Abstract
The mechanisms responsible for coated pit formation in cells remain unknown, but indirect evidence has argued both for and against a critical role of receptor cytoplasmic domains in the process. If the endocytic motifs of receptors are responsible for recruiting AP2 to the plasma membrane, thereby driving coated pit formation, then the level of constitutively internalized receptors at the membrane would be expected to govern the steady-state level of coated pits in cells. Here we directly test this hypothesis for broad classes of receptors containing three distinct constitutive internalization signals. Chimeric proteins consisting of an integral membrane reporter protein (Tac) coupled to cytoplasmic domains bearing tyrosine-, di-leucine-, or acidic cluster/casein kinase II-based internalization signals were overexpressed to levels that saturated the internalization pathway. Quantitative confocal immunofluorescence microscopy indicated that the number of plasma membrane clathrin-coated pits and the concentration of their structural components were invariant when comparing cells expressing saturating levels of the chimeric receptors to nonexpressing cells or to cells expressing only the Tac reporter lacking cytoplasmic internalization signals. Biochemical analysis showed that the distribution of coat proteins between assembled coated pits and soluble pools was also not altered by receptor overexpression. Finally, the cellular localizations of AP2 and AP1 were similarly unaffected. These results provide a clear indication that receptor endocytic signals do not determine coated pit levels by directly recruiting AP2 molecules. Rather, the findings support a model in which coated pit formation proceeds through recruitment and activation of AP2, likely through a limited number of regulated docking sites that act independently of endocytic signals.
INTRODUCTION
The initial steps in the formation of clathrin-coated pits (CPs) at the plasma membrane and trans-Golgi network (TGN) involve the recruitment to the membrane of clathrin and either AP2 (at the plasma membrane) or AP1 (in the TGN). Substantial cytosolic pools of these proteins exist in cells, often equaling or exceeding the membrane-bound forms (Goud et al., 1985). Several studies have shown that hormonal stimulation can lead to rapid and sustained several-fold increases in plasma membrane CPs under certain conditions, consistent with mobilization of these soluble pools (Connolly et al., 1981, 1984; Fisher and Rebhun, 1983). Nonetheless, little is known about the details of CP formation at the plasma membrane.
The finding that AP2 binds to specific internalization signals (endocytic motifs) in the cytoplasmic domains of certain transmembrane receptors (Pearse, 1988; Glickman et al., 1989) led to the speculation that the receptors themselves may initiate CP assembly by recruiting AP2 to the membrane (Pearse and Crowther, 1987; Brodsky, 1988). This concept has received experimental support from subsequent demonstrations that AP2 can bind directly to other receptor cytosolic domains (Glickman et al., 1989; Beltzer and Spiess, 1991; Sorkin et al., 1995) and to isolated sorting signals (Boll et al., 1996; Heilker et al., 1996; Honing et al., 1996). Among the signals described thus far, the best characterized conform to either of two tyrosine-based motifs (YXXφ or NXXY, where X is any amino acid and φ is an amino acid with a bulky hydrophobic group), the di-leucine motif, and the acidic cluster/casein kinase II site (Trowbridge, 1991; Trowbridge et al., 1993; Pond et al., 1995; Voorhees et al., 1995). Tyrosine-based motifs have been shown to interact with the μ2 and μ1 subunits of AP2 and AP1, respectively (Ohno et al., 1995, 1996; Boll et al., 1996). There is also evidence that di-leucine–based (Heilker et al., 1996; Salamero et al., 1996; Dietrich et al., 1997) and acidic cluster signals (Mauxion et al., 1996; Dittie et al., 1997) interact with AP complexes, providing a plausible mechanism by which these receptors might recruit CP components.
Although the affinities of soluble APs for these isolated receptor endocytic motifs are comparatively low when measured in vitro (Ohno et al., 1995, 1996; Page and Robinson, 1995; Boll et al., 1996), the interactions would be expected to be stronger when constrained to movement in two dimensions at the membrane surface. Indeed, early morphological studies in stably transformed cells suggested that very high levels of transferrin receptor expression seemed to correlate with the appearance of increased flat coated membranes (although not necessarily CPs) (Iacopetta et al., 1988; Miller et al., 1991). More recently, contrasting results were obtained when transient expression of lower levels of transferrin receptor, while still saturating the internalization machinery, failed to show any evidence for detectable changes in CP levels (Warren et al., 1997). Finally, locally high concentrations of a single activated receptor (FcεRI) generated by a receptor immobilization regimen also did not induce increased CP formation in intact RBL-2H3 cells (Santini and Keen, 1996).
While the latter findings provide information about a single receptor that enters CPs only after activation, under basal conditions the bulk of receptor-mediated endocytosis occurs by way of constitutively internalized receptors. If constitutively internalized receptors play a critical role in nucleating CPs, we hypothesized that a saturating level of internalization signals at the plasma membrane should stimulate CP formation, with concomitant recruitment to the membrane of soluble coat components from the cytosol. Alternatively, if CP formation is not directly responsive to the presence of receptor endocytic domains at the plasma membrane, then cells should maintain a homeostatic level of plasma membrane CPs in the face of saturating levels of receptors. We therefore sought a system in which a high density of constitutively internalized proteins could be achieved at the plasma membrane.
For this purpose, we chose to study HeLa cells overexpressing chimeric proteins in which different cytosolic internalization signals are appended to a monomeric integral membrane reporter protein, the interleukin-2 receptor α-chain (Tac). Expressed at low levels, these chimeric proteins are efficiently internalized with kinetics comparable to that of the parent receptor from which the endocytic signals were derived (Letourneur and Klausner, 1992; Marks et al., 1995; Voorhees et al., 1995). On overexpression, chimeric Tac proteins containing cytosolic di-leucine and YXXφ signals independently saturate the sorting machinery and accumulate at the plasma membrane (Marks et al., 1996). In this report we demonstrate that uptake of chimeric proteins bearing the acidic cluster/casein kinase II internalization motif reflects a third, independently saturable process. Collectively, these characteristics allowed us to test the role of these three distinct internalization signals in the nucleation of plasma membrane clathrin CPs in intact cells.
MATERIALS AND METHODS
Antibodies and Reagents
X22 and AP.6 were gifts from Dr. F.M. Brodsky (University of California, San Francisco) and have been previously characterized (Beck et al., 1992). Rabbit anti-Tac serum 1 was prepared by M.S.M. and J. S. Bonifacino (NIH, Bethesda, MD) by immunization with Tac affinity purified from stably transfected HeLa cells, and rabbit anti-Tac serum 2, which has been previously characterized (Leonard et al., 1984), was a gift of Dr. W. Greene, (University of California, San Francisco). Other antibodies were from the following sources: 100/3 and 100/2 monoclonal anti-γ-adaptin and anti-α-adaptin (Ahle et al., 1988) antibodies, respectively, were obtained from Sigma Chemical (St. Louis, MO); monoclonal anti-clathrin heavy chain (TD.1) (Nathke et al., 1992) and 7G7.B6 monoclonal anti-Tac were from ATCC (Rockville, MD); mAb H4A3 anti-Lamp1 was from Developmental Studies Hybridoma Bank, Johns Hopkins University (Baltimore, MD); fluorescein isothiocyanate-conjugated and rhodamine-lissamine–conjugated affinity-purified donkey or goat anti-rabbit or anti-mouse polyclonal antibodies were from Jackson ImmunoResearch (West Grove, PA); fluorescein- and phycoerythrin-conjugated anti-Tac, fluorescein-conjugated anti-CD4, and fluorescein-conjugated anti-CD63 were from Immunotech (Westbrook, ME). All other chemicals were reagent grade or better.
Plasmids
All cDNA constructs were cloned into pCDM8.1 (Bonifacino et al., 1990), a modified version of pCDM8 (Seed, 1987). Table 1 lists the inserts used and their sources. The TTM.GSTYΔ construct (Marks et al., 1995), containing a portion of the cytosolic domain of H-2Mb, was used in some immunofluorescence experiments instead of a chimera with the full-length cytosolic domain (TTMb [Marks et al., 1995]) because the latter is partially retained within the endoplasmic reticulum in HeLa cells (Marks, unpublished observations).
Table 1.
Cytoplasmic tails and constructs used in this manuscript
| Constructa | Source of cytoplasmic tailb | Sequence of cytoplasmic tailc | Ref.d |
|---|---|---|---|
| Tac | Tac | TM-RRQRKSRRTI | Bonifacino et al. (1990) |
| TTMb | Murine DMβ (Mb) | TM-RWRGHSSSYTPLSGSTYPEGRH-COOH | Marks et al. (1995) |
| TTM.GSTYΔ | Murine DMβ (Mb) | TM-RWRGHSSSYTPLSGSTY-COOH | Marks et al. (1995) |
| TTγt3-t2 | Tac/Mouse CD3γ | TM-RRQRKSRRT/DKQTLL-COOH | Letourneur and Klausner (1992) |
| TacF766-93 | Tac/Furin | TM-RRQRKSRRT/WQEECPSDSEEDEGRGERTAFIKDQSAL-COOH | Voorhees et al. (1995) |
| CD4 | Human CD4 | TM-RCRHRRRQAERMSQIKRLLSEKKTCQCPHRFQKTCSPI-COOH | Marks et al. (1996) |
Construct name indicates the name used in this study.
Source of cytoplasmic tail: For Tac chimeric proteins, this indicates the source of the novel non-Tac cytosolic sequences.
Sequence of cytoplasmic tail: Letters correspond to the single amino acid code: TM denotes transmembrane region; -COOH denotes the carboxyl terminus of the protein. Letters in bold type indicate sequences with known sorting activity.
Reference indicates the original reference describing the use of the construct.
Cell Culture, Transient Transfections, and Flow Cytometry
RBL-2H3 cells were a gift from Dr. M. A. Beaven (NIH, Bethesda, MD) and were cultured as described (Santini and Keen, 1996); for chilling experiments, cells were incubated in a glucose-saline piperazine-N,N′-bis(2-ethanesulfonic acid)-buffered medium (pH 7.2) with 0.1% BSA and 1 mM CaCl2 for 1 h on ice or at 37oC. HeLa cells (ATCC, Rockville, MD) were transfected by the calcium phosphate precipitation method with 10–20 μg of plasmid consisting of the indicated insert in pCDM8.1 as described (Marks et al., 1996), while COS-1 cells (ATCC) were transfected using lipofectAMINE and 15 μg of plasmid (Goodman et al., 1996). Control cells were transfected with pCDM8.1 with no insert. Cells were trypsinized 24–40 h posttransfection, replated on dishes containing coverslips for 8–16 h, and removed and processed for indirect immunofluorescence microscopy as described below. For flow cytometry, cells growing on dishes were released with PBS/10 mM EDTA, stained with directly conjugated antibodies as described (Marks et al., 1996), and analyzed on a Becton Dickinson FACScan using the CellQuest 3.01 software (San Jose, CA) to confirm expression at the plasma membrane. For experiments demonstrating saturability, parallel samples were analyzed by immunofluorescence microscopy, and cells expressing CD4, Tac, both, or neither were counted to determine cotransfection efficiencies.
Immunofluorescence, Confocal, and Electron Microscopy
For quantitative immunofluorescence microscopy experiments, 48-h posttransfection cells that were grown overnight on coverslips were fixed with 2% (wt/vol) formaldehyde in PBS and processed for immunostaining as previously described (Goodman et al., 1996; Santini and Keen, 1996). AP2 was detected using the mAB AP.6 (20 μg/ml) while AP1 was visualized by using monoclonal 100/3 (1:100). Tac-chimeric proteins were detected using rabbit anti-Tac serum 2 (1:2000); Lamp1 was detected with H4A3 (1:100); CD63 was detected with fluorescein-anti-CD63 (1:30); and transferrin receptor was detected with B3/25 (1:50). The primary antisera were detected with appropriate tagged second antibodies (1:100). Confocal analysis was performed on a Bio-Rad MRC-600 laser scanning confocal microscope (Cambridge, MA) running CoMos 7.0a software and interfaced to a Zeiss Axiovert 100 microscope with Zeiss Plan-Apo 63X 1.40 NA objective (Carl Zeiss, Thornwood, NY). All dual labeled samples were analyzed using simultaneous excitation at 488 nm and 568 nm.
For ultrastructural studies, samples were fixed and processed using a standard protocol employing glutaraldehyde fixation, osmication, uranyl acetate staining, and bismuth subnitrate counterstaining (Smith et al., 1985). Measurements of CP length were obtained by examining plasma membrane surfaces of randomly selected cells (n = 17 from each group) at 13,500–27,000× final magnification.
Evaluation of Tac-Chimera Expression and Plasma Membrane CP Density
Quantitation of CP density was estimated in cells imaged by confocal ‘x-z’ scans by plotting AP2 pixel intensity against the pixel intensity of transfected tagged proteins. AP2 was chosen as the CP marker rather than clathrin because of its lower background and the absence of Golgi staining; however, AP2 staining always coincided with clathrin on the plasma membrane (Santini and Keen, 1996).
To be able to evaluate signals spanning a greater range than the 8-bit capacity of the confocal framestore board, for each experiment a calibration curve of intensity as a function of photomultiplier gain for each channel was prepared using MultiSpeck fluorescence microscopy bead standards (M-7900, Molecular Probes Inc., Eugene, OR). Keeping the aperture vernier setting constant (generally at 4) and varying only the photomultiplier gain, images were acquired whose pixel intensity fell entirely within the measurable output range, i.e., 1–254. The integrated pixel intensity was determined within a box of 30 × 45 pixels drawn around each 5-μm bead (in each case being certain that ≤0.2% of the pixels had reached saturation), and was corrected for background by intensity measurements of similar areas between beads. Rescanning of individual beads at the conclusion of a series of measurements indicated that bleaching had reduced the intensity by less than 5% in all cases. The integrated pixel intensity of the individual beads, expressed as percent of maximum, was plotted as a function of the photomultiplier gain, and the resultant curves were used in the analysis of samples to normalize the pixel intensity readings obtained at different gain settings. Samples were then analyzed with the same constant aperture vernier setting and appropriate gain so as to ensure that the pixel intensity fell entirely within the measurable photomultiplier output range. Images were corrected for background, obtained with a blocked excitation beam, using the “subtract” function.
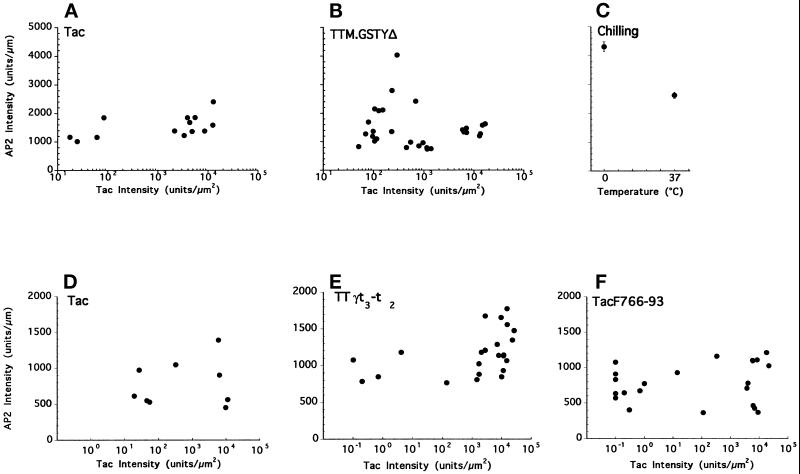
Vertical ‘x-z’ sections were processed as follows: the cell perimeter was measured using the length/profile function, and an area was then drawn that outlined the cell using the histogram function. The integrated pixel intensity within the cell area was obtained by using the banding features of the histogram, so that only image pixels with intensity above the background were counted. To obtain the level of Tac-chimera expression, the integrated pixel intensity was divided by the cell area (to give a measure of expression in intensity/μm2), while CP level was obtained by dividing integrated pixel intensity by cell perimeter. It should be noted that the results presented in Figure 3 show data collected entirely within single experiments, representative of the number of independent experiments indicated in the figure legend.
Figure 3.
Plasma membrane coated pit levels in cells expressing chimeric receptors containing tyrosine-, di-leucine-, or acidic cluster-based internalization signals. Quantitative analysis of AP2 and Tac signals in HeLa cells expressing Tac control (panel A) or TTM.GSTYΔ (panel B) from one experiment (representative of four). In a second experiment (representative of three independent assays) AP2 and Tac signals in cells expressing Tac control (panel D), TTγt3-t2 (panel E), or TacF766–93 (panel F) are shown. Data points were obtained by analyzing images similar to those shown in Figures 2 and 5, as described in detail in MATERIALS AND METHODS. For each cell measured, the relative AP2 signal intensity (per linear μm of cell perimeter, y-axis) is plotted against the overall Tac expression level (per μm2 of cell area, x-axis). In panel C, the level of plasma membrane-associated AP2 signal observed in RBL-2H3 cells that had been chilled on ice is compared with that in cells that had been kept at 37°C. This change correlates with known increases in CP density induced on cooling (see text for details). Error bars represent SE with n = 53 for 37°C and n = 38 for 0°C.
For determination of CP number (Table 2), AP2 immunofluorescent spots on the same cell sections were manually counted and divided by the cell perimeter. In presenting the data, cells were considered as expressing only if their Tac pixel intensity/μm2 value was above 100.
Table 2.
The number of plasma membrane clathrin coated pits is not affected by overexpression of Tac, TTM.GSTYΔ, TTγt3-t2, or TacF766-93
| Coated Pits No./μm | SEM | (n) | |
|---|---|---|---|
| Tac | |||
| Nonexpressing | 0.24 | ±0.02 | (15) |
| Expressing | 0.24 | ±0.01 | (19) |
| TTM.GSTYΔ | |||
| Nonexpressing | 0.26 | ±0.01 | (23) |
| Expressing | 0.23 | ±0.01 | (43) |
| TTγt3-t2 | |||
| Nonexpressing | 0.25 | ±0.01 | (18) |
| Expressing | 0.27 | ±0.01 | (44) |
| TacF766-93 | |||
| Nonexpressing | 0.26 | ±0.01 | (36) |
| Expressing | 0.27 | ±0.01 | (59) |
Selection of Overexpressing Cells
For biochemical analysis of AP2, AP1, and clathrin distribution, transiently transfected HeLa cells were separated into pools on the basis of surface expression of the lumenal domain of the Tac reporter. Cells transfected with Tac, TTM.GSTYΔ (Marks et al., 1995), or TTγt3-t2 (Letourneur and Klausner, 1992) were stained for 45–90 min at 4°C with hybridoma supernatant containing the 7G7.B6 monoclonal anti-Tac antibody. After three washes with DMEM/5% (vol/vol) FBS, cells (4–8 × 106) were resuspended in 80 μl of MACS buffer (PBS/5% FBS/5 mM EDTA), and 20 μl of MACS goat anti-mouse IgG magnetic microbeads (Miltenyi Biotec, Auburn, CA) were added to each sample. After 15 min at 4°C, cells were washed twice with MACS buffer and separated into positive and negative expressing cell fractions on a midi-MACS separator using AS separation columns as described by the manufacturer. Positively selected cell samples were collected, resuspended in DMEM/10% FBS, and incubated at 37°C for 20–45 min before subsequent analyses. Samples of each cell fraction were collected, stained with fluorescein-anti-Tac, and analyzed by flow cytometry on a Becton Dickinson FACScan using the CellQuest 3.01 software to determine the efficiency of separation.
Lysis, Gel Electrophoresis, and Immunoblotting
Mock transfected cells and positively and negatively selected cell populations were processed to determine the distribution of clathrin, AP2, and AP1 by detergent extraction at pH 6.5 as described (Santini and Keen, 1996). Under these conditions, CPs remain sedimentable, and the insoluble fraction accordingly reflects the extent of assembled clathrin coat protein (Pearse, 1982). Unassembled coat proteins appear in the soluble fraction, which may also contain membrane-bound coat proteins not fully incorporated into coat structures. Briefly, identical cell equivalents (0.5–1.5 × 106) were solubilized in 150 μl buffer 1 (100 mM 2-(N-morpholino)propane sulfonic acid, pH 6.5, 0.5% Triton X-100, 0.5 mM magnesium chloride, 1 mM EGTA) containing phosphatase (10 mM sodium fluoride, 1 mM sodium orthovanadate) and proteinase inhibitors (33 μg/ml aprotinin, 10 μg/ml leupeptin, 5 μg/ml E-64, 5 μg/ml pepstatin A, 1 mM PMSF) and lightly homogenized in microcentrifuge tubes with a mini pestle. After 10 min on ice, cells were pelleted for 10 min at 75,000 rpm in a TLA 120–2 rotor (approximately 245,000 × g) using a Beckman Optima TLX ultracentrifuge at 4°C. Supernatants and pellets were separately frozen on dry ice and stored at −80°C until further use. Pellets were resuspended in 150 μl buffer 2 (0.5 M Tris, pH 7.4, 150 mM sodium chloride, 1% Triton X-100, 0.02% sodium azide) containing phosphatase and proteinase inhibitors for 10 min on ice, and insoluble material was removed by centrifugation in a microcentrifuge. After boiling in SDS sample buffer, equal aliquots (10% of total) of each sample were fractionated by mini-SDS-PAGE using 7.5% acrylamide separating gels. Proteins were transferred onto supported nitrocellulose membranes and clathrin, AP2, and AP1 were detected using mAb TD-1, 100/2, and 100/3, respectively. Tac chimeras were detected using polyclonal rabbit anti-Tac serum 1. Antibody binding was visualized and quantitated using radioiodinated secondary antibodies or protein A (Amersham, Arlington Heights, IL) and PhosphorImager analysis using ImageQuant software (Molecular Dynamics, Sunnyvale, CA). In separate experiments using these extraction techniques with RBL-2H3 cells, immunoblotting, and ECL techniques with appropriate standards, we detected a 1.9-fold increase (from 45 to 85%) in the amount of assembled clathrin in chilled cells, in good agreement with previously published results (Anderson et al., 1977; Goldstein et al., 1979) and our ultrastructural measurements (our unpublished observations).
RESULTS
Coated Pits at the Plasma Membrane Are Unchanged in Response to Overexpression of a Chimeric Protein Containing the Tyrosine-based Internalization Motif of H-2Mb
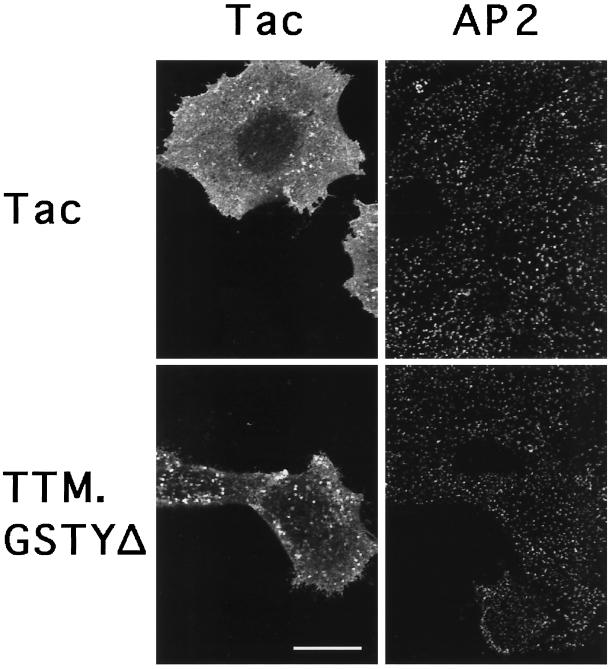
HeLa cells were transiently transfected to overexpress a control reporter protein, the Tac antigen (Tac), or a chimeric protein (TTM.GSTYΔ) consisting of the lumenal and transmembrane domain of Tac appended to a portion of the cytosolic tail of the β chain of the lysosomal resident protein, H-2Mb. The latter construct includes the tyrosine-based signal, YTPL, that is both necessary and sufficient for internalization from the plasma membrane and for lysosomal targeting of H-2M in HeLa cells (Lindstedt et al., 1995; Marks et al., 1995). The cells were then analyzed by flow cytometry and by immunofluorescence microscopy with antibodies against Tac and AP2 (Figure 1, left and right panels, respectively). Intact Tac, which has no internalization signal, accumulated at the plasma membrane in all transfected cells as expected from previous work (Marks et al., 1996). Also as previously observed, TTM.GSTYΔ was found in intracellular compartments, but its overexpression saturated the tyrosine-based sorting machinery and resulted in its appearance at the plasma membrane (Figure 1 and our unpublished results).
Figure 1.
Plasma membrane coated pit distribution in cells overexpressing a chimeric receptor containing a tyrosine-based internalization signal. Confocal immunofluorescence microscopy images showing distribution of the Tac reporter (left panels) and AP2 (right panels) visualized using rabbit anti-Tac serum and monoclonal AP.6, respectively, in Tac and TTM.GSTYΔ transiently transfected HeLa cells. Images were taken with a focal plane parallel to and near the substrate. Note AP2 staining in both transfected and untransfected cells. Bar, 20 μm.
There are substantial levels of soluble, unassembled clathrin and AP2 in HeLa cells, comparable or greater in amount to that present in assembled CPs (see below). Accordingly, recruitment of these cytosolic components would be expected to result in an approximate doubling in the number of CPs. However, when the localization of AP2 was evaluated as an indicator of the distribution of CPs (see MATERIALS AND METHODS), the pattern in cells overexpressing TTM.GSTYΔ appeared indistinguishable from that of cells in the same field that were not expressing the transgene, or of cells that had been transfected with intact Tac (Figure 1, right panels).
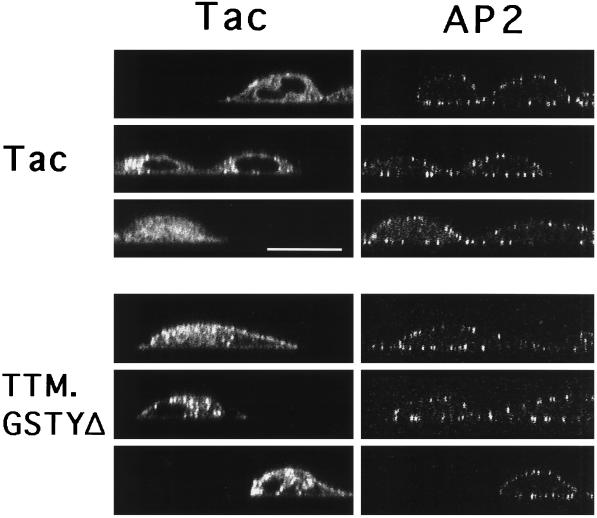
We next examined these samples by confocal microscopy using a focal plane perpendicular to the cell monolayer (‘x-z’ sections). By laser irradiation of only a narrow (≈0.4 μm wide) band through the cell, variations in CP numbers are more readily revealed (Santini and Keen, 1996). These images (Figure 2) provided a clear indication that there were no differences in the relative density of CPs in TTM.GSTYΔ-expressing cells in comparison with nonexpressing cells (cells negative for the Tac signal) or to the control (Tac). This was quantitatively confirmed by determining the number of discrete AP2-containing CPs in these and other cell profiles (Table 2).
Figure 2.
Confocal microscopy ‘x-z’ images of Tac reporter expression and coated pit distribution in cells expressing chimeric receptors with tyrosine-based internalization signals. HeLa cells transiently transfected with Tac or TTM.GSTYΔ were stained as indicated in Figure 1, and vertical confocal ‘x-z’ images were collected of Tac (left panels) and AP2 (right panels). Note AP2 staining in both transfected and untransfected cells. Bar, 20 μm.
Could expression of TTM.GSTYΔ and its cytosolic internalization signals have caused increased recruitment of CP components from the cytosol to the plasma membrane without discernibly altering the overall CP number, e.g. by enlarging preexisting CPs? To address this question, we used ‘x-z’ images of individual cells to compare the intensity of the AP2 signal at the plasma membrane with the overall level of expression of the Tac or TTM.GSTYΔ chimeras (Figure 3, A and B) as described in MATERIALS AND METHODS. The expression level of both the Tac control and TTM.GSTYΔ proteins in transfected cells spanned several orders of magnitude, confirming results previously obtained by flow cytometry (Figure 6, and Marks et al., 1996). The variations in endogenous AP2 intensity at the plasma membrane were much smaller in comparison (note the linear scale on the y-axis). Importantly, the AP2 levels at the plasma membrane showed no correlation with the level of expression of either Tac or TTM.GSTYΔ (Figure 3, A and B, respectively). Indeed, similar variations in CP density were observed in untransfected cells. These results indicate that AP2 recruitment to the plasma membrane and the number of plasma membrane CPs are independent of the level of tyrosine-based internalization signals at the plasma membrane.
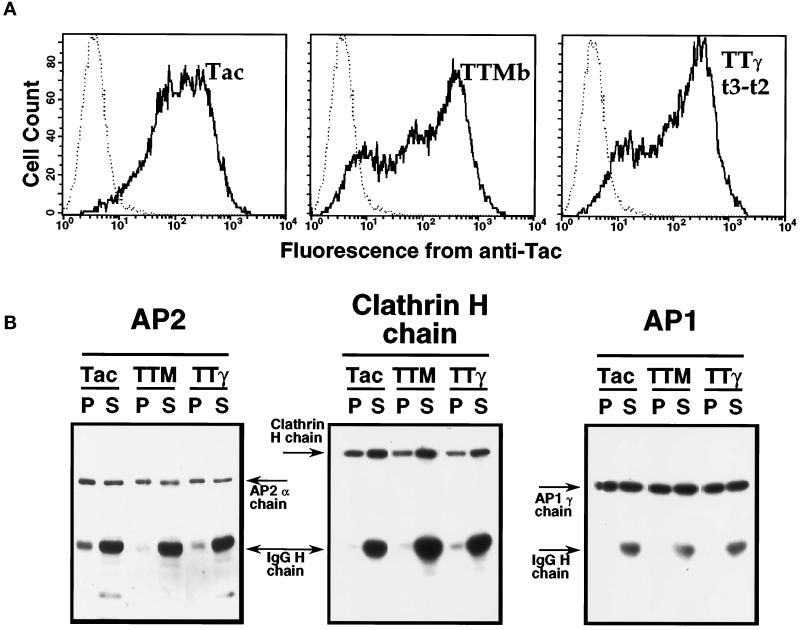
Figure 6.
The distribution of AP2, clathrin, and AP1 between soluble and insoluble pools in cells overexpressing chimeric receptors with tyrosine- or di-leucine-based internalization signals. HeLa cells transiently transfected with plasmids encoding Tac, TTMb, or TTγt3-t2 were subjected to positive selection as described in MATERIALS AND METHODS. (A) A fraction of the cells (106 in this representative experiment) was removed and stained with fluorescein-conjugated anti-Tac and then analyzed by flow cytometry. Fluorescence intensity profiles from the three positively selected pools of cells (thick lines) and from mock-transfected cells (dotted lines) are shown. (B) The remaining cells were lysed and fractionated into Triton X-100-soluble (S) and insoluble (P) pools, and equal aliquots (derived from 106 cells/lane) were subjected to SDS-PAGE and immunoblotted with antibodies to AP2, clathrin heavy chain, and AP1 as described in MATERIALS AND METHODS. The migration of each band, as well as the heavy chain from the anti-Tac antibody used for the cell isolation, were visualized using radioiodinated antibody and are indicated on the figure. TTM, TTMb; TTγ, TTγt3-t2.
Coated Pit Changes in Intact Cells Induced by Chilling Can Be Readily Detected by Confocal Microscopy and Biochemical Fractionation
We have previously found that confocal microscopy and, in particular, x-z scans of clathrin- or AP2-labeled RBL-2H3 cells yielded estimates of clathrin CP density in excellent quantitative agreement with published measurements obtained by electron microscopy (Santini and Keen, 1996). To ensure that this approach can also detect changes in clathrin CP assembly levels in intact cells, we exploited the observation of Anderson and colleagues (Anderson et al., 1977; Goldstein et al., 1979) that chilling of some cell types (but not of others [Foti et al., 1997a]) results in a substantial increase in CP levels. Analysis of RBL-2H3 cells treated in this manner by confocal microscopy revealed a 1.7-fold increase in the intensity of the plasma membrane AP2 signal in the chilled cells compared with controls (Figure 3C), while ultrastructural analysis of the same cells yielded a 1.6-fold increase in the amount of coated membrane surface (from 33 to 53 nm/μm). Similarly treated cells were also analyzed biochemically by lysis and fractionation to yield a detergent-insoluble fraction containing assembled clathrin and a detergent-soluble fraction containing unassembled protein (see below). Immunoblots of the two fractions revealed a 1.9-fold increase (from 45% to 85%) in the proportion of clathrin in the assembled fraction (our unpublished results). Collectively, these results validate the ability of both the confocal microscopy and biochemical (see below) techniques to detect changes in clathrin CP levels in intact cells.
Coated Pit Levels Are Maintained upon Overexpression and Plasma Membrane Accumulation of Proteins Containing Di-leucine–based Internalization Signals
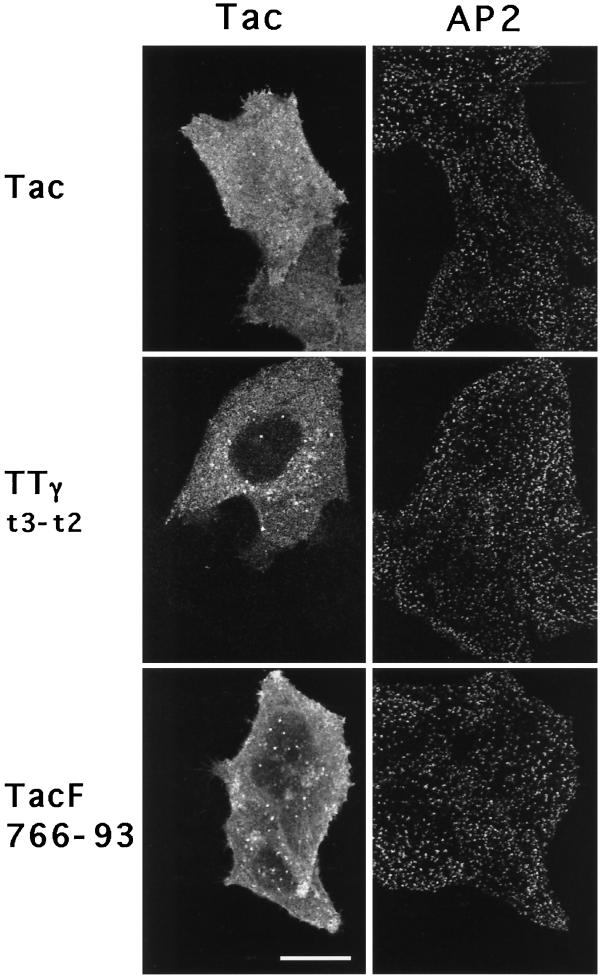
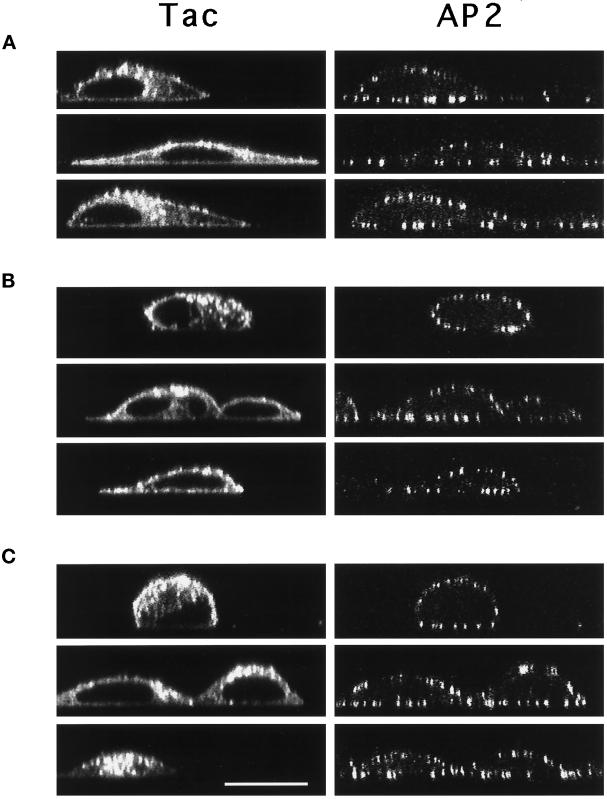
Other classes of sorting signals distinct from the tyrosine-based motifs have been shown to mediate internalization from the plasma membrane. One of these is the di-leucine motif (see Pond et al. [1995] and references therein) which has also been suggested to bind in vitro to AP2 and to the related AP1 complex (Heilker et al., 1996; Salamero et al., 1996; Dietrich et al., 1997). Protein sorting mediated by di-leucine–based signals is saturable through a limiting component distinct from that involved in sorting of tyrosine-based signals (Marks et al., 1996). Considering the inability of the tyrosine-based motifs to induce CP formation at the cell surface, we reasoned that overexpression of chimeric proteins with a di-leucine motif might, through a different mechanism, affect coat component recruitment. To test this hypothesis, we transfected cells with sufficient DNA to overexpress Tac chimeric proteins containing the di-leucine signal of CD3γ (TTγt3-t2 [Letourneur and Klausner, 1992]). As expected from previous work (Marks et al., 1996), overexpression resulted in cell surfact accumulation of TTγt3-t2 (Figure 10 in Marks et al., 1996; and see Figure 6A, right panel), consistent with saturation of the di-leucine–based internalization machinery. However, on staining to visualize AP2 and Tac (Figure 4), cells overexpressing TTγt3-t2 (middle panels) showed no obvious differences in the density of CPs when compared with cells expressing intact Tac (upper panels) or nonexpressing cells (negative for anti-Tac). These perceptions were substantiated more compellingly by confocal ‘x-z’ images of CPs (Figure 5, A and B).
Figure 4.
Coated pit distributions in cells overexpressing chimeric receptors containing di-leucine- or acidic cluster-based internalization signals. Confocal microscopy ‘x-y’ images showing the distribution of CPs (AP2, right panels) in HeLa cells transiently transfected to overexpress Tac, TTγt3-t2, and TacF766–93 (Tac, left panels). Images were acquired using a focal plane parallel to and near the substrate. Note AP2 staining in both transfected and untransfected cells. Bar, 20 μm.
Figure 5.
Confocal microscopy ‘x-z’ images of Tac reporter expression and coated pit distribution in cells overexpressing chimeric receptors with di-leucine- or acidic cluster-based internalization motifs. Confocal x-z micrographs of transfected HeLa cells expressing Tac (A), TTγt3-t2 (B), and TacF766–93 (C) stained for Tac (left panels) and AP2 (right panels). Bar, 20 μm.
Quantitation of the AP2 signal intensity at the plasma membrane and comparison with the intensity of total Tac staining in the same cells revealed that, as observed with TTM.GSTYΔ, the signal intensity for staining of TTγt3-t2 spanned several orders of magnitude (Figure 3, D and E). However, the distributions of the AP2 signal intensities were similar among untransfected cells and cells expressing either intact Tac or TTγt3-t2, and there was no correlation between the AP2 signal intensity and the level of the chimeric protein expressed. Finally, quantitative analysis of the number of CPs in profiles of cells expressing the TTγt3-t2 chimera were not significantly different from nonexpressing cells in the same field (Table 2). These results demonstrate that overexpression of di-leucine–based targeting signals at the plasma membrane to a level saturating the internalization machinery does not detectably alter CP profiles in cells.
The Distribution of AP2, Clathrin, and AP1 between Insoluble (Assembled) and Soluble (Unassembled) Pools Is Independent of Overexpression of YXXφ or Di-leucine Signals
To extend the immunofluorescence microscopy results, we analyzed the distribution of AP2 complexes between assembled and unassembled pools biochemically using cell fractionation. After transient transfection with plasmids encoding reporter alone (Tac), or chimeric receptor containing Tac reporter with tyrosine- (TTMb), or di-leucine–based signals (TTγt3-t2) at levels sufficient to induce saturation, overexpressing cells were isolated by using anti-Tac antibody-coated magnetic beads as described in MATERIALS AND METHODS. Positively selected cells with surface Tac expression (Figure 6A) were then lysed and fractionated to yield a detergent-insoluble fraction containing assembled clathrin coat structures (Pearse, 1982) and a soluble fraction containing unassembled coat proteins. Each fraction was analyzed by SDS-PAGE and immunoblotting with antibodies to the Tac transgene, the α-chain of AP2, the γ-chain of AP1, and the clathrin heavy chain. Examples of the resulting immunoblot profiles are shown in Figure 6B. The distribution of the AP2 α-chain and the clathrin heavy chain between soluble and insoluble pools was not significantly different among all positively selected pools of cells, regardless of whether the cells were expressing Tac transgenes containing or lacking an internalization signal. Furthermore, the distribution of the AP1 γ-chain was also identical among all samples, even though chimeric proteins containing tyrosine- or di-leucine–based endocytic motifs can be sorted to lysosomes by what is thought to be an AP1-dependent process (see below) and had accumulated in these structures. The Tac reporter was found nearly exclusively in the soluble fraction in all transfected cell samples (88–98% soluble; data not shown), suggesting that the majority of the reporter proteins were excluded from clathrin-coated membrane regions. These data confirm the immunofluorescence microscopy results and provide a biochemical demonstration that the proportion of coat proteins in detergent-insoluble CPs is essentially unchanged and that substantial soluble pools of AP2, AP1, and clathrin remain upon overexpression and accumulation of tyrosine- or di-leucine–based targeting signals at the plasma membrane.
Overexpression of an Acidic Cluster/Casein Kinase II Site Internalization Signal Does Not Induce Clathrin-coated Pit Formation
A third class of internalization signals has recently been described that comprises a series of acidic amino acids, often preceded by a serine residue that serves as a substrate for phosphorylation by casein kinase II. This type of acidic cluster/casein kinase II site signal has been demonstrated to mediate internalization from the cell surface as well as TGN targeting of the endoproteinase furin (Jones et al., 1995; Schäfer et al., 1995; Voorhees et al., 1995) and is involved in protein sorting of mannose 6-phosphate (M6P) receptors (Mauxion et al., 1996) and several other receptor proteins (Jewell-Motz and Liggett, 1995; Alconada et al., 1996; Goldman et al., 1996). Furthermore, the acidic clusters in the cation-dependent M6P receptor and furin have been suggested to be involved in AP1 recruitment to the TGN membrane (Mauxion et al., 1996) and secretory granules (Dittie et al., 1997), respectively.
Given these features of acidic cluster signals, we sought to determine whether their overexpression could recruit AP2 to the plasma membrane. Accordingly, we expressed a chimeric protein (denoted TacF766–93, Table 1) that contains the Tac reporter and the C-terminal half of the furin cytosolic domain; the latter moiety contains the acidic cluster/casein kinase II site (Voorhees et al., 1995) but lacks the tyrosine-based motif present in the N-terminal half. Flow cytometry indicated that cell surface expression of TacF766–93 was essentially undetectable at low expression levels, but became substantial at elevated expression levels (our unpublished results). Parallel analysis of cells by immunofluorescence microscopy showed similar transfection efficiencies among all populations and demonstrated efficient TGN localization of TacF766–93 in cells transfected with lower amounts of DNA. These results demonstrate that internalization and TGN localization mediated by the acidic cluster signal of furin is, indeed, saturable.
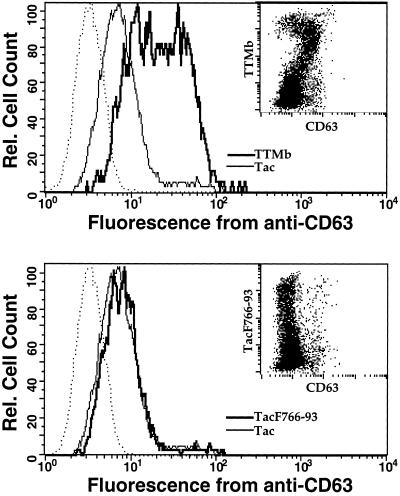
We next sought to determine whether internalization of proteins containing the acidic cluster signal is mediated by the same limiting factor(s) responsible for uptake of tyrosine-based internalization signals. HeLa cells were transiently transfected with high levels of plasmid coding for chimeric proteins containing reporter alone (intact Tac), tyrosine- (TTMb), or acidic cluster-based (TacF766–93) endocytic signals. The cells were then stained with antibodies to Tac and CD63, an endogenous lysosomal protein with a tyrosine-based sorting signal (Metzelaar et al., 1991), and analyzed by flow cytometry. Overexpression of TTMb resulted in induction of surface expression of CD63, proportional to TTMb surface expression; expression of the intact Tac reporter construct did not (Figure 7, upper panel and inset). In contrast, overexpression of TacF766–93 failed to induce surface expression of CD63 beyond that seen in untransfected cells or in cells transfected with intact Tac (Figure 7, lower panel and inset). Thus, whereas internalization mediated by the acidic cluster signal of furin is saturable, it does not compete with components that recognize tyrosine-based motifs.
Figure 7.
Tyrosine-based and acidic cluster signals utilize distinct saturable components for internalization from the plasma membrane. HeLa cells were transiently transfected with 20 μg of plasmid encoding Tac, TTMb (upper panel), or TacF766–93 (lower panel). After 40 h, cells were harvested, stained on ice with phycoerythrin-anti-Tac and fluorescein-antiCD63, and analyzed by flow cytometry. Inset, dot plot showing phycoerythrin staining (anti-Tac) on the y-axis and fluorescein staining (anti-CD63) on the x-axis for cells transfected with TTMb or TacF766–93. Each dot represents a single counted cell. Main graph, The dark line indicates CD63 staining on cells gated for surface expression above background for either TTMb or TacF766–93. The thin lines show CD63 staining on cells expressing similar levels of the noninternalized Tac. This level of staining is identical to that obtained with untransfected cells and likely represents the steady state expression level of CD63 under nonsaturating, physiological conditions. The dotted lines represent staining with a nonspecific control antibody (anti-CD4-FITC).
We then tested whether overexpression of TacF766–93 resulted in AP2 recruitment to the plasma membrane. HeLa cells transfected with high amounts of plasmid driving expression of TacF766–93 were analyzed by confocal immunofluorescence microscopy after double staining with antibodies to Tac and to AP2. Analysis of conventional (Figure 4, bottom panel) and ‘x-z’ images (Figure 5C) showed that overexpression of TacF766–93 resulted in cell surface expression of the Tac reporter, but failed to alter the distribution of AP2 relative to untransfected cells or cells overexpressing Tac with no internalization signal. Furthermore, quantitative analysis of the number of CPs (Table 2) and of the staining intensity of AP2 (Figure 3F) showed no correlation with the levels of TacF766–93 expression. Together with the other results shown in Figure 3 and Table 2, these findings demonstrate that CP components are not recruited from the cytosol upon overexpression of any of the three well characterized internalization signals.
Normal Targeting of AP1 and AP2 Is Observed Despite the Overexpression of YXXφ, Di-leucine, or Acidic Internalization Signals
Clathrin functions in integral membrane protein sorting not only at the plasma membrane, but also at the TGN. Here, clathrin assembly is thought to be mediated largely by the AP1 complex. Previous results have provided evidence for interaction of tyrosine-, di-leucine-, and acidic-based motifs with AP1 (Ohno et al., 1995; Boll et al., 1996; Honing et al., 1996; Mauxion et al., 1996; Ohno et al., 1996; Salamero et al., 1996; Dietrich et al., 1997; Dittie et al., 1997; Le Borgne and Hoflack, 1997). Furthermore, overexpression of TGN38/41, which contains a tyrosine-based TGN localization signal, appeared to induce the redistribution of AP1 to peripheral vesicular structures in COS cells (Reaves and Banting, 1994). We therefore examined the possibility that overexpression of proteins containing these internalization signals might affect the intracellular distribution of AP1.
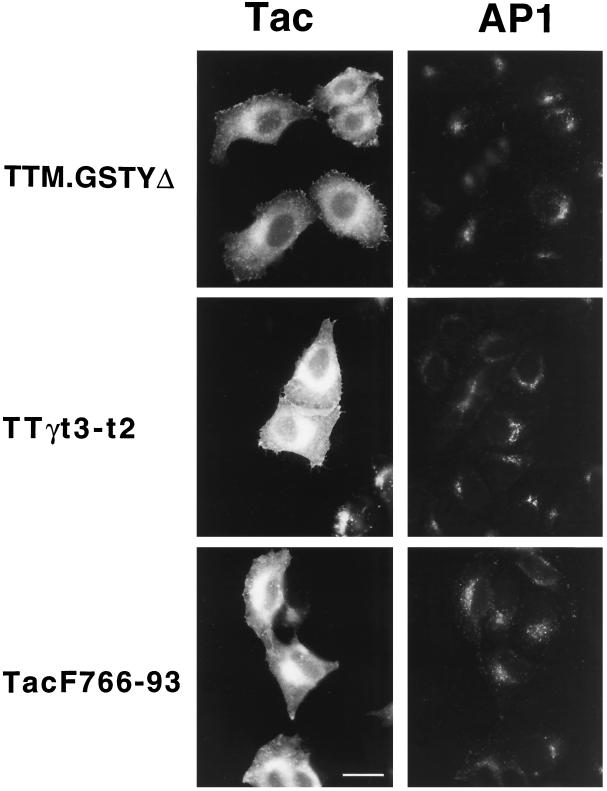
HeLa cells overexpressing TTM.GSTYΔ, TTγt3-t2, or TacF766–93 were costained with antibodies specific for Tac and for the γ-chain of AP1 (Figure 8). The characteristic perinuclear TGN localization of AP1 was clearly retained in cells expressing either the tyrosine-based signal of TTM.GSTYΔ (upper panels), the di-leucine signal of TTγt3-t2 (middle panels), or the acidic signal of TacF766–93 (lower panels). These results indicate that overexpression of these three classes of internalization and intracellular targeting signals fails to alter the intracellular distribution of AP1. It is also important to note that AP2 retained its plasma membrane localization and was not mistargeted upon overexpression of Tac, TTM.GSTYΔ, TTγt3-t2, or TacF766–93 (Figures 2 and 5), despite accumulation of the reporter protein at both the plasma membrane and in endocytic/lysosomal compartments. Finally, in similar overexpression experiments of TTM.GSTYΔ and TTγt3-t2 in COS-1 cells, no aberrant localization of AP1 or AP2 was observed (our unpublished results).
Figure 8.
Overexpression of chimeric receptors containing tyrosine-, di-leucine-, or acidic cluster/casein kinase II-based endocytic motifs does not affect the perinuclear localization of the Golgi- associated AP1. Distribution of AP1 visualized with 100/3 mAb (right panels) in HeLa cells in which expression of TTM.GSTYΔ (upper panels), TTγt3-t2 (middle panels), or TacF766–93 (lower panels) is revealed by staining with anti-Tac antibody (left panels). Note the AP1 distribution in both transfected and untransfected cells. Bar, 20 μm.
DISCUSSION
Receptor-mediated endocytosis is a dynamic process in which the first readily identifiable step is the concentration of cell surface receptors in plasma membrane CPs. After invagination of the pits, receptors are internalized into coated endocytic vesicles, the coat proteins are rapidly released and are subsequently recruited for new CP formation on the membrane. Consistent with this scheme, soluble pools of clathrin and AP2 can be readily detected, comprising up to 75% of the total complement depending on cell type (Goud et al., 1985; Corvera et al., 1989; Chakrabarti et al., 1993). It is known that CP formation can be rapidly and substantially induced in response to certain agents (e.g., EGF, nerve growth factor, carbachol) in some cells (Connolly et al., 1981, 1984; Geisow et al., 1985), suggesting that the recruitment process can be readily activated. Nevertheless, the factors regulating the initial steps of CP formation under either stimulated or basal conditions remain unknown.
APs manifest both receptor binding (Pearse, 1988; Ohno et al., 1995; Sorkin et al., 1995) and clathrin assembly activities (Keen et al., 1979; Zaremba and Keen, 1983). These properties have led to the plausible suggestion that receptors play a critical role in CP formation by initially recruiting APs through binding sites within their cytosolic domains (Pearse, 1988; Chang et al., 1993). Alternatively, one might envision these AP–receptor interactions occurring predominantly after CP formation, resulting in the recruitment or retention of receptors in the assembled CP. A distinguishing characteristic of the former hypothesis is that cell surface expression of elevated numbers of receptors that contain internalization sequences should result in the recruitment of soluble coat components and the formation of new CPs. Here we test this hypothesis by exploring the effects on CP formation of overexpression of functional receptors at levels sufficient to saturate the internalization machinery. Further, we use a generalized approach to examine the ability of surface chimeric receptors containing three distinct and independently acting classes of cytosolic internalization signals to recruit soluble coat components and induce CP formation.
The results obtained indicate that the number, extent, and distribution of plasma membrane CPs were indistinguishable between control (nonexpressing) cells, cells expressing Tac with no internalization signal, or cells overexpressing chimeric Tac receptors with tyrosine-, di-leucine-, or acidic cluster/casein kinase II-based internalization signals. Furthermore, there is no evidence that soluble pools of coat components are limiting, or even detectably altered, in the face of saturating levels of expression of these receptors at the plasma membrane. We have extended these studies by examining the effects of overexpression of chimeric receptors containing tyrosine- and di-leucine–based internalization signals in COS-1 cells. The results obtained are entirely in agreement with our observations in HeLa cells in that each signal is taken up by an independently saturable pathway, and there are no detectable effects on CP distribution (our unpublished observations).
Collectively, these and our earlier findings (Santini and Keen, 1996) demonstrate that plasma membrane clathrin CP levels remain remarkably constant regardless of the receptor density, the nature of its internalization motif, and whether receptor internalization occurs constitutively or only subsequent to ligand activation. We have also obtained similar results upon overexpression of transferrin receptor in this HeLa cell system, confirming the results of Warren and colleagues (Warren et al., 1997). Related findings have also been obtained upon overexpression of Lamp1 in two other cell systems (Uthayakumar and Granger, 1995). Taken together, these findings are inconsistent with a general model in which receptor internalization signals alone recruit plasma membrane AP2.
In this context it is worth noting that earlier observations of increased lattice formation on overexpression of transferrin receptor to extremely high levels were not seen in intact cells, but only upon examination of replicas of the upper surface of broken open cells, and then only in a very small percentage of the cell population (Miller et al., 1991). However, clathrin lattice assembly under optimal conditions is known to proceed extremely rapidly (Van Jaarsveld et al., 1981), raising the possibility that the anomalous flat lattices observed may have formed only during or subsequent to shearing of the upper membrane and breakage.
With respect to the TGN, Hoflack and co-workers found that overexpression of M6P receptors did not induce increased AP1 recruitment to the TGN beyond basal levels, and that soluble pools of AP1 persisted (Le Borgne and Hoflack, 1997). However, in cells in which the M6P receptor genes had been functionally inactivated, a loss of AP1 binding in vitro and in vivo was observed (Le Borgne et al., 1996; Le Borgne and Hoflack, 1997; Mauxion et al., 1996; Salamero et al., 1996). The latter findings may be a reflection of fundamental differences in the mechanisms of CP formation at the plasma membrane and in the TGN (West et al., 1997), with TGN clathrin CP assembly requiring both receptors and an additional, limiting factor. However, in cells completely lacking M6P receptor expression, there is extensive membrane redistribution in the TGN/lysosomal/endosomal compartments (Ludwig et al., 1994), which raises the possibility that loss of AP1 binding in this experimental system could be a consequence of mistargeting or dilution of a separate, limiting factor. In fact, other workers have reported continuing clathrin-coated vesicle formation and budding from the TGN despite inhibition of packaging of the cation-independent M6P receptor by wortmannin treatment (Gaffet et al., 1997). Finally, to the extent that the general mechanisms of coated vesicle formation in cells are likely to be similar, the formation of COPII-coated vesicles in the yeast endoplasmic reticulum has also been reported to be independent of the presence of transmembrane cargo molecules (Yeung et al., 1995). Thus, the available evidence supports a mechanism for CP formation that is generally independent of receptor sorting signals.
Nonetheless, under certain conditions clathrin CP formation at the plasma membrane is known to be responsive to specific effectors, including extracellular ligands such as EGF (Connolly et al., 1984) or intracellular molecules such as Nef that have been proposed to act as ‘connectors’ (Foti et al., 1997; Mangasarian et al., 1997). Similarly, recent studies by Hsu and coworkers (Aoe et al., 1997, 1998) have provided strong evidence that COPI coat levels can be modulated by multiple ligands acting through ERD2 receptor. Clearly, mechanisms exist for rapid recruitment of coat proteins and the formation of lattices, suggesting that certain effectors, perhaps acting as specific sensors of their microenvironment, may be uniquely able to regulate coat protein recruitment, retention, or release.
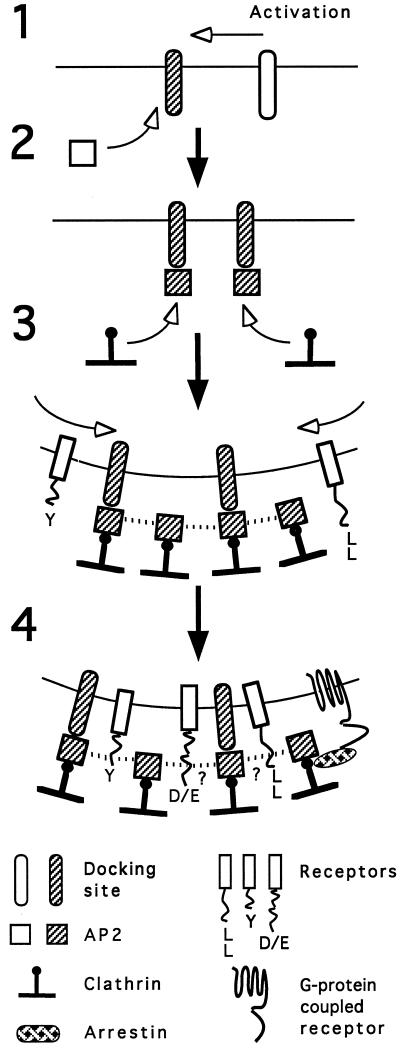
A model consistent with these observations for the clathrin CP system is presented in Figure 9. This scheme envisions regulation of CP formation resulting from tight control over AP activation, possibly through the action of a discrete membrane-bound AP docking site that initiates AP recruitment to the membrane (Figure 9, steps 1 and 2). Expression of the clathrin- and receptor-binding activity (step 3) of the membrane-bound AP results in coat assembly and receptor association with the growing CP (steps 3 and 4). Limiting numbers of these docking sites, and/or tight control of their activation (step 1), would then explain why excess receptors, regardless of their internalization motif or activation state, do not generally alter CP levels in cells. Though the AP-docking sites may be limited, receptor interaction sites on lattices are evidently more plentiful and can account for the multiple, independently saturable uptake pathways that have been observed. For example, the clathrin terminal domain serves as a recognition site for the arrestins that mediate internalization of many G protein-coupled receptors (Goodman et al., 1996, 1997; Barak et al., 1997).
Figure 9.
A scheme for clathrin-coated pit assembly at the plasma membrane. Docking sites for AP2 at the plasma membrane become exposed or activated (step 1), resulting in the recruitment of AP2 molecules from the cytosol (step 2). Once bound to the docking site, the clathrin-binding activity of the AP2 is expressed, clathrin trimers are recruited, and lattice assembly begins (step 3), perhaps promoted by AP2 self-association (dotted lines [Beck and Keen, 1991]). Transmembrane receptors then become associated with the growing or completed coat via internalization motifs in their cytoplasmic domains (step 4). For receptors with tyrosine-based signals, this association likely occurs through interaction with the μ2 subunit of the AP2 complex; interaction sites within the coat for receptors with di-leucine and acidic cluster/casein kinase II substrate signals are not certain. For some G protein-coupled receptors, arrestins mediate binding of the receptor to the terminal domains of the clathrin triskelia (Goodman et al., 1996, 1997).
This model is similar to earlier proposals (Moore et al., 1987; Anderson, 1993; Robinson, 1994) and is supported by evidence of specific AP binding to membranes (Moore et al., 1987; Mahaffey et al., 1990; Peeler et al., 1993; Zhang et al., 1994; Mallet and Brodsky, 1996; Seaman et al., 1996). Conversely, APs are also known to possess discrete determinants implicated in membrane targeting, distinct from the AP domains involved in receptor interaction (Robinson, 1993; Seaman et al., 1993; Ohno et al., 1995; Page and Robinson, 1995; Boll et al., 1996). These determinants may be responsible for recognition of specific docking sites. Identification of this docking site(s) and elucidation of the mechanism of its regulation remain to be rigorously established.
ACKNOWLEDGMENTS
We acknowledge Drs. J.S. Bonifacino, J.C. Cook, and A. Dancis for discussions; Dr. D. Frank, B. Bieler, and P. Hingorani for technical assistance; Dr. V.W. Hsu (Harvard Medical School) for communication of unpublished results; and the Diabetes and Endocrinology Research Center of the University of Pennsylvania for electron microscopy services. This work reflects comparable contributions by the Keen and Marks laboratories and was supported by NIH grant GM-28526 to J.H.K. and American Cancer Society grants RPG-97–003-01-BE and IRG-135Q to M.S.M.; F.S. was supported by NRSA award 5-T32-CA09678.
Footnotes
Abbreviations used: CP, Coated pit; M6P, mannose-6-phosphate; TGN, trans-Golgi network.
REFERENCES
- Ahle S, Mann A, Eichelsbacher U, Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988;7:919–29. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- Anderson RGW. Dissecting clathrin-coated pits. Trends Cell Biol. 1993;3:177–179. doi: 10.1016/0962-8924(93)90205-f. [DOI] [PubMed] [Google Scholar]
- Anderson RGW, Brown MS, Goldstein JL. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell. 1977;10:351–364. doi: 10.1016/0092-8674(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Aoe T, Cukierman E, Lee A, Cassel D, Peters PJ, Hsu VW. The KDEL receptor, Erd2, regulates intracellular traffic by recruiting a GTPase-activating protein For Arf1. EMBO J. 1997;16:7305–7316. doi: 10.1093/emboj/16.24.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoe T, Lee AJ, van Donselaar E, Peters PJ, Hsu VW. Modulation of intracellular transport by transported proteins: insight from regulation of COPI-mediated transport: Proc. Natl Acad Sci USA. 1998;95:1624–1629. doi: 10.1073/pnas.95.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak LS, Ferguson SS, Zhang G J, Caron MG. A Beta-arrestin green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J Biol Chem. 1997;272:27497–27500. doi: 10.1074/jbc.272.44.27497. [DOI] [PubMed] [Google Scholar]
- Beck KA, Chang M, Brodsky FM, Keen JH. Clathrin assembly protein AP-2 induces aggregation of membrane vesicles: a possible role for AP-2 in endosome formation. J Cell Biol. 1992;119:787–796. doi: 10.1083/jcb.119.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KA, Keen JH. Self-association of the plasma membrane-associated clathrin assembly protein AP-2. J Biol Chem. 1991;266:4437–4441. [PubMed] [Google Scholar]
- Beltzer JP, Spiess M. In vitro binding of the asialoglycoprotein receptor to the beta adaptin of plasma membrane coated vesicles. EMBO J. 1991;10:3735–3742. doi: 10.1002/j.1460-2075.1991.tb04942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll W, Ohno H, Zhou SY, Rapoport I, Cantley LC, Bonifacino JS, Kirchhausen T. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 1996;15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Suzuki CK, Klausner RD. A peptide sequence confers retention and rapid degradation in the endoplasmic reticulum. Science. 1990;247:79–82. doi: 10.1126/science.2294595. [DOI] [PubMed] [Google Scholar]
- Brodsky FM. Living with clathrin: its role in intracellular membrane traffic. Science. 1988;242:1396–1402. doi: 10.1126/science.2904698. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Joly M, Corvera S. Redistribution of clathrin-coated vesicle adaptor complexes during adipocytic differentiation of 3T3–L1 cells. J Cell Biol. 1993;123:79–87. doi: 10.1083/jcb.123.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MP, Mallet WG, Mostov KE, Brodsky FM. Adaptor self-aggregation, adaptor-receptor recognition and binding of alpha-adaptin subunits to the plasma membrane contribute to recruitment of adaptor (AP2) components of clathrin-coated pits. EMBO J. 1993;12:2169–2180. doi: 10.1002/j.1460-2075.1993.tb05865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JL, Green SA, Greene LA. Pit formation and rapid changes in surface morphology of sympathetic neurons in response to nerve growth factor. J Cell Biol. 1981;90:176–180. doi: 10.1083/jcb.90.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JL, Green SA, Greene LA. Comparison of rapid changes in surface morphology and coated pit formation of PC12 cells in response to nerve growth factor, epidermal growth factor, and dibutyryl cyclic AMP. J Cell Biol. 1984;98:457–65. doi: 10.1083/jcb.98.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera S, Bartels JL, Capocasale RJ, Cichowski K, Moore JS. Increased assembly of clathrin occurs in response to mitogenic activation of murine lymphocytes. J Biol Chem. 1989;264:12568–12572. [PubMed] [Google Scholar]
- Dietrich J, Kastrup J, Nielsen BL, Odum N, Geisler C. Regulation and function of the CD3gamma DxxxLL motif: a binding site for adaptor protein-1 and adaptor protein-2 in vitro. J Cell Biol. 1997;138:271–281. doi: 10.1083/jcb.138.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittie AS, Thomas L, Thomas G, Tooze SA. Interaction of furin in immature secretory granules from neuroendocrine cells with the Ap-1 adaptor complex is modulated by casein kinase Ii phosphorylation. EMBO J. 1997;16:4859–4870. doi: 10.1093/emboj/16.16.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GW, Rebhun LI. Sea urchin egg cortical granule exocytosis is followed by a burst of membrane retrieval via uptake into coated vesicles. Dev Biol. 1983;99:456–472. doi: 10.1016/0012-1606(83)90295-6. [DOI] [PubMed] [Google Scholar]
- Foti M, Carpentier JL, Aiken C, Trono D, Lew DP, Krause KH. Second-messenger regulation of receptor association with clathrin-coated pits: a novel and selective mechanism in the control of CD4 endocytosis. Mol Biol Cell. 1997;8:1377–1389. doi: 10.1091/mbc.8.7.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti M, Mangasarian A, Piguet V, Lew DP, Krause KH, Trono D, Carpentier JL. Nef-mediated clathrin-coated pit formation. J Cell Biol. 1997;139:37–47. doi: 10.1083/jcb.139.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffet P, Jones AT, Clague MJ. Inhibition of calcium-independent mannose 6-phosphate receptor incorporation into trans-Golgi nework-derived clathrin-coated vesicles by wortmannin. J Biol Chem. 1997;272:24170–24175. doi: 10.1074/jbc.272.39.24170. [DOI] [PubMed] [Google Scholar]
- Geisow MJ, Childs J, Burgoyne RD. Cholinergic stimulation of chromaffin cells induces rapid coating of the plasma membrane. Eur J Cell Biol. 1985;38:51–56. [PubMed] [Google Scholar]
- Glickman JN, Conibear E, Pearse BMF. Specificity of binding of clathrin adaptors to signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO J. 1989;8:1041–1047. doi: 10.1002/j.1460-2075.1989.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman PS, Schlador ML, Shapiro RA, Nathanson NM. Identification of a region required for subtype-specific agonist-induced sequestration of the m2 muscarinic acetylcholine receptor. J Biol Chem. 1996;271:4215–4222. doi: 10.1074/jbc.271.8.4215. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Anderson RG, Brown MS. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979;279:679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta(2)-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Gurevich VV, Benovic JL, Keen JH. Arrestin/clathrin interaction. Localization of the arrestin binding locus to the clathrin terminal domain. J Biol Chem. 1997;272:15017–15022. doi: 10.1074/jbc.272.23.15017. [DOI] [PubMed] [Google Scholar]
- Goud B, Huet C, Louvard D. Assembled and unassembled pools of clathrin: a quantitative study using an enzyme immunoassay. J Cell Biol. 1985;100:521–527. doi: 10.1083/jcb.100.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilker R, Manningkrieg U, Zuber JF, Spiess M. In vitro binding of clathrin adaptors to sorting signals correlates with endocytosis and basolateral sorting. EMBO J. 1996;15:2893–2899. [PMC free article] [PubMed] [Google Scholar]
- Honing S, Griffith J, Geuze HJ, Hunziker W. The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO J. 1996;15:5230–5239. [PMC free article] [PubMed] [Google Scholar]
- Iacopetta BJ, Rothenberger S, Kühn LC. A role for the cytoplasmic domain in transferrin receptor sorting and coated pit formation during endocytosis. Cell. 1988;54:485–489. doi: 10.1016/0092-8674(88)90069-4. [DOI] [PubMed] [Google Scholar]
- Jewell-Motz EA, Liggett SB. An acidic motif within the third intracellular loop of the alpha2C2 adrenergic receptor is required for agonist-promoted phosphorylation and desensitization. Biochemistry. 1995;34:11946–11953. doi: 10.1021/bi00037a036. [DOI] [PubMed] [Google Scholar]
- Jones BG, Thomas L, Molloy SS, Thulin CD, Fry MD, Walsh KA, Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen JH, Willingham MC, Pastan IH. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979;16:303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Griffiths G, Hoflack B. Mannose 6-phosphate receptors and ADP-ribosylation factors cooperate for high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2162–2170. doi: 10.1074/jbc.271.4.2162. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Hoflack B. Mannose 6-phosphate receptors regulate the formation of clathrin-coated vesicles in the TGN. J Cell Biol. 1997;137:335–345. doi: 10.1083/jcb.137.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WJ, Depper JM, Crabtree GR, Rudikoff S, Pumphrey J, Robb RJ, Kronke M, Svetlik PB, Peffer NJ, Waldmann TA, Greene WC. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984;311:626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Letourneur F, Klausner RD. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD63 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- Lindstedt R, Liljedahl M, Péléraux A, Peterson PA, Karlsson L. The MHC class II molecule H2-M is targeted to an endosomal compartment by a tyrosine-based targeting motif. Immunity. 1995;3:561–572. doi: 10.1016/1074-7613(95)90127-2. [DOI] [PubMed] [Google Scholar]
- Ludwig T, Munier-Lehmann H, Bauer U, Hollinshead M, Ovitt C, Lobel P, Hoflack B. Differential sorting of lysosomal enzymes in mannose 6-phosphate receptor-deficient fibroblasts. EMBO J. 1994;13:3430–3437. doi: 10.1002/j.1460-2075.1994.tb06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey DT, Peeler JS, Brodsky FM, Anderson RG. Clathrin-coated pits contain an integral membrane protein that binds the AP-2 subunit with high affinity. J Biol Chem. 1990;265:16514–16520. [PubMed] [Google Scholar]
- Mallet WG, Brodsky FM. A membrane-associated protein complex with selective binding to the clathrin coat adaptor AP1. J Cell Sci. 1996;109:3059–3068. doi: 10.1242/jcs.109.13.3059. [DOI] [PubMed] [Google Scholar]
- Mangasarian A, Foti M, Aiken C, Chin D, Carpentier JL, Trono D. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity. 1997;6:67–77. doi: 10.1016/s1074-7613(00)80243-5. [DOI] [PubMed] [Google Scholar]
- Marks MS, Roche PA, van Donselaar E, Woodruff L, Peters PJ, Bonifacino JS. A lysosomal targeting signal in the cytoplasmic tail of the beta chain directs HLA-DM to MHC class II compartments. J Cell Biol. 1995;131:351–369. doi: 10.1083/jcb.131.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Woodruff L, Ohno H, Bonifacino JS. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J Cell Biol. 1996;135:341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauxion F, Le Borgne R, Munier-Lehmann H, Hoflack B. A casein kinase II phosphorylation site in the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor determines the high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2171–2178. doi: 10.1074/jbc.271.4.2171. [DOI] [PubMed] [Google Scholar]
- Metzelaar MJ, Wijngaard PL, Peters PJ, Sixma JJ, Nieuwenhuis HK, Clevers HC. CD63 antigen.A novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J Biol Chem. 1991;266:3239–3245. [PubMed] [Google Scholar]
- Miller K, Shipman M, Trowbridge IS, Hopkins CR. Transferrin receptors promote the formation of clathrin lattices. Cell. 1991;65:621–632. doi: 10.1016/0092-8674(91)90094-f. [DOI] [PubMed] [Google Scholar]
- Moore MS, Mahaffey DT, Brodsky FM, Anderson RG. Assembly of clathrin-coated pits onto purified plasma membranes. Science. 1987;236:558–563. doi: 10.1126/science.2883727. [DOI] [PubMed] [Google Scholar]
- Nathke IS, Heuser J, Lupas A, Stock J, Turck CW, Brodsky FM. Folding and trimerization of clathrin subunits at the triskelion hub. Cell. 1992;68:899–910. doi: 10.1016/0092-8674(92)90033-9. [DOI] [PubMed] [Google Scholar]
- Ohno H, Fournier MC, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Page LJ, Robinson MS. Targeting signals and subunit interactions in coated vesicle adaptor complexes. J Cell Biol. 1995;131:619–630. doi: 10.1083/jcb.131.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse BM. Coated vesicles from human placenta carry ferritin, transferrin, and immunoglobulin G. Proc Natl Acad Sci USA. 1982;79:451–455. doi: 10.1073/pnas.79.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse BM. Receptors compete for adaptors found in plasma membrane coated pits. EMBO J. 1988;7:3331–3336. doi: 10.1002/j.1460-2075.1988.tb03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse BM, Crowther RA. Structure and assembly of coated vesicles. Annu Rev Biophys Biophys Chem. 1987;16:49–68. doi: 10.1146/annurev.bb.16.060187.000405. [DOI] [PubMed] [Google Scholar]
- Peeler JS, Donzell WC, Anderson RG. The appendage domain of the AP-2 subunit is not required for assembly or invagination of clathrin-coated pits. J Cell Biol. 1993;120:47–54. doi: 10.1083/jcb.120.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond L, Kuhn LA, Teyton L, M.-Schutze P, Tainer JA, Jackson MR, Peterson PA. A role for acidic residues in di-leucine motif-based targeting to the endocytic pathway. J Biol Chem. 1995;270:19989–19997. doi: 10.1074/jbc.270.34.19989. [DOI] [PubMed] [Google Scholar]
- Reaves B, Banting G. Overexpression of TGN38/41 leads to mislocalisation of gamma-adaptin. FEBS Lett. 1994;351:448–456. doi: 10.1016/0014-5793(94)00813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. Assembly and targeting of adaptin chimeras in transfected cells. J Cell Biol. 1993;123:67–77. doi: 10.1083/jcb.123.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. The role of clathrin, adaptors and dynamin in endocytosis. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Salamero J, Le Borgne R, Saudrais C, Goud B, Hoflack B. Expression of major histocompatibility complex class II molecules in HeLa cells promotes the recruitment of AP-1 golgi-specific assembly proteins on golgi membranes. J Biol Chem. 1996;271:30318–30321. doi: 10.1074/jbc.271.48.30318. [DOI] [PubMed] [Google Scholar]
- Santini F, Keen JH. Endocytosis of activated receptors and clathrin-coated pit formation: deciphering the chicken or egg relationship. J Cell Biol. 1996;132:1025–1036. doi: 10.1083/jcb.132.6.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer W, Stroh A, Berghofer S, Seiler J, Vey M, Kruse ML, Kern HF, Klenk HD, Garten W. Two independent targeting signals in the cytoplasmic domain determine trans-Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO J. 1995;14:2424–2435. doi: 10.1002/j.1460-2075.1995.tb07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MNJ, Ball CL, Robinson MS. Targeting and mistargeting of plasma membrane adaptors in vitro. J Cell Biol. 1993;123:1093–1105. doi: 10.1083/jcb.123.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MNJ, Sowerby PJ, Robinson MS. Cytosolic and membrane-associated proteins involved in the recruitment of Ap-1 adaptors onto the trans-Golgi network. J Biol Chem. 1996;271:25446–25451. doi: 10.1074/jbc.271.41.25446. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. Nature. 1987;329:840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Smith RM, Cobb MH, Rosen OM, Jarett L. Ultrastructural analysis of the organization and distribution of insulin receptors on the surface of 3T3–L1 adipocytes: rapid microaggregation and migration of occupied receptors. J Cell Physiol. 1985;123:167–179. doi: 10.1002/jcp.1041230204. [DOI] [PubMed] [Google Scholar]
- Sorkin A, McKinsey T, Shih W, Kirchhausen T, Carpenter G. Stoichiometric interaction of the epidermal growth factor receptor with the clathrin-associated protein complex AP-2. J Biol Chem. 1995;270:619–625. doi: 10.1074/jbc.270.2.619. [DOI] [PubMed] [Google Scholar]
- Trowbridge IS. Endocytosis and signals for internalization. Curr Opin Cell Biol. 1991;3:634–641. doi: 10.1016/0955-0674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Uthayakumar S, Granger BL. Cell surface accumulation of overexpressed hamster lysosomal membrane glycoproteins. Cell Mol Biol Res. 1995;41:405–420. [PubMed] [Google Scholar]
- Van Jaarsveld PP, Nandi PK, Lippoldt RE, Saroff H, Edelhoch H. Polymerization of clathrin protomers into basket structures. Biochemistry. 1981;20:4129–4135. doi: 10.1021/bi00517a028. [DOI] [PubMed] [Google Scholar]
- Voorhees P, Deignan E, van Donselaar E, Humphrey J, Marks MS, Peters PJ, Bonifacino JS. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RA, Green FA, Enns CA. Saturation of the endocytic pathway for the transferrin receptor does not affect the endocytosis of the epidermal growth factor receptor. J Biol Chem. 1997;272:2116–2121. doi: 10.1074/jbc.272.4.2116. [DOI] [PubMed] [Google Scholar]
- West MA, Bright NA, Robinson MS. The role of ADP-ribosylation factor and phospholipase D in adaptor recruitment. J Cell Biol. 1997;138:1239–1254. doi: 10.1083/jcb.138.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T, Barlowe C, Schekman R. Uncoupled packaging of targeting and cargo molecules during transport vesicle budding from the endoplasmic reticulum. J Biol Chem. 1995;270:30567–30570. doi: 10.1074/jbc.270.51.30567. [DOI] [PubMed] [Google Scholar]
- Zaremba S, Keen JH. Assembly polypeptides from coated vesicles mediate reassembly of unique clathrin coats. J Cell Biol. 1983;97:1339–1347. doi: 10.1083/jcb.97.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Davletov BA, Südhof TC, Anderson RGW. Synaptotagmin I is a high affinity receptor for clathrin AP-2: implication for membrane recycling. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]