Abstract
The actin cytoskeleton plays a major role in cell motility that is essential for the function of phagocytes. Calponin is an actin-associated regulatory protein. Here we report the finding of significant levels of the h2 isoform of calponin in peripheral blood cells of myeloid lineage. To study the functional significance, h2-calponin gene (Cnn2) interrupted mice were constructed. Germ line transmission of the Cnn2-flox-neo allele was obtained in chimeras from two independent clones of targeted embryonic stem cells. The insertion of the neoR cassette into intron 2 of the Cnn2 gene resulted in a significant knockdown of h2-calponin expression. Removing the frt-flanked neoR cassette by FLP1 recombinase rescued the knockdown effect. Cre recombinase-induced deletion of the loxP-flanked exon 2 eliminated the expression of h2-calponin protein. H2-calponin-free mice showed reduced numbers of peripheral blood neutrophils and monocytes. H2-calponin-free macrophages demonstrated a higher rate of proliferation and faster migration than that of h2-calponin-positive cells, consistent with a faster diapedesis of peripheral monocytes and neutrophils. H2-calponin-free macrophages showed reduced spreading in adhesion culture together with decreased tropomyosin in the actin cytoskeleton. The lack of h2-calponin also significantly increased macrophage phagocytotic activity, suggesting a novel mechanism to regulate phagocyte functions.
Leukocytes are mobile cells and their actin cytoskeleton plays a central role in the locomotion, transmigration, and phagocytosis. These activities are essential for the function of myeloid cells, including neutrophils, monocytes, and macrophages, in defensive and autoimmune responses (1). Despite the significant biological and medical importance, the regulation of actin cytoskeleton in myeloid cells is not well understood.
Calponin is an actin filament-associated protein of 34–37 kDa (292–330 amino acids) found in smooth muscle (2) as well as non-muscle cells (3,4). Through high affinity binding to F-actin, calponin inhibits the actin-activated myosin MgATPase (5–8) and motor activity (9–11). The association of calponin with actin filaments and its regulatory function have led to a model wherein calponin may represent a thin filament regulatory mechanism modulating smooth muscle contraction (12).
Three isoforms of calponin (h1, h2, and acidic) have been identified in higher vertebrates as the products of three homologous genes (13–17). The three isoforms of calponin have distinct theoretical isoelectric points (pI values): H1-calponin is basic (pI 9.4), h2-calponin is near neutral (pI 7.5), whereas the acidic calponin has a lower pI of 5.2. H1-calponin is the predominant isoform specifically expressed in differentiated smooth muscle cells and its role in regulating smooth muscle contractility (9) is a focus of ongoing research. The majority of previous structural and functional studies of calponin were obtained from chicken gizzard calponin that is equivalent to the mammalian h1-calponin. The acidic calponin has been found in smooth muscle (16) and brain (17) and its function remains to be investigated.
H2-calponin is found in smooth muscle and certain non-muscle cells (3, 4, 18). We previously demonstrated that h2-calponin is expressed at higher levels in developing and remodeling smooth muscles and its overexpression inhibited the rate of cell proliferation (18). We further found that h2-calponin is expressed in epidermal keratinocytes, lung alveolar epithelial cells, and fibroblasts and plays a role in stabilizing the actin cytoskeleton. The expression and degradation of h2-calponin are both under the regulation of cytoskeletal tension built by myosin motors (3, 4).
The function of h2-calponin in regulating the actin cytoskeleton of non-muscle cells suggests its role in multiple cellular activities. In the present study, we found significant levels of h2-calponin in peripheral blood cells of myeloid lineage. To study the functional significance, h2-calponin gene (Cnn2) interrupted mice were constructed through embryonic stem (ES)2 cell gene targeting (19). Germ line transmission of the Cnn2-flox-neo allele was successful in chimeric mice from two independent clones of targeted embryonic stem cells. The insertion of the neoR selection cassette into intron 2 of the Cnn2 gene resulted in a significant knockdown of h2-calponin expression. Removing the neoR cassette by FLP1 recombinase completely rescued the knockdown effect. The Cre recombinase-induced deletion of exon 2 eliminated the expression of h2-calponin protein. H2-calponin-free mice had reduced numbers of peripheral blood neutrophils and monocytes. H2-calponin-free cells showed a higher rate of proliferation and faster migration than that of wild type cells, consistent with a faster migration of blood myeloid cells into tissues. The h2-calponin-free macrophages demonstrated reduced spreading in adherent culture together with decreased tropomyosin in the actin cytoskeleton. The lack of h2-calponin also significantly increased phagocytotic activity, suggesting a novel mechanism to regulate macrophage function.
MATERIALS AND METHODS
All animal procedures were approved by the Institutional Animal Care and Use Committees and were conducted in accordance with the Guiding Principles in the Care and Use of Animals, as approved by the Council of the American Physiological Society.
Cloning of Mouse Cnn2 Genomic DNA—From a 129SvJ strain mouse genomic DNA library in λ DASHII phage vector (20, 21), clones bearing genomic DNA segments containing the h2-calponin gene were isolated using 32P-labeled mouse h2-calponin cDNA probe. The plating of λ phage, making of nylon membrane replicas, hybridization, and autoradiography were carried out as described previously (21). The plaque-purified positive phages were amplified in XL-1B MFA P2 Escherichia coli and purified by centrifugation through CsCl density gradients. The recombinant phage DNA was isolated by phenol/chloroform extraction and subcloned into plasmid vectors as overlapping restriction endonuclease fragments. Restriction mapping, Southern analysis, and partial sequencing were carried out to verify the cloned Cnn2 genomic DNA in comparison with the mouse genomic DNA sequence in the data base (Gene ID MGI: 105093).
Development of H2-Calponin Gene Knock-out Mice—A mouse Cnn2-targeted conditional mutagenesis construct was generated using the pPNT4 vector (22), provided by Dr. Marcus Conrad, Institute of Clinical Molecular Biology and Tumor Genetics GSF-Research Centre for Environment and Health, Germany). In the Cnn2 gene targeting construct, two loxP sequences were inserted in intron 1 and intron 2 in tandem orientation to allow Cre recombinase-catalyzed deletion of exon 2. The deletion of exon 2 not only removes a portion of the coding sequence but also results in a reading frameshift in the downstream mRNA after codon number 21. The shifted reading frame is terminated by a stop codon after encoding 11 missense amino acids. This Cre-mediated deletion of exon 2 can be induced in whole animals as well as in tissues or in cultured cells.
A neoR cassette adjacent to the downstream loxP sequence as constructed in the pPNT4 vector was inserted into intron 2 of the Cnn2 targeting construct for neomycin selection of the transfected ES cells. The neoR cassette is flanked by two frt sequences and can be deleted by FLP1-catalyzed recombination (23). This mechanism allows the removal of the neoR cassette after establishing the targeted loxP mutagenesis to avoid the effect of neoR insertion on h2-calponin expression. The induction of neoR removal can be done in whole animals, tissues, or cells.
Long flanking arms (4.6 and 5.5 kb) were placed in the gene targeting construct to provide sufficient regions for homologous recombination. Transfection of mouse HM-1 ES cells (x,y) with the h2-calponin gene targeting DNA construct using electroporation was carried out at the Northwestern University Transgenic and Targeted Mutagenesis Laboratory. Colonies of the transfected ES cells were selected by the acquisition of neomycin resistance conferred by the gene targeting construct. Genomic DNA from the drug-resistant ES cell colonies was extracted by protease K digestion and screened by Southern blotting using cloned 5′- and 3′-flanking genomic DNA probes for the homologous recombination generated change of BamHI restriction pattern.
Ten μg each of the ES cell genomic DNA was digested by BamHI, separated by 0.8% agarose gel electrophoresis, and transferred to the nylon membrane by capillary action using standard Southern blotting method. The membrane was prehybridized at 55 °C for 2 h in 0.25 m Na2HPO4, 14 mm H3PO4, 1 mm EDTA, 1% bovine serum albumin, 5% SDS, 0.1 mg/ml mechanically sheared salmon sperm DNA, 20% formamide. The 5′ and 3′ DNA probes were labeled with [32P]dCTP, heat-denatured, and added to the rolling hybridization flask together with 5% (w/v) dextran sulfate for incubation at 55 °C for 16 h. The membrane was then washed repeatedly by 40 mm sodium phosphate buffer containing 1% SDS and 1 mm EDTA to gradually reach 60 °C and examined by autoradiography.
Two original Cnn2-targeted mouse ES cell clones identified by Southern screening, 21C7 and 21H2, were used to produce chimeric mice. The blastocyst injection and embryo re-implantation were carried out at the Northwestern University Gene Targeting and Transgenic Core Facility. The 129SvJ-originated (albino) ES cells are injected into C57BL/6 (black) mouse blastocysts to produce chimeras. High chimerism males from the two targeted ES cell lines were mated with C57BL/6 females to test germ line transmission. The ES cell-originated offspring was first selected by the brown coat color in contrast to the pure C57BL/6 black littermates. The presence of the targeted Cnn2 allele in the ES cell-originated pups was then genotyped by PCR on genomic DNA extracted from tail biopsies. Two pairs of PCR primers were designed to identify the presence of the upstream loxP and the neoR cassette, respectively. Mice bearing the targeted Cnn2 allele were selected to mate with C57BL/6 for 7 to 9 generations to obtain a uniformed genetic background.
Disruption of the h2-calponin gene through deletion of the exon 2 region was obtained by crossing the Cnn2-flox mouse line with the Zp3-cre mouse line (The Jackson Laboratory) that expresses Cre recombinase in the female germ line. Removal of the neoR cassette inserted in intron 2 was achieved by crossing the Cnn2-flox-neo line with a Gt(ROSA)26Sor-FLP transgenic mouse line (The Jackson Laboratory) that expresses FLP1 recombinase in most tissue types, including the developing germ line.
SDS-PAGE and Western Blotting—Representative tissue samples were obtained from adult (4–5 months old) wild type and Cnn2-targeted mice. Immediately after euthanasia, tissues were rapidly dissected on ice and briefly rinsed in cold phosphate-buffered saline (PBS). Total proteins were extracted from the tissues by mechanical homogenization in SDS-PAGE sample buffer containing 2% SDS and heated at 80 °C for 5 min. SDS-PAGE samples of isolated or cultured cells were prepared similarly, omitting the mechanical homogenization step.
The protein extracts were examined by SDS-PAGE using the Laemmli buffer system and Coomassie Blue R-250 staining. Duplicate gels were electrically blotted on nitrocellulose membrane using a Bio-Rad semi-dry transfer apparatus for Western analysis. After blocking with 1% bovine serum albumin or 5% powdered skim milk in Tris-buffered saline, the membrane was incubated with a rabbit antiserum, RAH2, which was raised against mouse h2-calponin immunogen and has a weak cross-reaction to h1-calponin (24), a mouse anti-h1-calponin monoclonal antibody (mAb) CP1 (25), mouse anti-h2-calponin mAbs CP21 and 1D2 (3, 18), or anti-tropomyosin mAb LC24 (26) (provided by Prof. Jim Lin, University of Iowa) in Tris-buffered saline containing 0.1% bovine serum albumin or 0.5% powdered skim milk to examine the expression of calponin and tropomyosin. As described previously (4), the blots were washed with Tris-buffered saline containing 0.05% Tween 20 and incubated with alkaline phosphatase-labeled anti-rabbit IgG or anti-mouse IgG second antibody (Sigma). After the final washes, the blots were processed for 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium chromogenic substrate reaction (4).
Densitometry analysis of SDS gels and Western blots were performed on digital images scanned at 600 dots/inch using the NIH Image software version 1.61. Quantification of the SDS gel and Western blots was done by normalization to the amount of actin, total cellular protein, or histones determined in parallel SDS gels.
InVitro Differentiation of Human Peripheral Monocytes into Macrophages—Human monocytes were isolated by elutriation from peripheral blood of anonymous healthy donors to start primary cell cultures. This investigation was determined to be exempted research by the Northwestern University Institutional Review Board. The purified peripheral monocytes were suspended in serum-free RPMI 1640 medium, and allowed to adhere to cell culture dishes at 37 °C in 5% CO2 for 1 h before change to medium containing 20% fetal bovine serum (FBS), 100 μg/ml penicillin, 50 μg/ml streptomycin, and 1 μg/ml of polymyxin B. The adherent cells were allowed to differentiate into macrophages in culture for up to 7 days as described previously (27–33).
Collection and Immunophenotyping of Mouse Peritoneal Cells—Peritoneal residential cells were lavaged with PBS from wild type and h2-calponin knock-out mice. Mouse peritoneal cells were also elicited by injection of 2 ml of 3% thioglycollate broth 12 and 72 h prior to lavage. A fixed volume (8 ml) of PBS was used for each animal so the total number of cells lavaged could be compared.
Cell types are identified by immunophenotyping with florescence-conjugated antibodies recognizing specific cell surface markers of myeloid cell (Mac-1), macrophage (F4/80), and granulocyte (Gr-1) followed by flow cytometry analysis. 5 × 105 cells from each mouse were first incubated with anti-mouse CD16/CD32 antibody (BD Biosciences) to block the cell surface Fc III/II receptor and then stained with fluorescein isothiocyanate (FITC)-conjugated anti-Mac1 (Mac1-FITC, Serotec), allophycocyanin-conjugated anti-F4/80 (F4/80-allophycocyanin, Serotec), and phycoerythrin-Cy7 (PEcy7)-conjugated anti-Gr1 (Gr1-PEcy7, eBioscience, CA) antibodies at room temperature for 30 min. After washing away the unbound antibodies, the cells were analyzed using a BD LSR II flow cytometer (BD Biosciences) with BD FACSDIVA software. Using the FCS Express software (DeNovo Software, Los Angeles, CA), macrophages were identified as strong Mac1-positive, F4/80-positive, and Gr1-negative, whereas granulocytes were identified as strong Mac1-positive, F4/80-negative, and Gr1-positive.
In Vitro Wound Healing Assay—Residential mouse peritoneal cells were collected in RPMI 1640 medium containing 10% FBS, 100 μg/ml penicillin, and 50 μg/ml streptomycin and seeded in 12-well culture plates at 1 × 106 per well to allow high density adhesion of macrophages. After removing the floating cells, the adherent cells were incubated at 37 °C in 5% CO2 for 24 h to form a confluent monolayer. The macrophage monolayer was wounded by scratching with a thin pipette tip and examined at a series of time points under a phase-contrast microscope. The width of the wounds was measured from photographs to evaluate the rate of cell migration during the course of healing.
In Vitro Proliferation of Mouse Bone Marrow Cells—Bone marrow cells were flushed out from femora and tibias of 3-month-old wild type and h2-calponin knock-out mice. The cells were induced to differentiate into macrophages in DMEM containing 10% FBS, 20% L929 cell-conditioned media (34), 5% horse serum, 1% sodium pyruvate, 2 mm l-glutamine, 100 μg/ml penicillin, and 50 μg/ml streptomycin at 37 °C in 5% CO2. After 4 days of culture, the adherent cells were collected with a cell scraper and re-seeded in 96-well plates at 2.5 × 103 cells per well in 200 μl of the same media. The rate of cell proliferation in culture was examined in quadruplet wells at a series of time points by Crystal Violet staining of nuclei as described previously (18).
Transfection of RAW Cells—RAW264.7 mouse macrophage cells (35) (ATCC TIB-71) were seeded at 2 × 106 cells/100-mm dish in DMEM containing 10% FBS, 100 μg/ml penicillin, and 50 μg/ml streptomycin at 37 °C in 5% CO2 and cultured for 24 h prior to transfection with recombinant pcDNA3.1 plasmid DNA expressing mouse h2-calponin under the cytomegalovirus promoter (18). Two μg of DNA in 50 μl of DMEM was mixed with 5 μl of Lipofectamine (Invitrogen) and incubated at room temperature for 20 min before being gently mixed with 5 ml of DMEM and added to the RAW264.7 cell culture. After incubation at 37 °C in 5% CO2 for 6 h, 5 ml of DMEM containing 20% FBS was added to the dish and the culture was further incubated for 18 h before change to fresh media.
To establish RAW264.7 cell lines stably expressing h2-calponin, the transfected cells were selected by G418 (300 μg/ml) and drug-resistant single colonies were individually picked as described previously (18). The integration of the sense and antisense h2-calponin cDNA transgenes in the stably transfected RAW cell lines was verified by PCR. The expression of h2-calponin in the sense cDNA-transfected cells was examined by Western blotting using the RAH2 antibody.
RAW264.7 cells stably transfected with sense and antisense h2-calponin cDNA and non-transfected cells were seeded in 96-well culture plates at 1 × 103 cells per well for cell proliferation assay as described above. Multiple stable transfected cell lines were examined to avoid line to line differences.
Immunofluorescence Microscopy—To examine the cellular localization of h2-calponin in macrophages and the effect of Cnn2 knock-out on the structure of macrophage actin cytoskeleton, residential mouse peritoneal macrophages were cultured as a monolayer on glass coverslips for 24 h and stained with anti-h2-calponin antibody RAH2 and normal rabbit serum control, followed by TRITC- or FITC-labeled anti-rabbit IgG second antibody (Sigma) as described previously (18). Actin filaments were stained with TRITC-phalloidin. Tropomyosin was stained with mAb CG3 (36) hybridoma supernatant and SP2/0 myeloma supernatant control followed by TRITC- or FITC-labeled anti-mouse IgG second antibody. The results were observed under a Zeiss Axiovert 100H epifluorescence microscope and photographed.
Phagocytosis Assay—Phagocytosis was assessed by the uptake of fluorescent latex beads (37–40). After opsonization by incubation in 50% pooled normal mouse serum diluted in Krebs-Ringer PBS (pH 7.4) for 30 min, red fluorescence carboxyl microspheres (1.0 μm diameter, excitation/emission maxima at 580 nm/605 nm, Molecular Probes) were incubated with mouse residential and elicited peritoneal cells (1 × 106) at a particle to cell ratio of 50:1 in 0.5 ml of RPMI 1640 medium containing a final concentration of 5% mouse serum at 37 °C in 5% CO2 for 1 h. The cells were then thoroughly washed with PBS four times to remove the free and extracellularly bound particles and immediately stained with F4/80-allophycocyanin and Gr1-PEcy7 antibodies as above. After washing to remove the unbound antibodies, the cells were fixed with 1% formalin in PBS. Uptake of the fluorescent beads was measured using a BD FACSArray flow cytometer at 532 nm laser excitation. Phagocytotic activity was analyzed by the FCS Express software.
Data Analysis—All quantitative data are presented as mean ± S.D. or S.E. as noted in the figure legends. Statistical analysis was done with Student's t test using Microsoft Excel (two-tail assays unless noted in the figure legends).
RESULTS
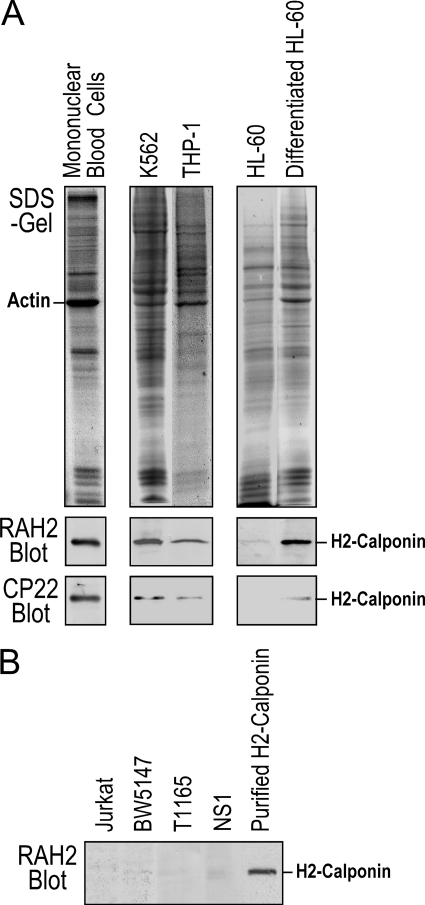
Significant Levels of H2-calponin in Myeloid Cells—We found that h2-calponin is highly expressed in human peripheral blood mononuclear cells, human myelogenous leukemia cell line K562 (ATCC CCL-243), and human monocyte line THP-1 (ATCC TIB-202) (Fig. 1A). Although h2-calponin is minimally detectable in undifferentiated human promyeloblast line HL-60 (ATCC CCL-240), the expression is significantly up-regulated 5 days after dimethyl sulfoxide-induced differentiation in culture (41). In contrast, h2-calponin is not detectable by RAH2 polyclonal antibody in the human T lymphocyte line Jurkat (ATCC TIB-152), mouse T lymphocyte line BW5147 (ATCC TIB-47), mouse plasmacytoma cell line T1165 (42), and mouse myeloma cell line NS-1 (43) (Fig. 1B). The data demonstrated a myeloid cell-specific expression of h2-calponin.
FIGURE 1.
Expression of h2-calponin in myeloid cells. A, Western blots using anti-h2-calponin polyclonal antibody RAH2 and mAb CP22 on total protein extracted from human peripheral mononuclear cells, human myelogenous leukemia cell line K562, human monocyte line THP-1, and in vitro differentiated human myeloid leukemia cell line HL-60 detected significant amounts of h2-calponin. B, no calponin expression was detectable in the human T lymphocyte line Jurkat, mouse T lymphocyte line BW5147, mouse plasmacytoma cell line T1165, and mouse myeloma cell line NS-1 by RAH2 Western blot.
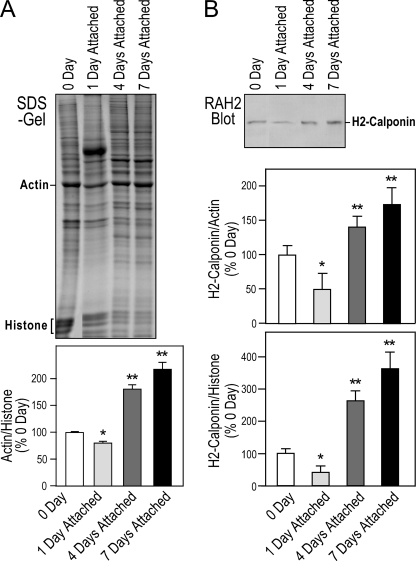
Regulation of H2-calponin during Monocyte-Macrophage Differentiation—To investigate the function of h2-calponin in differentiated myeloid cells, we examined the expression of h2-calponin during monocyte to macrophage differentiation. Employing histone as a control for the amount of chromosomal DNA and, therefore, the number of cells, SDS-PAGE showed that cellular actin contents significantly increased during macrophage differentiation (Fig. 2A), consistent with increased cytoskeleton volume and function. H2-calponin decreased early during macrophage differentiation (1 day in the adherent culture) and significantly increased after differentiation for 4–7 days into mature macrophage, when normalized against the level of actin or histone (Fig. 2B). The lower level of h2-calponin in monocytes versus that in macrophages may reflect the established functional differences between the two cell types, such as the highly mobile nature of peripheral monocytes versus the fact that macrophages largely remain in tissues (44).
FIGURE 2.
Expression of h2-calponin during monocyte-macrophage differentiation. H2-calponin expression was examined during adhesion-dependent differentiation of human peripheral monocytes. Fresh isolated monocytes adhered to plastic dishes were cultured to differentiate in vitro into macrophages. A, the total cellular protein extracts from the cells before (0 day) and 1, 4, and 7 days in culture were examined by SDS-PAGE. Gel densitometry analysis demonstrates a decrease followed by significant increases in actin as normalized to histone levels that reflects cell numbers. *, p < 0.05, and **, p < 0.01 versus the day 0 level. B, Western blots using RAH2 antibody and densitometry quantification demonstrated that the expression of h2-calponin normalized to the level of cellular actin or histone showed a decrease in h2-calponin at the early stage of monocyte-macrophage differentiation followed by significant increases during the later stages of differentiation. The quantification was done from three sets of samples and two repeated analyses. *, p < 0.005, and **, p < 0.001 versus the day 0 level.
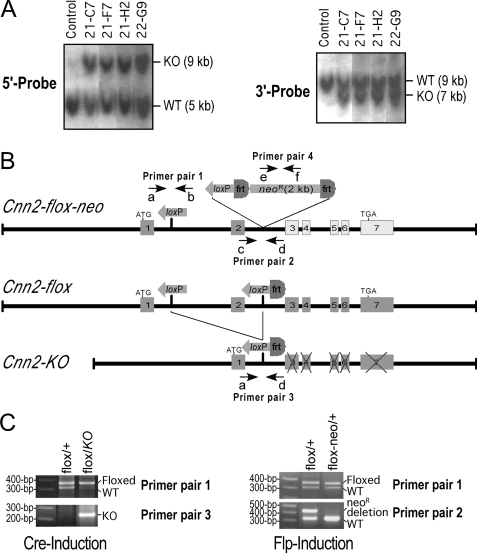
Cnn2-targeted Mouse Lines—Using the gene targeting construct derived from 129SvJ mouse genomic DNA that is syngenic to the HM-1 mouse ES cells, highly successful targeting of the Cnn2 gene was obtained. More than 20 Cnn2-targeted ES cell clones were obtained from ∼190 candidate clones that survived G418 selection. The Southern blots in Fig. 3A using the 5′ and 3′ genomic DNA probes showed representative genotyping results of the h2-calponin gene-targeted mouse ES cell clones.
FIGURE 3.
Genotyping of Cnn2-targeted mouse ES cells. A, Southern blot screening of the targeted Cnn2 allele in mouse ES cells. Genomic DNA of transfected ES cell clones was digested by BamHI and hybridized with 32P-labeled 5′- and 3′-flanking genomic DNA probes. The left panel shows that the 5′ probe detected a 9-kb band from the targeted allele (KO) in four representative positive clones. The presence of the 5-kb band from the wild-type (WT) allele indicates these cells are heterozygotes for the targeted Cnn2 allele. The right panel shows the same blot (after stripping the 5′ probe) re-probed with the 3′-flanking probe to detect the targeted allele as a 7-kb fragment together with the ∼9-kb WT allele band. B, maps of three modified Cnn2 alleles: Cnn2-flox-neo is the original Cnn2-targeted allele. The expression of exons 3–7 is negatively affected by the neoR cassette inserted in intron 2, resulting in a knockdown of h2-calponin expression. The Cnn2-flox allele is derived from the Cnn2-flox-neo allele by FLP1-induced removal of the neoR cassette, which restores the normal expression of h2-calponin. The Cnn2-KO allele is derived by further deletion of exon 2 through Cre-induced recombination, resulting in a reading frameshift in exons 3–7 and knock-out the expression of h2-calponin. The positions of 6 primers used as 4 pairs in PCR genotyping are outlined on the maps. C, PCR genotyping of the Cnn2-targeted alleles. The left panel shows agarose gels of PCR detections of the insertion of loxP sequence in intron 1 and the Cre-induced deletion of exon 2 (the PCR conditions for Primer pair 3 would detect the short product of Cnn2-KO allele but not the long segment in Cnn2-flox allele). The right panel shows agarose gels of PCR identification of the FLP1-induced removal of the neoR cassette (the PCR conditions for Primer pair 2 would detect the short product of Cnn2-flox allele but not the long segment in Cnn2-flox-neo allele) together with detection of the loxP insertion. The presence or absence of neoR cassette was confirmed by PCR using primer pair 4 (data not shown).
The two independent targeted ES cell clones selected for blastocyst injection both produced chimeric mice with high frequency germ line transmission. The development of the Cnn2 gene-targeted founder mouse lines from two original targeted ES cell clones allows phenotypic characterizations avoiding line-to-line variations. PCR genotyping of the ES cell-originated offspring showed Mendelian segregation of the Cnn2-flox-neo allele. PCR genotyping further demonstrated that the neoR selection marker can be effectively removed by crossing with the Gt(ROSA)26Sor-FLP mouse line to produce the Cnn2-flox allele (Fig. 3B and 3C). Crossing with the Zp3-cre mouse line effectively produced deletion of exon 2, generating the Cnn2-KO allele (Fig. 3, B and C).
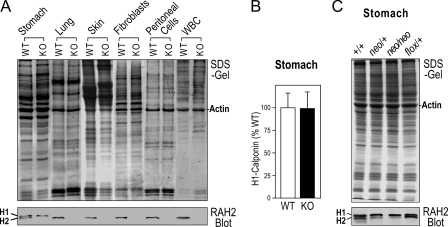
Knock-out and Knockdown of H2-calponin in Cnn2-targeted Mice—Mice homozygous for the Cnn2-KO allele survive to adulthood and are fertile. The Western blots in Fig. 4A showed complete loss of h2-calponin in representative tissue and cell types that are known to normally express h2-calponin (i.e. smooth muscle that also expresses h1-calponin (18), lung alveolar cells (4), epidermal keratinocytes (3), fibroblasts (3), peritoneal residential cells, and peripheral white blood cells (Figs. 1 and 2). Although the deletion of exon 2 retained a short 5′ reading frame in the mRNA encoding the first 21 amino acids of h2-calponin followed by 11 missense amino acids, no corresponding protein fragment was detected by the polyclonal anti-h2-calponin antibody RAH2 in Cnn2-targeted mouse tissues (data not shown), confirming the complete knock-out effect. It is important to note that the level of h1-calponin was not significantly changed in the h2-calponin null smooth muscle tissues (Fig. 4B), indicating that the calponin isoforms are non-redundant regulatory proteins.
FIGURE 4.
Knock-out and reversible knockdown of h2-calponin in Cnn2 gene-targeted mice. A, knock-out of h2-calponin in representative mouse tissue and cell types. The SDS-PAGE and Western blots showed that h2-calponin was absent in stomach (smooth muscle), lung (alveolar cells), skin (keratinocytes), fibroblasts, peritoneal residential cells, and peripheral white blood cells (WBC) of Cnn2-knock-out (KO) mice in contrast to these tissue and cell types in wild type (WT) mice, which express h2-calponin at significant levels. B, densitometry quantification of multiple Western blots of total protein extracts showed very similar levels of h1-calponin in the stomachs of wild type and h2-calponin knock-out mice, indicating no compensatory up-regulation of h1-calponin when h2-calponin ceases expression. C, SDS-PAGE and Western blot showed that the level of h2-calponin significantly decreased in the stomach of a Cnn2-flox-neo heterozygous mouse and further down in Cnn2-flox-neo homozygotes, demonstrating a dominant knockdown effect. FLP1-induced removal of the neoR cassette from the intron 2 of Cnn2 effectively restored the expression of h2-calponin to the wild type levels. +, wild type Cnn2 allele.
A valuable side effect is that the insertion of the neoR cassette into intron 2 of the h2-calponin gene (Fig. 3C) resulted in a significant knockdown of h2-calponin expression (Fig. 4C), likely due to an interruption of the transcription of full-length h2-calponin pre-mRNA. Showing various extents of decreases in the h2-calponin level in tissue types that normally express h2-calponin, the h2-calponin knockdown mice also survive to adulthood and are fertile. Whereas Cnn2-flox-neo homozygotes exhibited significant knockdown of h2-calponin expression, Cnn2-flox-neo/+ heterozygotes showed intermediate decreases in the h2-calponin level (Fig. 4C). This observation suggests that h2-calponin expression is dependent on the gene dosage, indicating a non-redundant function.
Deletion of the neoR cassette by FLP1 recombinase produced an effective rescue of the h2-calponin gene expression. The Western blot in Fig. 4C showed a complete restoration of the h2-calponin protein level. In addition to demonstrating the effectiveness of removing the neoR cassette from the genome of the Cnn2-targeted mice, the results showed that the Cnn2-flox-neo allele provides an experimental system of reversible h2-calponin knockdown for investigating the functional effects of rescuing h2-calponin expression on tissue and cell phenotypes.
Decreased Number of Monocytes and Neutrophils in Peripheral Blood of H2-calponin Knockdown and Knock-out Mice—To investigate the physiological effects of h2-calponin deficiency in myeloid cells, we examined peripheral blood white cells. The results in Table 1 showed decreased numbers of peripheral monocytes and neutrophils in adult h2-calponin knockdown and knock-out mice as compared with age-matched wild type controls. The ratios of eosinophils and basophils were not significantly decreased. Consistently, the minimal level of h2-calponin detected in undifferentiated HL-60 human promyeloblast cells (41) (Fig. 1A) suggests that h2-calponin may be expressed in only a subset of differentiated myeloid cells in the granulocyte-monocyte progenitor lineage.
TABLE 1.
Decreased peripheral monocytes and neutrophils in h2-calponin knockdown and knock-out mice
Peripheral blood smears of wild type, h2-calponin knockdown, and h2-calponin knock-out mice were stained with Giemsa and white blood cells were counted under a microscope. Two hundreds cells were randomly counted for each mouse and 4 mice were examined in each group. The values shown are the percentage of total white blood cells (mean ± S.D.). Decreased numbers of monocytes and neutrophils were found in the h2-calponin knockdown and knock-out mice.
| Wild type | Cnn2 knockdown | Cnn2 knock-out | |
|---|---|---|---|
| Monocytes | 3.7 ± 0.3 | 2.2 ± 0.5a | 2.1 ± 0.5a |
| Neutrophils | 10.5 ± 0.7 | 6.4 ± 1.2a | 6.3 ± 0.8a |
| Eosinophils | 1.7 ± 0.3 | 1.7 ± 0.4 | 1.7 ± 0.3 |
| Basophils | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Lymphocytes | 84.1 ± 1.1 | 89.7 ± 1.8 | 89.9 ± 1.2 |
p < 0.001 compared with the wild type control.
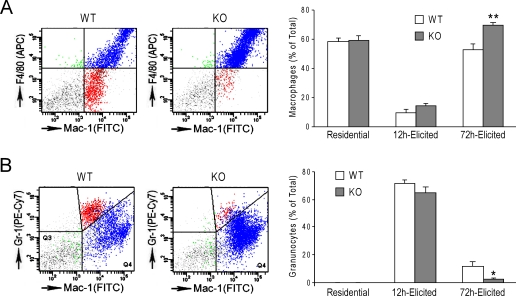
Peritoneal Macrophages Are Increased and Granulocytes Decreased in H2-calponin Knock-out Mice—The decreased numbers of neutrophil and monocyte in the peripheral blood of h2-calponin knockdown and knock-out mice could result from reduced production, more rapid turn over, or faster migration into tissues. Therefore, we examined the total number of myeloid cells as well as the percentage of F4/80+ macrophages and Gr-1+ granulocytes in residential and thioglycollate-elicited peritoneal cells from wild type and h2-calponin knock-out mice. The residential and elicited total cell counts and the number of Mac-1+ myeloid cells were not significantly different between the knock-out and wild type mice (data not shown). However, the results showed that 72 h after thioglycollate stimulation when macrophages are the dominant cell type in the peritoneum, the ratio of macrophage in total peritoneal cells was higher in the knock-out mice than that in the wild type mice (Fig. 5A). In contrast, the number of peritoneal granulocytes was significantly lower 12 h after thioglycollate stimulation (9.9 ± 1.4 × 106 versus 16.9 ± 1.5 × 106, p < 0.01) when granulocytes are the dominant cell type, and this trend remained 72 h after stimulation (1.9 ± 0.5% versus 7.5 ± 2.8%, although statistic significance was not reached). The same trend was also shown by the percent of granulocytes in total peritoneal cells (Fig. 5B). The reduced number of granulocytes in the peritoneum may correlate to the decreased number of neutrophils in peripheral blood (Table 1). On the other hand, the increased numbers of macrophages, despite the reduced numbers of monocytes in the peripheral blood, suggests increased diapedesis although increased tissue survival or local proliferation cannot be excluded.
FIGURE 5.
Increased diapedesis of h2-calponin-free macrophages. The left panels show representative flow cytometry histograms of 72-h elicited peritoneal cells for the identification of macrophages (A) and granulocytes (B). The Mac-1+ population is indicated on the x axis (FITC) and the Gr-1 (phycoerythrin-Cy7) or F4/80 (allophycocyanin) is indicated on the y axis. Macrophages were identified by strong Mac1-positive, F4/80-positive, and Gr1-negative stains, whereas granulocytes were identified by strong Mac1-positive, F4/80-negative, and Gr1-positive stains. The upper right quadrant represents the double positive cells that represent macrophages (A) and granulocytes (B). The right panels show the summarized results in which the percent of 72-h elicited macrophages was significantly increased in h2-calponin knock-out (KO) mice compared with the wild type (WT) control (**, p < 0.01 in two-tail Student's t test) (A), whereas the percent of 72-h elicited granulocytes showed a corresponding decrease in h2-calponin knock-out mice than that in WT mice (B) (*, p < 0.05 in one-tail Student's t test). n = 7 mice in each experimental group except for n = 5 or the 72 h elicited h2-calponin knock-out group.
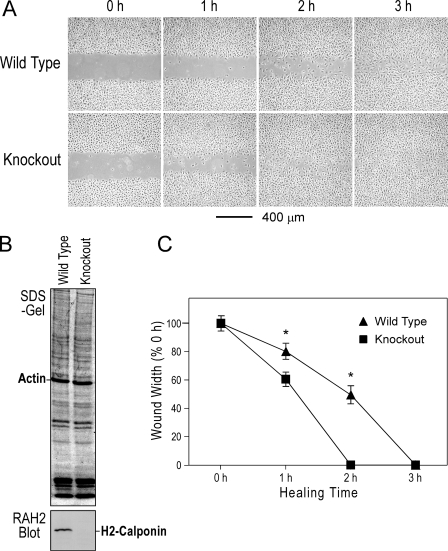
Increased Motility of H2-calponin-free Macrophages—We further examined the intrinsic motility of h2-calponin-free macrophages in the absence of chemotactic stimulation. In vitro wound healing assay in monolayer cells provides direct measurement for the rate of two-dimensional cell migration. The results from testing cultures of mouse peritoneal residential macrophages showed a significantly earlier closure of the scratch wound in h2-calponin-free cells versus the wild type control (Fig. 6). This result demonstrates a faster migration rate of h2-calponin-free macrophages and is consistent with the function of h2-calponin as an inhibitor for the motility of macrophages. Therefore, the increased diapedesis of h2-calponin-free myeloid cells may correlate to altered function of the actin cytoskeleton. The results demonstrated a role of h2-calponin in regulating macrophage motility that is an essential component of immune responses.
FIGURE 6.
Faster migration of h2-calponin-free macrophages during in vitro wound healing. A, scratch wounds were made in monolayer cultures of peritoneal residential macrophage 24 h after plating. Healing of the wound by cell migration was monitored for 6 h. The micrographs showed an earlier closure of the wound in the h2-calponin-free macrophage culture than the wild type control. B, SDS-PAGE and Western blot using RAH2 antibody on total protein extracts from cells collected at the end of the wound healing experiments confirmed the presence and absence of h2-calponin in the wild type and Cnn2 knock-out cells, respectively. C, quantification of the wound healing data demonstrates the faster migration rate of the h2-calponin knockout macrophages than the wild type control. *, p < 0.05.
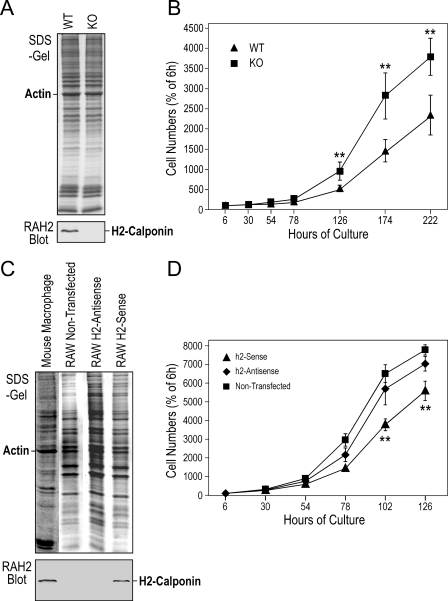
Faster Proliferation of H2-calponin-free Macrophages—Since macrophages might proliferate in tissues (45) and h2-calponin is known to inhibit cell proliferation (18), it is worth investigating the effect of h2-calponin on macrophage proliferation. Examining the proliferation of mouse bone marrow cells cultured in macrophage differentiation media showed that the cells from h2-calponin knock-out mice proliferated significantly faster than the wild type controls (Fig. 7, A and B).
FIGURE 7.
Faster proliferation of h2-calponin-free cells. A, Western blots showed that bone marrow cells isolated from adult wild type (WT) and h2-calponin knock-out (KO) mice had positive and negative, respectively, expression of h2-calponin. B, the rate of bone marrow cell proliferation in L929 cell-conditioned media was examined by Crystal Violet staining and the results showed a significantly faster proliferation of h2-calponin-free bone marrow cells than that of wild type cells. C, Western blots showed that the RAW264.7 mouse macrophage line had lost endogenous h2-calponin expression in contrast to primary mouse peritoneal macrophages. Stable transfective expression of mouse h2-calponin cDNA under the cytomegalovirus promoter produced a significant level of h2-calponin. D, RAW264.7 cells stably transfected with sense or antisense h2-calponin cDNA expression vector and non-transfected control were examined for the rate of cell proliferation as in B. The results demonstrated a significant decrease in the h2-calponin sense cDNA-transfected cells in comparison to that of the antisense-transfected and non-transfected RAW cells. The slightly lower proliferation rate of the antisense cDNA-transfected cells than that of the non-transfected cells reflected the effect of G418 in the culture of the transfected cells. **, p < 0.001.
Considering that in vitro differentiated bone marrow cells may not represent a uniform proliferating population, we also investigated an extensively studied mouse macrophage cell line, RAW264.7 (35). In contrast to freshly isolated macrophages, we found that the RAW264.7 cell line does not express endogenous h2-calponin (Fig. 7C). Therefore, RAW264.7 cells stably transfected with sense or antisense h2-calponin cDNA expression constructs were examined. The forced expression of h2-calponin in sense h2-calponin cDNA transfected cells significantly inhibited the rate of cell proliferation, whereas the antisense cDNA control maintained a proliferation rate similar to that of the untransfected cells (Fig. 7D). Because h2-calponin is specifically expressed in differentiated myeloid cells (Fig. 1), the effect of h2-calponin on the differentiation state of RAW cells remains to be investigated.
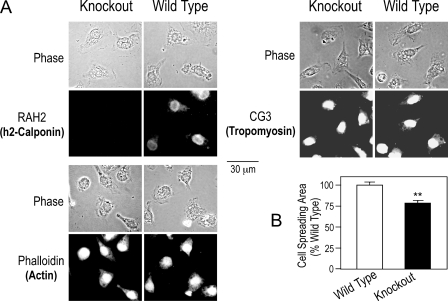
Loss of H2-calponin in Macrophages Reduces Cell Spreading in Adhesion Culture—To investigate the mechanisms for h2-calponin to regulate macrophage function, we examined the cell morphology and actin cytoskeleton structure of h2-calponin-free mouse macrophages. The phase-contrast and immunofluorescence micrographs in Fig. 8A demonstrate that the h2-calponin-free macrophages have a reduced spreading area compared with the wild type cells in the adherent culture (Fig. 8B), with apparently identical morphology of the actin cytoskeleton. The cell spreading in adhesion culture reflects cell adhesion to the substrate as well as the mechanical force generated in the cytoskeleton (46). We previously showed that h2-calponin increases the stability of actin filaments (3). Therefore, the reduced spreading of h2-calponin-free macrophages suggests a role of h2-calponin in macrophage adhesion, in addition to the regulation of cytoskeleton dynamics suggested by the cell migration experiments (Fig. 6).
FIGURE 8.
Removal of h2-calponin from macrophages reduces cell spreading area in adhesion cultures. A, immunofluorescence microscopy using anti-h2-calponin RAH2 and anti-tropomyosin CG3 antibodies followed by TRITC-conjugated secondary antibodies and TRITC-phalloidin staining of F-actin examined the cellular localization of h2-calponin in macrophages and the effect of its removal on the actin cytoskeleton. The phase-contrast and fluorescence images demonstrate that h2-calponin-free macrophages had a smaller spreading area, although the structure of actin cytoskeleton was not significantly altered. B, the reduced spreading area of h2-calponin-free macrophages in adherent culture was quantified using NIH Image software version 1.61. **, p < 0.01.
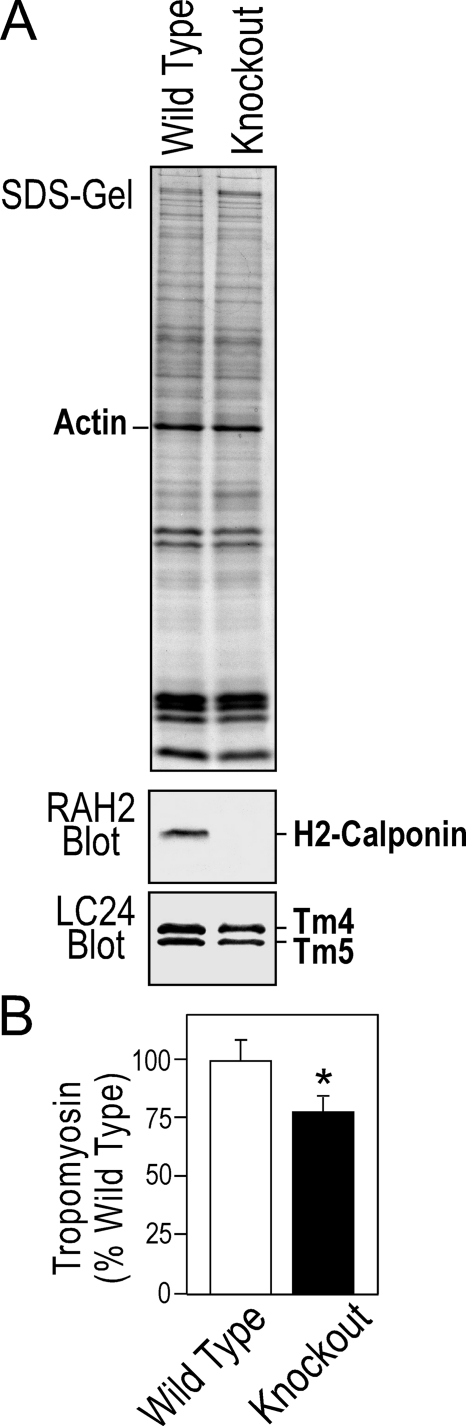
It has been shown by previous studies that macrophages express only low molecular weight tropomyosin (47). Consistently, non-muscle tropomyosin isoforms 4 and 5 (Tm4 and Tm5) (48) were detected in both wild type and h2-calponin-free macrophages (Figs. 8A and 9A). No high molecular weight tropomyosin isoforms was detectable (data not shown). H2-calponin-free macrophages showed a decreased level of tropomyosin than that in the wild type cells (Fig. 9B). This observation is consistent with our previous finding that the level of h2-calponin determines the level of tropomyosin in the cell (3).
FIGURE 9.
Removal of h2-calponin resulted in a decrease of tropomyosin in macrophages. A, Western blots showed a lower level of tropomyosin versus actin in the h2-calponin-free macrophages than that in wild type cells. B, this decrease in the tropomyosin level was quantitatively demonstrated by densitometry analysis (*, p < 0.05).
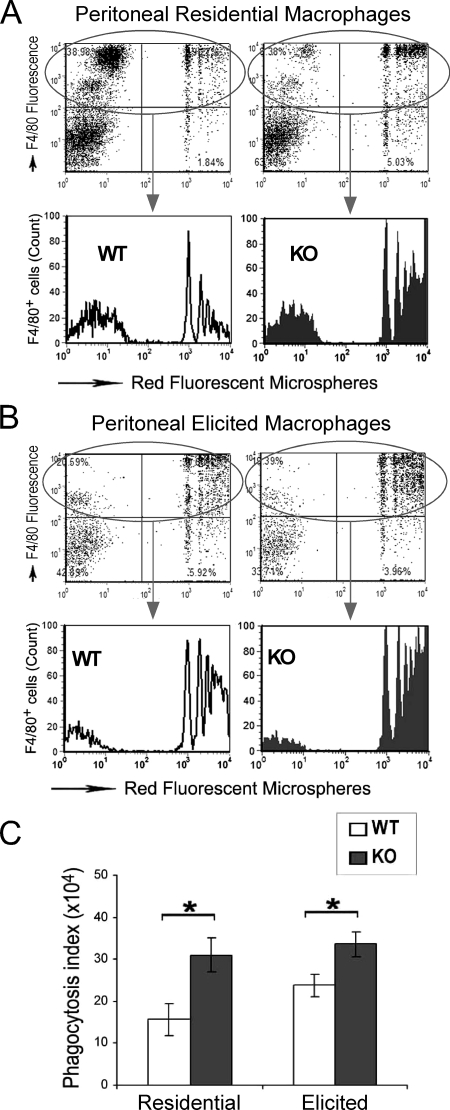
Increased Phagocytosis of H2-calponin-free Macrophages—We further examined the phagocytosis function of h2-calponin-free macrophages in vitro using fluorescent latex beads. The results demonstrated that the lack of h2-calponin resulted in enhanced phagocytic activity of both residential (Fig. 10A) and 72-h elicited (Fig. 10B) mouse peritoneal macrophages. In contrast, h2-calponin-free and wild type granulocytes showed no difference in phagocytotic activity at any of the time points examined (data not shown). The increased phagocytosis activity of h2-calponin-free macrophages is consistent with a more active actin cytoskeleton function as suggested by the cell motility studies. Although h2-calponin is a negative regulator of phagocytosis, it remains to be investigated why its expression increases when monocytes differentiate into macrophages (Fig. 2) and become more phagocytic. A hypothesis is that when monocytes differentiated into macrophages with increased phagocytotic activity, a higher level of h2-calponin is needed for an effective regulation of the cytoskeleton activity: a faster car would need more powerful brakes.
FIGURE 10.
H2-calponin-free macrophages had enhanced phagocytosis. Residential (A) and elicited (B) peritoneal cells isolated from h2-calponin knock-out (KO) and wild type (WT) mice were incubated with red fluorescent carboxyl microspheres to test phagocytosis activity. In the representative flow cytometric histograms of the F4/80-positive WT and KO macrophages, the x axis represents the fluorescent intensity indicating phagocytosis of the beads and the y axis indicates the gating for macrophages (upper panels) and the relative cell counts (lower panels). The peaks from left to right represent cell populations with no bead-ingested, 1 bead ingested, and 2 to more than 5 bead ingested. C, the phagocytosis activities measured on peritoneal residential (n = 7 for both WT and KO) and 72-h elicited (n = 7 for WT, n = 5 for KO) macrophages are summarized as phagocytosis index (percent of beads-ingested cells × mean florescence intensity of cells containing beads). The data demonstrated an enhanced phagocytosis activity of both residential and elicited macrophages when h2-calponin was absent (*, p < 0.05).
DISCUSSION
Calponin has been extensively studied for its function in the regulation of smooth muscle contractility. In contrast to the smooth muscle-specific h1 isoform, h2-calponin is found in both smooth muscle (18) and non-muscle cells, such as epidermal keratinocytes, fibroblasts, and lung alveolar cells (3, 4). The present study reports for the first time the high level expression of h2-calponin in the monocyte-granulocyte lineage of myeloid cells with an up-regulation during monocyte to macrophage differentiation. To understand the function of h2-calponin in the regulation of myeloid cell motility, we constructed h2-calponin gene-targeted mice and examined the effects of h2-calponin deficiency on the function of macrophages. Several novel findings are discussed as follows.
Up-regulated Expression of H2-calponin in Differentiated Granulocytes and Mature Macrophages—The finding of high levels of h2-calponin expression in myeloid cells but not in T and B lymphocytes (Fig. 1) suggests its role in a unique function of myeloid cells. While the h2-calponin expression is dependent on the differentiation of granulocytes, up-regulation of h2-calponin expression also occurs during the differentiation of peripheral blood monocytes into macrophages (Figs. 1 and 2). Monocytes and macrophages are extremely dynamic cells with intensive membrane trafficking, fusion, and fission. Actin cytoskeleton plays a central role in macrophage locomotion, phagocytosis and endocytosis (49), and change in cell shape required for transmigration (1). Considering that h2-calponin is an inhibitor in a balanced function of the actin cytoskeleton (9), the differential expression of h2-calponin in different leukocyte lineages may reflect their cytoskeleton activities. For example, the increased h2-calponin inhibition in macrophages may correspond to its reduced mobility versus that in monocytes (50, 51).
It is commonly observed that the differentiation of monocytes into macrophages in culture is dependent on adhesion to the culture dish (52). We have previously demonstrated that the expression of h2-calponin is dependent on the tension built in the actin cytoskeleton against the stiffness of the cultural substrate (3). In the present study, we found an increase in h2-calponin expression during adhesion-dependent differentiation of monocytes into mature macrophages (Fig. 2). This correlation suggests that the attachment of monocytes to the substrate increases cytoskeleton tension that up-regulates the expression of h2-calponin, providing a lead to further investigate cell signaling during the adhesion-dependent monocyte-macrophage differentiation. The early dip of the h2-calponin level after monocyte adhesion to the culture dish (Fig. 1) suggests a lag between the cell adhesion and h2-calponin up-regulation, which may correspond to the high compliance of differentiating monocytes that undergo transendothelial migration after in vivo adhesion to the vascular wall (53).
The association of h2-calponin regulation and function to the substrate adherence-dependent differentiation of monocytes into mature macrophages is a novel finding. Considering that calponin plays an inhibitory role in the regulation of cell motility, the up-regulation of h2-calponin expression during monocyte-macrophage differentiation may reflect a need of more active regulation for the more dynamic activity of the actin filaments that is required in a balanced function of macrophages. In addition, the finding that increased h2-calponin expression in mature macrophages from this study is associated with its reduced motility compared with that of monocytes (50, 51). In other words, the presence of a sufficiently high level of h2-calponin in macrophages may be a critical factor that balances the function of macrophages to maintain a physiological level of activity and normal immune function.
H2-calponin Regulation of Cell Motility as a Potential Target to Control Macrophage Function—We have previously demonstrated that forced expression of h2-calponin inhibited the proliferation rate of smooth muscle cells (18). In the present study, we found that L929 cell-conditioned media-stimulated bone marrow cells from h2-calponin knock-out mice had an increased proliferation rate in culture as compared with h2-calponin expressing wild type cells. The effect of h2-calponin on macrophage proliferation was confirmed by comparing RAW cells lacking endogenous h2-calponin with that transfectively expressing h2-calponin (Fig. 7). The results demonstrated that consistent with the observation from smooth muscle cell studies, h2-calponin plays a role in regulating myeloid cell proliferation. Together with its effect on inhibiting macrophage migration (Fig. 6) and phagocytosis (Fig. 10), these results demonstrate that h2-calponin may play multiple roles in regulating macrophage function during the activation of macrophage responses.
The volume of actin cytoskeleton increases during monocyte-macrophage differentiation as shown by the significantly lower amount of histone detected in SDS gels in which the loadings were normalized by the amount of actin (Fig. 2A). This observation is consistent with the notion that the actin cytoskeleton is a critical component in the function of mature macrophages. Our data that removal or addition of h2-calponin can alter the proliferation, migration, and phagocytosis of macrophages suggest that calponin-mediated inhibition of actin cytoskeleton activity is a potential target for controlling macrophage function. For example, enhancing the inhibitory function of h2-calponin may provide a treatment for autoimmune diseases, whereas reducing the inhibition may strengthen immune defenses. We have previously demonstrated that h2-calponin gene expression and protein turnover are both regulated by mechanical tension built in the actin cytoskeleton by the myosin II motor depending on the mechanical stiffness of the substrates (3, 4). H2-calponin is known to increase the stability of the actin filaments (3). Therefore, the removal of h2-calponin from macrophages may make the actin cytoskeleton more dynamic, consistent with the increased macrophage migration and phagocytosis. On the other hand, it is anticipated that constitutive expression of h2-calponin or preventing h2-calponin degradation to increase the inhibition on actin-based cell motility might be able to suppress macrophage function for the treatment of autoimmune diseases.
A previous study showed that unregulated force expression of h2-calponin-enhanced cell migration and treatment of antisense h2-calponin RNA reduced chemotaxis in human umbilical vein endothelial cells (54). Therefore, h2-calponin may play different roles in chemotaxis and intrinsic motility (the present study) of different cells. Considering that rapidly remodeling smooth muscle tissues express higher levels of h2-calponin (18), these observations suggest that the capacity of calponin regulation needs is positively related to the degree of cytoskeleton activity. Therefore, a proper level of h2-calponin might be critical to maintain the physiological balance of cell motility, which could be different in different cell types and requires further investigation.
Possible Mechanism for the Regulation of Actin Cytoskeleton by H2-calponin—The removal of h2-calponin from macrophages did not alter the apparent structure of actin filaments in cultured monolayer cells (Fig. 8A), similar to that seen in fibroblasts from Cnn2 knock-out mice (data not shown) and SM3 smooth muscle cells lacking or force-expressing h2-calponin (18). Tropomyosin is an actin filament-associated protein highly conserved through evolution (48). Multiple isoforms of tropomyosin are present in non-muscle cells. Functional significance of the tropomyosin isoforms is not fully understood. A switching from high molecular weight to low molecular weight tropomyosin isoforms in tumor cells suggested functional significance in cellular phenotypes (55). It has been reported that macrophage intrinsically lacks the high molecular weight tropomyosin isoforms presumably related to its high mobility (47). We previously showed that cytomegalovirus promoter-directed overexpression of h2-calponin in a smooth muscle cell line that lacks endogenous calponin increased the level of tropomyosin corresponding to increased stability of the actin cytoskeleton (3). The effect of removing h2-calponin on decreasing the level of tropomyosin in macrophages (Fig. 9) suggests that h2-calponin may function together with tropomyosin to regulate macrophage cell motility. In the absence of h2-calpoin, the decrease in tropomyosin may destabilize the actin cytoskeleton, which might also contribute to the increased phagocytotic activity (Fig. 10). Nonetheless, the up-regulation of h2-calponin during monocyte-macrophage differentiation indicates that the inhibitory regulation by h2-calponin seems critical to physiologically controlled activity of macrophages.
In conclusion, our study revealed high levels of h2-calponinin in myeloid cells. Functional characterization of macrophages from h2-calponin knock-out mice showed a role of h2-calponin in inhibiting myeloid cell proliferation, migration, and phagocytosis. The role of h2-calponin in the function of monocytes, macrophages, and neutrophils demonstrates a novel mechanism in the regulation of myeloid cell activity in immune responses via the function of actin cytoskeleton. In addition, our investigation using a representative mobile non-muscle cell system provided valuable information for the role of calponin in regulating non-muscle cell motility that has broad physiological and pathological significance.
Acknowledgments
We thank Dr. Marcus Conrad for the pPNT4 vector, Prof. Jim Lin for providing the anti-tropomyosin mAbs, and Dr. Lynn Doglio, Northwestern University Transgenic and Targeted Mutagenesis Laboratory, for helpful consultations during the development of Cnn2-targeted mouse lines.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-086720 (to J.-P. J.) and AR-049217 and AR048269 (to R. M. P.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ES cell, embryonic stem cell; Cnn2, mouse h2-calponin gene; FBS, fetal bovine serum; FITC, fluorescein isothiocyanate; mAb, monoclonal antibody; PBS, phosphate-buffered saline; DMEM, Dulbecco's modified Eagle's medium; TRITC, tetramethylrhodamine isothiocyanate.
References
- 1.Jones, G. E. (2000) J. Leukocyte Biol. 68 593–602 [PubMed] [Google Scholar]
- 2.Takahashi, K., Hiwada, K., and Kobuku, T. (1986) Biochem. Biophys. Res. Commun. 141 20–26 [DOI] [PubMed] [Google Scholar]
- 3.Hossain, M. M., Crish, J. F., Eckert, R. L., Lin, J. J.-C., and Jin, J.-P. (2005) J. Biol. Chem. 280 42442–42453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hossain, M. M., Smith, P. G., Wu, K., and Jin, J.-P. (2006) Biochemistry 45 15670–15683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winder, S., and Walsh, M. P. (1990) J. Biol. Chem. 265 10148–10155 [PubMed] [Google Scholar]
- 6.Abe, M., Takahashi, K., and Hiwada, K. (1990) J. Biochem. (Tokyo) 108 835–838 [DOI] [PubMed] [Google Scholar]
- 7.Horiuchi, K. Y., and Chacko, S. (1991) Biochem. Biophys. Res. Commun. 176 1487–1493 [DOI] [PubMed] [Google Scholar]
- 8.Winder, S. J., Allen, B. G., Fraser, E. D., Kang, H.-M., Kargacin, G. J., and Walsh, M. P. (1993) Biochem. J. 296 827–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh, M. P. (1991) Biochem. Cell Biol. 69 771–800 [DOI] [PubMed] [Google Scholar]
- 10.Shirinsky, V. P., Birynkov, K. G., Hettasch, J. M., and Sellers, J. R. (1992) J. Biol. Chem. 267 15886–15892 [PubMed] [Google Scholar]
- 11.Haeberle, J. R. (1994) J. Biol. Chem. 269 12424–12431 [PubMed] [Google Scholar]
- 12.Allen, B. G., and Walsh, M. P. (1994) Trends Biochem. Sci. 19 362–368 [DOI] [PubMed] [Google Scholar]
- 13.Takahashi, K., and Nadal-Ginard, B. (1991) J. Biol. Chem. 266 13284–13288 [PubMed] [Google Scholar]
- 14.Nishida, W., Kitami, Y., and Hiwada, K. (1993) Gene (Amst.) 130 297–302 [DOI] [PubMed] [Google Scholar]
- 15.Strasser, P., Gimona, M., Moessler, H., Herzog, M., and Small, J. V. (1993) FEBS Lett. 330 13–18 [DOI] [PubMed] [Google Scholar]
- 16.Applegate, D., Feng, W., Green, R. S., and Taubman, M. B. (1994) J. Biol. Chem. 269 10683–10690 [PubMed] [Google Scholar]
- 17.Trabelsi-Terzidis, H., Fattoum, A., Represa, A., Dessi, F., Ben-Ari, Y., and der Terrossian, E. (1995) Biochem. J. 306 211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hossain, M. M., Hwang, D.-Y., Huang, Q.-Q., Sasaki, Y., and Jin, J.-P. (2003) Am. J. Physiol. 284 C156–C167 [DOI] [PubMed] [Google Scholar]
- 19.Thomas, K. R., and Capecchi, M. R. (1987) Cell 51 503–512 [DOI] [PubMed] [Google Scholar]
- 20.Gao, J., Hwang, J. M., and Jin, J.-P. (1996) Biochem. Biophys. Res. Commun. 218 292–297 [DOI] [PubMed] [Google Scholar]
- 21.Huang, Q.-Q., Chen, A., and Jin, J.-P. (1999) Gene (Amst.) 229 1–10 [DOI] [PubMed] [Google Scholar]
- 22.Conrad, M., Brielmeier, M., Wurst, W., and Bornkamm, G. W. (2003) BioTechniques 34 1136–1138, 1140 [DOI] [PubMed] [Google Scholar]
- 23.Meyers, E. N., Lewandoski, M., and Martin, G. R. (1998) Nat. Genet. 18 136–141 [DOI] [PubMed] [Google Scholar]
- 24.Nigam, R., Triggle, C. R., and Jin, J.-P. (1998) J. Muscle Res. Cell Motil. 19 695–703 [DOI] [PubMed] [Google Scholar]
- 25.Jin, J.-P., Walsh, M. P., Resek, M. E., and McMartin, G. A. (1996) Biochem. Cell Biol. 74 187–196 [DOI] [PubMed] [Google Scholar]
- 26.Warren, K. S., Lin, J. L., McDermott, J. P., and Lin, J. J. (1995) J. Cell Biol. 129 697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, Q.-Q., Ma, Y. Y., Adebayo, A., and Pope, R. M. (2007) Arthritis Rheum. 56 2192–2201 [DOI] [PubMed] [Google Scholar]
- 28.Liu, H., Perlman, H., Pagliari, L. J., and Pope, R. M. (2001) J. Exp. Med. 194 113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, H., Ma, Y., Cole, S. M., Zander, C., Chen, K. H., Karras, J., and Pope, R. M. (2003) Blood 102 344–352 [DOI] [PubMed] [Google Scholar]
- 30.Liu, H., Eksarko, P., Temkin, V., Haines, G. K., 3rd, Perlman, H., Koch, A. E., Thimmapaya, B., and Pope, R. M. (2005) J. Immunol. 175 8337–8345 [DOI] [PubMed] [Google Scholar]
- 31.Ma, Y., Liu, H., Tu-Rapp, H., Thiesen, H. J., Ibrahim, S. M., Cole, S. M., and Pope, R. M. (2004) Nat. Immunol. 5 380–387 [DOI] [PubMed] [Google Scholar]
- 32.Pagliari, L. J., Perlman, H., Liu, H., and Pope, R. M. (2000) Mol. Cell Biol. 20 8855–8865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perlman, H., Pagliari, L. J., Georganas, C., Mano, T., Walsh, K., and Pope, R. M. (1999) J. Exp. Med. 190 1679–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies, J. Q., and Gordon, S. (2005) in Basic Cell Culture Protocols (Helgason, C. D., and Miller, C. L., eds) Third Edition, Vol. 290, pp. 91–103, Humana Press Inc., Totowa, NJ [Google Scholar]
- 35.Raschke, W. C., Baird, S., Ralph, P., and Nakoinz, I. (1978) Cell 15 261–267 [DOI] [PubMed] [Google Scholar]
- 36.Lin, J. J.-C., Chou, C.-S., and Lin, J. L.-C. (1985) Hybridoma 4 223–242 [DOI] [PubMed] [Google Scholar]
- 37.Oda, T., and Maeda, H. (1986) J. Immunol. Methods 88 175–183 [DOI] [PubMed] [Google Scholar]
- 38.Ichinose, M., Asai, M., Imai, K., and Sawada, M. (1995) Immunopharmacology 30 217–224 [DOI] [PubMed] [Google Scholar]
- 39.Scott, R. S., McMahon, E. J., Pop, S. M., Reap, E. A., Caricchio, R., Cohen, P. L., Earp, H. S., and Matsushima, G. K. (2001) Nature 411 207–211 [DOI] [PubMed] [Google Scholar]
- 40.Aziz, A., Vanhille, L., Mohideen, P., Kelly, L. M., Otto, C., Bakri, Y., Mossadegh, N., Sarrazin, S., and Sieweke, M. H. (2006) Mol. Cell. Biol. 26 6808–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins, S. J., Ruscetti, F. W., Gallagher, R. E., and Gallo, R. C. (1978) Proc. Natl. Acad. Sci. U. S. A. 75 2458–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le, J., Reis, L. F., and Vilcek, J. (1988) Lymphokine Res. 7 99–106 [PubMed] [Google Scholar]
- 43.Yeh, M. Y., Hellström, I., Brown, J. P., Warner, G. A., Hansen, J. A., and Hellström, K. E. (1979) Proc. Natl. Acad. Sci. U. S. A. 76 2927–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tacke, F., and Randolph, G. J. (2006) Immunobiology 211 609–618 [DOI] [PubMed] [Google Scholar]
- 45.Gordon, S. (2007) Eur. J. Immunol. 37 S9–S17 [DOI] [PubMed] [Google Scholar]
- 46.Discher, D. E., Jammey, P., and Wang, Y. (2005) Science 310 1139–1143 [DOI] [PubMed] [Google Scholar]
- 47.Nakamura, Y., Sakiyama, S., and Takenaga, K. (1995) Cell Motil. Cytoskeleton 31 273–282 [DOI] [PubMed] [Google Scholar]
- 48.Lin, J. J., Warren, K. S., Wamboldt, D. D., Wang, T., and Lin, J. L. (1997) Int. Rev. Cytol. 170 1–38 [DOI] [PubMed] [Google Scholar]
- 49.Beningo, K. A., and Wang, Y. L. (2002) J. Cell Sci. 115 849–856 [DOI] [PubMed] [Google Scholar]
- 50.Wilkinson, P. C. (1982) Immunobiology 161 376–384 [DOI] [PubMed] [Google Scholar]
- 51.Wilkinson, P. C., and Allan, R. B., (1980) in Mononuclear Phagocytes, Functional Aspects (van Furth, I., ed) Part I, pp. 475–500, Martinus Nijhoff Publishers, Boston, MA
- 52.Liu, H., Bo, S., Huang, C.-C., Eksarko, P., and Pope, R. M. (2008) Immunol. Lett. 117 70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ley, K., Laudanna, C., Cybulsky, M. I., and Nourshargh, S. (2007) Nat. Rev. Immunol. 7 678–689 [DOI] [PubMed] [Google Scholar]
- 54.Tang, J., Hu, G., Hanai, J.-I., Yadlapalli, G., Lin, Y., Zhang, B., Galloway, J., Bahary, N., Sinha, S., Thisse, B., Thisse, C., Jin, J.-P., Zon, L. I., and Sukhatme, V. P. (2006) J. Biol. Chem. 281 6664–6672 [DOI] [PubMed] [Google Scholar]
- 55.Miyado, K., Kimura, M., and Taniguchi, S. (1996) Biochem. Biophys. Res. Commun. 225 427–435 [DOI] [PubMed] [Google Scholar]