Turmeric has been used for centuries in Ayurvedic medicine as a treatment for inflammatory disorders including arthritis. Based on this traditional usage, dietary supplements containing turmeric rhizome and turmeric extracts are also being used in the western world for arthritis treatment and prevention. However, to our knowledge, no data are available regarding anti-arthritic efficacy of complex turmeric extracts similar in composition to those available for use as dietary supplements. Therefore, the studies described here were undertaken to determine the in vivo efficacy of well characterized curcuminoid-containing turmeric extracts in the prevention or treatment of arthritis using streptococcal cell wall (SCW) induced arthritis, a well-described animal model of rheumatoid arthritis (RA). Arthritic index, a clinical measure of joint swelling, was used as the primary endpoint for assessing the effect of extracts on joint inflammation. An essential oil-depleted turmeric fraction containing 41% of the three major curcuminoids was efficacious in preventing joint inflammation when treatment was started before, but not after, the onset of joint inflammation. A commercial sample containing 94% of the three major curcuminoids was more potent in preventing arthritis than the essential oil-depleted turmeric fraction when compared by total curcuminoid dose per body weight. In conclusion, these data (1) document the in vivo anti-arthritic efficacy of an essential oil-depleted turmeric fraction and (2) suggest that the three major curcuminoids are responsible for this anti-arthritic effect, while the remaining compounds in the crude turmeric extract may inhibit this protective effect.
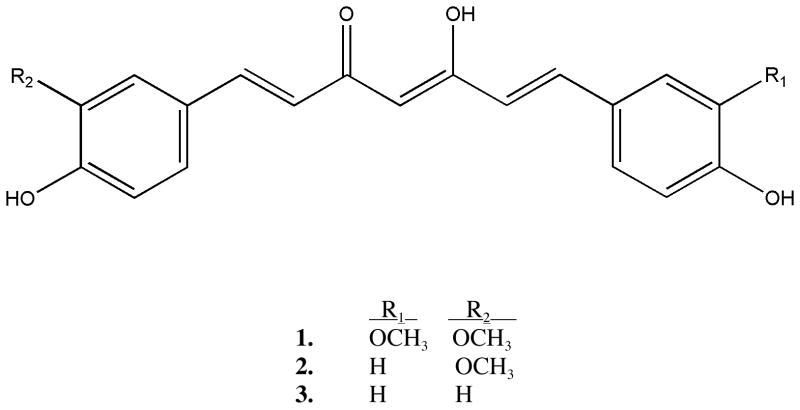
Turmeric (Curcuma longa L. [Zingiberaceae]) is a perennial native to Asia where its rhizome is used as a spice (being responsible for the yellow color of curry), a pigment dye for textiles or for skin in religious ceremonies, and a traditional medicine, primarily used for the treatment of inflammatory disorders.1 Typical dietary intake of turmeric is estimated to be as high as 2.5 g/d (approximately 100 mg curcumin/d) in some countries,2 while up to 8 g/d of curcumin, one of the three major curcuminoids (curcumin [1], demethoxycurcumin [2] and bis-demethoxycurcumin [3], see Figure 1) that constitute 3% of the total rhizome, is without evidence of side effects in Phase I clinical trials.3
Figure 1.
Chemical structures of curcumin (1), demethoxycurcumin (2) and bis-demethoxycurcumin (3).
Organic extracts of the rhizomes of turmeric have previously been shown to have anti-inflammatory effects in vitro.4–8 However, analysis of these published reports is confounded by the use of extracts that are neither well characterized nor uniform in their composition. Most of these studies have evaluated the effects of various uncharacterized extracts designated as “curcumin” that inhibit production of TNF-α and IL-1β, as well as PGE2.4–8. The major site of action of these curcumin extracts is thought to be inhibition of transcription factor activation, including activation of NF-κB.4,5,9 NF-κB activation during inflammation leads to increased gene expression of cytokines (TNF-α, IL-1β), chemokines (MCP-1) and other inflammatory proteins (COX-2) that are critical mediators of joint inflammation in rheumatoid arthritis (RA).4,10–12
Analysis of the scant number of publications in the literature investigating the anti-arthritic efficacy of turmeric in animal or human studies is again confounded by the lack of characterization of the extracts tested, as well as the variety of models studied. In rodents, an uncharacterized curcumin extract (10–80 mg/kg orally) has been reported to reduce carrageenin-induced paw edema, a model of acute inflammation rather than actual destructive arthritis,13 as well as paw swelling in adjuvant-induced arthritis (100–300 mg/kg/d orally).14,15 In humans, one small double blind crossover study evaluating a single dose of an uncharacterized curcumin extract (1.2g/day) or phenylbutazone in RA (n=18 patients) reported some success in decreasing disease symptoms.16 In none of these reports was a chemical analysis of what one would assume to be curcumin-enriched extracts presented. In addition, to our knowledge, there are no reports on the anti-arthritic effect of unpurified, chemically complex turmeric extracts more analogous in composition to dietary turmeric supplements available for over-the-counter use.
Previous evaluation by our group of randomly chosen, over-the-counter turmeric dietary supplements (n=9), demonstrated that the majority of these extracts do not contain essential-oils but are still very complex extracts that are composed of less than 50% curcuminoids (median value, 19.3%).17 We therefore prepared, chemically characterized, and then tested the in vitro anti-inflammatory efficacy, followed by the in vivo anti-arthritic efficacy, of a complex turmeric extract depleted in essential oils and containing less than 50% curcuminoids which we isolated from a commercial source of ground turmeric rhizome. The efficacy of this chemically complex turmeric extract was compared to that of a commercially available curcumin product (greater than 90% curcuminoids by HPLC analysis). The animal model used, streptococcal cell wall (SCW)-induced arthritis, is a well-characterized animal model of rheumatoid arthritis.18–20 In this model, over a 28 day course, female Lewis rats develop an initial acute phase of joint swelling followed by a chronic phase of inflammation that is associated with actual joint destruction. Joint histopathology in this model is very similar to that seen in rheumatoid arthritis (RA), a disease typified by recurrent cycles of joint inflammation and gradual joint destruction.21,22 In addition, a granulomatous inflammatory response, similar to that responsible for inactivating tuberculosis bacilli by walling off the invading bacteria, occurs in the liver and spleen of these animals at sites of SCW deposition.18–20
Results and Discussion
Chemical and in vitro Biological Analysis of Turmeric
HPLC analysis of the essential oil-depleted turmeric extract (henceforth referred to as turmeric fraction) that was used in the in vivo arthritis model revealed a curcuminoid content of 41% and a negligible amount of essential oils (Table 1 and Figure S1B, Supporting Data). The chemical composition of this essential-oil depleted turmeric fraction can be compared to that of the crude turmeric extract in Table 1 and Figure S1A. The in vivo anti-arthritic effect of the essential-oil depleted turmeric fraction was compared to that of a commercial curcumin product labeled as “98+% curcumin” (henceforth referred to as purified curcuminoids) that was found on HPLC analysis to be composed of a 94% mixture of three curcuminoids with no evidence of essential oils ( Table 1 and Figure S1C). In vitro biological screening for anti-inflammatory efficacy revealed a 10-fold lower IC50 for the two chemically complex turmeric extracts as compared to the purified curcuminoids with respect to inhibition of PGE2 production (Table 1), suggesting that the complex extracts could be more potent anti-inflammatory agents.
Table 1.
Chemical and Biological Characterization of Turmeric Samples
| Curcuminoid Content (% by weight)
|
In vitro PGE2 Inhibition IC50 (μg extract/mL) | ||||
|---|---|---|---|---|---|
| Extract | total | curcumin (1) | demethoxy-curcumin (2) | bis-demethoxy-curcumin (3) | |
| Crude Turmeric Extract | 33.7 | 21.4 | 7.2 | 5.1 | 0.13 |
| Essential Oil-Depleted Turmeric Fraction | 40.6 | 25.7 | 8.7 | 6.2 | 0.48 |
| Purified Curcuminoids (commerical curcumina) | 93.6 | 74.2 | 14.9 | 4.5 | 2.66 |
Although labeled as “98+% curcumin”, this product contained all three curcuminoids with total curcuminoid content < 98%.
In vivo Anti-arthritic Efficacy of Intraperitoneal Turmeric Fraction Treatment
Daily intraperitoneal (ip) administration of the turmeric fraction (23 mg total curcuminoids/kg/d), beginning 4 days prior to SCW administration, significantly inhibited joint inflammation in both the acute inflammatory and chronic destructive phases of arthritis (64% and 72% inhibition, respectively; Figure S2A). In contrast, when the start of treatment was delayed until day 8, i.e., after the resolution of the acute phase but prior to initiation of the chronic phase of arthritis, this same dose of turmeric fraction was without a clinically significant effect on joint swelling (Figure S2B). Daily treatment with a lower dose of turmeric fraction (4 mg curcuminoids/kg/d) beginning 4 days prior to SCW injection was also without significant effect on joint swelling (Figure S2C). In all experiments, which also included vehicle- and turmeric fraction-treated non-arthritic animals (n=5–8/group), there were no signs of toxicity as measured by weight gain, ALT or creatinine in normal or SCW-treated animals receiving turmeric fraction treatment (data not shown). However, there was a 5–7% mortality in turmeric-treated normal and SCW animals (vs. no deaths in vehicle-treated animals) of unknown etiology that was not statistical significant by Fisher’s Exact Test (data not shown).
These results provide the first in vivo evidence of anti-arthritic efficacy of a chemically complex turmeric product similar in chemical composition to commercially available dietary supplements. Because pretreatment was required to prevent joint inflammation, these data only support the use of curcuminoid-containing turmeric products for arthritis prevention and not for disease treatment in the face of active inflammation. Mechanistic studies to be presented elsewhere suggest that early treatment with the curcuminoid-containing turmeric fraction may be needed to prevent activation of NF-κB, a transcription factor that induces the expression of key mediators of joint inflammation (Funk et al., unpublished data). It should also be stated, however, that while an 8-day delay before start of treatment with turmeric fraction was not efficacious, the possible effect of a somewhat earlier treatment (eg. day 3 in the SCW model), as might occur clinically at the first signs of inflammation, was not yet examined.
In vivo Anti-arthritic Efficacy of Intraperitoneal Purified Curcuminoid Treatment
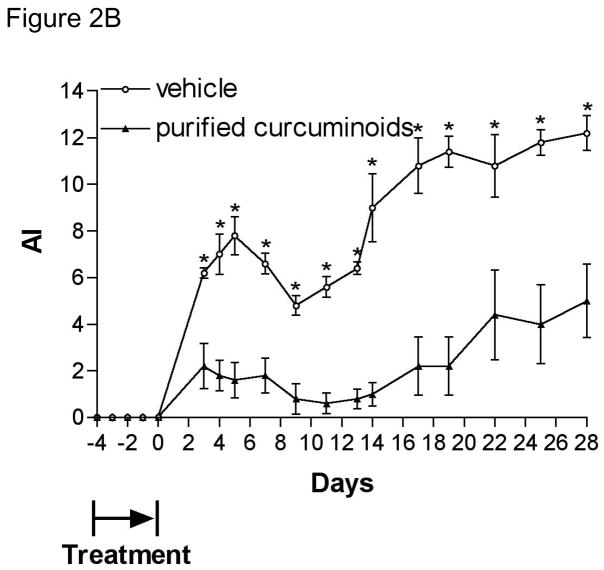
In contrast to the ineffectiveness of low dose turmeric fraction on joint inflammation (Figure S2C), daily ip administration of the same low dose of purified curcuminoids (4 mg total curcuminoids/kg/d) beginning 4 days prior to SCW administration significantly inhibited joint inflammation in both the acute and chronic phases (75% and 68% inhibition, respectively; Figure 2A, closed circles). The degree of arthritis inhibition by the purified curcuminoids, as demonstrated by arthritic index (Figure 2A) or arthritis incidence (Figure 3), was similar to that caused by a six-fold higher (by curcuminoid content) dose of turmeric fraction (Figures S2A and Figure 3). These results suggest that the remaining components of the turmeric fraction, other than the three major curcuminoids (compounds 1–3), may actually inhibit the protective, anti-arthritic effect of the curcuminoids, a finding that runs counter to the prevailing notion that complex botanical agents may provide increased efficacy for disease treatment. The identification of these other components is currently under investigation (Timmermann et al., unpublished data). Interestingly, pre-treatment only with the purified curcuminoids for 5 days prior to SCW injection (Figure 2B) was sufficient to prevent joint inflammation over the subsequent 28-day course of the experiment (65% and 59% inhibition of acute and chronic inflammation, respectively), conferring a degree of protection similar to that seen with daily treatment (Figure 2A). This finding is particularly notable as we are unaware of any other agents that have been shown in this model to have such lasting inhibitory effects on joint inflammation. Moreover, our demonstration of a prolonged protective effect from a brief period of pre-treatment with either low dose purified curcuminoids (Figure 2B) or high dose turmeric fraction (data not shown) most definitely supports the possible use of curcuminoid-containing turmeric dietary supplements for arthritis prevention and also raises the question of whether continuous dosing would be needed and/or what effect less-than-perfect compliance might have on disease prevention.
Figure 2.
Effect of purified curcuminoids on joint inflammation. Female Lewis rats were injected on day 0 with SCW (25 μg/g) or vehicle. Joint swelling was assessed by daily calculation of the arthritic index (mean ± SEM) with statistical significance was determined by Students’ T test as described in methods. (A) Purified curcuminoids (4 mg curcuminoids/kg/d) or vehicle alone ip injections were begun 4 days prior to SCW administration (n=11 animals/group) and continued on a daily basis until 10 days after SCW injection at which time treatment frequency decreased to 5 days/week. * p < 0.02. (B) Purified curcuminoids (4 mg/kg/d) or vehicle alone ip injections were begun 4 days prior to SCW administration (n=5 animals/group) and continued daily for only 5 days with last dose 6 hours prior to SCW administration. * p < 0.01. (C) Purified curcuminoids (117 mg/kg/d) or vehicle alone were administered orally 4 days prior to SCW administration (n=10–11 animals/group) and continued on a daily basis until 10 days after SCW injection at which time treatment frequency decreased to 5 days/week. * p < 0.05.
Figure 3.

Comparison of effect of turmeric fraction (TF) vs. purified curcuminoids (CURC) on incidence of arthritis vs. hepatic granuloma formation. Female Lewis rats were injected on day 0 with SCW (25 μg/g) or vehicle. Botanical extracts or vehicle alone ip injections were begun 4 days prior to SCW administration and continued on a daily basis until 10 days after SCW injection at which time treatment frequency decreased to 5 days/week. Incidence of joint swelling at day 28 was assessed by calculation of the arthritic index (n=11–34 animals/group). The incidence of granuloma formation was assessed histologically as described in methods (n=11–23 animals/group). Statistical significance was determined by Fisher’s Exact Testing. * p < 0.05 vs. untreated. ** p <.007 vs. untreated.
In vivo Anti-arthritic Efficacy of Oral Purified Curcuminoid Treatment
Although dietary supplements are obviously taken orally, our initial experiments used an intraperitoneal mode of extract delivery in order to maximize delivery so that botanical bioactivity could be determined and compared independent of possible differential rates of gastrointestinal absorption and further metabolism. Subsequent examination of the effect of daily oral (po) treatment with a 30-fold higher dose of purified curcuminoids to account for a possible lower degree of gastrointestinal absorption was also significantly protective when treatment was begun 4 days prior to SCW administration (Figure 2C). Statistically significant protection was demonstrated in this oral dosing study up until day 23 (48% inhibition on day 3 and 45% inhibition on day 23) after which time the lower degree of protection occurring in this study was no longer statistically significant (42% inhibition on day 28, p = 0.06). This oral dose of curcuminoids corresponds to approximately 1 g curcuminoids/d in a 70 kg person after correcting for the surface area of rats vs. humans.23 a dose well below the 8 g/d curcumin dose previously reported to be without side effects in humans.3 The effect of higher oral curcumin doses, or the addition of other botanically-derived compounds, such as piperine, that can enhance curcumin absorption by 50% and 20-fold in rats and humans, respectively,24 has not yet been tested in this arthritis model. In contrast to the increased mortality demonstrated with ip turmeric fraction treatment, there was no mortality, or other signs of toxicity, in normal or SCW animals treated ip or orally with purified curcuminoids (data not shown).
Effect of Turmeric Fraction or Purified Curcuminoids on Granuloma Formation
Dose dependent prevention of granulomatous inflammation by the turmeric fraction paralleled the efficacy of this extract in preventing joint inflammation; high dose turmeric fraction prevented hepatic granulomas while low dose did not (Figure 3). In contrast, there was a dissociation in the anti-inflammatory effects of low dose purified curcuminoids, as this extract prevented joint inflammation while having no effect on hepatic granuloma formation (Figure 3). The ability of the complex turmeric fraction, but not purified curcuminoids, to inhibit SCW-induced granuloma formation could be due to a requirement for (1) a higher dose of the three major curcuminoids for this anti-inflammatory effect (i.e., 23 vs. 4 mg total curcuminoids/kg/d) and/or (2) additional anti-inflammatory effects of the remaining components of the turmeric fraction. In either case, these results demonstrate that a therapeutic window may exist for using purified curcuminoids for arthritis prevention without increasing the risk of reactivating latent granulomatous infections, such as tuberculosis, a disease made quiescent by granuloma formation around the invading microorganism. To state the converse, these results also suggest that anti-arthritic doses of more complex turmeric extracts could increase the risk of reactivation of latent granulomatous infections, as has been reported in RA patients with latent tuberculosis infections when treated with TNF inhibitors, another potent anti-inflammatory agent.25,26
Conclusions
The results of these translational studies provide compelling evidence to support further clinical testing of curcuminoid-containing turmeric dietary supplements for the prevention of rheumatoid arthritis (RA) or RA flares. Moreover, these studies suggest that purified curcuminoids may be safer and more efficacious for this use than complex curcuminoid-containing turmeric extracts. However, several caveats should be mentioned when extrapolating these results to clinical use. To our knowledge, no clinical trials in RA patients have yet been done to document the safety or anti-arthritic efficacy of specific turmeric products available for over the counter use. We are also not aware of any studies in animals or humans investigating the use of turmeric products for the treatment or prevention of other types of arthritis, such as osteoarthritis. At the same time, the studies presented here do provide strong evidence supporting the need for well designed in vivo preclinical trials and clinical trials when testing specific anti-inflammatory effects of well-characterized botanical extracts. For example, our in vitro biological screening PGE2 assay suggested that purified curcuminoids might be 10-fold less potent anti-inflammatory agents, while our in vivo studies demonstrated the exact opposite with respect to anti-arthritic efficacy. In short, the chemical complexity of bioactive botanical extracts perhaps serves as a mirror for the complexity that may also be associated with their rational clinical use.
Experimental Section
General Experimental Procedures
Turmeric rhizome powder was purchased from San Francisco Herb and Natural Food, SF, CA. Commercial curcumin was obtained from Fisher Scientific (ACROS Organics, curcumin 98+%, # 218580100, Lot A017528901) and subjected to quantitative HPLC analysis before use in vitro or in vivo. Extract analyses were performed with an Agilent 1100 series HPLC system with quaternary pump, degasser, thermostatted column compartment, thermostatted autosampler, diode array detector and ChemStation for LC 3D, Rev. A.09.03 (1417) software for system control and data acquisition (Agilent Technologies Inc., Palo Alto, CA).
Sample Preparation
The crude turmeric extract was prepared by mechanically stirring turmeric powder (500g) with methanol (1.8 L) at 25 °C for 24 h. The mixture was filtered through a fritted funnel and, after washing the marc with fresh methanol, the solvent from the combined filtrate and washings was stripped off under vacuum using 20l Laborota 20 rotary evaporator. The resulting residue, after leaving under vacuum for 48 h, weighed 47.5g (9.5%). The curcuminoid-containing turmeric fraction was prepared by mechanically stirring turmeric powder (1 kg) with n-hexane (3.5 L) at 25 °C for 24 h. The mixture was filtered as above and the marc washed thoroughly with fresh n-hexane. The hexane-washed marc, after drying under the hood overnight, was extracted with methanol (3.5 L) by stirring mechanically at 25 °C for 24 h, filtered, and the marc washed with methanol. The solvent from the combined filtrate and washings was stripped off and the residue was left under vacuum overnight as described above. The residue was re-dissolved in methanol and the solvent stripped off. The resulting residue, after leaving under vacuum overnight, was powdered in a mortar and left in vacuum oven set at 45 °C for a minimum of 48 h. The yield of turmeric fraction was 31.3 g (3.1%).
Chemical and Biological Analyses
For HPLC analyses, triplicates of approximately 1 mg sample were weighed using an analytical balance, dissolved in 5 mL methanol, sonicated for 1 min and diluted with HPLC grade methanol in a 10 mL volumetric flask. One mL from each solution was transferred into an amber autosampler vial and 3 × 10 μL were injected onto the HPLC column. Stock solutions of pure curcumin, demethoxycurcumin and bis-demethoxycurcumin were prepared individually and their purity was confirmed by HPLC, MS, NMR and elemental analysis. Samples were injected onto a Synergy, 4μ, Hydro-RP 250×4.6 mm column coupled with SecurityGuard AJO-4287 guard column (Phenomenex, Torrance, CA 90501). The mobile phases used were as follows: A: 500 μL acetic acid/L of Nanopure water; B: 500 μL acetic acid/L of acetonitrile. The organic mobile phase was filtered through a 0.2 μm PTFE membrane filter and the water based mobile phase was filtered through a 0.2 μm cellulose nitrate filter. The mobile phases were thoroughly degassed before analysis and throughout the analysis. The eluent was monitored at 425 nm, 370 nm, 280 nm and 250 nm; the flow rate was 1 mL/min. In vitro screening for anti-inflammatory activity of extracts, as determined by inhibition of prostaglandin E2 (PGE2) production, was conducted before use in animals to ensure reproducibility of extract preparation. Briefly, U937 cells (ATCC, CRL-1593.2), cultured in RPMI 1640 with 25 mM HEPES, 10 % FBS were plated; differentiated with 10 nM of PMA (Sigma) for 24h; washed with media; and then treated with LPS (1 μg/mL) with or without varying concentrations of curcuminoid-containing extracts for an additional 24h. Culture supernatants were collected and stored at −80 °C prior to PGE2 immunoassay (R & D Systems, Minneapolis, MN).
Animal Procedures
Following standard protocols18–20 conducted in accordance with institutional guidelines, female Lewis rats (Harlan, Indianapolis, IN) were administered a single ip injection of vehicle (normal saline) or peptidoglycan-polysaccharide polymers (25 μg rhamnose/g body weight) isolated from the sonicated cell wall of Group A Streptococcus pyogenes (Lee Laboratories, Grayson, GA). At the indicated times, control and SCW-treated animals received an intraperitoneal (ip) injection of botanical sample or vehicle (0.5–1 μL/g DMSO) or oral delivery of botanical sample or vehicle (0.5 μL/g 8% saccharine in DMSO). Intraperitoneal treatments with turmeric fraction or purified curcuminoids were administered either beginning 4 days prior to SCW administration (pretreatment protocols) or 8 days after SCW administration (delayed treatment) and were continued on a daily or 5X weekly basis as indicated. For oral dosing studies, purified curcuminoids were administered orally by syringe beginning 4 days prior to SCW administration and were continued on a daily basis until day 14 at which time dosing was switched to 5X weekly. For comparison of anti-arthritic response to the two samples, dosing was normalized and expressed as mg total curcuminoids/g body weight. Joint inflammation was determined in a blinded fashion by daily assessment of arthritic index (AI) in each distal limb using standard criteria (0=normal; 1=slight erythema and edema; 2=increased edema with loss of landmarks; 3=marked edema; 4=marked edema with ankylosis on flexion). 18–20 Where indicated, inhibitory effects of treatments on AI were calculated on day 3 for the acute phase or day 28 for the chronic phase of joint swelling. To monitor for possible toxic effects of treatments in normal or SCW-treated animals, daily weights were recorded, and serum creatinine and alamine aminotransferase (ALT) levels in blood samples obtained 28 days after injection of SCW (or vehicle) were determined using a Hemagen Diagnostics Endocheck Plus Chemistry Analyzer to monitor for possible renal- or hepato-toxicity, respectively.
Histology
Liver specimens obtained 28 days after SCW injection were fixed in 10% formalin and tissues were embedded in paraffin. Granuloma formation was assessed in hematoxylin and eosin (H&E) stained sections of liver using standard criteria.18–20
Statistical Analyses
Values are presented as mean ± the SEM with statistical significance determined by ANOVA with post hoc testing or by Fishers Exact Test, as appropriate, using Instat software (Graphpad, San Diego, CA).
Supplementary Material
HPLC analyses of the turmeric extracts used for the in vivo studies as well as data demonstrating the in vivo anti-arthritic efficacy of the turmeric fraction are available in Figures S1(A,B, and C) and S2(A,B and C), respectively. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This work was supported by the Office of Dietary Supplements (ODS) and the National Center for Complementary and Alternative Medicine (NCCAM) of the National Institutes of Health (5 P50 AT000474). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS or National Institutes of Health.
References and Notes
- 1.Aggarwal BB, Kumar A, Aggarwal MS, Shishodia S. In: Phytopharmaceuticals in Cancer Chemoprevention. Bagchi D, Preus HG, editors. CRC Press; Boca Raton, FL: 2004. pp. 349–387. Chapter 23. [Google Scholar]
- 2.Chainani-Wu N. J Altern Comp Med. 2003;9(1):161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, et al. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 4.Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. J Immunol. 1999;163:3474–3483. [PubMed] [Google Scholar]
- 5.Grandjean-Laquerriere A, Gangloff SC, Le Naour R, Trentesaux C, Hornebeck W, Guenounou M. Cytokine. 2002;18:168–177. doi: 10.1006/cyto.2002.0888. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, Dannenberg AJ. Carcinogen. 1999;20:445–451. doi: 10.1093/carcin/20.3.445. [DOI] [PubMed] [Google Scholar]
- 7.Abe Y, Hashimoto S, Horie T. Pharmacol Res. 1999;39:41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- 8.Chan MM. Biochem Pharmacol. 1995;49:1551–1556. doi: 10.1016/0006-2952(95)00171-u. [DOI] [PubMed] [Google Scholar]
- 9.Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Thomas P, Dannenberg AJ. Am J Physiol. 2003;284:G321–327. doi: 10.1152/ajpgi.00230.2002. [DOI] [PubMed] [Google Scholar]
- 10.Liu SF, Ye X, Malik AB. Circulation. 1999;100:1330–1337. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- 11.Umezawa K, Ariga A, Matsumoto N. Anti-Canc Drug Des. 2000;15:239–244. [PubMed] [Google Scholar]
- 12.Makarov SS. Arth Res. 2001;3:200–206. doi: 10.1186/ar300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukhopadhyay A, Basu N, Ghatak N, Gujral PK. Agents Actions. 1982;12:508–515. doi: 10.1007/BF01965935. [DOI] [PubMed] [Google Scholar]
- 14.Joe B, Rao UJ, Lokesh BR. Mol Cell Biochem. 1997;169:125–134. doi: 10.1023/a:1006877928703. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee M, Tripathi LM, Srivastava VM, Puri A, Shukla R. Immunopharmacol Immunotoxicol. 2003;25:213–224. doi: 10.1081/iph-120020471. [DOI] [PubMed] [Google Scholar]
- 16.Deodhar SD, Sethi R, Srimal RC. Ind J Med Res. 1980;7:632–634. [PubMed] [Google Scholar]
- 17.Roberts AS, Rodriguez VP, Timmermann BN, Solyom AM. Council on Undergraduate Research, Undergraduate Posters on the Hill; April 20, 2004; Washington, DC. [Google Scholar]
- 18.Funk JL, Chen J, Downey KJ, Davee S, Stafford G. Arth Rheum. 2003;48:1721–1731. doi: 10.1002/art.10985. [DOI] [PubMed] [Google Scholar]
- 19.Geratz JD, Tidwell RR, Schwab JH, Anderle SK, Pryzwansky KB. Am J Pathol. 1990;136:909–921. [PMC free article] [PubMed] [Google Scholar]
- 20.Allen JB, Malone DG, Wahl SM, Calandra GB, Wilder RL. J Clin Invest. 1985;76:1042–1056. doi: 10.1172/JCI112057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choy EH, Panayi GS. New Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 22.Aletaha D, Ward M. Ann Rheum Dis. 2005 doi: 10.1136/ard.2005.038513. (June 23 published online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Canc Chemother Rep. 1966;50:219–234. [PubMed] [Google Scholar]
- 24.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) MMWR Morb Mort Weekly Rep. 2004;53:683–686. [Google Scholar]
- 26.Ehlers S. J Rheumatol-Supp. 2005;74:35–39. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HPLC analyses of the turmeric extracts used for the in vivo studies as well as data demonstrating the in vivo anti-arthritic efficacy of the turmeric fraction are available in Figures S1(A,B, and C) and S2(A,B and C), respectively. This material is available free of charge via the Internet at http://pubs.acs.org.