Abstract
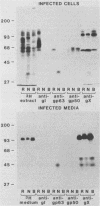
The pseudorabies virus vaccine strains Norden and Bartha each have been reported to have deletions in the small unique component of the genome (B. Lomniczi, M. L. Blankenship, and T. Ben-Porat, J. Virol. 49:970-979, 1984). The deletion in Norden was shown to delete the entire coding region for gI but not any of the coding sequences for gp63. However, gp63 in Norden-infected cells was only 36 kilodaltons, and a 44-kilodalton form of gp63 was released into the medium. In Bartha, the deletion removed the coding region for all but 89 amino acids of gp63, and no gp63 was detected in either Bartha-infected cells or medium.
Full text
PDF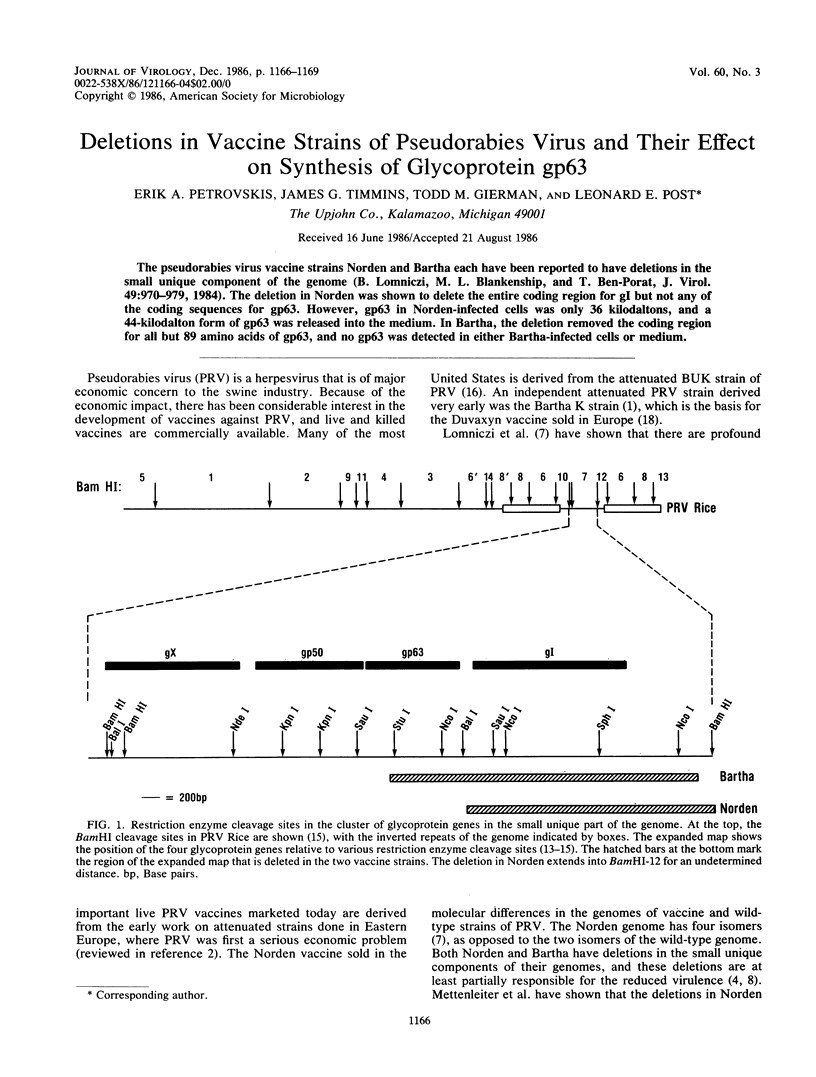


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., DeMarchi J., Pendrys J., Veach R. A., Kaplan A. S. Proteins specified by the short unique region of the genome of pseudorabies virus play a role in the release of virions from certain cells. J Virol. 1986 Jan;57(1):191–196. doi: 10.1128/jvi.57.1.191-196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns A., van den Ouweland A., Quint W., van Oirschot J., Gielkens A. Presence of markers for virulence in the unique short region or repeat region or both of pseudorabies hybrid viruses. J Virol. 1985 Jan;53(1):89–93. doi: 10.1128/jvi.53.1.89-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Construction of influenza haemagglutinin genes that code for intracellular and secreted forms of the protein. Nature. 1982 Dec 16;300(5893):598–603. doi: 10.1038/300598a0. [DOI] [PubMed] [Google Scholar]
- Lomniczi B., Blankenship M. L., Ben-Porat T. Deletions in the genomes of pseudorabies virus vaccine strains and existence of four isomers of the genomes. J Virol. 1984 Mar;49(3):970–979. doi: 10.1128/jvi.49.3.970-979.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Watanabe S., Ben-Porat T., Kaplan A. S. Genetic basis of the neurovirulence of pseudorabies virus. J Virol. 1984 Oct;52(1):198–205. doi: 10.1128/jvi.52.1.198-205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Roizman B. Generation of an inverting herpes simplex virus 1 mutant lacking the L-S junction a sequences, an origin of DNA synthesis, and several genes including those specifying glycoprotein E and the alpha 47 gene. J Virol. 1986 May;58(2):583–591. doi: 10.1128/jvi.58.2.583-591.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C., Lukacs N., Rziha H. J. Mapping of the structural gene of pseudorabies virus glycoprotein A and identification of two non-glycosylated precursor polypeptides. J Virol. 1985 Jan;53(1):52–57. doi: 10.1128/jvi.53.1.52-57.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Lukàcs N., Rziha H. J. Pseudorabies virus avirulent strains fail to express a major glycoprotein. J Virol. 1985 Oct;56(1):307–311. doi: 10.1128/jvi.56.1.307-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Armentrout M. A., Marchioli C. C., Yancey R. J., Jr, Post L. E. DNA sequence of the gene for pseudorabies virus gp50, a glycoprotein without N-linked glycosylation. J Virol. 1986 Aug;59(2):216–223. doi: 10.1128/jvi.59.2.216-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Post L. E. Use of lambda gt11 to isolate genes for two pseudorabies virus glycoproteins with homology to herpes simplex virus and varicella-zoster virus glycoproteins. J Virol. 1986 Oct;60(1):185–193. doi: 10.1128/jvi.60.1.185-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea T. J., Timmins J. G., Long G. W., Post L. E. Mapping and sequence of the gene for the pseudorabies virus glycoprotein which accumulates in the medium of infected cells. J Virol. 1985 Apr;54(1):21–29. doi: 10.1128/jvi.54.1.21-29.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd D., McFerran J. B. Control of Aujeszky's disease. Vet Rec. 1985 Dec 14;117(24):647–647. doi: 10.1136/vr.117.24.647-e. [DOI] [PubMed] [Google Scholar]
- Wathen M. W., Wathen L. M. Isolation, characterization, and physical mapping of a pseudorabies virus mutant containing antigenically altered gp50. J Virol. 1984 Jul;51(1):57–62. doi: 10.1128/jvi.51.1.57-62.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Oirschot J. T., Gielkens A. L. Some characteristics of four attenuated vaccine virus strains and a virulent strain of Aujeszky's disease virus. Vet Q. 1984 Sep;6(4):225–229. doi: 10.1080/01652176.1984.9693940. [DOI] [PubMed] [Google Scholar]