Abstract
Somatic histone H1 reduces both the rate and extent of DNA replication in Xenopus egg extract. We show here that H1 inhibits replication directly by reducing the number of replication forks, but not the rate of fork progression, in Xenopus sperm nuclei. Density substitution experiments demonstrate that those forks that are active in H1 nuclei elongate to form large tracts of fully replicated DNA, indicating that inhibition is due to a reduction in the frequency of initiation and not the rate or extent of elongation. The observation that H1 dramatically reduces the number of replication foci in sperm nuclei supports this view. The establishment of replication competent DNA in egg extract requires the assembly of prereplication complexes (pre-RCs) on sperm chromatin. H1 reduces binding of the pre-RC proteins, XOrc2, XCdc6, and XMcm3, to chromatin. Replication competence can be restored in these nuclei, however, only under conditions that promote the loss of H1 from chromatin and licensing of the DNA. Thus, H1 inhibits replication in egg extract by preventing the assembly of pre-RCs on sperm chromatin, thereby reducing the frequency of initiation. These data raise the interesting possibility that H1 plays a role in regulating replication origin use during Xenopus development.
INTRODUCTION
After fertilization, Xenopus laevis eggs undergo rapid and synchronous cell divisions until the early embryos reach the midblastula transition (MBT). At the MBT, slower, asynchronous cell division cycles appear accompanied by the onset of transcription (Newport and Kirschner, 1982). In the early cleavage stages, S-phase lasts only about 15 min but lengthens substantially after the embryo reaches the MBT. This lengthening of S-phase is thought to result from a reduction in the number of active origins of replication (Hyrien and Mechali, 1993; Hyrien et al., 1995). The mechanisms governing origin use during development are at present unclear, although at least two hypotheses have been proposed. According to one view, the increase in the nucleo-cytoplasmic ratio that occurs during early embryogenesis limits an unidentified factor that controls origin use. Less factor would result in a reduction in the number of initiations and an increase in replicon size (Newport and Kirschner, 1982). Increasing nuclear concentration in Xenopus egg extract results in a lower frequency of initiation and an extended S-phase within individual nuclei, supporting this view (Dasso and Newport, 1990; Walter and Newport, 1997). The second hypothesis contends that higher-order chromatin structure, which is established during embryogenesis (Wolffe, 1989; Dimitrov et al., 1993; Micheli et al., 1993), restricts the binding of initiation proteins to the DNA, thereby limiting origin use (Stillman, 1996). Higher-order chromatin structure can determine site-specific initiation of replication within the dihydrofolate reductase gene locus in egg extract (Lawlis et al., 1996), although precisely how specific origins are selected in this system is not clear. It has been suggested that other chromosomal elements, such as enhancers, may be required for origin selection after the MBT (Stillman, 1996). This view is supported by the fact that initiations within transcription units of ribosomal DNA are specifically repressed while intergenic origins are apparently unaffected at the late blastula stage. This suggests that chromatin is actively remodeled to tightly coordinate DNA replication with transcription, both temporally and spatially throughout the cell cycle (Hyrien and Mechali, 1993; Hyrien et al., 1995).
It is well documented that establishment of higher-order chromatin structure during development is mediated primarily by programmed changes in individual chromatin components (Dimitrov et al., 1993; Bouvet, et al., 1994; Kandolf, 1994). This developmental course can be reversed, however, by introducing nuclei from differentiated cells into Xenopus eggs (Gurdon and Uehlinger, 1966) or egg extracts (Dimitrov and Wolffe, 1996). These remodeled nuclei resemble embryonic nuclei both structurally and functionally. As an important structural and functional organizer of chromatin, individual linker histone variants are expressed under developmental control (reviewed by Khochbin and Wolffe, 1994). Xenopus cleavage-stage linker histone variant B4 (H1 M) appears late in oogenesis and persists in early embryonic chromatin through the MBT when somatic-type H1s gradually accumulate. By the end of gastrulation, somatic-type H1 has completely replaced B4 within embryonic chromatin (Dimitrov et al., 1993; Hock et al., 1993; Dworkin-Rastl et al., 1994). There is substantial evidence supporting the view that differential expression of linker histone genes during development may have important functional significance. The linker histone B4 and HMG1, another chromosomal protein found in early embryonic chromatin, bind to nucleosomal core and linker DNA at much lower affinity than somatic H1 variants and have been implicated in the genesis of more dynamic and extended embryonic chromatin to facilitate the exceptionally rapid early embryonic cell cycles (Dimitrov et al., 1994; Nightingale et al., 1996; Ura et al., 1996). Replacement of B4 and HMG1 with somatic linker histones fixes the position of nucleosomes on the DNA (Ura et al., 1995) and leads to chromatin compaction and the formation of higher-order structure (Wolffe, 1989). Such chromatin specifically represses 5S rRNA gene transcription by reducing accessibility of the oocyte genes to transcription factors (Chipev and Wolffe, 1992; Bouvet et al., 1994; Kandolf, 1994). Furthermore, recent work has shown that somatic linker histones cause the loss of mesodermal competence in Xenopus by the selective repression of regulatory genes required for mesodermal differentiation pathways (Steinbach et al., 1997). Alternatively, replacement of the somatic H1s with B4 and HMG1 reverses the repressive effects of the somatic H1s, thereby promoting transcriptional competence in nuclei from terminally differentiated cells (Dimitrov and Wolffe, 1996). Collectively, these data suggest that H1 variants can exert dominant effects on nuclear activity, such as transcription, by modulating chromatin structure during development.
What role, if any, the cleavage stage linker B4 and somatic H1s play in regulating DNA replication during Xenopus development is not clear. Differential effects of H1 variants on S-phase progression have been reported in avian and mammalian somatic cells (Bergman et al., 1988; Sun et al., 1989; Brown et al., 1996), although a direct role for H1s in regulating DNA replication in these systems has not been established. We have recently developed a system to determine the effects of somatic H1 variants on nuclear assembly and DNA replication using cell-free extracts derived from Xenopus eggs (Lu et al., 1997). Somatic H1s added to the extract are correctly assembled onto sperm chromatin, thereby preserving normal nucleosomal organization but promoting the formation of higher-order chromatin structure. H1-containing chromatin in the extract resembles somatic-type chromatin, both structurally and functionally, in many ways (Shimamura et al., 1989; Wolffe, 1989; Chipev and Wolffe, 1992). Under these conditions, H1 delays the initiation of replication within individual nuclei, at least in part, by slowing assembly of the nuclear lamina. However, H1 inhibits replication even when lamina assembly is complete, suggesting that H1 may also affect replication directly (Lu et al., 1997).
Somatic H1 could inhibit DNA replication in egg extract in at least three ways. First, it could reduce replication competence by limiting the assembly of prereplication complexes (pre-RCs) on sperm chromatin. The pre-RC is formed by the sequential binding of origin recognition complex (ORC) proteins, XCdc6, and the minichromosome maintenance (MCM) proteins to chromatin and is required for initiation of replication (Carpenter et al., 1996; Coleman et al., 1996; Romanowski et al., 1996; Rowles et al., 1996; for review see Romanowski and Madine 1996, 1997; and Stillman, 1996). Second, H1 could inhibit initiation through mechanisms that prevent cyclin-cdk activation. In this case, pre-RCs may be formed but they would remain inactive. Third, H1 could inhibit replication elongation, thereby arresting replication forks shortly after initiation.
In this report we have investigated the mechanism by which somatic H1 inhibits replication of sperm nuclei in egg extract. We find that H1 reduces the number of replication forks, but not the rate of fork progression, in preassembled Xenopus sperm nuclei. H1 does not inhibit second-strand synthesis on single-strand M13 DNA, which confirms that replication elongation is unaffected by this somatic linker histone. H1 reduces the assembly of pre-RCs on sperm chromatin and the number of replication foci after initiation. Replication competence is restored in these nuclei only under conditions that promote the loss of H1 from chromatin and licensing of the DNA. Thus, H1 inhibits replication in the extract by preventing pre-RC assembly on sperm chromatin and thereby reducing the frequency of initiation. These data raise the interesting possibility that H1 may play a role in regulating replication origin use during development.
MATERIALS AND METHODS
Purification of Histone H1
Histone H1c was purified from its overexpressing mouse cell line as described by Lu et al. (1997). Purity of isolated H1 was verified by silver staining of SDS-polyacrylamide gels. An extinction coefficient of 18.76 ml·mg−1·cm−1 at 210 nm was used to determine the protein concentration of each sample.
Preparation of Xenopus Egg Extracts and Permeable Sperm Nuclei
Metaphase-arrested and interphase extracts were prepared from Xenopus laevis eggs as previously described (Blow, 1993; Lu et al., 1997). For preparation of 6-dimethylaminopurine (6-DMAP)-treated interphase extract, metaphase extract was supplemented with 3 mM 6-DMAP, and then mixed with 0.3 mM CaCl2. Permeable Xenopus sperm nuclei (“sperm chromatin”) were prepared according to Lu et al. (1997).
In Vitro DNA Replication
Sperm chromatin was incubated at 1–3 ng DNA/μl extract supplemented with an energy-regenerating system (Blow and Laskey, 1986), 100 μg/ml cycloheximide, 2 mM ATP, and 5.68 μM histone H1c or an equivalent volume of water. The amount of H1 added to extract is comparable to the estimated amount of somatic linker histone present in a Xenopus embryo at the gastrula–neurula transition, i.e., >45 ng/embryo (Dworkin-Rastl et al., 1994). Deoxynucleoside triphosphates were added to a final concentration of 50 μM to readjust pool sizes after dilution (Blow and Laskey, 1986). All incubations were performed at 22°C. The extent of extract dilution was kept constant at 30% for all experiments. M13 replication was performed essentially as described above except that 10 ng M13 mp19 phage (GIBCO/BRL, Gaithersburg, MD) was used per microliter of extract. Nascent DNA was detected by adding 100 μCi/ml [α-32P]deoxy-ATP (dATP) or 20 μM 5-biotin-16-deoxyuridine triphosphate (Biotin-dUTP; Boehringer Mannheim, Indianapolis, IN) to the extract. Quantitation of DNA replication was carried out as previously described (Lu et al., 1997).
For nuclear transfer, samples were diluted with 50-fold ice-cold buffer A and underlayed with fresh extract. Diluted nuclei were centrifuged into fresh extract at 770 g for 10 min at 4°C in a Jouan CR4–22 swing-out centrifuge. The supernatant was carefully removed, and the remaining extract was gently mixed to promote suspension of the chromatin or nuclei. Samples were then incubated at 22°C for various times at indicated in Figure 7.
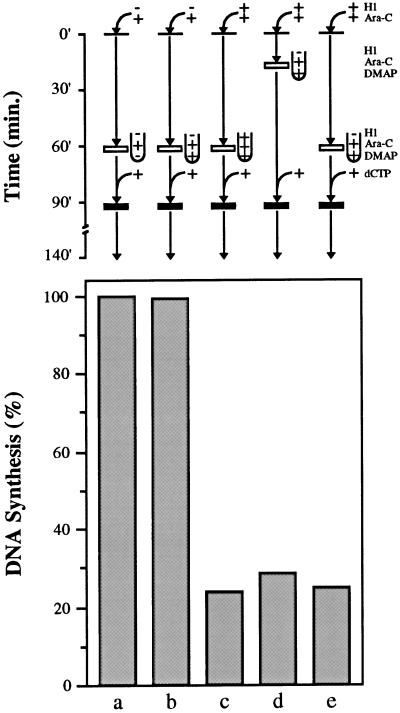
Figure 7.
H1 nuclei do not replicate in H1-free extract that lacks licensing activity. Sperm chromatin was incubated in extract in the presence of [α-32P]dATP and Ara-CTP either without H1 (lanes a and b) or with H1 (lanes c, d, and e) for 20 (lane d) or 60 min (lanes a, b, c, and e). Sperm chromatin (lane d) or nuclei (lanes a, b, c, and e) were then transferred to untreated extract without H1 (lane a), to 6-DMAP extract without H1 (lanes b, d, and e) or to 6-DMAP extract with H1 (lane c), all containing Ara-CTP. After 90 min, all samples were released from Ara-CTP arrest with dCTP and incubated for an additional 50 min. DNA replication is expressed as a percentage of the control sample (lane a).
Cytosine β-d-arabinofuranoside 5′-triphosphate (Ara-CTP) was added to egg extract to a final concentration of 200 μM. Stalled forks were released by addition of deoxycytosine triphosphate (dCTP) to a final concentration of 1 mM. Density substitution experiments were performed essentially as described by Leno and Munshi (1994). Sperm chromatin was incubated at 1 ng DNA/μl extract supplemented with 0.25 mM bromodeoxyuridine triphosphate (BrdUTP) and 100 μCi/ml [α-32P]dATP for 3 h at 22°C. Samples were stopped by digesting with stop mix C containing 500 μg/ml proteinase K for 1 h at 37°C. The DNA was extracted three times with phenol-chloroform and separated by centrifugation to equilibrium in a cesium chloride gradient. Individual fractions were collected, and the refractive index of every fifth sample was determined. All fractions were counted by liquid scintillation.
Immunofluorescence Microscopy
Control and H1 samples were diluted 50-fold with ice-cold buffer A (Leno and Laskey, 1991), spun through 30% sucrose in buffer A onto polylysine-coated coverslips, and incubated in buffer A containing 0.5% Triton X-100 for 10 min. Samples were rinsed in PBS, fixed in PBS containing 4% paraformaldehyde for 10 min, and blocked for 10 min in PF buffer (0.75×PBS, 0.1% Triton X-100, 0.02% SDS, 2% BSA) containing 10% FCS. Coverslips were incubated in primary antibody in PF buffer for 1 h. Affinity-purified antibodies recognizing XOrc2, XCdc6, and Mcm3 were used at a dilution of 1:500, 1:50, and 1:1000, respectively. The coverslips were then washed twice in PF buffer, incubated with fluorescein-conjugated donkey anti-rabbit secondary antibody (Amersham, Arlington Heights, IL) for 1 h, and finally washed twice in PF buffer. Bulk DNA was visualized with Hoechst 33258 (Sigma, St. Louis, MO). Incorporated biotin-dUTP was detected with Texas-red streptavidin (Lu et al., 1997). Samples were viewed with an Odyssey laser confocal microscope (Noran Instruments, Middleton, WI).
Alkaline and Neutral Agarose Gel Electrophoresis
Alkaline agarose gel electrophoresis was performed as described previously (Mahbubani et al., 1997; Walter and Newport, 1997) with minor modifications. Replication reactions were mixed with 500 μl of ice-cold buffer A, and nuclei were pelleted by centrifugation at 770 × g for 5 min at 4°C in a Juan CR4–22 swing-out centrifuge. Pellets were digested with stop mix N (20 mM Tris-HCl, 200 mM NaCl, 5 mM EDTA, 0.5% SDS, pH 8.0) supplemented with 2 μg/ml RNase A and 200 μg/ml Proteinase K for 1 h at 37°C. DNA was extracted twice with phenol-chloroform, then with chloroform, and then precipitated with ethanol. Dried samples were dissolved in alkaline gel loading buffer (50 mM NaOH, 1 mM EDTA, 1.25% Ficoll, 0.0125% bromocresol green) and separated on a 0.8% alkaline gel as described by Mahbubani et al. (1997). Autoradiographs were scanned as previously described (Lu et al., 1997).
Neutral agarose gel electrophoresis was performed as described by Jackson et al. (1995). Briefly, replication reactions were incubated with stop mix C containing 0.5 mg/ml Proteinase K for 1 h at 37°C. DNA was then extracted twice with phenol-chloroform, then with chloroform, and precipitated with ethanol. Dried samples were dissolved in Tris-EDTA buffer containing 1 μg/μl RNase A and incubated at 37°C for 1 h. DNA fragments were resolved on a 1.0% nusieve agarose gel (FMC BioProducts, Rockland, ME) using a 1×Tris-borate-EDTA buffer system run at 6 V/cm.
RESULTS
H1 Reduces the Rate and Extent of DNA Replication but Does Not Affect the Length of S-Phase in Egg Extract
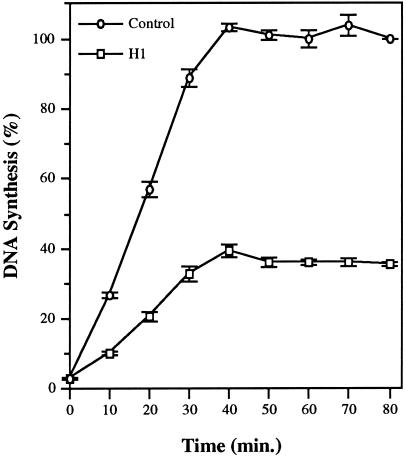
Somatic histone H1 slows assembly of the nuclear lamina and delays the initiation of DNA replication within individual sperm nuclei in Xenopus egg extract (Lu et al., 1997). This H1-induced delay in lamina assembly is responsible, at least in part, for the delay in initiation because preassembled H1 nuclei initiate replication at the same time as control nuclei. However, H1 inhibits replication even when lamina assembly is complete, suggesting that H1 modulates replication through multiple pathways in egg extract (Lu et al., 1997). As an initial step toward determining the mechanism by which H1 inhibits replication of Xenopus sperm nuclei in egg extract, we have used the elongation inhibitor Ara-CTP, a dCTP analog (Cozzarelli, 1977), to arrest nuclei early in S-phase and subsequently determine the effects of H1 on S-phase progression following release from inhibition. Specifically, sperm chromatin was incubated in egg extract supplemented with Ara-CTP and [α-32P]dATP either without (Control) or with histone H1 (H1) for 90 min. In parallel experiments, >95% of both control and H1 nuclei initiated replication during this initial incubation period, as judged by the incorporation of biotin-dUTP into nascent DNA (our unpublished observations). Stalled replication forks were then released by the addition of excess dCTP to the extract, and the extent of replication was measured by incorporation of radioactivity into acid-insoluble material at various times. The results from three separate experiments in which three different extracts were used are shown in Figure 1. Control nuclei completed replication 40 min after release with dCTP; however, the rate of elongation after release is approximately 2.25-fold lower than that observed in the absence of Ara-CTP (Walter and Newport, 1997) and, therefore, the actual duration of S-phase in our extracts, in the absence of Ara-CTP, is approximately 18 min. This is very similar to the length of S-phase in the embryo before the MBT (i.e., from 10 to 15 min; Hyrien and Mechali, 1993). The rate of replication in H1 nuclei is markedly reduced relative to control nuclei, and the extent of synthesis after release from elongation arrest is only 40% of the input DNA. Interestingly, the presence of H1 on sperm chromatin does not increase the length of S-phase in the extract. Thus, H1 reduces both the rate and extent of DNA replication without affecting the length of S-phase in egg extract.
Figure 1.
H1 reduces the rate and extent of DNA replication but does not affect the length of S-phase in egg extract. Xenopus sperm chromatin was incubated for 90 min at 1 ng DNA/μl egg extract supplemented with 200 μM Ara-CTP, [α-32P]dATP, and with histone H1 (H1) or without H1 (Control). Parallel experiments showed that >95% of both control and H1 nuclei initiated replication during this initial incubation period, as judged by the incorporation of biotin-dUTP into nascent DNA (our unpublished observations). Stalled replication forks were then released by addition of dCTP to a final concentration of 1 mM, and the extent of DNA synthesis was determined at 10-min intervals up to 80 min. DNA replication is expressed as a percentage of the control sample at 80 min. Shown are the mean values, along with the SEM, from three separate experiments in which three different extracts were used.
The amount of H1 added to extract in these experiments is comparable to the estimated amount of somatic linker histones present in a Xenopus embryo at the gastrula–neurula transition stage, i.e., >45 ng/embryo (Dworkin-Rastl et al., 1994). However, the final concentration of H1 in the extract is in excess of the final concentration of DNA (5.7 μM H1 vs. ∼20 nM DNA in nucleosome lengths). Addition of excess H1 is necessary for its assembly onto sperm chromatin, presumably because of the high concentration of nucleoplasmin and RNA in the extract. The molecular chaperone nucleoplasmin removes somatic H1 variants from somatic chromatin (Dimitrov and Wolffe, 1996) whereas RNA has been shown to compete with DNA for the binding of somatic H1 (Halmer and Gruss, 1995). However, this excess of H1 does not cause large-scale precipitation or aggregation of sperm chromatin since normal nucleosomal organization is preserved (Lu et al., 1997) and DNA replication does take place in H1 chromatin (Figure 1).
H1 Reduces the Frequency of Initiation but Not the Rate of Elongation in Egg Extract
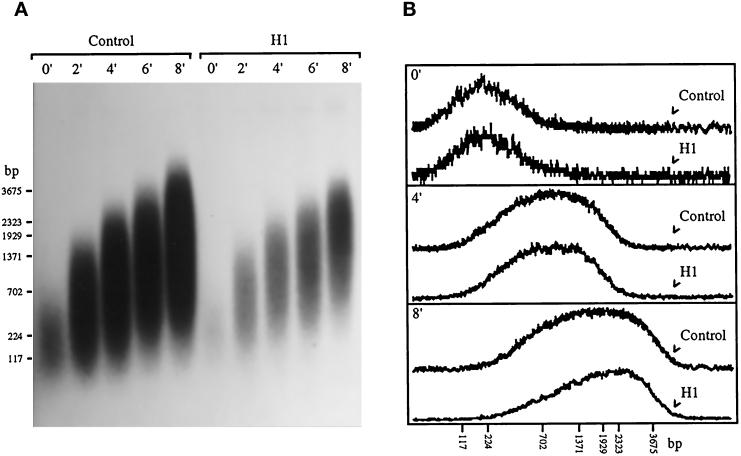
H1 could reduce the rate of DNA replication by reducing the number of active replication forks or by reducing the rate at which individual forks elongate, although the latter seems less likely given the fact that H1 does not increase the length of S-phase. To determine which of these two possibilities is correct, we incubated sperm chromatin in egg extract under the conditions described in Figure 1 and collected samples, at 2-min intervals, after release with dCTP. Nascent DNA was then resolved by alkaline agarose gel electrophoresis, and the resultant gel was subjected to autoradiography (Figure 2A). The relative amount of radioactivity in the center of each lane was determined by densitometry, and these values were plotted as a function of molecular size in kilobases (kb). Data from samples collected immediately after dCTP addition to extract (0′), and after a 4-min (4′) and 8-min (8′) release are shown in Figure 2B. The amplitudes of the control samples on the graph were reduced to facilitate comparison with the H1 samples.
Figure 2.
H1 reduces the number of replication forks in sperm nuclei but not the rate of fork movement. (A) Xenopus sperm chromatin was incubated for 90 min at 1 ng DNA/μl egg extract supplemented with Ara-CTP, [α-32P]dATP, and histone H1 (H1) or without H1 (Control). Stalled replication forks were then released by addition of dCTP to a final concentration of 1 mM, and samples were collected at 2-min intervals for up to 8 min. The purified 32P-labeled replication products were resolved on a 0.8% alkaline agarose gel that was subjected to autoradiography. The positions of DNA molecular mass markers are indicated. (B) The relative amount of radioactivity in the center of each lane in panel A was determined by densitometry, and these values were plotted as a function of molecular size in kilobases (kb). Data from samples collected immediately after dCTP addition to extract (0′), and after a 4-min (4′) and 8-min (8′) release are shown. The amplitudes of the control samples on the graph were reduced to facilitate comparison with the H1 samples.
The rate of replication fork movement, calculated to be approximately 7 ± 1 nucleotides per second, was virtually identical in both control and H1 nuclei (Figure 2B). This value is in close agreement with the fork rates previously reported for Xenopus embryos, egg extracts, and Xenopus somatic cells, i.e., 8–10 ± 2 nucleotides per second (Callan, 1972; Mahbubani et al., 1992; Hyrien and Mechali, 1993). The fact that H1 does not alter the rate of fork progression argues that the reduction in the rate of DNA replication that we observe (Figure 1) is due to a reduction in the number of replication forks in sperm nuclei (Figure 2A). The relative amount of radioactivity incorporated into nascent DNA in this experiment was determined by excising and combining all control or H1 lanes from the agarose gel after autoradiography (Figure 2A) and counting the samples by liquid scintillation. Incorporation in the H1 sample was ∼30% of that observed in the control sample, indicating that ∼70% fewer replication forks are active in H1 nuclei than in control nuclei. Within individual sperm nuclei, all initiation events occur synchronously, at the beginning of S-phase, in egg extract (Blow and Watson, 1987). The fact that H1 does not increase the length of S-phase in our experiments (Figure 1) suggests that all active origins in H1 nuclei also fire in synchrony with one another. Furthermore, this synchrony of initiation coupled with a virtually identical rate of elongation (Figure 2B) argues that the average size of replicons in H1 nuclei are the same as that in control nuclei. Indeed, we estimate the average replicon size in both control and H1 nuclei to be approximately 15 kb, within the range determined previously for replication in Xenopus eggs and egg extracts, i.e., 7–20 kb (Mahbubani et al., 1992; Hyrien and Mechali, 1993; Walter and Newport, 1997).
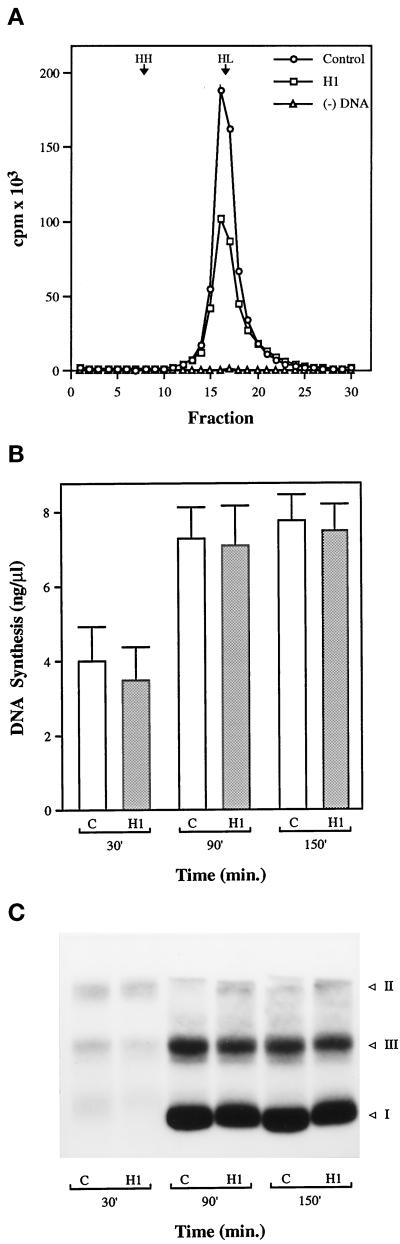
When nascent DNA from both control and H1 nuclei is substituted with the thymidine analog BrdUTP and labeled with [α-32P]dATP, a single peak of radioactivity is observed at a density of ∼1.75 g/ml after centrifugation to equilibrium in a cesium chloride gradient (Figure 3A). The appearance of a single heavy/light (HL) peak demonstrates that the DNA in H1 nuclei undergoes a single round of semiconservative replication in the extract. Thus, those origins that do fire in the presence of H1 produce forks that elongate to form large tracts of fully replicated DNA, supporting the view that H1 inhibits initiation, but not elongation, of replication in the extract. Second-strand synthesis on single-strand (ss) M13 DNA mimics replication elongation on double-strand DNA after replication origins unwind to form single-stranded replication bubbles (Mechali and Harland, 1982; Jackson et al., 1995). Although it might not be expected a priori that H1 would influence second-strand synthesis on ss M13, we nevertheless tested this possibility by incubating M13 DNA in egg extract, with or without H1, for up to 150 min in the presence of [α-32P]dATP. The extent of DNA synthesis, from three separate experiments in which three different extracts were used, is shown in Figure 3B. Virtually no differences in the rate or extent of DNA synthesis were observed between control and H1-containing extract. Nascent DNA was purified from the samples shown in Figure 3B and resolved by neutral agarose gel electrophoresis, after which the resultant gels were subjected to autoradiography. A typical autoradiograph is shown in Figure 3C. Virtually identical replication products, including double-stranded supercoiled (I), double-stranded circular relaxed (II), and double-stranded linear (III) forms of DNA, were observed in both control and H1 samples. Taken together, these data indicate that H1 does not inhibit replication elongation, but rather reduces the number of active replication forks in sperm nuclei, possibly by inhibiting a step that precedes the unwinding of replication origins.
Figure 3.
Histone H1 does not inhibit replication elongation. (A) Sperm chromatin was incubated in egg extract containing BrdUTP, [α-32P]dATP, and H1 (H1), or without H1 (Control), for 3 h. A separate sample without sperm chromatin was included as a control (−DNA). DNA was purified from each sample and centrifuged to equilibrium in a cesium chloride gradient. Fractions from each sample were analyzed by liquid scintillation, and the counts per min (cpm) were determined. The expected densities of heavy-light (HL, 1.75 g/ml) and heavy-heavy (HH, 1.79 g/ml) DNA are indicated. (B) Single-stranded M13 DNA was incubated at 10 ng/μl in control extract (lane C) or extract containing H1 (lane H1) supplemented with [α-32P]dATP for various times as indicated. DNA replication is expressed as nanograms of DNA synthesized per microliter of extract. Shown are the mean values along with the SEM from three separate experiments in which three different extracts were used. (C) DNA from an experiment shown in panel B was purified and resolved on a 1% neutral agarose gel, which was then subjected to autoradiography. The position of different forms of double-stranded DNA are indicated: form I, double-stranded supercoiled; form II, double-stranded circular relaxed; form III, double-stranded linear.
Histone H1 Reduces the Number of Replication Foci in Sperm Nuclei
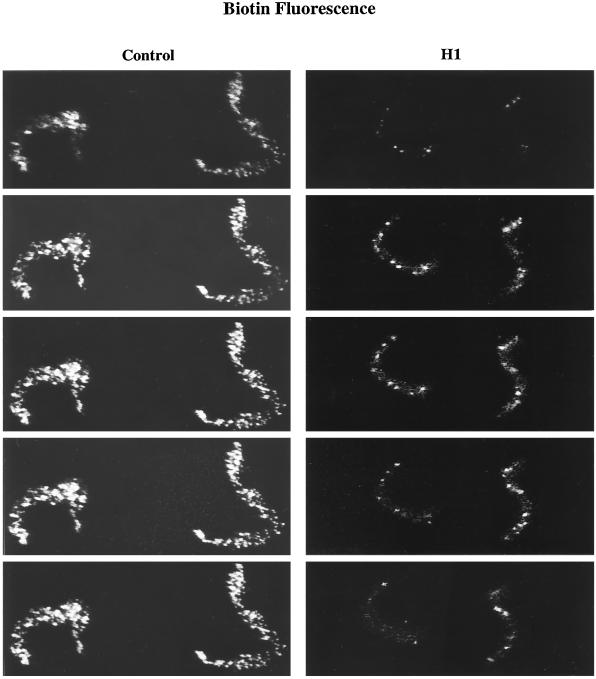
Replication forks are clustered in discrete sites or foci, uniformly distributed throughout nuclei replicating in Xenopus egg extract (Mills et al., 1989; Leno and Laskey, 1991; Cox and Laskey, 1991). It has been estimated that there are from 300 to 1000 forks clustered within each replication focus and from 100 to 300 foci within each sperm nucleus (Mills et al., 1989). Our data suggest that H1 may reduce the number of replication forks in sperm nuclei by as much as 70% and, therefore, we sought to determine whether H1 also reduces the number of replication foci in these nuclei. To visualize replication foci, control (Control) and H1 (H1) chromatin was incubated with biotin-dUTP for 3 h in egg extract treated with the protein kinase inhibitor 6-DMAP. Even though 6-DMAP inhibits most initiation events in unlicensed sperm nuclei (Blow, 1993; Mahbubani et al., 1997; Munshi and Leno, 1998), those origins that escape inhibition are distributed in approximately the same number of foci present throughout nuclei incubated in extract without kinase inhibition (Munshi and Leno, 1998). Furthermore, replication foci were more easily resolved, microscopically, within nuclei incubated in 6-DMAP-treated extract than in untreated extract, presumably because of the reduced number of initiations within each focus in 6-DMAP extract (Munshi and Leno, 1998). Figure 4 shows a confocal series of optical sections taken at 0.5-μm intervals through two control nuclei and two H1 nuclei stained with Texas-Red streptavidin. H1 nuclei are highly condensed (Lu et al., 1997) and consistently contained far fewer replication foci than control nuclei. Taken together, these data demonstrate that H1 reduces the number of replication forks and the number of replication foci within sperm nuclei incubated in egg extract.
Figure 4.
H1 reduces the number of replication foci in sperm nuclei. Metaphase-arrested egg extracts were treated with the protein kinase inhibitor 6-DMAP and released into interphase by Ca2+ addition. Sperm chromatin was then added to released extract supplemented with biotin-dUTP and H1 (H1) or without H1 (Control) and incubated for 3 h. A confocal series of optical sections taken at 0.5-μm intervals through two control nuclei and two H1 nuclei stained with Texas-Red streptavidin are shown.
Replication protein A (RP-A) is localized within foci on sperm chromatin incubated in egg extract (Adachi and Laemmli, 1992). These RP-A–containing foci are thought to be the precursors of replication foci in this system (Adachi and Laemmli, 1992, 1994; Yan and Newport, 1995). We found that H1 reduces the number of RP-A–containing foci in untreated extract (our unpublished observations) to vitrually the same extent as it reduces replication foci in 6-DMAP–treated extract (Figure 4), indicating that H1 limits both prereplication and replication focus formation on sperm chromatin.
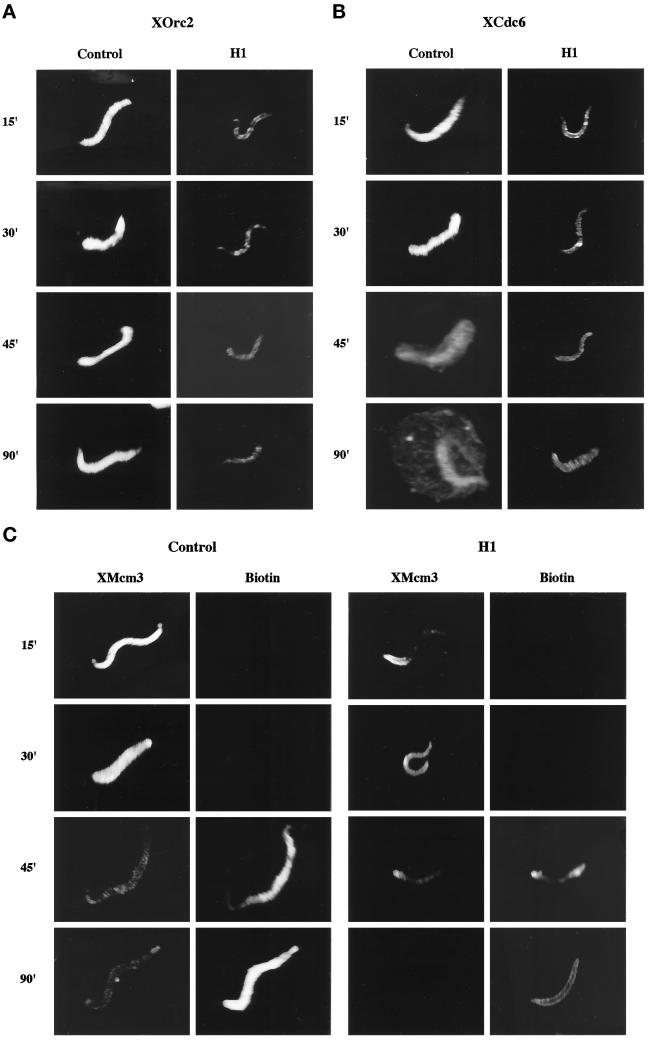
H1 Restricts Prereplication Complex Assembly on Sperm Chromatin
A reduction in the number of replication forks implies a reduction in the number of active replication origins. One way in which H1 could restrict origin use in the extract is by preventing the formation of pre-RCs on sperm chromatin. The pre-RC is formed by the sequential binding of ORC proteins, Cdc6, and the MCM proteins to chromatin (Carpenter et al., 1996; Coleman et al., 1996; Romanowski et al., 1996; Rowles et al., 1996), and it has been shown that assembly of this complex is essential for establishing replication-competent DNA ( Chong et al., 1995; Kubota et al., 1995; Madine et al., 1995a,b; Coleman et al., 1996; Romanowski et al., 1996). To determine whether H1 affects pre-RC assembly, sperm chromatin was incubated in egg extract supplemented with biotin-dUTP, with H1 (H1), or without H1 (Control), for up to 90 min. The nuclei from each sample were then probed with Texas-Red streptavidin, to detect nascent DNA, and with antibodies to three components of the pre-RC, namely, XOrc2 (Figure 5A), XCdc6 (Figure 5B), or XMcm3 (Figure 5C). All three associated with sperm chromatin within 15 min in the control sample (Figure 5, A–C, Control 15′). By 45 min, most control nuclei had entered S-phase (Figure 5C, Control Biotin 45′), which was accompanied by the apparent redistribution of XCdc6 to the nuclear envelope (Figure 5B, Control 45′) and the loss of XMcm3 from the chromatin (Figure 5C, Control 45′) consistent with previous reports (Carpenter et al., 1996; Coleman et al., 1996; Romanowski et al., 1996; Rowles et al., 1996). The presence of H1 dramatically reduced the association of all three pre-RC proteins with sperm chromatin after 15 min in the extract (Figure 5, A–C, H1 15′). Even after 30 min, when nuclear assembly was complete, the levels of XOrc2, XCdc6, and XMcm3 were still markedly reduced in H1 nuclei (Figure 5, A–C, H1 30′). This difference in pre-RC protein levels between control and H1 nuclei was confirmed by Western blot, although XMcm3 levels were higher than expected in both control and H1 nuclei at all time points examined (our unpublished observations). H1 did not inhibit replication completely, nor did it prevent the loss of XMcm3 from sperm chromatin after S-phase entry (Figure 5C, H1 XMcm3 45′ and 90′) demonstrating that pre-RCs assembled in the presence of H1 are functional. These data raise the interesting possibility that H1 limits origin use, and the extent of DNA replication, by restricting the assembly of pre-RCs on sperm chromatin.
Figure 5.
H1 restricts the assembly of prereplication complexes on sperm chromatin. Xenopus sperm chromatin was incubated at 1 ng DNA/μl in extract containing biotinylated dUTP and H1 (H1) or without H1 (Control) for up to 90 min. After 15, 30, 45, and 90 min in extract, the samples were processed as described in MATERIALS AND METHODS. XOrc2 (A), XCdc6 (B), and XMcm3 (C) were visualized by indirect immunofluorescence, and biotin incorporation was detected with fluorescent streptavidin (C). Samples were examined by confocal microscopy.
Restricting Pre-RC Formation Limits the Replication Capacity of H1 Nuclei
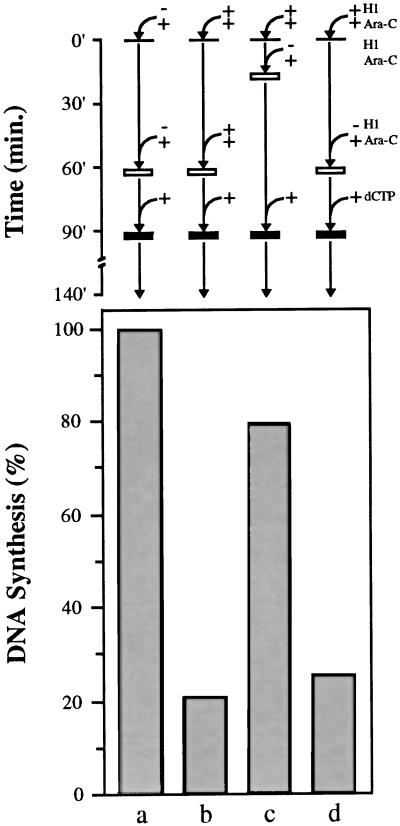
H1 restricts the assembly of pre-RCs on sperm chromatin by egg extract (Figure 5, A–C). In light of the essential role that pre-RCs play in establishing replication competence in this system, we sought to determine whether reduced pre-RC formation is responsible for the observed reduction in DNA replication in H1 nuclei (Figure 1). To answer this question, we carried out experiments as shown in Figure 6. Sperm chromatin was incubated in egg extract supplemented with Ara-CTP and [α-32P]dATP without H1 (lane a) or with H1 (lanes b–d). After 20 min (lane c) or 60 min (lanes a, b, and d) in extract, four volumes of extract containing Ara-CTP and [α-32P]dATP without H1 (lanes a, c, and d), or with H1 (lane b) were added. After a total incubation time of 90 min, dCTP was added to all samples, which were then incubated for an additional 50 min. Control nuclei replicated completely under these experimental conditions (lane a) whereas replication was reduced nearly 5-fold in nuclei containing H1 (lane b) consistent with our earlier results (Figures 1 and 2A). However, replication was restored to ∼80% of control levels when H1 chromatin was exposed to fresh H1-free extract after an initial 20-min incubation (lane c). H1 is rapidly lost from sperm chromatin after dilution with extract lacking H1 (Lu et al., 1997), demonstrating that loss of H1 from chromatin is associated with the acquisition of replication competence (lane c). If H1 chromatin is incubated in the extract for 60 min before dilution with H1-free extract, however, replication is not restored (lane d), demonstrating that the timing of extract addition is critical for establishing replication competence in H1 nuclei.
Figure 6.
H1 nuclei replicate when diluted with fresh extract if fresh extract lacks H1 and dilution occurs before nuclear envelope assembly. Sperm chromatin was incubated in extract supplemented with [α-32P]dATP and Ara-CTP either without H1 (lane a) or with H1 (lanes b, c, and d) for 20 (lane c) or 60 min (lanes a, b, and d). Four volumes of fresh extract supplemented with [α-32P]dATP and Ara-CTP either without H1 (lanes a, c, and d) or with H1 (lane b) were then added. By 90 min, all samples were released from Ara-CTP arrest with dCTP and incubated for an additional 50 min. DNA replication is expressed as a percentage of the control sample (lane a).
Under the experimental conditions described here, sperm chromatin does not possess an intact nuclear envelope after 20 min in the extract; however, by 60 min, envelope assembly is complete in virtually all nuclei (our unpublished observations). Therefore, it is possible that an intact nuclear envelope prevents the restoration of replication competence in H1 nuclei. One way in which an intact nuclear envelope can prevent replication in egg extract is by preventing the licensing of unlicensed DNA, i.e., DNA that lacks essential components of the pre-RC (Blow, 1993; Kubota et al., 1995; Madine et al., 1995b; Romanowski et al., 1996). We have already established that H1 reduces the extent to which one licensing protein, namely XMcm3, binds to sperm chromatin (Figure 5C); however, it is not clear whether reduced levels of XMcm3, or other pre-RC proteins, contribute to the reduction in replication capacity that we observe in H1 nuclei (Figure 1). To investigate this possibility, we performed experiments essentially as described in Figure 6, except that control and H1 chromatin (20 min) or nuclei (60 min) were transferred to fresh extracts treated with 6-DMAP (Figure 7). Metaphase-arrested egg extracts treated with 6-DMAP and subsequently released into interphase are incapable of completely replicating nuclei with unlicensed DNA, but these extracts are fully capable of replicating nuclei with licensed DNA (Blow, 1993; Chong et al., 1995; Mahbubani et al., 1997). In theory, if the H1-induced reduction in pre-RC assembly is responsible for the inhibition of replication in egg extract, and if an intact nuclear envelope prevents complete pre-RC assembly on DNA by H1-free extract, then replication should be inhibited in 6-DMAP extract even if H1 chromatin is transferred before nuclear envelope assembly is complete. On the other hand, if H1 chromatin contains sufficient pre-RCs for complete replication of all sperm DNA, and other features associated with an intact nuclear envelope prevent replication of H1 nuclei (Figure 6, lane d), then H1 chromatin should replicate when the chromatin is transferred before nuclear envelope assembly even in 6-DMAP extracts. Our results clearly show that replication is inhibited in 6-DMAP extract even when H1 chromatin is transferred to H1-free extract before nuclear envelope assembly (Figure 7, lane d). Thus, when H1 chromatin is transferred to H1-free extract, replication will only occur if that extract is capable of assembling complete pre-RCs on the DNA (compare Figure 7, lane d, with Figure 6, lane c). Taken together, these data indicate that H1 inhibits DNA replication in the extract by preventing the assembly of pre-RCs on sperm chromatin.
DISCUSSION
We have recently developed a system to assemble somatic H1 histones onto Xenopus sperm chromatin using cell-free extracts derived from activated Xenopus eggs. Somatic H1s added to the extract are correctly assembled onto sperm chromatin, thereby preserving normal nucleosomal organization but promoting the formation of higher-order chromatin structure (Lu et al., 1997). Somatic histone H1 reduces both the rate and extent of DNA replication in Xenopus egg extract (Figure 1). We have investigated the mechanism(s) by which H1 exerts these effects on sperm chromatin and found that although H1 has little or no effect on replication elongation (Figures 2 and 3), it does reduce the frequency of initiation events (Figure 2), presumably by affecting a step before the formation of replication bubbles (Figure 3). H1 also reduces the assembly of pre-RCs on sperm chromatin (Figure 5) and the number of replication foci within sperm nuclei (Figure 4). This reduction in pre-RC assembly is responsible for the inhibition of replication by H1 inasmuch as replication competence is restored only under conditions that promote the loss of H1 from chromatin and the licensing of sperm DNA (Figures 6 and 7). Taken together, these data raise the interesting possibility that H1 reduces the replication capacity of sperm nuclei in the extract by limiting the assembly of pre-RCs on sperm chromatin and thereby reducing the frequency of initiation within the genome.
Studies with Xenopus egg extracts have shown that assembly of pre-RCs is a highly coordinated process involving a number of essential replication proteins. The multisubunit ORC is first assembled onto sperm chromatin, thereby facilitating the sequential binding of XCdc6 and the MCM complex of proteins (Chong et al., 1995; Kubota et al., 1995; Madine et al., 1995a,b; Coleman et al., 1996; Romanowski et al., 1996; Rowles et al., 1996; Thommes et al., 1997). It is now well established that replication competence is dependent upon the assembly of pre-RCs on chromatin (reviewed by Romanowski and Madine, 1996, 1997) as immunodepletion of ORC, XCdc6, or MCM proteins from extract renders sperm nuclei incompetent for initiation (Madine et al., 1995a,b; Carpenter et al., 1996; Coleman et al., 1996; Romanowski et al., 1996). Furthermore, studies in the yeast Saccharomyces cerevisiae show that reduced levels of functional ORC and Cdc6 lead to a reduction in origin use, suggesting that pre-RC assembly determines the frequency of initiation events throughout the genome (Liang et al., 1995). The mechanism(s) by which H1 limits pre-RC assembly on sperm chromatin is not clear. However, given the sequential binding of pre-RC proteins to sperm chromatin, it seems reasonable to speculate that the reduced binding of XCdc6 and XMcm3 that we observe (Figure 5, B and C) is due to the reduction in chromatin-bound ORC (Figure 5A), and, therefore, H1 limits replication competence by reducing the binding of ORC to chromatin. One way in which H1 could limit ORC binding is by masking replication origins. This masking could result from changes in chromatin structure, such as compaction of the DNA (Figures 4 and 5; also see Lu et al., 1997). H1-induced changes in chromatin structure are known to regulate transcription by modulating transcription factor binding in egg extracts (Shimamura et al., 1989; Chipev and Wolffe, 1992; Bouvet et al., 1994; Kandolf, 1994; Trieschmann et al., 1995). In Xenopus eggs or egg extracts, DNA replication initiates at multiple sites that are randomly, but evenly, distributed on chromatin, apparently due to a relaxed DNA sequence requirement (Mechali and Kearsey, 1984; Mahbubani et al., 1992). Moreover, immunodepletion of the embryonic linker histone B4 has no apparent effect on DNA replication in egg extract (Dasso et al., 1994), implying that B4, unlike somatic H1, may not restrict the binding of pre-RC proteins to embryonic chromatin. This may be due to the fact that B4-chromatin already adopts a dynamic and extended structure (Dimitrov et al., 1994; Nightingale et al., 1996; Ura et al., 1996) in which potential binding sites are easily accessible for assembly of pre-RCs.
Immunodepletion of ORC from egg extract causes a substantial reduction in the rate of DNA replication and an extended S-phase due to a reduction in the number of initiation events in sperm nuclei (Walter and Newport, 1997). Furthermore, an increase in nucleo–cytoplasmic ratio also reduces the number of initiations in sperm chromatin, leading to an increase in replicon size (Walter and Newport, 1997). However, replicon size increases even when ORC, MCM, and cdk2-cyclin E are not limiting, which suggests that another positive activity determines replicon size in this system and that not all ORC binding sites are used as replication origins in sperm nuclei (Walter and Newport, 1997). Thus, both ORC density and an unidentified positive activity could regulate the frequency of initiation and replicon size in egg extract. The H1-induced reduction in pre-RC binding to sperm chromatin that we observe (Figure 5) is accompanied by a reduction in the number of initiation events (Figure 2), a reduction in the number of replication foci (Figure 4), and an overall reduction in the extent of DNA replication (Figure 1) in egg extract. However, no change in the length of S-phase was observed (Figure 1). Thus, the reduction in the frequency of initiation within H1 nuclei occurs without a corresponding increase in replicon size (Figures 2 and 3). These results are surprising, especially in light of the work of Walter and Newport (1997). One possible explanation for our results is that H1 is not uniformly distributed along sperm chromatin and that replication is inhibited where H1 is present above a critical threshold level but unaffected where H1 levels fall below this threshold. In theory, this differential binding by H1 could lead to regional changes in chromatin structure that could serve to regulate the timing of initiation within large chromatin domains. It is important to note, however, that the failure of any DNA to replicate during S-phase would be lethal to the early embryo; therefore, other factors, absent from egg extract but present at the MBT, may regulate H1 binding, resulting in a reduction in origin use but complete replication of all DNA.
H1 reduces the number of foci that contain RP-A (our unpublished observations), the precursors of replication foci (Adachi and Laemmli, 1992, 1994; Yan and Newport, 1995). H1 also reduces the number of replication foci within sperm nuclei incubated in egg extract (Figure 4). Precisely how H1 affects replication focus formation is not clear. One possibility is that a reduction in pre-RC formation leads to a reduction in the number of initiations, and fewer initiations may limit the extent of DNA synthesis by imposing topological constraints upon the replication machinery. In theory, such constraints could also reduce the number of replication foci in sperm nuclei. However, while 6-DMAP dramatically reduces the levels of XMcm3 (Chong et al., 1995; Rowles et al., 1996; Mahbubani et al., 1997), the number of initiation events (Mahbubani et al., 1997), and the extent of DNA replication in sperm nuclei (Blow, 1993), it does not substantially reduce the number of replication foci within these nuclei (Munshi and Leno, 1998; also compare Figure 4 [Control] in this paper with Mills et al., 1989). Thus, incomplete pre-RC assembly and a reduced frequency of initiation cannot account for the reduced number of replication foci that we observe in H1 nuclei (Figure 4). An alternate possibility is that H1 induces global changes in chromatin structure and that these changes may limit the formation of prereplication foci and replication foci as well as the assembly of pre-RCs.
The results presented here raise the interesting possibility that linker histones play a role in regulating the initiation of DNA replication during Xenopus development. Somatic H1 could modulate replication origin use by promoting changes in chromatin structure after the MBT. In theory, localized changes in chromatin structure could be part of a general mechanism that serves to coordinately regulate the timing of DNA replication between multiple adjacent replicons in somatic cells. Further investigation into the mechanisms by which H1 restricts pre-RC assembly and origin use, along with the identification of other factors that participate in this process, will help us understand how DNA replication is regulated during development.
ACKNOWLEDGMENTS
We thank Rajan Munshi for providing metaphase extract, Dr. Peter Jackson for providing RP-A antiserum, Jennifer Johns for technical assistance, and Dr. Susan Wellman for helpful comments on the manuscript. This work was supported by a grant from the National Science Foundation (MCB-9506280) to G.H.L.
Footnotes
Abbreviations used: Ara-CTP, Cytosine β-d-arabinofuranoside 5′-triphosphate; 6-DMAP, 6-dimethylaminopurine; MCM, minichromosome maintenance; ORC, origin recognition complex; Pre-RC, pre-replication complex; RP-A, replication protein A.
REFERENCES
- Adachi Y, Laemmli UK. Identification of nuclear pre-replication centers poised for DNA synthesis in Xenopus egg extracts: immunolocalization study of replication protein A. J Cell Biol. 1992;119:1–15. doi: 10.1083/jcb.119.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi Y, Laemmli UK. Study of the cell cycle-dependent assembly of the DNA pre-replication centers in Xenopus egg extracts. EMBO J. 1994;13:4153–4164. doi: 10.1002/j.1460-2075.1994.tb06733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman MG, Wawra E, Winge M. Chicken histone H5 inhibits transcription and replication when introduced into proliferating cells by microinjection. J Cell Sci. 1988;91:201–209. doi: 10.1242/jcs.91.2.201. [DOI] [PubMed] [Google Scholar]
- Blow JJ. Preventing re-replication of DNA in a single cell cycle: evidence for a replication licensing factor. J Cell Biol. 1993;122:993–1002. doi: 10.1083/jcb.122.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ, Laskey RA. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986;47:577–587. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
- Blow JJ, Watson JV. Nuclei act as independent and integrated units of replication in a Xenopus cell-free DNA replication system. EMBO J. 1987;6:1997–2002. doi: 10.1002/j.1460-2075.1987.tb02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet P, Dimitrov S, Wolffe AP. Specific regulation of Xenopus chromosomal 5S rRNA gene transcription in vivo by histone H1. Genes Dev. 1994;8:1147–1159. doi: 10.1101/gad.8.10.1147. [DOI] [PubMed] [Google Scholar]
- Brown DT, Alexander BT, Sittman DB. Differential effect of H1 variant overexpression on cell cycle progression and gene expression. Nucleic Acids Res. 1996;24:486–493. doi: 10.1093/nar/24.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan HG. Replication of DNA in the chromosomes of eukaryotes. Proc R Soc Lond B. 1972;181:19–41. doi: 10.1098/rspb.1972.0039. [DOI] [PubMed] [Google Scholar]
- Carpenter PB, Muller PR, Dunphy WG. Role for a Xenopus Orc2-related protein in controlling DNA replication. Nature. 1996;379:357–360. doi: 10.1038/379357a0. [DOI] [PubMed] [Google Scholar]
- Chipev CC, Wolffe AP. Chromosomal organization of Xenopus laevis oocyte and somatic 5S rRNA genes in vivo. Mol Cell Biol. 1992;12:45–55. doi: 10.1128/mcb.12.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JPJ, Mahbubani HM, Khoo C, Blow JJ. Purification of an MCM-containing complex as a component of the DNA replication licensing system. Nature. 1995;375:418–421. doi: 10.1038/375418a0. [DOI] [PubMed] [Google Scholar]
- Coleman TR, Carpenter PB, Dunphy WG. The Xenopus cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell. 1996;87:53–63. doi: 10.1016/s0092-8674(00)81322-7. [DOI] [PubMed] [Google Scholar]
- Cox LS, Laskey RA. DNA replication occurs at discrete sites in pseudonuclei assembled from purified DNA in vitro. Cell. 1991;66:271–275. doi: 10.1016/0092-8674(91)90617-8. [DOI] [PubMed] [Google Scholar]
- Cozzarelli NR. The mechanism of action of inhibitors of DNA synthesis. Annu Rev Biochem. 1977;46:641–668. doi: 10.1146/annurev.bi.46.070177.003233. [DOI] [PubMed] [Google Scholar]
- Dasso M, Dimitrov S, Wolffe AP. Nuclear assembly is independent of linker histones. Proc Natl Acad Sci USA. 1994;91:12477–12481. doi: 10.1073/pnas.91.26.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso M, Newport JW. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell. 1990;61:811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Almouzni G, Dasso M, Wolffe AP. Chromatin transitions during early Xenopus embryogenesis: changes in histone H4 acetylation and in linker histone type. Dev Biol. 1993;160:214–227. doi: 10.1006/dbio.1993.1299. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Dasso MC, Wolffe AP. Remodeling sperm chromatin in Xenopus laevis egg extracts: the role of core histone phosphorylation and linker histone B4 in chromatin assembly. J Cell Biol. 1994;126:591–601. doi: 10.1083/jcb.126.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S, Wolffe AP. Remodeling somatic nuclei in Xenopus laevis egg extracts: molecular mechanisms for the selective release of histones H1 and H10 from chromatin and the acquisition of transcriptional competence. EMBO J. 1996;15:5897–5906. [PMC free article] [PubMed] [Google Scholar]
- Dworkin-Rastl E, Kandolf H, Smith RC. The maternal histone H1 variant, H1M (B4 protein), is the predominant H1 histone in Xenopus pregastrula embryos. Dev Biol. 1994;161:425–439. doi: 10.1006/dbio.1994.1042. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Uehlinger V. “Fertile” intestine nuclei. Nature. 1966;210:1240–1241. doi: 10.1038/2101240a0. [DOI] [PubMed] [Google Scholar]
- Halmer L, Gruss C. Influence of histone H1 on the in vitro replication of DNA and chromatin. Nucleic Acids Res. 1995;23:773–778. doi: 10.1093/nar/23.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock R, Moorman A, Fischer D, Scheer U. Absence of somatic histone H1 in oocytes and preblastula embryos of Xenopus laevis. Dev Biol. 1993;158:510–522. doi: 10.1006/dbio.1993.1209. [DOI] [PubMed] [Google Scholar]
- Hyrien O, Maric C, Mechali M. Transition in specification of embryonic metazoan DNA replication origins. Science. 1995;270:994–997. doi: 10.1126/science.270.5238.994. [DOI] [PubMed] [Google Scholar]
- Hyrien O, Mechali M. Chromosomal replication initiates and terminates at random sequences but at regular intervals in the ribosomal DNA of Xenopus early embryos. EMBO J. 1993;12:4511–4520. doi: 10.1002/j.1460-2075.1993.tb06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PK, Chevalier S, Philippe M, Kirschner MW. Early events in DNA replication require cyclin E and are blocked by p21cip1. J Cell Biol. 1995;130:755–769. doi: 10.1083/jcb.130.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandolf H. The H1A histone variant is an in vivo repressor of oocyte-type 5S gene transcription in Xenopus laevis embryos. Proc Natl Acad Sci USA. 1994;91:7257–7261. doi: 10.1073/pnas.91.15.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khochbin S, Wolffe AP. Developmentally regulated expression of linker-histone variants in vertebrates. Eur J Biochem. 1994;225:501–510. doi: 10.1111/j.1432-1033.1994.00501.x. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Mimura S, Nishimoto S, Takisawa H, Nojima H. Identification of the yeast MCM3-related protein as a component of Xenopus DNA replication licensing factor. Cell. 1995;81:601–609. doi: 10.1016/0092-8674(95)90081-0. [DOI] [PubMed] [Google Scholar]
- Lawlis SJ, Keezer SM, Wu J, Gilbert DM. Chromosome architecture can dictate site-specific initiation of DNA replication in Xenopus egg extracts. J Cell Biol. 1996;135:1207–1218. doi: 10.1083/jcb.135.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leno GH, Laskey RA. The nuclear membrane determines the timing of DNA replication in Xenopus egg extracts. J Cell Biol. 1991;112:557–566. doi: 10.1083/jcb.112.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leno GH, Munshi R. Initiation of DNA replication in nuclei from quiescent cells requires permeabilization of nuclear membrane. J Cell Biol. 1994;127:5–14. doi: 10.1083/jcb.127.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Weinreich M, Stillman B. Orc and Cdc6 interact and determine the frequency on initiation of DNA replication in the genome. Cell. 1995;81:667–676. doi: 10.1016/0092-8674(95)90528-6. [DOI] [PubMed] [Google Scholar]
- Lu ZH, Sittman DB, Brown DT, Munshi R, Leno GH. Histone H1 modulates DNA replication through multiple pathways in Xenopus egg extract. J Cell Sci. 1997;110:2745–2758. doi: 10.1242/jcs.110.21.2745. [DOI] [PubMed] [Google Scholar]
- Madine MA, Khoo CY, Mills AD, Laskey RA. MCM3 complex required for cell cycle regulation of DNA replication in vertebrate cells. Nature. 1995a;375:421–424. doi: 10.1038/375421a0. [DOI] [PubMed] [Google Scholar]
- Madine MA, Khoo C, Mills AD, Musahl C, Laskey RA. The nuclear envelope prevents reinitiation of replication by regulating the binding of MCM3 to chromatin in Xenopus egg extracts. Curr Biol. 1995b;5:1270–1279. doi: 10.1016/s0960-9822(95)00253-3. [DOI] [PubMed] [Google Scholar]
- Mahbubani HM, Chong JPJ, Chevalier S, Thommes P, Blow JJ. Cell cycle regulation of the replication licensing system: involvement of a Cdk-dependent inhibitor. J Cell Biol. 1997;136:125–135. doi: 10.1083/jcb.136.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahbubani HM, Paull T, Elder JK, Blow JJ. DNA replication initiates at multiple sites on plasmid DNA in Xenopus egg extracts. Nucleic Acids Res. 1992;20:1457–1462. doi: 10.1093/nar/20.7.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechali M, Harland RM. DNA synthesis in a cell-free system from Xenopus eggs: priming and elongation on single-stranded DNA in vitro. Cell. 1982;30:93–101. doi: 10.1016/0092-8674(82)90015-0. [DOI] [PubMed] [Google Scholar]
- Mechali M, Kearsey S. Lack of specific sequence requirement for DNA replication in Xenopus eggs compared with high sequence specificity in Yeast. Cell. 1984;38:55–64. doi: 10.1016/0092-8674(84)90526-9. [DOI] [PubMed] [Google Scholar]
- Micheli G, Luzzatto ARC, Carri MT, Capoa A, Pelliccia F. Chromosome length and DNA loop size during early embryonic development of Xenopus laevis. Chromosoma. 1993;102:478–483. doi: 10.1007/BF00357103. [DOI] [PubMed] [Google Scholar]
- Mills AD, Blow JJ, White JG, Amos WB, Wilcock D, Laskey RA. Replication occurs at discrete foci spaced throughout nuclei replicating in vitro. J Cell Sci. 1989;94:471–477. doi: 10.1242/jcs.94.3.471. [DOI] [PubMed] [Google Scholar]
- Munshi, R., and Leno, G.H. (1998). Replication of nuclei from cycling and quiescent mammalian cells in 6-DMAP-treated Xenopus egg extract. Exp. Cell Res. (in press). [DOI] [PubMed]
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982;30:675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Nightingale K, Dimitrov S, Reeves R, Wolffe AP. Evidence for a shared structural role for HMG1 and linker histones B4 and H1 in organizing chromatin. EMBO J. 1996;15:548–561. [PMC free article] [PubMed] [Google Scholar]
- Romanowski P, Madine MA. Mechanisms restricting DNA replication to once per cell cycle: MCMs, pre-replicative complexes and kinases. Trends Cell Biol. 1996;6:184–188. doi: 10.1016/0962-8924(96)10015-5. [DOI] [PubMed] [Google Scholar]
- Romanowski P, Madine MA. Mechanisms restricting DNA replication to once per cell cycle: the role of Cdc6 and ORC. Trends Cell Biol. 1997;7:9–10. doi: 10.1016/S0962-8924(97)30077-4. [DOI] [PubMed] [Google Scholar]
- Romanowski P, Madine MA, Rowles A, Blow JJ, Laskey RA. The Xenopus origin recognition complex is essential for DNA replication and MCM binding to chromatin. Curr Biol. 1996;6:1416–1425. doi: 10.1016/s0960-9822(96)00746-4. [DOI] [PubMed] [Google Scholar]
- Rowles A, Chong JPJ, Brown L, Howell M, Evan GI, Blow JJ. Interaction between the origin recognition complex and the replication licensing system in Xenopus. Cell. 1996;87:287–296. doi: 10.1016/s0092-8674(00)81346-x. [DOI] [PubMed] [Google Scholar]
- Shimamura A, Sapp M, Rodriguez-campos A, Worcel A. Histone H1 represses transcription from minichromosomes assembled in vitro. Mol Cell Biol. 1989;9:5573–5584. doi: 10.1128/mcb.9.12.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach OC, Wolffe AP, Rupp RAW. Somatic linker histones cause loss of mesodermal competence in Xenopus. Nature. 1997;389:395–399. doi: 10.1038/38755. [DOI] [PubMed] [Google Scholar]
- Stillman B. Cell cycle control of DNA replication. Science. 1996;274:1659–1664. doi: 10.1126/science.274.5293.1659. [DOI] [PubMed] [Google Scholar]
- Sun J, Wiaderkiewicz R, Ruiz-Carrillo A. Histone H5 in the control of DNA synthesis and cell proliferation. Science. 1989;245:68–71. doi: 10.1126/science.2740916. [DOI] [PubMed] [Google Scholar]
- Thommes P, Kubota Y, Takisawa H, Blow JJ. The RLF-M component of the replication licensing system forms complexes containing all six MCM/P1 polypeptides. EMBO J. 1997;16:3312–3319. doi: 10.1093/emboj/16.11.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieschmann L, Alfonso PJ, Crippa MP, Wolffe AP, Bustin M. Incorporation of chromosomal proteins HMG-14/HMG-17 into nascent nucleosomes induces an extended chromatin conformation and enhances the utilization of active transcription complexes. EMBO J. 1995;14:1478–1489. doi: 10.1002/j.1460-2075.1995.tb07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ura K, Hayes JJ, Wolffe AP. A positive role for nucleosome mobility in the transcriptional activity of chromatin templates: restriction by linker histones. EMBO J. 1995;14:3752–3765. doi: 10.1002/j.1460-2075.1995.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ura K, Nightingale K, Wolffe AP. Differential association of HMG1 and linker histones B4 and H1 with dinucleosomal DNA: structural transitions and transcriptional repression. EMBO J. 1996;15:4959–4969. [PMC free article] [PubMed] [Google Scholar]
- Walter J, Newport JW. Regulation of replicon size in Xenopus egg extracts. Science. 1997;275:993–995. doi: 10.1126/science.275.5302.993. [DOI] [PubMed] [Google Scholar]
- Wolffe AP. Dominant and specific repression of Xenopus oocyte 5S RNA genes and satellite I DNA by histone H1. EMBO J. 1989;8:527–537. doi: 10.1002/j.1460-2075.1989.tb03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Newport J. FFA-1, a protein that promotes the formation of replication centers within nuclei. Science. 1995;269:1883–1885. doi: 10.1126/science.7569932. [DOI] [PubMed] [Google Scholar]