Abstract
Centromeric silencing and heterochromatin formation in Schizosaccharomyces pombe require the RNA interference (RNAi) machinery. Three factors that mediate this mechanism have been identified: 1) the RNA-dependent RNA polymerase complex RdRC, 2) the Argonaute-containing RITS (RNA-induced initiation of transcriptional silencing) complex, and 3) the endoribonuclease Dicer ortholog Dcr1. S. pombe mutants lacking a new factor described here, Ers1, are completely defective in RNAi-dependent silencing of centromeric regions but, importantly, not in RNAi-independent silencing at the mat3M or tel2R loci. ers1Δ cells likewise fail to convert centromeric pre-small interfering RNA transcripts into small interfering RNAs, are defective in histone H3 Lys9 methylation, and are unable to recruit the RITS complex to centromeric sequences. Surprisingly, Ers1 lacks obvious orthologs outside of the genus Schizosaccharomyces. Within this group, it is diverging rapidly, raising the possibility that it is coevolving with target RNA elements.
Heterochromatin formation and gene silencing in many eukaryotes require a core system of a histone H3 Lys9 (H3-K9) methyltransferase and a family of proteins (the HP1 family) that recognize this modification. A link between RNAi4 and transcriptional silencing was first suggested from studies of double-stranded RNA-dependent DNA methylation in plants (1). Landmark studies in the fission yeast Schizosaccharomyces pombe demonstrated that heterochromatic silencing at centromeres requires three canonical RNAi components, the Dicer endoribonuclease Dcr1, the RNA-dependent RNA polymerase Rdp1, and the Argonaute protein Ago1 (2). Mutation of these components, like mutations in the histone methyltransferase Clr4, results in a loss of centromeric silencing, accumulation of centromeric transcripts, a defect in histone H3 Lys9 methylation, and a defect in the formation of centromere-coded siRNAs (2). A role for RNAi in heterochromatin has also been found in Drosophila and mammals (3, 4). In S. pombe, Ago1 functions as part of a protein complex called RITS that contains an HP1 homolog, Chp1 (5). The recruitment of this complex to chromatin requires Dcr1, suggesting that Ago1 needs to be loaded with siRNAs to be targeted (5). Rdp1 likewise is part of a complex called RdRC that interacts with the RITS complex (6). Despite these important advances, how the RNAi machinery is recruited to specific sites, how it controls histone methylation, and how it mediates silencing are not clear. Since the initial studies, no factors essential for and specific to RNAi-dependent heterochromatin formation in S. pombe have been reported besides those polypeptides identified in the RITS and RdRC complexes.
EXPERIMENTAL PROCEDURES
S. pombe Genetic Methods—Homologous replacement of DNA was accomplished by lithium acetate transformation of PCR products containing 100 bp of targeting homology. YS medium (5 g/liter Difco yeast extract + 250 mg/liter each l-histidine, l-leucine, adenine, uracil, and l-lysine and 3% glucose) was used for all experiments. FOA medium contained 1 g/liter 5-fluoroorotic acid.
Chromatin Immunoprecipitation—Strains were grown in YS medium, harvested at A600 = 0.8–1.0, and cross-linked for 15 min with 1% formaldehyde at 30 °C with shaking. Quenching was done with 0.25 m glycine for 5 min at room temperature. Cells were harvested by centrifugation at 3500 rpm for 5 min at 4 °C and washed twice with 1× ice-cold Tris-buffered saline. Cells were resuspended in 1× Tris-buffered saline and transferred to a screw-cap tube. After centrifugation, the supernatant was discarded, and the pellet was flash-frozen and stored at –80 °C. Pellets were resuspended in 500 μl of lysis buffer (50 mm Hepes/KOH (pH 7.5), 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, and 1× Sigma fungal protease inhibitor mixture), and cells were lysed by bead beating three times for 1 min with 2-min rests on ice. Tubes were punctured and spun into tubes for 2 min at 1000 rpm. The flow-through was transferred to a microcentrifuge tube and centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatant was discarded, and the pellet was resuspended in 1.4 ml of lysis buffer and sonicated six times for 40 s (50% duty cycle) using a Branson Sonifier 450 microtip sonicator. After centrifugation at 14,000 rpm for 10 min at 4 °C, the supernatant was transferred to a new tube. 0.1 ml was set aside as the input control. 100 μl of the lysate was added to add 400 μl of lysis buffer plus 2 μl of anti-H3 (Abcam ab1791) or anti-H3K9me2 (mAbcam ab1220) antibody or 1 μl of anti-H3K9me3 (Upstate 07-523) antibody and agitated on a Nutator for 3 h at 4 °C. 30 μl of a 50% slurry of protein A-Sepharose washed with lysis buffer was added to the antibody incubations and agitated on a Nutator for 3 h. Beads was washed twice for 5 min with lysis buffer, twice for 5 min with high salt lysis buffer (lysis buffer with 500 mm NaCl), once for 5 min with wash buffer (10 mm Tris-HCl (pH 8.0), 0.25 m LiCl, 0.5% Nonidet P-40, 0.5% sodium deoxycholate, and 1 mm EDTA), and once for 5 min with 10 mm Tris-HCl (pH 8.0) and 1 mm EDTA. Samples were eluted by addition of 100 μl of elution buffer (50 mm Tris (pH 8.0), 10 mm EDTA, and 1% SDS) and incubation for 20 min at 70 °C. 2.5 μl of 20 mg/ml proteinase K was added to the input and eluates and incubated overnight at 65 °C. Samples were purified using a Qiagen PCR purification kit. DNAs were quantified by qPCR. The primers used are listed in the supplemental material.
RNA Extraction and RT-qPCR Analysis—Cultures (A = 0.4–0.5) were harvested by centrifugation, washed twice with ice-cold water, and flash-frozen. Pellets were resuspended in 1 ml of TRIzol reagent and transferred to screw-cap tubes containing zirconia-silica beads. Lysis was accomplished by two cycles of bead beading for 2.5 min. Following centrifugation at 13,500 rpm for 10 min at 4 °C, the supernatant was transferred to a microcentrifuge tube, extracted once with chloroform, and precipitated with isopropyl alcohol. Following resuspension and reprecipitation with isopropyl alcohol, pellets were resuspended in 100 μl of water. 10 μg of RNA was used in standard RT reactions using oligo[(dT)20-N] primers. Following RNA hydrolysis and cleanup using a Zymo Research DNA Clean & Concentrator-5 column, samples were analyzed by qPCR. The primers used are listed in the supplemental material. siRNA Northern Hybridization—Centromeric siRNA analysis was performed exactly as described (7).
RESULTS AND DISCUSSION
To identify new factors required for RNAi-dependent heterochromatin formation, we sought to generate and test knockout mutants of genes coding for proteins that localize to heterochromatin. To enrich for such factors, we took advantage of work that determined the subcellular localization of yellow fluorescent protein fusions for nearly all S. pombe open reading frames (8). Because S. pombe contains only three centromeres that cluster/associate with the spindle pole body during interphase (9, 10), we systematically disrupted genes encoding proteins annotated as having a “nuclear dot” or “spindle pole body” localization. This was performed in a strain harboring a centromeric reporter gene, imr1L::ura4+ (details of the work will be described elsewhere).5 Here, we describe a new gene, ERS1 (essential for RNAi-dependent silencing 1), identified using this approach. We found that ERS1 is specifically required for RNAi-dependent heterochromatin formation and silencing.
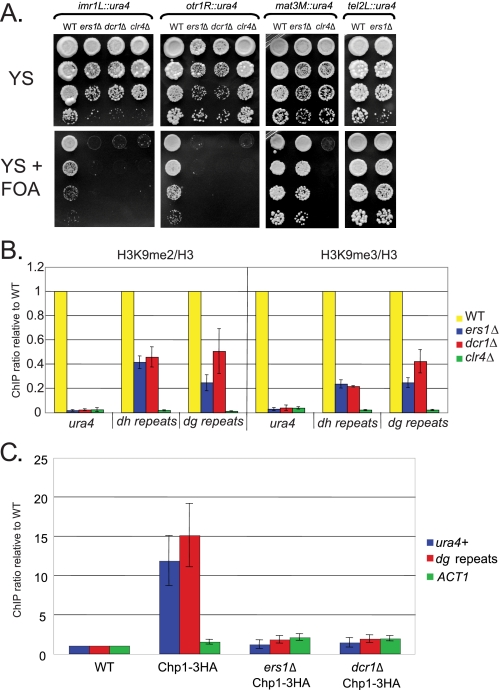
ERS1 corresponds to SPCC1393.05, which encodes a 957-amino acid protein with no identifiable domains. Using FOA medium, which selects against ura4+ expression, we examined the effects of the ers1Δ mutant on reporter gene silencing. Like the dcr1Δ mutant, the ers1Δ mutant is completely defective in the silencing of ura4+ genes placed in the inner or outer repeats of cen1. Critically, ers1Δ mutants display no defect in silencing of ura4+ reporter genes placed at mat3M or tel2R, where RNAi-dependent mechanisms act redundantly with RNAi-independent silencing mechanisms. This result distinguishes Ers1 from factors also required for RNAi-independent silencing such as Clr4.
Mutants in the RNAi machinery have been reported previously to reduce histone H3 Lys9 methylation at endogenous centromeric sequences and to abolish it at ura4+ reporter genes inserted at the centromeres (2, 7, 11). We found that ers1Δ cells display a defect in both H3 Lys9 dimethylation and trimethylation (both normalized for nucleosome density) at the endogenous dh and dg regions of the centromeric outer repeats (Fig. 1B). The phenotypes were identical to those of dcr1Δ cells. Likewise, a complete defect in histone H3 Lys9 methylation at an imr1L::ura4 gene was observed in both ers1Δ and dcr1Δ cells, comparable with that in cells lacking the histone H3-K9 methyltransferase Clr4 (Fig. 1B).
FIGURE 1.
Ers1 is essential for RNAi-dependent silencing and heterochromatin formation in S. pombe. A, reporter gene assays. Serial dilutions of cells of the indicated deletion and reporter genotypes were plated on the indicated media and incubated at 30 °C. B, ChIP analysis of histone methylation. Cells of the indicated genotypes (constructed in an imr1L::ura4+ background) were examined for H3 Lys9 dimethylation and trimethylation modifications. Samples were normalized for nucleosome density by performing ChIP with antibodies to H3. C, ChIP analysis of RITS recruitment. ChIP was performed using anti-hemagglutinin (HA) antibodies on strains of the indicated genotypes (constructed in an imr1L::ura4+ background). Signals were normalized to the untagged reporter strain. WT, wild-type.
To determine whether Ers1 acts upstream or downstream of the recruitment of the RITS complex, we tagged the Chp1 subunit with a triple-hemagglutinin epitope and examined its association with imr1L::ura4 and the dg repeats. As shown in Fig. 1C, recruitment of RITS to both sites was abolished in ers1Δ cells. Immunoblot analysis indicated that the failure in Chp1 recruitment could not be attributed to a reduction in its accumulation in mutant cells (supplemental Fig. S1). More generally, the silencing defect of ers1Δ cells was not due to a lack of expression of any known RNAi component because RT-qPCR analysis revealed normal levels of DCR1, RDP1, HRR1, CID12, AGO1, CHP1, and TAS3 mRNAs in mutant cells (supplemental Fig. S2).
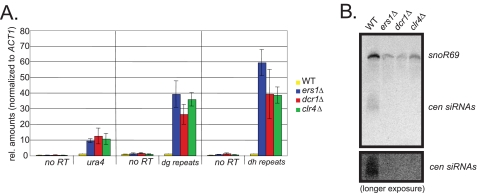
Using RT-qPCR analysis, we found that the FOA phenotype of ers1Δ cells correlated with an accumulation of the imr1L::ura4+ transcript (Fig. 2A). As observed previously for dcr1Δ and clr4Δ mutants, RT-qPCR analysis revealed robust accumulation of the centromeric dg transcript in ers1Δ cells (Fig. 2A). Finally, we examined the role of ERS1 in the production of siRNAs derived from the centromeric repeats using Northern hybridization. We observed an apparently complete defect in siRNA production (Fig. 2B). Although cells lacking general heterochromatin factors such as Clr4 also display defects in RNAi due to poorly understood feedback mechanisms (12), our biochemical data, together with the observed lack of a silencing defect of ers1Δ cells in RNAi-independent regions, demonstrate that it is specific to the RNAi pathway. Although we have been unable to observe enrichment of Ers1 at centromeres using ChIP (supplemental Fig. S3), we note that this is also true for components of the RdRC complex (6).
FIGURE 2.
Ers1 is required for processing of centromeric transcripts into siRNAs. A, transcript analysis. RT-qPCR analysis was performed on total RNA extracted from cells of the indicated genotypes constructed in an imr1L::ura4+ background. ACT1 was used for normalization. B, siRNA analysis. Small RNAs were analyzed by Northern hybridization using radiolabeled probes against 10 different dg-dh region siRNAs and a control small nucleolar (sno) RNA. A longer exposure is shown to demonstrate the complete lack of siRNAs in the ers1Δ mutant. rel., relative; WT, wild-type; cen, centromeric.
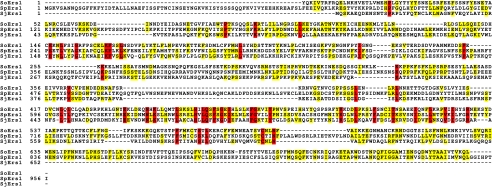
Despite the fact that ers1Δ displays all of the hallmarks of a bona fide essential component of the RNAi-dependent heterochromatin machinery, its sequence offers no clues to its function, as no orthologs are apparent in the current non-redundant NCBI Database. However, sequence assemblies of the genomes of Schizosaccharomyces japonicus and Schizosaccharomyces octosporus have recently been released (www.broad.mit.edu/annotation/genome/schizosaccharomyces_group/MultiHome.html). We identified orthologs of Ers1 using the available gene models (S. japonicus) or TBLASTN (S. octosporus) and observed a remarkable degree of divergence despite the close phylogenetic relationship between these species. (Fig. 3) The Schizosaccharomyces class belongs to the Taphrinomycotina early branching subphylum of Ascomycota (13). Another member of this subphylum whose genome sequence is available is the human pathogen Pneumocystis carinii. Searches of its sequence yielded no detectable orthologs of Ers1. The absence of orthologs outside of the Schizosaccharomyces clade, together with its divergence within this group, suggests that Ers1 may be a rapidly evolving protein. Interestingly, core RNAi components have been shown to be rapidly evolving and subject to positive selection in closely related Drosophila species (14). Thus, an intriguing albeit speculative possibility is that Ers1 is a recognition factor for the RNAi machinery that coevolving parasitic RNA targets.
FIGURE 3.
Ers1 is a rapidly diverging protein. Shown is a ClustalW alignment of predicted Ers1 sequences from the indicated Schizosaccharomyces species (S. octosporus (So), S. pombe (Sp), and S. japonicus (Sj)). Identical residues between two species are colored yellow, and invariant residues are colored red. Note that exons encoding the N- and C-terminal sequences of the S. octosporus ortholog could not be predicted with confidence, and therefore, these regions are not present in the alignment.
Supplementary Material
Acknowledgments
We thank Danesh Moazed and Karl Ekwall for strains and protocols.
This work was supported, in whole or in part, by National Institutes of Health Grant R01GM063670 (to H. D. M.). This work was also supported by an Opportunity grant from the Sandler Foundation (to H. D. M.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3, Table 1, and primer sequences.
Footnotes
The abbreviations used are: RNAi, RNA interference; siRNA, small interfering RNA; FOA, 5-fluoroorotic acid; qPCR, quantitative PCR; RT, reverse transcription; ChIP, chromatin immunoprecipitation.
S. Braun, M. Rowley, S. Shankar, and H. D. Madhan, manuscript in preparation.
References
- 1.Mette, M. F., Aufsatz, W., van der Winden, J., Matzke, M. A., and Matzke, A. J. (2000) EMBO J. 19 5194–5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volpe, T. A., Kidner, C., Hall, I. M., Teng, G., Grewal, S. I., and Martienssen, R. A. (2002) Science 297 1833–1837 [DOI] [PubMed] [Google Scholar]
- 3.Pal-Bhadra, M., Leibovitch, B. A., Gandhi, S. G., Rao, M., Bhadra, U., Birchler, J. A., and Elgin, S. C. (2004) Science 303 669–672 [DOI] [PubMed] [Google Scholar]
- 4.Deshpande, G., Calhoun, G., and Schedl, P. (2005) Genes Dev. 19 1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verdel, A., Jia, S., Gerber, S., Sugiyama, T., Gygi, S., Grewal, S. I., and Moazed, D. (2004) Science 303 672–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motamedi, M. R., Verdel, A., Colmenares, S. U., Gerber, S. A., Gygi, S. P., and Moazed, D. (2004) Cell 119 789–802 [DOI] [PubMed] [Google Scholar]
- 7.Buhler, M., Haas, W., Gygi, S. P., and Moazed, D. (2007) Cell 129 707–721 [DOI] [PubMed] [Google Scholar]
- 8.Matsuyama, A., Arai, R., Yashiroda, Y., Shirai, A., Kamata, A., Sekido, S., Kobayashi, Y., Hashimoto, A., Hamamoto, M., Hiraoka, Y., Horinouchi, S., and Yoshida, M. (2006) Nat. Biotechnol. 24 841–847 [DOI] [PubMed] [Google Scholar]
- 9.Uzawa, S., and Yanagida, M. (1992) J. Cell Sci. 101 267–275 [DOI] [PubMed] [Google Scholar]
- 10.Appelgren, H., Kniola, B., and Ekwall, K. (2003) J. Cell Sci. 116 4035–4042 [DOI] [PubMed] [Google Scholar]
- 11.Sadaie, M., Iida, T., Urano, T., and Nakayama, J. (2004) EMBO J. 23 3825–3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noma, K., Sugiyama, T., Cam, H., Verdel, A., Zofall, M., Jia, S., Moazed, D., and Grewal, S. I. (2004) Nat. Genet. 36 1174–1180 [DOI] [PubMed] [Google Scholar]
- 13.Lumbsch, H. T., and Hunhndorf, S. M. (2007) Myconet 13 1403–1418 [Google Scholar]
- 14.Obbard, D. J., Jiggins, F. M., Halligan, D. L., and Little, T. J. (2006) Curr. Biol. 16 580–585 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.