, Y. (1997) Jpn. J. Pharmacol. 73 251-254 [DOI] [PubMed] [Google Scholar]
- 35.Pritchard, L. M., Logue, A. D., Hayes, S., Welge, J. A., Xu, M., Zhang, J., Berger, S. P., and Richtand, N. M. (2003) Neuropsychopharmacology 28 100-107 [DOI] [PubMed] [Google Scholar]
- 36.Millan, M. J., Seguin, L., Gobert, A., Cussac, D., and Brocco, M. (2004) Psychopharmacology 174 341-357 [DOI] [PubMed] [Google Scholar]
- 37.Rubinstein, M., Gershanik, O., and Stefano, F. J. (1988) Eur. J. Pharmacol. 148 419-426 [DOI] [PubMed] [Google Scholar]
- 38.Starr, M. S., and Starr, B. S. (1989) Pharmacol. Biochem. Behav. 33 41-44 [DOI] [PubMed] [Google Scholar]
- 39.Alexander, G. E., and Crutcher, M. D. (1990) Trends Neurosci. 13 266-271 [DOI] [PubMed] [Google Scholar]
- 40.Albin, R. L., Young, A. B., and Penney, J. B. (1989) Trends Neurosci. 12 366-375 [DOI] [PubMed] [Google Scholar]
- 41.Surmeier, D. J., Ding, J., Day, M., Wang, Z., and Shen, W. (2007) Trends Neurosci. 30 228-235 [DOI] [PubMed] [Google Scholar]
- 42.Bordet, R., Ridray, S., Carboni, S., Diaz, J., Sokoloff, P., and Schwartz, J. C. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 3363-3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiorentini, C., Busi, C., Gorruso, E., Gotti, C., Spano P., and Missale, C. (2008) Mol. Pharmacol. 74 59-69 [DOI] [PubMed] [Google Scholar]