Abstract
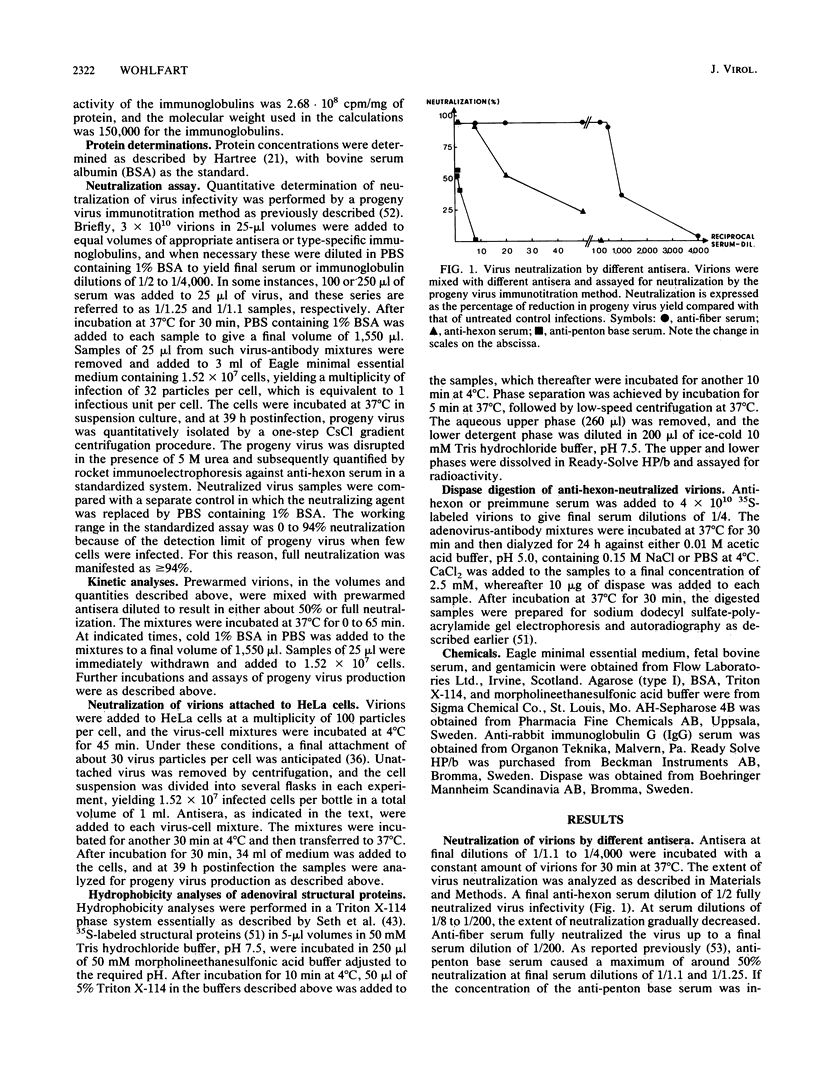
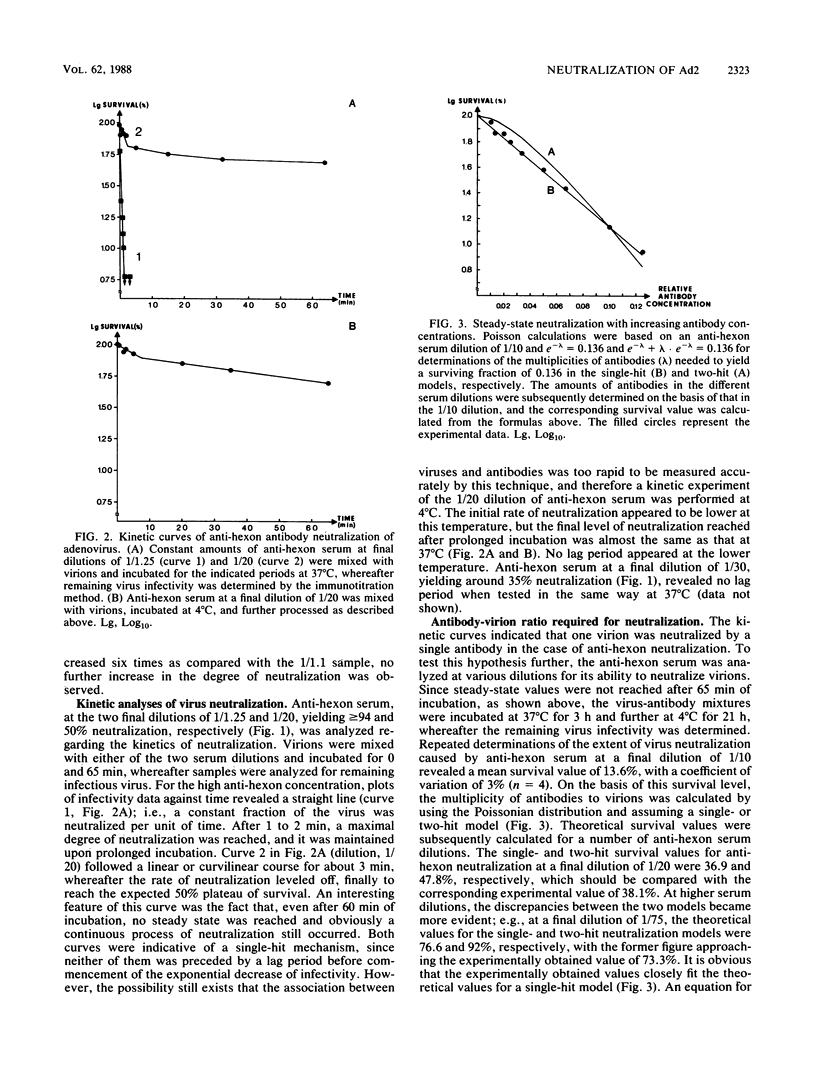
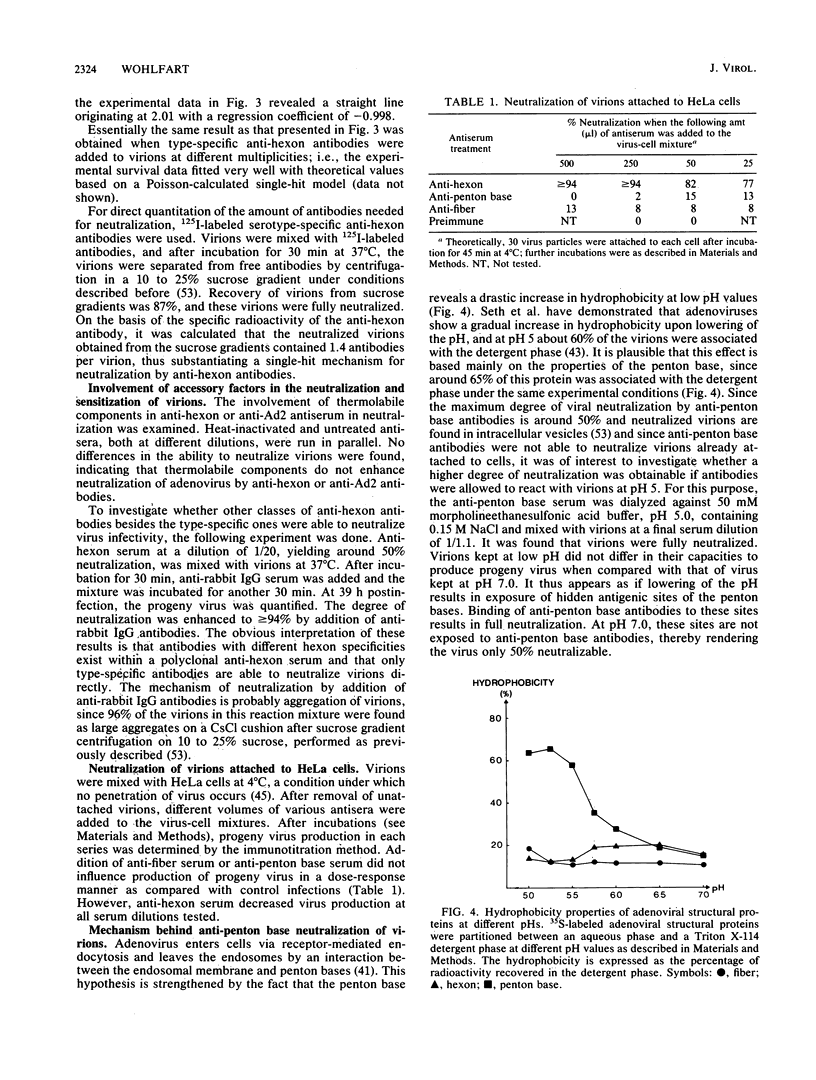
Kinetic curves for neutralization of adenovirus type 2 with anti-hexon serum revealed no lag periods even when the serum was highly diluted or when the temperature was lowered to 4 degrees C, thus indicating a single-hit mechanism. Multiplicity curves determined with anti-hexon serum displayed a linear correlation between the degree of neutralization and dilution of antiserum. Neutralization values experimentally obtained under steady-state conditions fully fitted a single-hit model based on Poisson calculations. Quantitation of the amount of 125I-labeled type-specific anti-hexon antibodies needed for full neutralization of adenovirus showed that 1.4 antibodies were attached per virion under such conditions. Virions already attached to HeLa cells at 4 degrees C were, to a large extent, neutralizable by anti-hexon serum, whereas anti-fiber and anti-penton base antisera were negative. It is suggested that adenovirus may be neutralized by two pathways: aggregation of the virions (extracellular neutralization) as performed by anti-fiber antibodies and blocking of virion entrance from the acidic endosomes into the cytoplasm (intracellular neutralization). The latter effect could be obtained by (i) covering of the penton bases, as performed by anti-penton base antibodies, thereby preventing interaction between the penton bases and the endosomal membrane, which results in trapping of virions within endosomes, and (ii) inhibition of the low-pH-induced conformational change of the viral capsid, which seems to occur in the endosomes and is necessary for proper exposure of the penton bases, as performed by anti-hexon antibodies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks P. A., Kasel J. A., Huber M., Alford R. H., Knight V. Persistence of neutralizing antibody in adult volunteers immunized with adnovirus soluble antigens. Proc Soc Exp Biol Med. 1966 Jan;121(1):240–243. doi: 10.3181/00379727-121-30747. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Burlingham B. T. Penetration of host cell membranes by adenovirus 2. J Virol. 1973 Aug;12(2):386–396. doi: 10.1128/jvi.12.2.386-396.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew P. The surface nature of proteins of a bovine enterovirus, before and after neutralization. J Gen Virol. 1976 Jul;32(1):17–23. doi: 10.1099/0022-1317-32-1-17. [DOI] [PubMed] [Google Scholar]
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970 Mar;40(3):462–477. doi: 10.1016/0042-6822(70)90189-3. [DOI] [PubMed] [Google Scholar]
- Cremer N. E., Riggs J. L., Lennette E. H. Neutralization kinetics of western equine encephalitis virus by antibody fragments. Immunochemistry. 1975 Jul;12(6-7):597–601. doi: 10.1016/0019-2791(75)90092-0. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M., STRICKLAND A. G. A study of the basic aspects of neutralization of two animal viruses, western equine encephalitis virus and poliomyelitis virus. Virology. 1956 Apr;2(2):162–205. doi: 10.1016/0042-6822(56)90017-4. [DOI] [PubMed] [Google Scholar]
- Della-Porta A. J., Westaway E. G. A multi-hit model for the neutralization of animal viruses. J Gen Virol. 1978 Jan;38(1):1–19. doi: 10.1099/0022-1317-38-1-1. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Kao S. Y., Lewis A. J., Crainic R., Wimmer E. Functional basis of poliovirus neutralization determined with monospecific neutralizing antibodies. J Virol. 1983 May;46(2):466–474. doi: 10.1128/jvi.46.2.466-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Ostapchuk P., Wimmer E. Bivalent attachment of antibody onto poliovirus leads to conformational alteration and neutralization. J Virol. 1983 Nov;48(2):547–550. doi: 10.1128/jvi.48.2.547-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Lutter L., Philipson L. Structural proteins of adenoviruses. XII. Location and neighbor relationship among proteins of adenovirion type 2 as revealed by enzymatic iodination, immunoprecipitation and chemical cross-linking. Virology. 1975 Sep;67(1):197–208. doi: 10.1016/0042-6822(75)90417-1. [DOI] [PubMed] [Google Scholar]
- Everitt E., Meador S. A., Levine A. S. Synthesis and processing of the precursor to the major core protein of adenovirus type 2. J Virol. 1977 Jan;21(1):199–214. doi: 10.1128/jvi.21.1.199-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Philipson L. Mechanism of the arginine requirement for adenovirus synthesis. I. Synthesis of structural proteins. J Virol. 1971 Nov;8(5):742–753. doi: 10.1128/jvi.8.5.742-753.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald D. J., Padmanabhan R., Pastan I., Willingham M. C. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983 Feb;32(2):607–617. doi: 10.1016/0092-8674(83)90480-4. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- GRANOFF A. THE INTERACTION OF NEWCASTLE DISEASE VIRUS AND NEUTRALIZING ANTIBODY. Virology. 1965 Jan;25:38–47. doi: 10.1016/0042-6822(65)90249-7. [DOI] [PubMed] [Google Scholar]
- Hahon N. Neutralization of residual infectivity of Venezuelan equine encephalomyelitis virus by anti-gamma globulin. J Gen Virol. 1970 Mar;6(3):361–372. doi: 10.1099/0022-1317-6-3-361. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Icenogle J., Shiwen H., Duke G., Gilbert S., Rueckert R., Anderegg J. Neutralization of poliovirus by a monoclonal antibody: kinetics and stoichiometry. Virology. 1983 Jun;127(2):412–425. doi: 10.1016/0042-6822(83)90154-x. [DOI] [PubMed] [Google Scholar]
- Iorio R. M., Bratt M. A. Neutralization of Newcastle disease virus by monoclonal antibodies to the hemagglutinin-neuraminidase glycoprotein: requirement for antibodies to four sites for complete neutralization. J Virol. 1984 Aug;51(2):445–451. doi: 10.1128/jvi.51.2.445-451.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASEL J. A., HUBER M., LODA F., BANKS P. A., KNIGHT V. IMMUNIZATION OF VOLUNTEERS WITH SOLUBLE ANTIGENS OF ADENOVIRUS TYPE 1. Proc Soc Exp Biol Med. 1964 Oct;117:186–190. doi: 10.3181/00379727-117-29531. [DOI] [PubMed] [Google Scholar]
- KJELLEN L. Studies on the interactions of adenovirus, antibody, and host cells in vitro. Virology. 1962 Nov;18:448–456. doi: 10.1016/0042-6822(62)90035-1. [DOI] [PubMed] [Google Scholar]
- Kjellén L., Pereira H. G. Role of adenovirus antigens in the induction of virus neutralizing antibody. J Gen Virol. 1968 Jan;2(1):177–185. doi: 10.1099/0022-1317-2-1-177. [DOI] [PubMed] [Google Scholar]
- Kroll J. Line immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:61–67. [PubMed] [Google Scholar]
- LAFFERTY K. J. THE INTERACTION BETWEEN VIRUS AND ANTIBODY. I. KINETIC STUDIES. Virology. 1963 Sep;21:61–75. doi: 10.1016/0042-6822(63)90305-2. [DOI] [PubMed] [Google Scholar]
- MANDEL B. Neutralization of viral infectivity: characterization of the virus-antibody complex, including association, dissociation, and host-cell interaction. Ann N Y Acad Sci. 1960 Jan 13;83:515–527. doi: 10.1111/j.1749-6632.1960.tb40925.x. [DOI] [PubMed] [Google Scholar]
- Majer M. Virus sensitization. Curr Top Microbiol Immunol. 1972;58:69–84. doi: 10.1007/978-3-642-65357-5_2. [DOI] [PubMed] [Google Scholar]
- Mandel B. Characterization of type 1 poliovirus by electrophoretic analysis. Virology. 1971 Jun;44(3):554–568. doi: 10.1016/0042-6822(71)90369-2. [DOI] [PubMed] [Google Scholar]
- Mandel B. Neutralization of poliovirus: a hypothesis to explain the mechanism and the one-hit character of the neutralization reaction. Virology. 1976 Feb;69(2):500–510. doi: 10.1016/0042-6822(76)90480-3. [DOI] [PubMed] [Google Scholar]
- Mautner V., Willcox H. N. Adenovirus antigens: a model system in mice for subunit vaccination. J Gen Virol. 1974 Dec;25(3):325–336. doi: 10.1099/0022-1317-25-3-325. [DOI] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Mednis B. Structure and development of viruses as observed in the electron microscope. V. Entry and uncoating of adenovirus. J Virol. 1969 Nov;4(5):777–796. doi: 10.1128/jvi.4.5.777-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson R., Svensson U., Everitt E. Virus receptor interaction in the adenovirus system. II. Capping and cooperative binding of virions on HeLa cells. J Virol. 1983 Jun;46(3):956–963. doi: 10.1128/jvi.46.3.956-963.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possee R. D., Schild G. C., Dimmock N. J. Studies on the mechanism of neutralization of influenza virus by antibody: evidence that neutralizing antibody (anti-haemagglutinin) inactivates influenza virus in vivo by inhibiting virion transcriptase activity. J Gen Virol. 1982 Feb;58(Pt 2):373–386. doi: 10.1099/0022-1317-58-2-373. [DOI] [PubMed] [Google Scholar]
- RUBIN H., FRANKLIN R. M. On the mechanism of Newcastle disease virus neutralization by immune serum. Virology. 1957 Feb;3(1):84–95. doi: 10.1016/0042-6822(57)90025-9. [DOI] [PubMed] [Google Scholar]
- Seth P., Fitzgerald D., Ginsberg H., Willingham M., Pastan I. Evidence that the penton base of adenovirus is involved in potentiation of toxicity of Pseudomonas exotoxin conjugated to epidermal growth factor. Mol Cell Biol. 1984 Aug;4(8):1528–1533. doi: 10.1128/mcb.4.8.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Willingham M. C., Pastan I. Adenovirus-dependent release of 51Cr from KB cells at an acidic pH. J Biol Chem. 1984 Dec 10;259(23):14350–14353. [PubMed] [Google Scholar]
- Seth P., Willingham M. C., Pastan I. Binding of adenovirus and its external proteins to Triton X-114. Dependence on pH. J Biol Chem. 1985 Nov 25;260(27):14431–14434. [PubMed] [Google Scholar]
- Smith K. O., Trousdale M., Gehle W. Adenovirus-antibody agglutination reactions. J Immunol. 1965 Nov;95(5):810–817. [PubMed] [Google Scholar]
- Svensson U., Persson R., Everitt E. Virus-receptor interaction in the adenovirus system I. Identification of virion attachment proteins of the HeLa cell plasma membrane. J Virol. 1981 Apr;38(1):70–81. doi: 10.1128/jvi.38.1.70-81.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson U. Role of vesicles during adenovirus 2 internalization into HeLa cells. J Virol. 1985 Aug;55(2):442–449. doi: 10.1128/jvi.55.2.442-449.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Pereira H. G. Antigens and structure of the adenovirus. J Mol Biol. 1965 Aug;13(1):13–20. doi: 10.1016/s0022-2836(65)80076-6. [DOI] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S. PRODUCTION OF SPECIFIC NEUTRALIZING ANTIBODY WITH SOLUBLE ANTIGENS OF TYPE 5 ADENOVIRUS. Proc Soc Exp Biol Med. 1963 Oct;114:37–42. doi: 10.3181/00379727-114-28579. [DOI] [PubMed] [Google Scholar]
- Wadell G. Sensitization and neutralization of adenovirus by specific sera against capsid subunits. J Immunol. 1972 Mar;108(3):622–632. [PubMed] [Google Scholar]
- Wohlfart C. E., Everitt E. Assessment of specific virus infectivity and virus neutralization by a progeny virus immunotitration method as exemplified in an adenovirus system. J Virol Methods. 1985 Jul;11(3):241–251. doi: 10.1016/0166-0934(85)90113-2. [DOI] [PubMed] [Google Scholar]
- Wohlfart C. E., Svensson U. K., Everitt E. Interaction between HeLa cells and adenovirus type 2 virions neutralized by different antisera. J Virol. 1985 Dec;56(3):896–903. doi: 10.1128/jvi.56.3.896-903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfart C., Everitt E. Human adenovirus 2 as immunogen in rabbits yields antisera with high titers of antibodies against the nonstructural 72K DNA-binding protein. Virus Res. 1985 Jul;3(1):77–85. doi: 10.1016/0168-1702(85)90043-7. [DOI] [PubMed] [Google Scholar]