Abstract
We examined effects of manganese on the nervous system and innervation of lateral cilia of Crassostrea virginica. While essential in trace amounts, tissue manganese accumulation is neurotoxic, inducing Manganism, a Parkinson’s-like disease in humans. Lateral cilia of the gill of C. virginica are controlled by a reciprocal serotonergic-dopaminergic innervation from their ganglia. Oysters were incubated 3 days in the presence of up to 1 mM manganese, followed by superfusion of the cerebral ganglia, visceral ganglia or gill with dopamine or serotonin. Beating rates of cilia were measured by stroboscopic microscopy of isolated gill preparations or gill preparations with the ipsilateral cerebral and/or visceral ganglia attached. Acute manganese treatments impaired the dopaminergic, cilio-inhibitory system, while having no effect on the serotonergic, cilio-excitatory system, which is in agreement with the proposed mechanism of manganese toxicity in humans. Manganese treatments also decreased endogenous dopamine levels in the cerebral and visceral ganglia, and gills, but not serotonin levels. We demonstrated that manganese disrupts the animal’s dopaminergic system, and also that this preparation can be used to investigate mechanisms that underlie manganese neurotoxcity. It also may serve as a model in pharmacological studies of drugs to treat or prevent Manganism and other dopaminergic cell disorders.
Keywords: cilia, Crassostrea virginica, dopamine, ganglia, gill, serotonin, manganese, Manganism
Introduction
Manganese is an element present in all animal tissues and required as an enzyme cofactor or activator for numerous reactions of metabolism (Cotzias, 1958). While essential in trace amounts, excessive manganese exposure can result in toxic accumulations in human brain tissue and resulting extrapyramidal symptoms similar to those seen in patients with Idiopathic Parkinson’s disease (Calne, et al., 1994; Aschner, 2000; Levy and Nassetta, 2003; Dobson, et al., 2004). This Parkinson-like neurological condition first described in 1837 in two manganese ore-crushing mill workers (Couper, 1837) has been referred to as Manganism (Mena, et al., 1967; Barbeau, 1984; Donaldson, 1987; Gorell, et al., 1999). Inhalation of manganese from the atmosphere is believed to be the primary cause of manganese toxicity (Andersen, et al., 1999). In addition to mining and manganese ore processing, high levels of airborne manganese are possible in a number of other occupational settings, including welding, dry battery manufacture, and use of certain organochemical fungicides like Maneb. (NAS, 1973; Meco, et al., 1994; Reidy, et al., 1992; Iregren, 1999; Olanow, 2004). Most recently, questions are being asked about the safety of ambient manganese in the general population and there is a growing concern that chronic, low-level occupational or increased environmental exposure to manganese may be a contributing factor in a variety of neurological conditions including the high numbers of people diagnosed with Parkinson’s disease in the United States and elsewhere (Mergler, 1999; Lucchini, et al., 1999; Davis, 1999; Kaiser, 2003; Levy and Nassetta, 2003; Jankovic, 2005; Ostiguy, et al., 2006; Dorman, et al., 2006).
Although manganese toxicity has been recognized for some time, the primary mechanism underlying its neurotoxic effects remains elusive. Clinically, Manganism resembles Idiopathic Parkinson’s disease, a dopaminergic cell disorder. Symptoms common to both disorders include gait imbalance, rigidity, tremors and bradykinesia (Mena, et al., 1967, 1970; Rosenstock, et al., 1971; Huang, et al., 1989), suggesting a similar etiology of neuronal damage in the substantia nigra with a resulting deficiency of the neurotransmitter dopamine for the striatum. However, compared to Parkinson’s, there are some differentiating features seen with Manganism including symmetry of effects, more prominent dystonia, a characteristic “cock walk,” an intention rather than resting tremor, earlier behavioral and cognitive dysfunction, difficulty turning, and a poor response to Levodopa (Barbeau, et al., 1976; Huang, et al, 1993, 1998; Calne, et al., 1994; Lu, et al., 1994; Koller, et al., 2004; Olanow, 2004; Jankovic, 2005; Cersosimo and Koller, 2006) suggesting different or more extensive damage in the basal ganglia or to the dopaminergic system. Human and animal studies have shown that toxic exposure to manganese results in metal accumulations in various areas of the basal ganglia and dysfunction of cells of both the striatum and the globus pallidus (Eriksson, et al., 1992; Calne, et al., 1994; Brenneman, et al., 1999; Nagatomo, et al., 1999; Newland, 1999; Pal, et al., 1999; Baek, et al., 2003). Other studies have shown that manganese selectively targets dopaminergic neurons in the human basal ganglia (Pal, et al., 1999; Olanow, 2004) and decreases dopamine levels in the striatum (Mena, et al., 1970; Parenti, et al., 1986; Eriksson, et al., 1987; Vescovi, et al., 1991; Sistrunk, et al., 2007). Considering the clinical similarities between Manganism and Parkinson’s Disease, and the fact that manganese accumulates in brain regions rich in dopaminergic neurons, it has long been suggested that manganese neurotoxicity involves a disruption in dopaminergic neurotransmission (Neff, et al., 1969; Hornykiewicz, 1972; Graham, 1984).
Bivalves and other marine invertebrates are often used in metal environmental toxicology studies because their tissues readily accumulate trace metals to concentrations that are usually much higher on a wet weight basis than what is present in the surrounding seawater (Rainbow, 1993; Phillips and Rainbow, 1993; Boening, 1999). Numerous reports have been made on the bioaccumulation of various heavy metals in the eastern oyster, Crassostrea virginica, and other oyster species (Capar and Yess, 1996; Bu-Olayan and Subrahmanyam, 1997; Scanes and Roach, 1999; Abbe et al., 2000; Fang et al., 2001; Spooner et al., 2003; Rodney et al., 2006). While concentrations of dissolved manganese in freshwaters, even that which is free of anthropogenic sources, can range from 10 to >10 000 µg/L (Reimer, 1999), manganese concentrations in open seawater tend to range from 0.4 to 10 µg/L (US EPA, 1984; Zeri et al., 2000) and rarely rise to over 200 µg/L except in areas of severe hypoxia or coastal regions with high river flows (Eaton, 1979). As an essential nutrient, manganese is actively assimilated and utilized by both plants and animals and studies show significant bioaccumulation of manganese by aquatic biota at lower trophic levels. (Folsom et al., 1963; Thompson et al., 1972; Bryan and Hummerstone, 1973; Pentreath, 1973; Rai and Chandra, 1992). Our lab reported that C. virginica, incubated in the presence of MnCl2, readily accumulated manganese into its tissues (Murray, et al., 2007). Despite its potential for bioaccumulation compared to other aquatic metals, manganese is believed to be one of the least toxic and only a few published reports exist on manganese toxicity in marine organisms. A 2004 IPCA report based upon available toxicity data, suggest that effects of total manganese on marine species of phytoplankton, invertebrates and fish have been observed in laboratory tests that range from a low of 1.5 mg/L based upon a 5-d EC 50 for the marine diatom Ditylum brightwellii (Canterford and Canterford, 1980) to a high of 300 mg/L based up a 7-d LC 50 for the adult clam, Mya arenaria (Eisler, 1977). The only published study involving C. virginica reported manganese toxicity at 16 mg/L based upon a 48 hr LC 50 for oyster embryos (Calabrese et al., 1973). Although there is no current marine quality guideline for manganese, the IPCS (2004) suggested a guidance value of 0.3 mg/L for the protection of 95% marine species with 50% confidence.
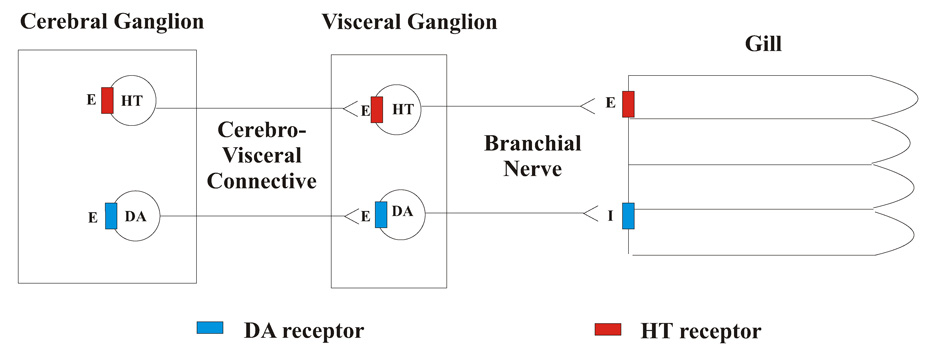
In this study we sought to use C. virginica as a model to study the physiological effects of manganese on a known dopaminergically innervated system. Dopamine, serotonin and other biogenic amines are present in the nervous tissue and gill of C. virginica (Downer, et al, 2006). The innervation of the lateral ciliated cells in the gill by the nervous system of C. virginica is by a cilio-excitatory serotonergic system and a cilio-inhibitory dopaminergic system, schematically shown in Figure 1 (Carroll and Catapane, 2007). The anatomy of the oyster showing the gill, cerebral ganglia and visceral ganglia is shown in Figure 2. Application of serotonin to the cerebral ganglia, visceral ganglia or gill activates quiescent cilia and increases their beating rates. Similar treatments with dopamine decrease the beating rates of the cilia of the lateral cells of the gill and causes cilio-stasis. It is postulated that the animal’s cerebral ganglia contain dopaminergic and serotonergic neurons, which synapse in the visceral ganglia with a second set of dopaminergic and serotonergic neurons, which peripherally innervate the gill via the branchial nerve. At each ganglion serotonin acts as an exciter of cilio-excitatory circuits while dopamine acts as an exciter of cilio-inhibitory circuits. Within the gill, the epithelial cells containing the lateral cilia have serotonin and dopamine receptors that when activated increase or decrease the beating rates of the cilia, respectively.
Figure 1. Schematic of Lateral Ciliary Innervation.
Schematic representation of the innervation of the lateral ciliated cells of the gill of Crassostrea virginica. Serotonin (HT), Dopamine (DA), E = excitatory neurotransmitter, I = inhibitory neurotransmitter.
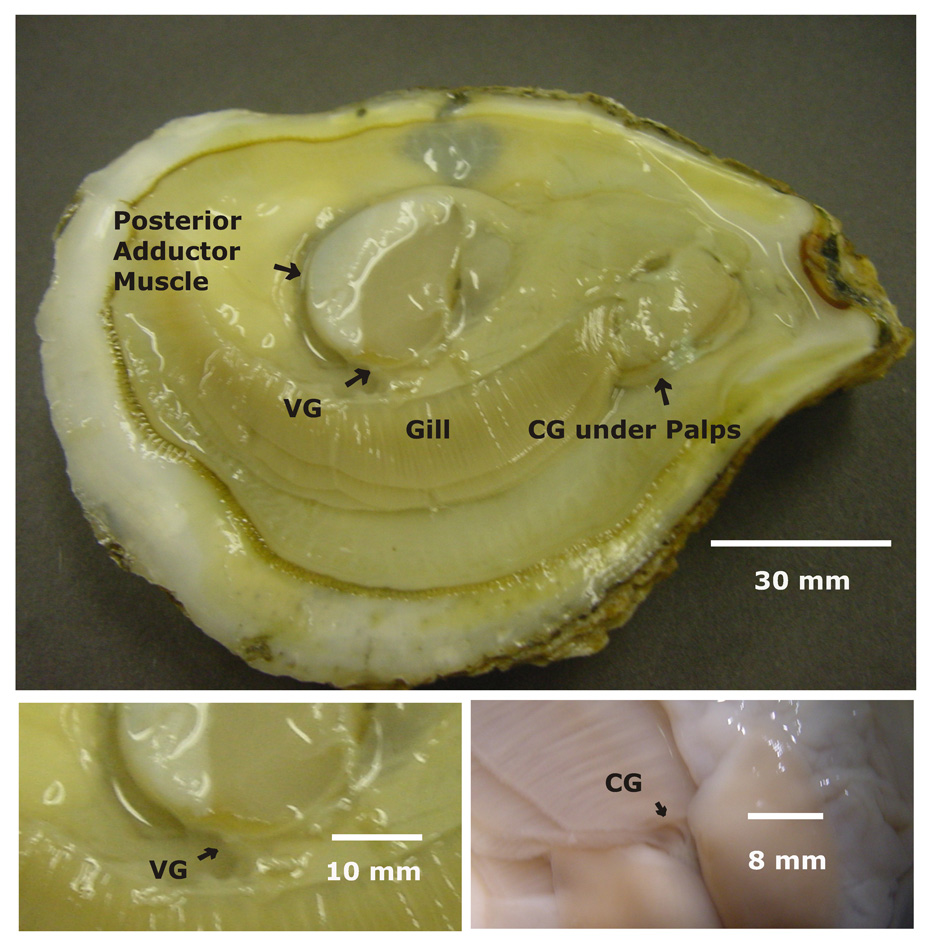
Figure 2.
A - Photograph of a shucked oyster showing the gill, posterior adductor muscle, visceral ganglia (VG) and the location of the cerebral ganglia (CG) under the palps. B - Closeup of the VG, C - Closeup of the CG after reflecting the palps.
The oyster, C. virginica provides a relatively simple nervous system with a serotonergic-dopaminergic innervation component that directs an observable and measurable physiological response, and may be useful in investigating the mechanisms that underlie both manganese neurotoxicity and other dopaminergic cell disorders.
Materials and Methods
Oysters (Crassostrea virginica) were incubated in Instant Ocean® artificial seawater (ASW) obtained from Aquarium Systems Inc. (Mentor, OH, USA). Dopamine, serotonin, 1-octanesulfonic acid (sodium salt, SigmaUltra) and HPLC standards were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other reagents including manganese chloride (MnCl2 • 4 H2O, ASC grade) were obtained from Fisher Scientific (Pittsburgh, PA, USA).
Adult C. virginica of approximately 80 mm shell length were obtained from Frank M. Flower and Sons Oyster Farm in Oyster Bay, NY, USA. They were maintained in the lab for up to two weeks in temperature-regulated aquaria in ASW at 16 – 18°C, specific gravity of 1.024 ± 0.001, salinity of 31.9 ppt, and pH of 7.2 ± 0.2. Each animal was tested for health prior to experimentation by the resistance it offered to being opened. Animals that fully closed in response to tactile stimulation and required at least moderate hand pressure to being opened were used for the experiments. In order to ensure that each oyster would receive equal manganese exposure during the experiment and not just close up, healthy specimens were shucked by removing their right shell before being placed in individual temperature-controlled aerated containers of ASW for 3 days in the presence of up to 1.0 mM manganese. Control animals were similarly prepared without exposure to added manganese. Both control and experimentally treated animals tolerated the 3-day treatment well. Survival was excellent and only animals with visible signs of heart pumping were used in subsequent experiments.
After 3 days, control and manganese-treated specimens were dissected and prepared for microscopic observation of the beating of the cilia of the lateral ciliated cells of the gill epithelium by removing the mantle and most of the internal organs from the gills and ganglia. Cerebral ganglion preparations (CG preparations) were prepared by dissecting the animals so that the gill with the ipsilateral branchial nerve, visceral ganglion, cerebrovisceral connective and cerebral ganglion attached was positioned in an observation chamber containing ASW. Visceral ganglion preparations (VG preparations) were prepared similarly except that the ipsilateral cerebral ganglion was excised. The observation chambers had a plexiglass barrier separating the cerebral ganglion (CG preparations) or visceral ganglion (VG preparations) from the gill so that serotonin or dopamine could be specifically applied to either ganglion or gill without leakage of the chemical to the other chambers (Catapane, et al., 1978). Isolated gill preparations, devoid of any ganglia, were also prepared from control and manganese-treated animals. Just prior to use serotonin was freshly dissolved in ASW, and dopamine was dissolved in ASW containing 10 mg% ascorbic acid buffered with sodium bicarbonate, pH 7.2, to retard dopamine oxidation as described by Malanga (1975). In order to observe lateral cilia beating, gills were positioned so that the cilia of the medial gill lamina were viewed at 100-200X magnification with an Olympus CK inverted microscope with transmitted stroboscopic light from a Grass Instruments PS 22 Photo Stimulator. The activity of the lateral cilia was measured by the method of Catapane, et al., (1978) by synchronizing the flashing rate of the stroboscope with the beating rate of the cilia. Because the lateral cilia beat in a metachronal wave pattern (Aiello and Sleigh, 1972) when synchronization is achieved the lateral cilial waves appear motionless in a characteristic horse-shoe like configuration. At all multiple synchronizing rates above the one corresponding to the true beating frequency, the wavelength of the beating cilia will appear to be a fraction of the true wavelength. The beating rates of the lateral cilia are expressed as beats/s ± sem. Statistical analysis comparing beating of lateral cilia of manganese treated animals to the controls was determined by a two-way ANOVA.
Endogenous serotonin and dopamine levels were measured using High Performance Liquid Chromatography with fluorescence detection based on the method of Fotopoulou and Ioannou (2002). Animals were treated for three days with or without manganese in the same manner as for the portion of the study on beating rates of the lateral cilia, after which time each animal’s cerebral ganglia, visceral ganglia and gill tissue were excised and prepared for HPLC measurements of amine levels. The tissues were homogenized with a Brinkman Polytron homogenizer with Omni International disposable probe tips in 0.4 M HCl. They were centrifuged at 12,000 g for 20 min, and then vacuum filtered through a 0.24 micron filter. Sample (20 µL) was injected into a Beckman System Gold 126/168 HPLC system fitted with a Phenomenex-Gemini (Torrance, CA, USA) 5µ C18 reverse phase, ion pairing column with a guard column. The mobile phase was 50 mM acetate buffer (pH 4.7) containing 1-octanesulfonic acid (1.1 mM) and EDTA (0.11 mM), mixed with methanol (85:15 v/v). All reagents were HPLC grade. The flow rate was 2 mL/min in isocratic mode. A Jasco FP 2020 Plus Spectrofluorometer was used for detection of native fluorescence (280 nm excitation, 320 nm emission) and was fitted with a 16 µL flow cell. HPLC results are reported as ng/g wet mass for gill and ng/ganglion for the ganglia. Statistical analysis comparing dopamine and serotonin levels in gill and ganglia of manganese treated animals to the controls was determined by a two-way ANOVA.
Results
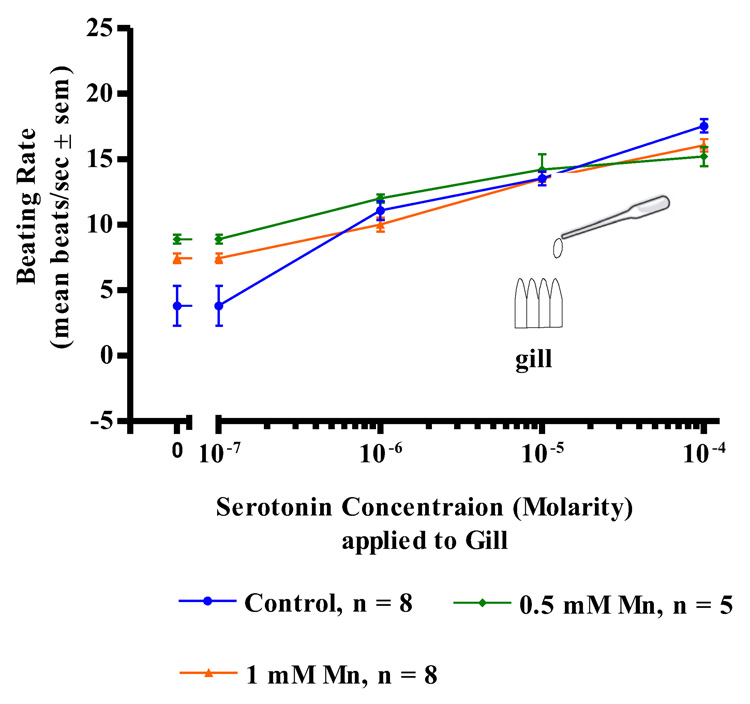
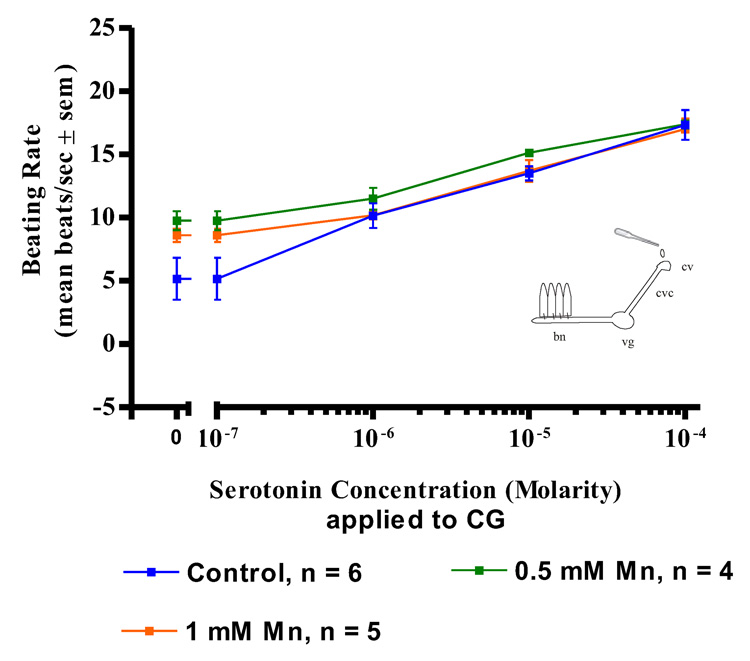
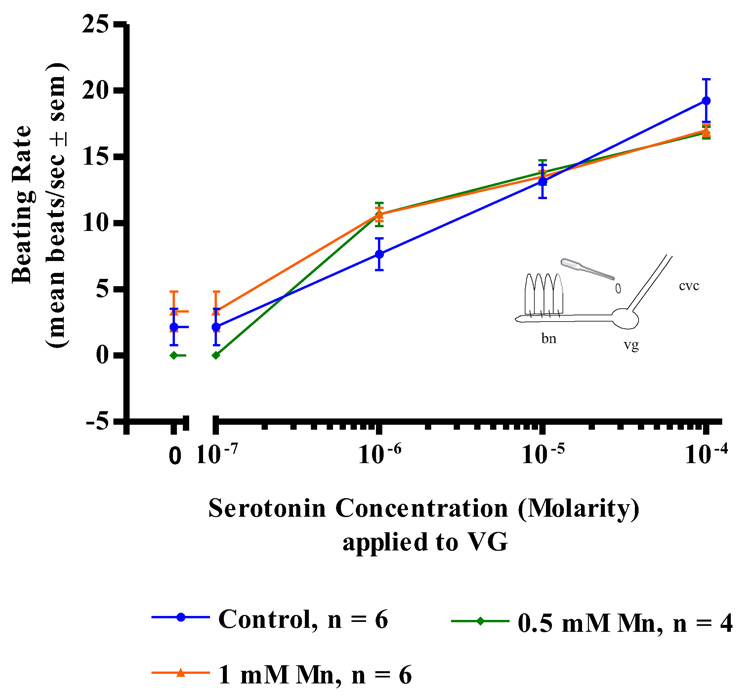
Manganese treatments (up to 1mM) had no effects on the actions of serotonin applications to either the cerebral ganglia, visceral ganglia or isolated gill preps. In both control and manganese-treated animals, serotonin additions (10−7 to 10−4 M) to isolated gill filaments activated the lateral cilia and produced a dose dependent increase in the beating rates of the cilia (Figure 3). Similar results were observed in both control and manganese-treated animals when serotonin was applied to the ganglia of either CG preparations (Figure 4) or VG preparation (Figure 5). In all cases, the dose dependent increases in the beating rates of the cilia that occurred upon serotonin additions were not statistically different for the manganese-treated animals as compared to controls.
Figure 3. Serotonin on Gill Preparations.
The changes in beating rates (mean beats/s ± sem) of lateral gill cilia in response to serotonin applied directly to excised gill of controls and animals treated with 0.5 and 1 mM Mn. Statistical analysis was determined by a two-way ANOVA and showed no significant differences among the treatments.
Figure 4. Serotonin on CG Preparations.
The changes in beating rates (mean beats/s) of lateral gill cilia in response to superfusion of serotonin to the cerebral ganglia of CG Preparations of controls and animals treated with 0.5 and 1 mM Mn. Statistical analysis was determined by a two-way ANOVA and showed no significant differences among the treatments. bn = branchial nerve, vg = visceral ganglion, cvc = cerebrovisceral connective, cv = cerebral ganglion.
Figure 5. Serotonin on VG Preaprations.
The changes in beating rates (mean beats/s) of lateral gill cilia in response to superfusion of serotonin to the visceral ganglia of VG Preparations of controls and animals treated with 0.5 and 1 mM Mn. Statistical analysis was determined by a two-way ANOVA and showed no significant differences among the treatments.
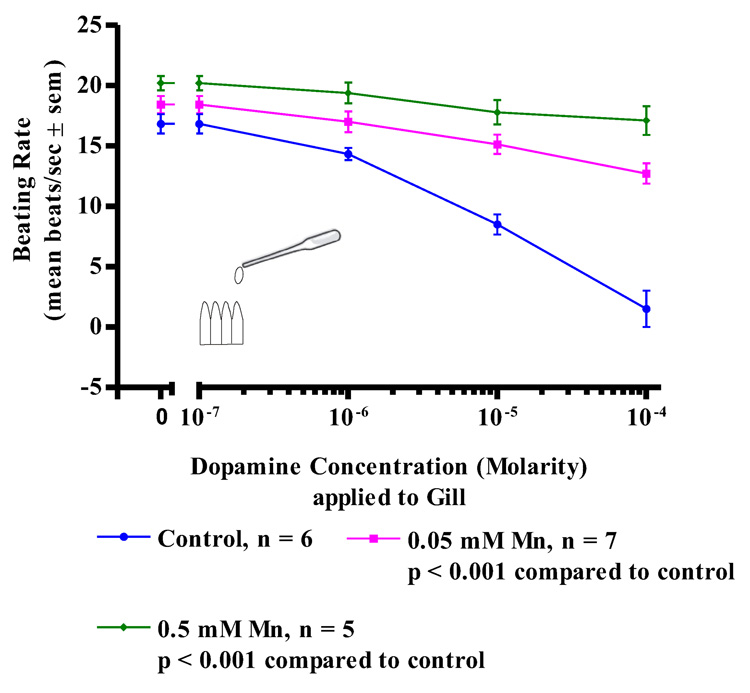
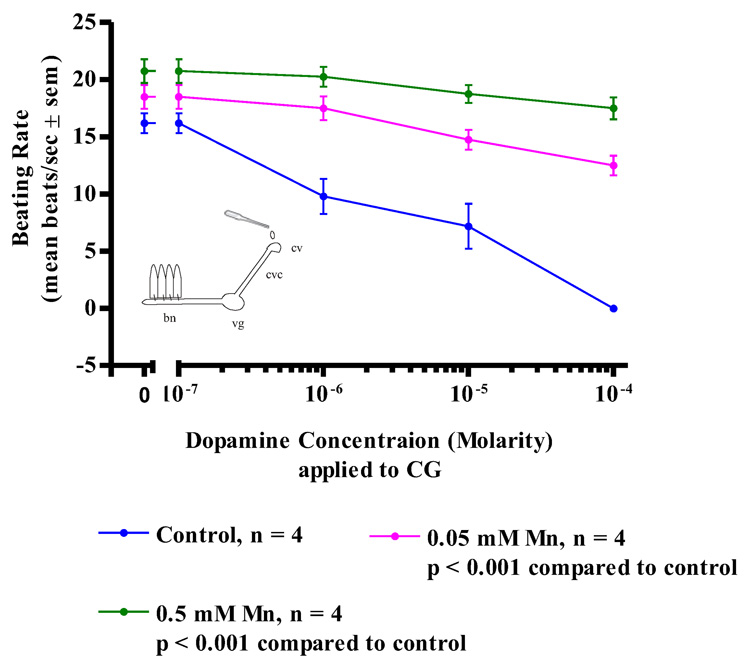
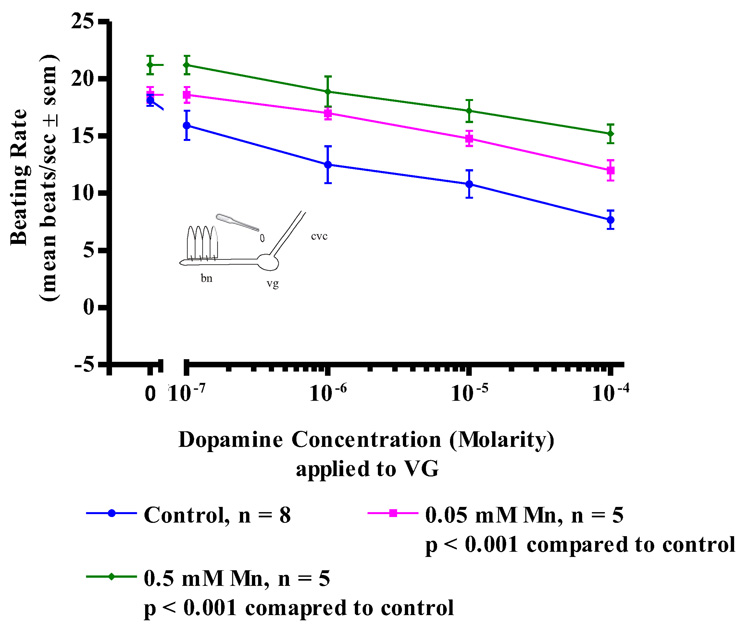
While acute treatments with manganese had no effect on the oyster’s serotonergic, cilio-excitatory system, there was noted impairment of the animal’s dopaminergic, cilio-inhibitory system. Since the basal beating rate of the lateral cilia tended to be quiescent at the start of the experiments, before testing for the cilio-inhibitory effects of applied dopamine to the cerebral ganglia, visceral ganglia or gill preparations, gill tissue was bathed in 10−5 M serotonin to activate the lateral cilia and allow it to achieve a steady beating rate of at least 15 beats/s. The serotonin remained in the bath for the duration of the experiments. In control animals, subsequent application of dopamine to isolated gill preparations (Figure 6) produced an expected dose dependant decrease in the beating rates of the lateral cilia, with 10−4 M dopamine causing almost complete cessation of beating. In contrast, gill preparations of manganese-treated animals showed a statistically significant reduction in the inhibition of beating rates of the lateral cilia in response to dopamine, with 0.5 mM manganese treatments producing the most reduction of effect. Similar differences in the response of control and manganese-treated animals to dopamine were obtained in experiments involving CG preparations (Figure 7) and VG preparations (Figure 8). In control animals, application of dopamine to either the cerebral or visceral ganglia caused the expected dose dependent reduction of lateral cilia beating rates, while in manganese-treated animals, dopamine application to either the cerebral or visceral ganglia was statistically less effective in reducing the beating rates of the cilia.
Figure 6. Dopamine on Gill Preperations.
The changes in beating rates (mean beats/s ± sem) of lateral gill cilia in response to dopamine applied directly to excised gill of controls and animals treated with 0.05 and 0.5 mM Mn. The gill was first activated with 10−5 M serotonin before dopamine applications. Statistical analysis was determined by a two-way ANOVA.
Figure 7. Dopamine on CG Preparations.
The changes in beating rates (mean beats/s ± sem) of lateral gill cilia in response to superfusion of dopamine to the cerebral ganglia of CG Preparations of controls and animals treated with 0.05, and 0.5 mM Mn. The gill was first activated with 10−5 M serotonin before dopamine applications. Statistical analysis was determined by a two-way ANOVA.
Figure 8. Dopamine on VG Preperations.
The changes in beating rates (mean beats/s ± sem) of lateral gill cilia in response to superfusion of dopamine to the visceral ganglia of VG Preparations of controls and animals treated with 0.05, and 0.5 mM Mn. The gill was first activated with 10−5 M serotonin before dopamine applications. Statistical analysis was determined by a two-way ANOVA.
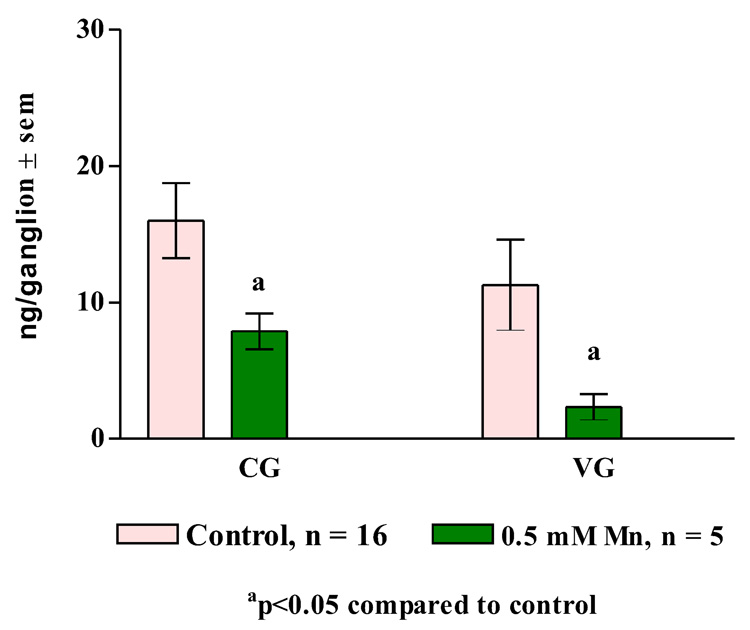
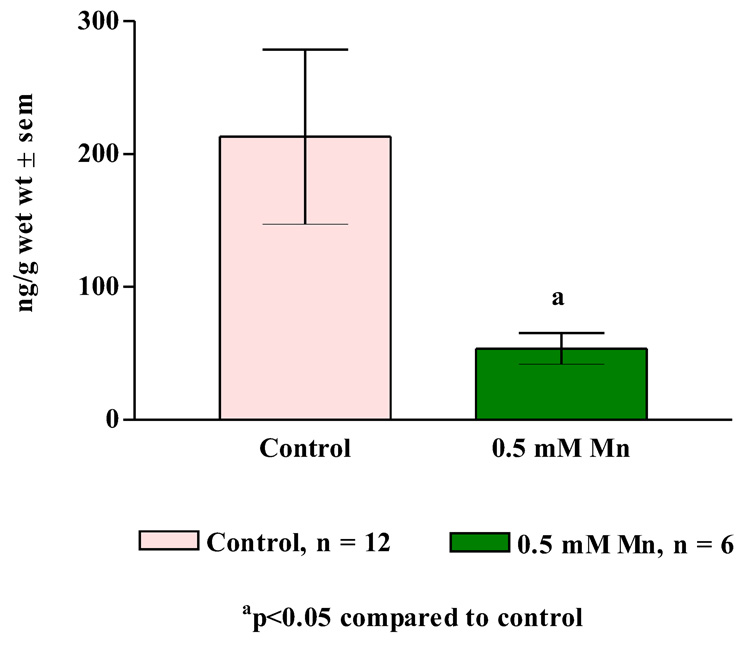
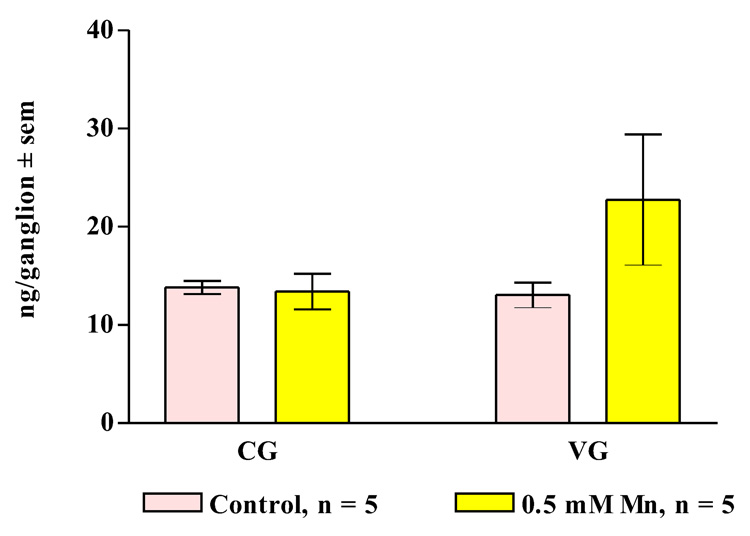
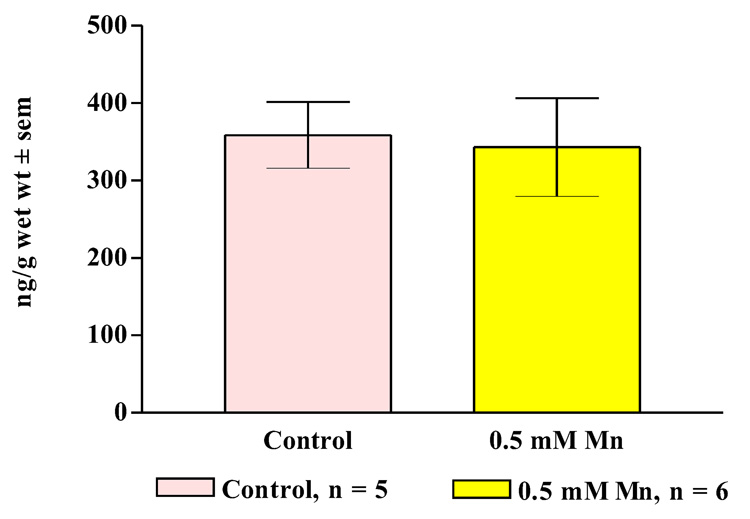
In other experiments, treating oysters for three days with 0.5 mM manganese resulted in statistically significant decreases of dopamine levels in the cerebral ganglia, visceral ganglia and gill of the animals (Figure 9, Figure 10) while not causing any significant change in serotonin levels (Figure 11, Figure 12).
Figure 9. Ganglion Dopamine Levels.
Endogenous dopamine levels (mean ± sem) in the cerebral ganglia (CG) and visceral ganglia (VG) of animals treated for 3 days with 0.5 mM manganese. Statistical analysis was determined by a two-way ANOVA.
Figure 10. Gill Dopamine Levels.
Endogenous dopamine levels (mean ± sem) in the gill of animals treated for 3 days with 0.5 mM manganese. Statistical analysis was determined by a two-way ANOVA.
Figure 11. Ganglion Serotonin Levels.
Endogenous serotonin levels (mean ± sem) in the cerebral ganglia (CG) and visceral ganglia (VG) of animals treated for 3 days with 0.5 mM manganese. Statistical analysis was determined by a two-way ANOVA and showed no significant differences between the treated and untreated animals.
Figure 12. Gill Serotonin Levels.
Endogenous serotonin levels (mean ± sem) in the gill of animals treated for 3 days with 0.5 mM manganese. Statistical analysis was determined by a two-way ANOVA and showed no significant differences between the treated and untreated animals.
Discussion
The study shows that a 3-day treatment of C. virginica with manganese disrupted the oyster’s dopaminergic, cilio-inhibitory mechanism, while not impairing the serotonergic cilio-excitatory mechanism. The actions of exogenous dopamine on the activity of the lateral cilia when applied to the cerebral ganglia, visceral ganglia or isolated gill were all reduced, and in all cases higher dose manganese treatments (0.5 mM) produced greater disruption of the cilio-inhibitory mechanism than lower dose treatments (0.05 mM.). In contrast, there was no significant difference in the response of the cilia in manganese-treated animals, compared to controls, when exogenous serotonin was applied to the cerebral ganglia, visceral ganglia or gill. The study also shows that similar treatments results in lower endogenous dopamine, but not serotonin levels, in the gill tissue, cerebral ganglia and visceral ganglia of manganese-exposed animals compared to controls. Taken together, the results indicate the specificity of manganese toxicity on the animal’s dopaminergic system.
The results indicate that the mechanism of action underlying manganese disruption of the cilio-inhibitory response to dopamine is likely to be primarily post-synaptic, at least at the level of the gill, because direct application of dopamine to isolated gill preparations did not reduce the beating rates of the cilia in manganese-treated animals as in did in untreated animals. While manganese treatments did reduce endogenous dopamine levels at the gill, the fact that subsequent applications of exogenous dopamine to gill preparations did not elicit a significant decrease in the beating rates of the lateral cilia indicates that a reduction in terminal dopamine release does not fully explain the lack of response of the lateral ciliated cells to dopamine in manganese-treated oysters. It is possible that manganese treatments damaged dopamine receptors on gill epithelium or disrupted the post-receptor signal transduction mechanism that slows the beating of the lateral cilia. Similar post-synaptic dopaminergic impairment also may be occurring at the cerebral and visceral ganglia. Besides the reduction in dopamine levels, manganese treatments may have had other or additional effects on dopaminergic neurons in the ganglia, including neuronal loss, decrease terminal release of dopamine or defective dopamine reuptake. While the actions of exogenous dopamine application to the cerebral or visceral ganglia of manganese treated animals were impaired, bath applications of drugs to the ganglia, which most likely contain heterogenous polysynaptic pathways, cannot finely discern these mechanisms and the toxic actions of manganese within the ganglia are likely to be more complex and involve multiple sites of action. While manganese treatments did reduce endogenous dopamine levels in both ganglia, compared to controls, the HPLC results do not allow us to determine whether this reduction was due to an actual loss of dopaminergic neurons or simply neuronal dysfunction at the pre or post-synaptic level. The mechanism by which manganese produces dopaminergic dysfunction in humans also is not fully resolved, but it is postulated that it may be more related to downstream neuronal pathways than to deficits in nigrostriatal functions (Calne, et al., 1994; Huang, et al.; 1998, Olanow, 2004). Still, Manganism is associated with reductions in nigrostriatal dopamine levels, and in a recent study, a progressive in vivo decrease in nigrostriatal dopamine release was correlated with subtle motor deficits in manganese exposed non-human primates (Guilarte, et al., 2006).
While the cellular and molecular mechanism of manganese toxicity remains unclear, several lines of evidence suggest that exposure to manganese or manganese-containing compounds induces oxidative stress-mediated dopaminergic cell death (Anantharam, et al., 2002; Stredrick, et al., 2004; Latchoumycandane, et al., 2005) which is in agreement with current theories on oxidative stress as a mediator of neuronal death in Parkinson’s disease and other neurodegenerative diseases (Fahn and Cohen, 1992; Albers and Beal, 2000; Schulz, et al, 2000; Dawson and Dawson, 2003; Emerit, et al., 2004). Oxidative stress is also suspected of being a factor in 1-methyl-4-phenyl-1,2,3,6-tetrahyrdopyridine (MPTP)-induced Parkinson’s disease because transgenic mice who overexpressed copper/zinc superoxide dismutase were protected from the dopaminergic neuronal degeneration caused by MPTP exposure (Przedborski, et al., 1992).
Dopaminergic neurons and dopamine-rich areas of the brain are particularly vulnerable to oxidative stress, because the enzymatic and non-enzymatic metabolism of dopamine can generate reactive oxygen species and various neurotoxic catecholamine metabolites such as 6-hydroxydopamine (Halliwell, 1992; Lotharius and Brundin, 2002; Cantuti-Castelvetri, et al., 2003; Dauer and Przedborski, 2003; Dawson and Dawson, 2003.) Even in bivalves, a previous study on Mytilus edulis, showed that treatments with 6-hydroxydopamine caused a reduction in the animal’s ganglionic levels of dopamine (Stefano, et al., 1976.) A recent study using transgenic mice provided in vivo evidence that chronic exposure to unregulated cytosolic dopamine alone was sufficient to cause neurodegeneration in striatal neurons and resulting motor dysfunction (Chen, et al., 2008).
Manganese is a trace element in animal systems required for normal carbohydrate, lipid, amino acid and protein metabolism, as well as a required cofactor for various antioxidant enzymes such as mitochondrial superoxide dismutase (Cotzias, 1958; Takeda, 2003). However when in excess, manganese is cytotoxic and has been shown to raise levels of reactive oxygen species (Ali, et al., 1995; Milatovic, et al., 2007), deplete glutathione (Shi and Dalal, 1990), impair energy metabolism (Brouillet, et al., 1993), and cause oxidation of catecholamine and other biological chemicals (Archibald and Tyree, 1987). The prooxidant character of excess manganese and the fact that metal accumulates in dopamine-rich areas of the brain strongly suggests that manganese toxicity is causing further oxidative stress on an already stressed dopaminergic system (Galvani, et al., 1995; Sloot, et al., 1996; Aschner, 1997; Takeda, 2003; HaMai and Bondy, 2004).
The present study demonstrates that the gill/ganglia preparations of C. virginica can be used to investigate the mechanism that underlies manganese neurotoxcity, and may also serve as a model in the pharmacological study of drugs to treat or prevent Manganism and perhaps other dopaminergic cell disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbe GR, Riedel GF, Sanders JG. Factors that influence the accumulation of copper and cadmium by transplanted eastern oysters (Crassostrea virginica) in the Patuxent River, Maryland. Mar Environ Res. 2000;49:377–396. doi: 10.1016/s0141-1136(99)00082-3. [DOI] [PubMed] [Google Scholar]
- Aiello E, Sleigh MA. The metachronal wave of lateral cilia of Mytilus edulis. J Cell Biol. 1972;54:493–506. doi: 10.1083/jcb.54.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers DS, Beal MF. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative diseases. J Neural Transm Suppl. 2000;59:133–154. doi: 10.1007/978-3-7091-6781-6_16. [DOI] [PubMed] [Google Scholar]
- Ali SF, Duhart HM, Newport GD, Lipe GW, Slikker W., Jr Manganese-induced reactive oxygen species: Comparison between Mn+2 and Mn+3. Neurodegeneration. 1995;4:329–334. doi: 10.1016/1055-8330(95)90023-3. [DOI] [PubMed] [Google Scholar]
- Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3-dependent proteolytic cleavage of protein kinase C delta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci. 2002;22:1738–1751. doi: 10.1523/JNEUROSCI.22-05-01738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ME, Gearhart JM, Clewell HJ., III Pharmacokinetic data needs to support risk assessments for inhaled and ingested manganese. Neurotoxicology. 1999;20:161–171. [PubMed] [Google Scholar]
- Archibald FS, Tyree C. Manganese poisoning and the attack of trivalent manganese upon catecholamines. Arch Biochem Biophys. 1987;256:638–650. doi: 10.1016/0003-9861(87)90621-7. [DOI] [PubMed] [Google Scholar]
- Aschner M. Metals and Oxidative Damage in Neurological Disorders. New York: Plenum Press; 1997. Manganese neurotoxicity and oxidative damage; pp. 77–93. [Google Scholar]
- Aschner M. Manganese in health and disease: from transport to neurotoxicity. In: Massaro E, editor. Handbook of Neurotoxicology. Totowa, NJ: Humana Press; 2000. pp. 195–209. [Google Scholar]
- Baek SY, Lee MJ, Jung HS, Kim HJ, Lee CR, Yoo C, Lee JH, Lee H, Yoon CS, Kim YH, Park J, Kim JW, Jeon BS, Kim Y. Effect of manganese exposure on MPTP neurotoxicities. Neurotoxicology. 2003;24:657–665. doi: 10.1016/S0161-813X(03)00033-0. [DOI] [PubMed] [Google Scholar]
- Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C Cotzias) Neurotoxicology. 1984;5:13–35. [PubMed] [Google Scholar]
- Barbeau A, Inoué N, Cloutier T. Role of manganese in dystonia. In: Eldridge R, Fahn S, editors. Advances in Neurol. vol 14. New York: Raven Press; 1976. pp. 339–352. [PubMed] [Google Scholar]
- Boening DW. An evaluation of bivalves as biomonitors of heavy metal pollution in marine waters. Environ Monit Assessmt. 1999;55:459–470. [Google Scholar]
- Brenneman KA, Cattley RC, Ali SF, Dorman DC. Manganese induced developmental neurotoxicity in the CD rat: is oxidative damage a mechanism of action? Neurotoxicology. 1999;20:477–488. [PubMed] [Google Scholar]
- Brouillet EPL, Shinobu U, McGarvey F, Hochberg F, Beal MF. Managnese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp Neurol. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Bryan GW, Hummerstone LG. Brown seaweed as an indicator of heavy metals in estuaries in south-west England. J Mar Biol Assoc UK. 1973;53:705–720. [Google Scholar]
- Bu-Olayan AH, Subrahmanyam MN. Accumulation of copper, nickel, lead and zinc by snail, Lunella coronatus and pearl oyster, Pinctada radiata from the Kuwait coast before and after the Gulf War oil spill. Sci Total Environ. 1997;197:161–165. doi: 10.1016/s0048-9697(97)05428-4. [DOI] [PubMed] [Google Scholar]
- Calabrese A, Collier RS, Nelson DA, MacInnes JR. The toxicity of heavy metals to embryos of the American oyster Crassostrea virginica. Mar Biol. 1973;18:162–166. [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic Parkinsonism: similarities and difference. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Canterford GS, Canterford DR. Toxicity of heavy metals to the marine diatom Ditylum brightwellii (West) Grunow: correlation between toxicity and metal speciation. J Mar Biol Assoc UK. 1980;60:227–242. [Google Scholar]
- Cantuti-Castelvetri I, Shukitt-Hale B, Joseph JA. Dopamine neurotoxicity: age dependent behavioral and histological effects. Neurobiol Aging. 2003;24:697–706. doi: 10.1016/s0197-4580(02)00186-0. [DOI] [PubMed] [Google Scholar]
- Capar SG, Yess NJ. US Food and Drug Administration survey of cadmium, lead and other elements in clams and oysters. Food Addit Contam. 1996;13:553–560. doi: 10.1080/02652039609374440. [DOI] [PubMed] [Google Scholar]
- Carroll MA, Catapane EJ. The nervous system control of lateral ciliary activity of the gill of the bivalve mollusc, Crassostrea virginica. Comp Biochem Physiol A. 2007;148:445–450. doi: 10.1016/j.cbpa.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catapane EJ, Stefano GB, Aiello E. Pharmacological study of the reciprocal dual innervation of the lateral ciliated gill epithelium by the CNS of Mytilus edulis (Bivalvia) J Exp Biol. 1978;74:101–113. [Google Scholar]
- Cersosimo MG, Koller WC. The diagnosis of manganese-induced parkinsonism. NeuroToxicology. 2006;27:340–346. doi: 10.1016/j.neuro.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Chen L, Ding Y, Cagniard B, Van Laar AD, Mortimer A, Chi W, Hastings TG, Kang UJ, Zhuang X. Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. J Neurosci. 2008;28:425–433. doi: 10.1523/JNEUROSCI.3602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotzias GC. Manganese in health and disease. Physiol Revs. 1958;38:503–532. doi: 10.1152/physrev.1958.38.3.503. [DOI] [PubMed] [Google Scholar]
- Couper J. On the effects of black oxide of manganese when inhaled into the lungs. Br Ann Med Pharmacol. 1837;1:41–42. [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Davis JM. Inhalation health risks of manganese: An EPA perspective. Neurotoxicology. 1999;20:511–518. [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann NY Acad Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- Donaldson J. The physiopathologic significance of manganese in brain: its relation to schizophrenia and neurodegenerative disorders. Neurotoxicology. 1987;8:451–462. [PubMed] [Google Scholar]
- Dorman DC, Melanie, Struve F, Clewell HJ, III, Andersen ME. Application of pharmacokinetic data to the risk assessment of inhaled manganese. NeuroToxicology. 2006;27:752–764. doi: 10.1016/j.neuro.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Downer N, Myrthil M, Nduka E, Lecky D, Catapane EJ. Effects of acute temperature stress on the distribution of biogenic amines in the American Oyster, Crassostrea virginica; Annals of the 2006 SICB Conference; 2006. abstract P2.100. [Google Scholar]
- Eaton A. The impact of anoxia on Mn fluxes in the Chesapeake Bay. Geochim Cosmochim Acta. 1979;43:429–432. [Google Scholar]
- Eisler R. Acute toxicities of selected heavy metals to the softshell clam, Mya arenaria. Bull Environ Contam Toxicol. 1977;17:137–144. doi: 10.1007/BF01685540. [DOI] [PubMed] [Google Scholar]
- Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dobson AW, Dorman DC, Aschner M. Manganese exposure and induced oxidative stress in the rat brain. Sci Total Environ. 2004;334–335:409–416. doi: 10.1016/j.scitotenv.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson H, Magiste K, Plantin LO, Fonnum F, Hedstrtm K, Theodorsson G, Norheim E, Kristensson K, Stalberg E, Heilbronn E. Effects of manganese oxide on monkeys as revealed by a combined neurochemical, histological and neurophysiological evaluation. Arch Toxicol. 1987;61:46–52. doi: 10.1007/BF00324547. [DOI] [PubMed] [Google Scholar]
- Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann Neurol. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- Fang ZQ, Cheung RYH, Wong MH. Heavy Metal concentrations in edible bivalves and gastropods available in the major markets of the Pearl River Delta. J Environ Sci. 2001;13:210–217. [PubMed] [Google Scholar]
- Folsom TR, Young DR, Johnson JN, Pillai KC. Manganese-54 and zinc-65 in coastal organisms of California. Nature. 1963;200:327–329. [Google Scholar]
- Fotopoulou MA, Ioannou PCP. Post-column terbium complexation and sensitized fluorescence detection for the determination of norepinephrine, epinephrine and dopamine using high-performance liquid chromatography. Anal Chim Acta. 2002;462:179–185. [Google Scholar]
- Galvani P, Fumagalli P, Santagostino A. Vulnerability of mitochondrial complex I in PC12 cells exposed to manganese. Eur J Pharmacol. 1995;293:377–383. doi: 10.1016/0926-6917(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Rybicki BA, Cole Johnson C, Peterson EL. Occupational metal exposures and the risk of Parkinson’s disease. Neuroepidemiology. 1999;18:303–308. doi: 10.1159/000026225. [DOI] [PubMed] [Google Scholar]
- Graham DG. Catecholamine toxicity: a proposal for the molecular pathogenesis of manganese neurotoxicity and Parkinson’s disease. NeuroToxicology. 1984;5:83–95. [PubMed] [Google Scholar]
- Guilarte TR, Ming-Kai C, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol. 2006;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- HaMai D, Bondy SC. Oxidative basis of manganese neurotoxicity. Ann NY Acad Sci. 2004;1012:129–141. doi: 10.1196/annals.1306.010. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine and extrapyramidal motor function and dysfunction. Res Publ Assoc Res Nerv Mental Dis. 1972;50:390–415. [PubMed] [Google Scholar]
- Huang CC, Chu NS, Lu CS, Wang JD, Tsai JL, Tzeng JL. Chronic manganese intoxication. Arch Neurol. 1989;46:1104–1106. doi: 10.1001/archneur.1989.00520460090018. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lu CS, Chu NS, Hochberg F, Lilienfeld D, Olanow W. Progression after chronic manganese exposure. Neurology. 1993;43:1479–1483. doi: 10.1212/wnl.43.8.1479. [DOI] [PubMed] [Google Scholar]
- Huang CC, Chu NS, Lu CS, Chen RS, Calne DB. Longterm progression in chronic manganism- Ten years follow-up. Neurology. 1998;50:698–700. doi: 10.1212/wnl.50.3.698. [DOI] [PubMed] [Google Scholar]
- International Program on Chemical Safety (IPCS) Manganese and its compounds: environmental aspects. Geneva, Switzerland: World Health Organization; Concise International Chemical Assessment Document (Cicads) 63. 2004 http://www.inchem.org/documents/cicads/cicads/cicad63.htm#app4.
- Iregren A. Manganese neurotoxicity in industrial exposures: proof of effects, critical exposure level, and sensitive tests. Neurotoxicology. 1999;20:315–324. [PubMed] [Google Scholar]
- Jankovic J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology. 2005;64:2021–2028. doi: 10.1212/01.WNL.0000166916.40902.63. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology. 2005;64:2021–2028. doi: 10.1212/01.WNL.0000166916.40902.63. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Manganese: A high octane dispute. Science. 2003;300:926–928. doi: 10.1126/science.300.5621.926. [DOI] [PubMed] [Google Scholar]
- Koller WC, Lyons KE, Truly W. Effect of levodopa treatment for Parkinsonism in welders. A double-blind study, Neurology. 2004;62:730–733. doi: 10.1212/01.wnl.0000113726.34734.15. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase C delta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J Pharmacol Exp Therap. 2005;313:46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- Levy BS, Nassetta WJ. Neurological effects of manganese in humans: a review. Int J Occup Environ Health. 2003;9:153–163. doi: 10.1179/oeh.2003.9.2.153. [DOI] [PubMed] [Google Scholar]
- Lotharius J, Brundin P. Pathogenesis of Parkinson's disease. Dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3:932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- Lu CS, Huang CC, Chum NS, Calne DB. Levodopa failure in chronic manganism. Neurology. 1994;44:1600–1602. doi: 10.1212/wnl.44.9.1600. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Apostoli P, Perrone C, Placidi D, Albini E, Migliorati P. Long-term exposure to “low levels’ of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicity. 1999;20:287–297. [PubMed] [Google Scholar]
- Malanga CJ. Dopaminergic stimulation of frontal ciliary activity in the gill of Mytilus edulis. Comp Biochem Physiol C. 1975;51:25–34. doi: 10.1016/0306-4492(75)90033-7. [DOI] [PubMed] [Google Scholar]
- Meco G, Bonfanti V, Vanacore N, Fabrizio E. Parkinsonism after chronic exposure to the fungicide maneb (manganese ethylene-bis-dithiocarbamate. Scand J Work, Environ Health. 1994;20:301–305. doi: 10.5271/sjweh.1394. [DOI] [PubMed] [Google Scholar]
- Mena I, Marin O, Fuenzalida S, Cotzias GC. Chronic manganese poisoning. Clinical picture and manganese turnover. Neurology. 1967;17:128–136. doi: 10.1212/wnl.17.2.128. [DOI] [PubMed] [Google Scholar]
- Mena I, Court J, Fuenzalida S, Papavasiliou PS, Cotzias GS. Modification of chronic manganese poisoning. N Engl J Med. 1970;282:5–10. doi: 10.1056/NEJM197001012820102. [DOI] [PubMed] [Google Scholar]
- Mergler D. Manganese: the controversial metal. At what levels can deleterious effects occur? Can J Neurol Sci. 1996;23:93–94. doi: 10.1017/s0317167100038774. [DOI] [PubMed] [Google Scholar]
- Mergler D. Neurotoxic effects of low level exposure to manganese in human populations. Environ Res. 1999;80:99–102. doi: 10.1006/enrs.1998.3902. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, Aschner JL, Aschner M. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol Sci. 2007;98:198–205. doi: 10.1093/toxsci/kfm095. [DOI] [PubMed] [Google Scholar]
- Murray S, Lovell A, Carroll MA, Catapane EJ. Distribution of manganese in the oyster Crassostrea virginica raised in Jamaica Bay, NY and its accumulations in oysters acutely exposed to high levels; Annals of the 2007 SICB Conference; 2007. [Google Scholar]
- Nagatomo S, Umehara F, Hanada K, Nobuhara Y, Takenaga S, Arimura K, Osame M. Manganese intoxication during total parenteral nutrition: report of two cases and review of the literature. J Neurolog Sci. 1999;162:102–105. doi: 10.1016/s0022-510x(98)00289-5. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences. Medical and biological effects of environmental pollutants: Manganese. Wash. DC: National academy Press; 1973. pp. 1–101. [Google Scholar]
- Neff NH, Barrett RE, Costa E. Selective depletion of caudate nucleus dopamine and serotonin during chronic manganese dioxide administration to squirrel monkeys. Experientia. 1969;25:1140–1141. doi: 10.1007/BF01900234. [DOI] [PubMed] [Google Scholar]
- Newland MC. Animal models of manganese's neurotoxicity. Neurotoxicology. 1999;20:415–432. [PubMed] [Google Scholar]
- Olanow CW. Manganese -induced parkinsonism and Parkinson's disease. Ann NY Acad Sci. 2004;1012:209–223. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- Ostiguy C, Asselin P, Malo S. The emergence of manganese-related health problems in Quebec: An integrated approach to evaluation, diagnosis, management and control. NeuroToxicology. 2006;27:350–356. doi: 10.1016/j.neuro.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- Parenti M, Flauto C, Parati E, Vescovi A, Groppetti A. Manganese neurotoxicity: effects of L-DOPA and pargyline treatments. Brain Res. 1986;367:8–13. doi: 10.1016/0006-8993(86)91571-4. [DOI] [PubMed] [Google Scholar]
- Pentreath RJ. The accumulation from water of 65Zn, 54Mn, 58Co, and 59Fe by the mussel, Mytilus edulis. J Mar Biol Assoc UK. 1973;53:127–143. [Google Scholar]
- Phillips D, Rainbow P. Biomonitoring of trace aquatic contaminants. London, New York: Elsevier Applied Science; 1993. p. 371. [Google Scholar]
- Przedborski S, Kostic V, Jackson-Lewis V, Naini AB, Simonetti S, Fahn S. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurosci. 1992;12:1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette BA, Tabbal SD, Jennings D, Good L, Perlmutter JS, Evanoff B. Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology. 2005;64:230–235. doi: 10.1212/01.WNL.0000149511.19487.44. [DOI] [PubMed] [Google Scholar]
- Rai UN, Chandra P. Accumulation of copper, lead, manganese and iron by field populations of Hydrodictyon reticulatum (Linn.) Lagerheim. Sci Total Environ. 1992;116:203–211. doi: 10.1016/0048-9697(92)90449-3. [DOI] [PubMed] [Google Scholar]
- Rainbow PS. The Significance of Trace Metal Concentrations in Marine Invertebrates. In: Dallinger R, Rainbow PS, editors. Ecotoxicology of Metals in Invertebrates. Boca Raton, Florida: Lewis Publishers; 1993. pp. 3–23. [Google Scholar]
- Reidy TJ, Bowler RM, Rauch SS, Pedroza GI. Pesticide exposure and neuropsychological impairment in migrant farm workers. Arch Clin Neuropsychol. 1992;7:85–95. [PubMed] [Google Scholar]
- Reimer PS. Environmental effects of manganese and proposed freshwater guidelines to protect aquatic life in British Columbia [MSc thesis] Vancouver, B.C.: University of British Columbia; 1999. [Google Scholar]
- Rodney E, Herrera P, Luxama J, Boykin M, Crawford A, Carroll MA, Catapane EJ. Bioaccumulation and Tissue Distribution of arsenic, cadmium, copper and zinc in Crassostrea virginica grown at two different depths in Jamaica Bay. Vol. 29. New York: In Vivo; 2007. pp. 16–27. [PMC free article] [PubMed] [Google Scholar]
- Rosenstock HA, Simons DG, Meyer JS. Chronic manganism. Neurologic and laboratory studies during treatment with levodopa. JAMA. 1971;217:1354–1358. doi: 10.1001/jama.217.10.1354. [DOI] [PubMed] [Google Scholar]
- Sadek AH, Rauch R, Schulz PE. Parkinsonism due to manganism in a welder. Int J Toxicol. 2003;22:393–401. doi: 10.1177/109158180302200511. [DOI] [PubMed] [Google Scholar]
- Scanes PR, Roach AC. Determining natural background concentrations of trace metals in oysters from New South Wales. Australia Environ Pollut. 1999;105:437–446. [Google Scholar]
- Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- Shi XL, Dalal NS. The glutathionyl radical formation in the reaction between manganese and glutathione and its neurotoxic implications. Med-Hypoth. 1990;33:83–87. doi: 10.1016/0306-9877(90)90184-g. [DOI] [PubMed] [Google Scholar]
- Sistrunk SC, Ross MK, Filipov NM. Direct effects of manganese compounds on dopamine and its metabolite DOPAC: An in vivo study. Environ Toxicol Pharmacol. 2007;23:286–296. doi: 10.1016/j.etap.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloot WN, Korf J, Koster JF, DeWit LEA, Gramsbergen JBP. Manganese-induced hydroxyl radical formation in rat striatum is not attenuated by dopamine depletion or iron chelation in vivo. Exp Neurol. 1996;138:236–245. doi: 10.1006/exnr.1996.0062. [DOI] [PubMed] [Google Scholar]
- Spooner DR, Maher W, Otway N. Trace metal concentrations in sediments and oysters of Botany Bay, NSW, Australia. Arch Environ Contam Toxicol. 2003;45:0092–0101. doi: 10.1007/s00244-002-0111-0. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Catapane EJ, Aiello E. Dopaminergic agents: Influence on serotonin in Molluscan nervous system. Science. 1976;194:539–541. doi: 10.1126/science.973139. [DOI] [PubMed] [Google Scholar]
- Stredrick DL, Stokes AH, Worst TJ, Freeman WH, Johnson EA, Lash LH, Aschner M, Vrana KE. Manganese-induced cytotoxicity in dopamine-producing cells. NeuroToxicology. 2004;25:543–553. doi: 10.1016/j.neuro.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Takeda A. Manganese action in brain function. Brain Res Revs. 2003;41:79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Thompson SE, Burton CA, Quinn DJ, Ng YC. Concentration factors of chemical elements in edible aquatic organisms. Livermore, CA: University of California, Lawrence Livermore Laboratory, Bio-Medical Division; 1972. [Google Scholar]
- US EPA. Health assessment document for manganese. Final draft. Cincinnati, OH: US Environmental Protection Agency, Office of Research and Development (Report No. EPA/600/8-83/013F); 1984. [Google Scholar]
- Vescovi A, Facheris L, Zaffaroni A, Malanca G, Parati EA. Dopamine metabolism alterations in a manganese-treated pheochromocytoma cell line (PC12) Toxicology. 1991;67:129–142. doi: 10.1016/0300-483x(91)90137-p. [DOI] [PubMed] [Google Scholar]
- Wedler FC. Biological significance of manganese in mammalian systems. Progr Med Chem. 1993;30:89–133. doi: 10.1016/s0079-6468(08)70376-x. [DOI] [PubMed] [Google Scholar]
- Zeri C, Voutsinou-Taliadouri F, Romanov AS, Ovsjany EI, Moriki A. A comparative approach of dissolved trace element exchange in two interconnected basins: Black Sea and Aegean Sea. Mar Pollut Bull. 2000;40:666–673. [Google Scholar]