Abstract
Aquaporin 0 (AQP0) and AQP1 are expressed in the lens, each in a different cell type, and their functional roles are not thoroughly understood. Our previous study showed that these two AQPs function as water transporters. In order to further understand the functional significance of these two different aquaporins in the lens, we investigated their initiation and continued expression. AQP0 transcript and protein were first detected at embryonic stage (E) 11.25 in the differentiating primary fiber cells of the developing lens; its synthesis continued through the adult stage in the secondary fiber cells. Low levels of AQP1 expression were first seen in lens anterior epithelial cells at E17.5; following postnatal day (P) 6.5, the expression gradually progressed towards the equatorial epithelial cells. In the postnatal lens, the increase in membrane water permeability of epithelial cells and lens transparency coincides with the increase in AQP1 expression. AQP1 expression reaches its peak at P30 and continues through the adult stage both in the anterior and equatorial epithelial cells. The enhancement in AQP1 expression concomitant with the increase in the size of the lens suggests the progression in the establishment of the lens microcirculatory system. In vitro and in vivo studies show that both aquaporins share at least one important function, which is water transport in the lens microcirculatory system. However, the temporal expression of these two AQPs suggests an apparently unique role/s in lens development and transparency. To our knowledge, this is the first report on the expression patterns of AQP0 and AQP1 during lens development and differentiation and their relation to lens transparency.
Keywords: aquaporins, AQP0, AQP1, MIP26, membrane permeability, cataract, cell-to-cell adhesion
INTRODUCTION
The ocular lens is a transparent, avascular and constantly growing unique tissue. Mammalian adult lenses have two major cell types; a monolayer of cuboidal epithelial cells on the anterior surface and multiple layers of elongated crystallin-rich fiber cells in the core of the lens. Near the equator is the germinative zone, where epithelial cells continuously divide and undergo remarkable morphological, biochemical, as well as physiological transformations. At the equator, these daughter cells elongate and differentiate to form a new layer of secondary fiber cells, covering the previously formed fiber cells. Each cuboidal epithelial cell elongates more than 100 times its original length generating substantial plasma membrane area, as it becomes a fiber cell (Coulombre and Coulombre, 1963).
The normal avascular lens generates a standing circulating current that enters at both poles, passes into and through the fiber cells, and exits at the equator; the circulating flux of ions causes a flow of water through aquaporins in the lens creating a microcirculation that compensates for the lack of vasculature (Mathias et al., 1997). The lens microcirculatory system delivers glucose and other nutrients to the fiber cells. Aquaporins (AQPs) thus play a major role in lens homeostasis (Mathias and Rae, 2004). The aquaporin superfamily of trans-membrane proteins facilitates the movement of water and certain neutral solutes across the plasma membrane (Agre et al., 2002; Verkman, 2005).
To date, thirteen AQP genes have been reported as being expressed in physiologically diverse mammalian tissues. Five different AQP proteins (AQP0 (MIP26), AQP1, AQP3, AQP4, and AQP5) have been identified in the mammalian eye and two of them (AQP0 and AQP1) are expressed in the lens (Patil et al., 1997; Hamann et al., 1998; Varadaraj et al., 2005). In the eye, AQP0 is exclusively expressed in the lens fiber cells suggesting that it is of fundamental significance for the normal functioning of the lens. No lens pathology has been associated with AQP1 deficiency in human (Preston et al., 1994) or mouse (Ma et al., 1998). However, three natural AQP0 mutations in human (Berry et al., 2000; Francis et al., 2000a,b; Geyer et al., 2006), and three in mice (Cataract Fraser, CatFr: Shields and Griffin, 1993; Shiels et al., 2000; Cataract lens opacity, Catlop: Shiels and Bassnett, 1996; Cataract Tohoku, CatTohm: Okamura et al., 2003) have been associated with autosomal dominant cataracts. Moreover, targeted inactivation of AQP0 in mice resulted in cataract (Shiels et al., 2001). These data on humans and mice illustrate the importance of AQP0 for lens transparency and homeostasis.
AQP0 is the first cloned mammalian aquaporin and constitutes more than 50% of the lens membrane protein. However, the function of AQP0 has been under speculation since its identification in the lens membrane. The discovery of AQP1 as a water channel in RBCs by Peter Agre’s lab (Preston et al., 1994) suggested the closely related AQP0 might also be a water channel. Subsequent in vitro expression of AQP0 in Xenopus oocytes showed that AQP0 does function as a water channel (Kushmerick et al., 1995; Mulders et al., 1995; Chandy et al., 1997; Francis et al., 2000a,b; Schey et al., 2000; Chepelinsky, 2003; Ball et al., 2003) and possibly also as a glycerol transporter (Kushmerick et al., 1998). Varadaraj et al. (1999,2005) investigated the function of AQP0 in the mammalian lens and corroborated its role as a water transporter but found no evidence for glycerol transport.
The water permeability of AQP0 can be regulated experimentally with pH and Ca2+ (Nemeth-Cahalan and Hall, 2000; Nemeth-Cahalan et al., 2004). Studies on AQP0 knockout mouse lens showed an 80% reduction in the fiber cell membrane water permeability compared to the wild type lens (Shiels et al., 2001). Several research groups have investigated the expression, localization (Patil et al., 1997; Hamann et al., 1998; Zampighi et al., 2003; Varadaraj et al., 2005), and interaction of this protein with other lens proteins (Zampighi et al., 2002; Tan et al., 2004; Yu and Jiang, 2004; Fan et al., 2005; Yu et al., 2005; Rose et al., 2006). However, a detailed study on aquaporin gene expression profiles and their functional significance during ocular lens development and differentiation is lacking.
The permeability of AQP0 is 40 times less than that of the AQP1 channel, which is expressed in the lens epithelial cells. This low water permeability may be compensated by the sheer abundance of AQP0 in fiber cell membranes. The high abundance and low water permeability may indicate unidentified functions for AQP0 in lens. Hence, the first and simplest hypothesis is that AQP0 is necessary to provide water transport for the lens fiber cells. However, this role does not explain why the lens switches the protein expression from AQP1 in the equatorial epithelial cells to AQP0 in the differentiating secondary fiber cells. In order to further delineate the functional significance of the temporal expression of AQP0 and AQP1 in two different cell types in the lens, we investigated their initiation and continued expression at transcriptional, translational, and functional levels during lens development and maturation.
RESULTS
Developmental Expression of AQP0 Transcripts
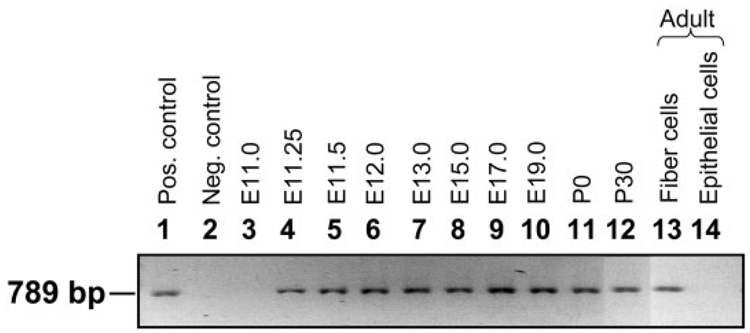
RNA samples extracted at different stages of lens development (as mentioned in the Experimental Procedures section), were subjected to RT-PCR in order to determine the stage at which AQP0 transcripts become detectable. The results indicate that AQP0 is not expressed during the initial stages (E9.0–E11.0) of lens development when the lens placode, lens pit, and lens vesicle are formed (Fig. 1, lane 3; data not shown for E9.0–E10.5). The transcripts begin to express at E11.25 (Fig. 1; lane 4) when the posterior epithelial cells of the lens differentiate into primary fiber cells, and continue to express throughout lens development as well as in the adult lens (Fig. 1; lanes 4–13). As expected, the transcripts were absent in the anterior epithelial cells of even 4-month-old adult mouse lenses (Fig. 1, lane 14). The AQP0 genomic DNA sequence has three introns between the start and stop codons. The total RNA was not contaminated with genomic DNA, since the RT-PCR amplified products of AQP0 showed a band of ~789 bp (Fig. 1, lanes 4–13), which is comparable to that of the PCR amplified product of cloned AQP0 cDNA (789 bp; Fig. 1, lane 1) used as a positive control. Also, there was no amplification in DNase-free RNase I treated RNA samples subjected to RT-PCR (Fig. 1, lane 2, negative control).
Fig. 1.
Developmental expression of AQP0 transcripts in mouse lens using reverse transcriptase polymerase chain reaction (RT-PCR). Lane 1, positive control; PCR product of mouse AQP0 full-length coding region of the cDNA (789-bp segment). Lane 2, negative control; adult lens fiber cell total RNA incubated with RNase I enzyme before RT-PCR. Lane 3, E11.0; Lane 4, E11.25; Lane 5, E11.5; Lane 6, E12.0; Lane 7, E13.0; Lane 8, E15.0; Lane 9, E17.0; Lane 10, E19.0; RNA from lens fiber cells; Lane 11, P0 RNA from lens fiber cells; Lane 12, P30 RNA from lens fiber cells; Lane 13, RNA from 4-month-old adult lens fiber cells; Lane 14, RNA from 4-month-old adult lens epithelial cells. Pos., positive; Neg., negative.
Developmental Expression of AQP0 Protein
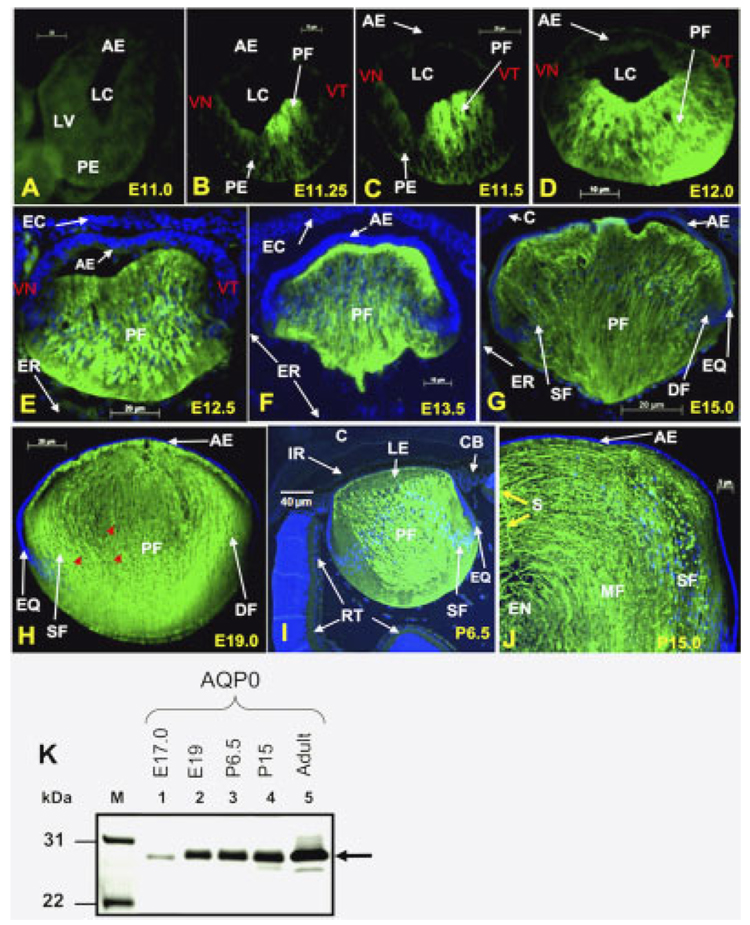
Identification of the stage and localization of the initial expression of AQP0 transcripts prompted us to find out the same for the AQP0 protein, and hence we performed immunocytochemistry (Fig. 2). There was no detectable level of specific anti-AQP0 antibody binding to cryosections of embryonic lens at E9.0 to E11.0. Figure 2A shows a representative image; to observe the sections, the green background was enhanced digitally. Sections of the developing lens at E11.25, E11.5, E12.0, E12.5, E13.5, E15.0, E19.0, and postnatal lens at P6.5 and P15 showed binding of anti-AQP0 antibody to fiber cells. Thus, it is evident that detectable levels of AQP0 protein are also present at E11.25, the stage at which AQP0 transcripts were first identified. The antibody did not bind to lens epithelial cells (Fig. 2B–J) but only to fiber cells, indicating specificity.
Fig. 2. AQP0 protein expression. Immunocytochemistry.
A: an optical section of a wild type embryonic lens at stage E11.0 with no detectable anti-AQP0 antibody binding to the lens vesicles or to the other parts of the developing eye. B–H: Developmental stages, E11.25, E11.5, E12.0, E12.5. E13.5, E15.0, and E19.0. I,J: Postnatal stages P6.5 and P15.0. B–J: Anti-AQP0 antibody binding (green) to the differentiating and differentiated primary and secondary fiber cells. E–I: No anti-AQP0 antibody binding to cornea, iris, ciliary body, or retina. Selected lens sections were stained with DAPI (Blue) nuclear stain. Red arrows indicate degrading nuclei in the maturing fiber cells. K: Western blot: Total lens membrane fractions were isolated and proteins extracted from embryonic, postnatal, and adult mouse lens, and loaded onto SDS-polyacrylamide gels (50 µg/lane). Western blotting was performed as described in Experimental Procedures section using rabbit anti-AQP0 primary antibody. Lane 1, E17; Lane 2, E19; Lane 3, P6.5; Lane 4, P15; Lane 5, 4-month-old adult lens fiber cells. M, Molecular weight marker Mark12 (Invitrogen). Arrow, aquaporin-specific band of ~28 kDa. AE, anterior epithelium; C, cornea; CB, ciliary body; DF, differentiating fiber cells; EC, embryonic cornea; EN, embryonic nucleus; ER, embryonic retina; EQ, equatorial epithelial cells; IR, iris; LC, lens cavity; LE, lens; LV, lens vesicle; MF, matured fiber cells; PE, posterior epithelial cells; PF, primary embryonic lens fiber cells; S, fiber cell suture; SF, secondary fiber cells; RT, retina; VT, ventrotemporal half of the lens vesicle; VN, ventronasal half of the lens vesicle.
Detectable levels of AQP0 expression start at E11.25 (Fig. 2B) during the initial stage of primary fiber cell differentiation, which starts in the ventrotemporal half of the lens vesicle. The expression continues as more and more primary fiber cells are differentiated from posterior embryonic lens epithelial cells. Anti-AQP0 antibody bound mainly to the differentiating primary fiber cells and not to any other tissue in the eye, including the embryonic cornea, ciliary body, and retina (Fig. 2F–I). Figure 2G shows differentiating secondary fiber cells. Nuclei in the primary fiber cells start to disintegrate from E17.0 onward (Fig. 2H, stage E19.0, red arrows). By P15, several layers of secondary fiber cells (SF) are added and some of the secondary fiber cells lost their nuclei to become mature fiber (MF) cells (Fig. 2J). Secondary fiber cells form the suture lines as indicated by the yellow arrow in Figure 2J.
Western blotting was performed to examine the lens aquaporin protein expression levels and modifications. Due to the technical difficulties in obtaining enough proteins for Western analysis at very early stages, samples were prepared from E17 onwards. Cell membrane proteins isolated from embryonic lens at E17.0, E19.0, postnatal lens at P6.5, P15.0, and adult lens showed specific binding of anti-AQP0 antibody to a major band of ~28-kDa (Fig. 2K, lanes 1–5); there was no antibody binding to lens epithelial cell membrane proteins of adult lens (data not shown). The fiber cell membrane protein fraction did not bind to anti-AQP3, AQP4, or AQP5 antibodies (data not shown), suggesting that these AQPs are not expressed.
We also observed that, during the initial stages of embryonic lens development, before and at the time of the primary fiber cell differentiation, the lens vesicle and embryonic retina are very close to each other (Fig. 2E). After primary fiber cell differentiation, the gap between the embryonic lens and retina significantly widens and the only area closer to the embryonic retina is the equatorial region of the lens, where lens secondary fiber cells differentiate continuously (Fig. 2F,G).
Developmental Expression of AQP1 Transcripts
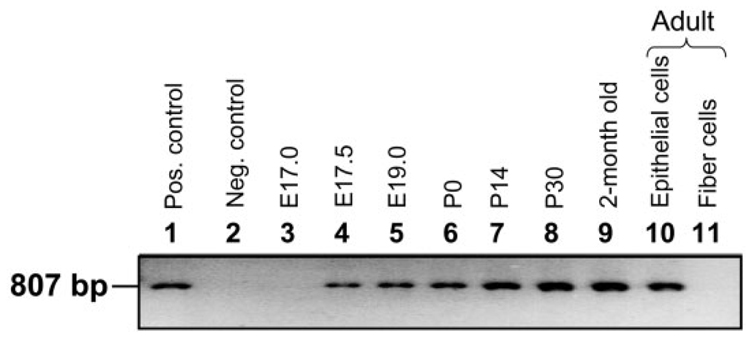
We sought to identify the initiation of expression of AQP1 transcript by performing RT-PCR at different stages of lens development (Fig. 3) as well as in developed lenses. AQP1 transcripts were not detected in the embryonic lens at E17.0 (Fig. 3, lane 3); however, the transcripts began to express at or around E17.5 (Fig. 3, lane 4) in the lens epithelial cells and continued to express throughout the adult life (Fig. 3, lane 10). In the adult mouse, the transcripts were present only in the lens epithelial layer (Fig. 3, lane 10) and, as anticipated, not in the lens fiber cells (Fig. 3, lane 11). The transcripts begin to express after capsule formation, which takes place at E17.0; also, this happens after AQP0 expression, which occurs at E11.25.
Fig. 3.
Developmental expression of AQP1 transcripts in mouse lens using RT-PCR. Lane 1, positive control; PCR product of mouse AQP1 full-length coding region of the cDNA (807-bp segment) using sense and antisense primers (see Experimental Procedures section). Lane 2, negative control; 4-month-old adult lens epithelial cell total RNA incubated with RNase I enzyme before RT-PCR; Lane 3, E17.0; Lane 4, E17.5; Lane 5, E19.0; Lane 6, P0; Lane 7, P14; Lane 8, P30; Lane 9, epithelial cells from 2-month-old mouse lenses; Lane 10, RNA from 4-month-old adult lens epithelial cells; Lane 11, RNA from 4-month-old adult lens fiber cells. Pos., positive; Neg., negative.
We have used positive and negative controls as described for AQP0 except substituting AQP1 cDNA. The size of the RT-PCR amplified product is similar to that of the cloned mouse AQP1 cDNA PCR fragment (807 bp; Fig. 3, lane 1). The total RNA is unlikely to be contaminated with genomic DNA, since the RT-PCR amplified products of AQP1 showed a band size of ~807 bp (Fig. 3, lanes 4–10). There were no RT-PCR amplified products in the DNase free RNase I treated samples (Fig. 3, lane 2), further confirming the integrity of the extracted RNA.
Developmental Expression of AQP1 Protein
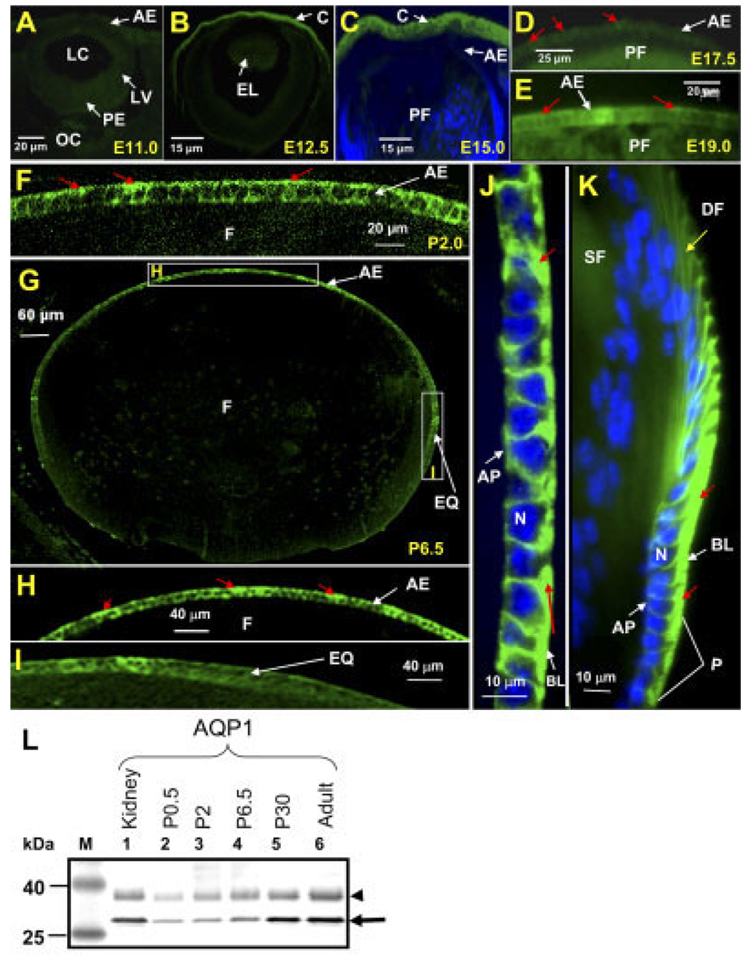
The expression and localization of AQP1 protein in the anterior and equatorial lens epithelial cells were studied using immunocytochemistry (Fig. 4). The anti-AQP1 antibody did not bind to the cryosections of the embryonic lens at E9.0 to E17.0. Representative data are shown in Figure 4A and B. However, there was AQP1 expression in the developing embryonic corneal endothelial cells at E12.5 (Fig. 4B) and this served as a positive control for anti-AQP1 specific binding. We have also investigated E13.5 to E17.0 of the embryonic lens. There was no detectable level of antibody binding at these stages (e.g., E15.0, Fig. 4C). Lens sections of E17.5, E19.0, P2.0, P6.5, and adult mouse (4 months of age) showed binding of anti-AQP1 only to the epithelial cells (Fig. 4D–K), indicating specificity. There was a low level of anti-AQP1 binding by the anterior epithelial cells at E17.5 (Fig. 4D). The antibody binding intensified in the anterior epithelium at E19.0 (Fig. 4E) and P2 (Fig. 4F). At P6.5, there was a very high level of anti-AQP1 antibody binding to the anterior epithelial cells (Fig. 4H) and a very low level of binding to the equatorial epithelial cells (Fig. 4I). We have also investigated AQP1 expression in P10 to P35 lenses; maximum expression in the anterior and equatorial epithelial cells occurred at ~P30 (data not shown). In the adult, the antibody binding was very strong in the anterior as well as equatorial epithelial cells (Fig. 4J and K), specifically on the basolateral side rather than the apical side (shown in red arrows). Intense anti-AQP1 binding was seen in the actively dividing equatorial epithelial cells (progenitors). The differentiating and elongating cells showed decreased levels of anti-AQP1 binding (shown in yellow arrow, Fig. 4K).
Fig. 4. AQP1 protein expression. Immunocytochemistry.
A–C: Optical sections of wild type embryonic lens at stages E11.0, E12.5, and E15.0 with no detectable levels of anti-AQP1 antibody binding to the epithelial cells. Note: B and C show AQP1-specific antibody binding to the developing cornea. D: Embryonic lens at E17.5, showing anti-AQP1 antibody binding (green) to the basolateral side (red arrows) of the anterior epithelial cells. E: Embryonic lens section at E19.0. F: Postnatal day 2 (P2), showing anti-AQP1 antibody binding (green) to most of the anterior epithelial cells, especially in the basolateral plasma membrane. G–I: Lens sections at P6.5; intense anti-AQP1 antibody binding (green) to most of the anterior epithelial cells and low levels to the equatorial epithelial cells; there was no nonspecific binding of anti-AQP1 antibody (to the fiber cells, marked as “F” in G and H). H,I: Digitally magnified regions of the anterior epithelium and equatorial epithelium, respectively, shown as boxed areas in figure G. J,K: Anterior and equatorial lens epithelium of adult mouse lens, respectively, showing intense binding of anti-AQP1 antibody in the basolateral side (red arrows). Selected sections were stained with DAPI (Blue) nuclear stain. L: Western blot: Total lens membrane proteins were extracted from embryonic, postnatal, and adult mouse lenses and loaded onto SDS-polyacrylamide gels (50 µg/lane). Western blotting was performed as described in Experimental Procedures section using rabbit anti-AQP1 primary antibody. Lane 1, mouse kidney (positive control); lane 2, P0.5; lane 3, P2; lane 4, P6.5; lane 5, P30; lane 6, adult lens epithelial cells. M, Molecular weight marker BenchMark Pre-stained (Invitrogen). Arrow, aquaporin specific protein band of ~28 kDa; arrowhead, AQP1 glycosylated form of ~35 kDa. AE, anterior epithelium; AP, apical side; BL, basolateral side; C, cornea; DF, differentiating fiber cells; EL, embryonic lens; EQ, equatorial epithelial cells; F, fiber cells; LC, lens cavity; LV, lens vesicle; N, Nucleus; OC, optic cup; P, progenitor epithelial cells; PE, posterior epithelial cells; PF, primary embryonic lens fiber cells; SF, secondary fiber cells.
Cell membrane preparations from postnatal day 0.5, 2.0, 6.5, 30.0, and 4-month-old adult lenses, subjected to Western blotting, showed specific anti-AQP1 antibody binding to a 28-kDa nonglycosylated, and a ~34-kDa glycosylated protein fraction from lens epithelial cell membrane (Fig. 4L, lanes 2–6) and not to lens fiber cell membrane protein (data not shown). AQP1 has two consensus sequences for N-linked glycosylation at amino acids 42 and 205 (Huebert et al., 2002; Moon et al., 1995). Glycosylation has been confirmed by the deglycosylation studies conducted by Preston et al. (1992) and Nejsum et al. (2002); moreover, the antibody supplier’s data sheet on “specificity of the antibody” has cited the binding of the antibody to glycosylated AQP1. The quantity of AQP1 protein increased steadily from P0.5 to P30.0. AQP1 expression levels increased severalfold from P0 to P30; in 4-month-old adult lenses, there was no significant difference in the AQP1 protein level compared to the P30 lenses. Western blots of epithelial cell membrane protein showed no anti-AQP3, AQP4, or AQP5 antibody binding (data not shown).
Functional Expression of Aquaporins in Lens Epithelial Cells and Fiber Cells
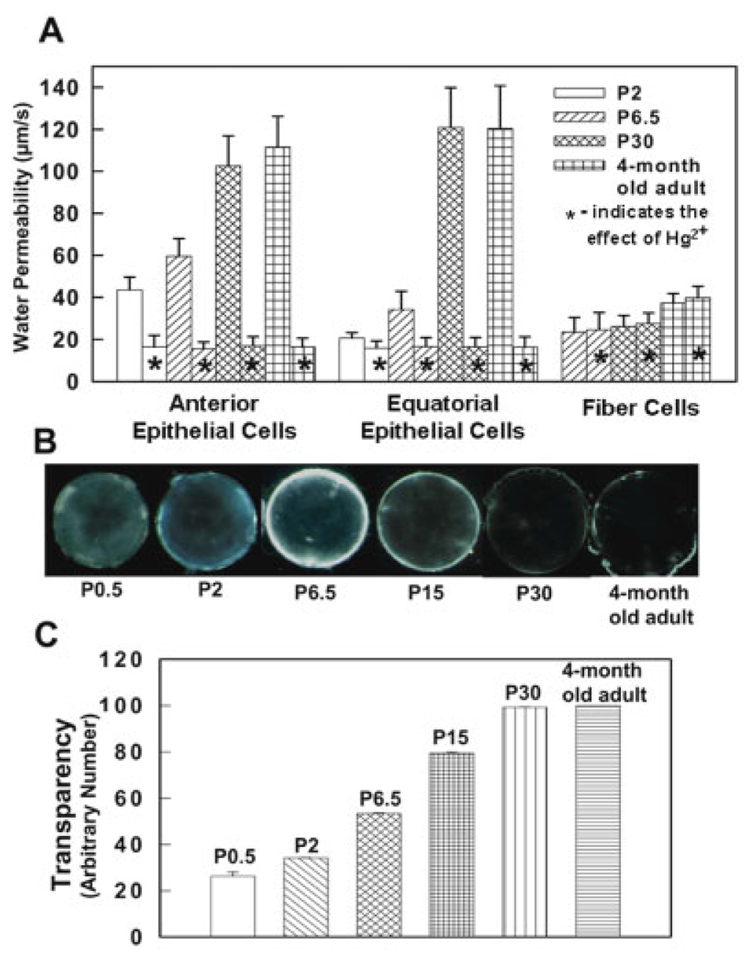
For functional comparison, water permeability measurements were carried out on individual anterior and equatorial epithelial cells, and fiber cell vesicles, from postnatal and adult mouse lenses. Water permeability of the anterior epithelial cells is significantly higher than that of the equatorial epithelial cells at P2 and P6.5 (Fig. 5A). However, anterior and equatorial epithelial cells from P30 and 4-month-old adult lens did not show any significant difference in membrane water permeability. There was no significant difference in fiber cell membrane water permeability at P2 and P6.5; however, there was a slight increase in fiber cell membrane water permeability in P30 and 4-month-old adult mouse (Fig. 5A). Hg2+ significantly reduced the membrane water permeability of the lens epithelial cells from both the anterior pole and equatorial regions expressing AQP1 (Fig 5); it did not affect the water permeability of the fiber cells, which express AQP0. These data are in accordance with the AQP1 and AQP0 expression pattern observed by immunostaining (Fig. 2, Fig 4, Fig 6).
Fig. 5. Membrane water permeability and lens transparency.
A: The water permeability of mouse lens anterior epithelial cells, equatorial epithelial cells, and fiber cell membrane vesicles at P2, P6.5, P30, and 4-month-old adult. *, Effect of Hg2+. B: A comparison of transparency at P0.5, P2, P6.5, P15, P30, and 4-month-old adult lenses. C: Quantification of lens transparency. The data on lens transparency are consistent with the AQP0 and AQP1 expression levels seen in Figure 2 and Figure 4.
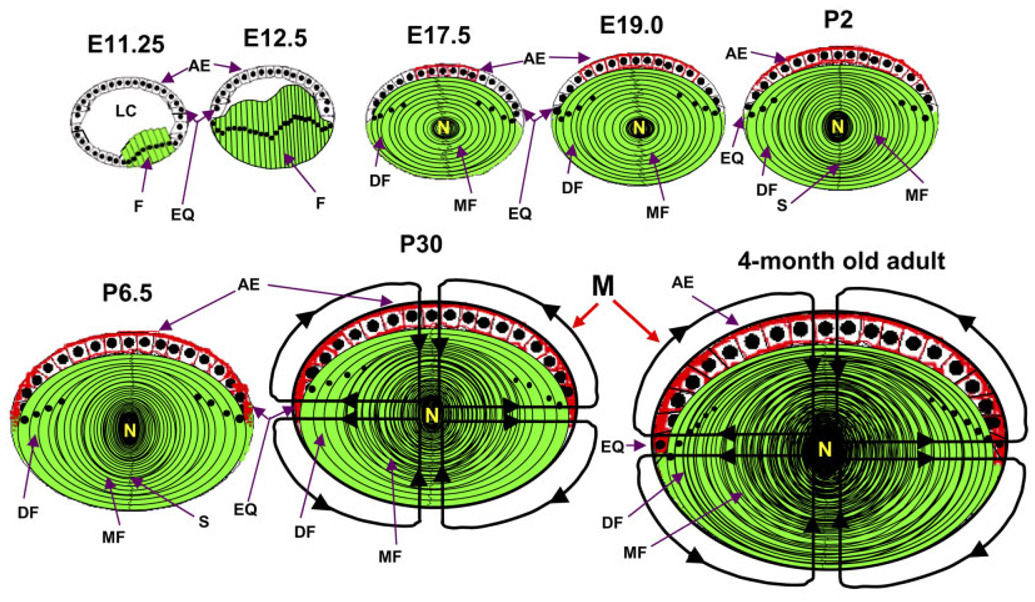
Fig. 6.
Schematic (not to scale) representation of the expression patterns of AQP0 and AQP1 in developing, postnatal and adult mouse lenses. At E11.25, fiber cells begin to express AQP0 (green). At E12.5, more fiber cells develop and express AQP0. At E17.5, AQP1 expression begins in the anterior epithelial cells (red); even more fiber cells are added and AQP0 expression continues. E19.0 and P2 show the gradual spreading of AQP1 expression toward the equatorial epithelial cells. P6.5 shows the expression of AQP1 in all of the epithelial cells and AQP0, in the fiber cells. P30 shows microcirculation; AQP1 expression reaches its maximum at P30. Microcirculation in the adult lens is depicted in the last picture of the series. E, embryonic stage; P, postnatal day; AQP0 in green; AQP1 in red; AE, anterior epithelial cells; DF, differentiating fiber cells; EQ, equatorial epithelial cells; F, fiber cells; LC, lens cavity; M, microcirculation; MF, matured fiber cells; N, lens nucleus.
Progress in Lens Transparency
We examined the lens transparency in relation to membrane water permeability and aquaporin expression levels. Postnatal mouse lenses from P0.5, P2, P6.5, P15, P30, and 4-month-old adult mouse lenses were investigated. Transparency of the lenses gradually increased from P0.5 to P30; however, at P6.5, there was a twofold increase in lens transparency compared to P0.5 (Fig. 5B,C) and a similar trend was seen in AQP1 protein expression (Fig. 4L). From P6.5 to P30, an increasing trend was observed for lens transparency (Fig. 5B and C) as well as epithelial membrane water permeability (Fig. 5A) and AQP1 protein expression (Fig. 4L). Lens transparency at P30, is not significantly different from that of 4-month-old lens (Fig. 5B and C), and the same is true for levels of AQP1 expression (Fig. 4L) and epithelial cell membrane water permeability (Fig. 5A).
DISCUSSION
The work presented here demonstrates the characteristic patterns of AQP0 and AQP1 expression in embryonic, postnatal, and adult mouse lenses. It also offers insights into the function/s of these proteins and provides clues for further investigation.
The normal lens shows asymmetry in development; posterior epithelial cells of a normally developing embryonic lens vesicle contain two subpopulations, which differentiate into primary lens fiber cells (Faber et al., 2002). At E11.25, posterior epithelial cells in the ventrotemporal half of the lens vesicle first begin to differentiate and simultaneously express AQP0; these cells elongate towards anterior epithelial cells as they differentiate into the primary fiber cells. At E12.0, the posterior epithelial cells in the ventronasal half of the lens vesicle start to elongate towards anterior epithelial cells and begin to express AQP0. The asymmetric expression of AQP0 protein at embryonic stages 11.25 to 12.5 is shown in Figure 2B–E. Differentiation signals emanating from the developing retina (Faber et al., 2002) may be responsible for initiating the expression of AQP0. After primary fiber cell differentiation, the only region that is closer to the retina is the equator, where further embryonic lens development and lens growth-related activities happen throughout life. Retinal involvement in lens development has been reported by several researchers (Freeman, 1963; Reeve and Wild, 1978; Bosco et al., 1980, 1993; Filoni et al., 1980, 1997).
Development of the mouse embryonic lens is normally complete by E14.0 (Bard et al., 1998) and the equatorial epithelial cells begin to differentiate into secondary fiber cells resulting in the enlargement of the lens. The primary fiber cells progressively move toward the center and thus the embryonic lens nucleus is retained throughout life. Very little was previously known about the expression pattern of AQP0 protein in the developing mouse lens. There is a report on the expression of AQP0 transcript at E11.5; the investigators used mouse embryonic head total RNA and performed RT-PCR (Zhou et al., 2002). Another report states that AQP0 protein expression begins at E12.5, in rat embryonic lens (Yancey et al., 1988). Based upon these two studies, Zhou et al. (2002) speculated that AQP0 transcripts might have been produced a day before the protein was actually synthesized. Our study shows that both AQP0 transcripts and protein are present in detectable quantities in the developing embryonic mouse lens at E11.25. AQP0 protein is not detected in the anterior epithelial cells or undifferentiated posterior epithelial cells at this stage. Binding of anti-AQP0 antibody to the fiber cells and not to the epithelial cells reveals the exclusive expression of AQP0 in the fiber cells.
AQP0 transcripts and protein are present at the time of first primary fiber cell differentiation at E11.25, whereas AQP1 expression begins only at E17.5 in the very anterior epithelial cells, after completion of embryonic lens development. This is interesting because lens anterior epithelial cells develop much earlier than the primary and secondary fiber cells. The observed temporal pattern of expression suggests that as the lens core becomes progressively larger with the addition of new fiber cells, there is an increasing demand for higher epithelial membrane water permeability in order to establish the microcirculation (Mathias and Rae, 2004). Figure 5 and Figure 6 summarize the membrane water permeability, lens transparency, and the expression patterns of AQP0 in the fiber cells and of AQP1 in the epithelial cells. Water permeability of the fiber cells did not show significant differences from postnatal to adult, and could not be blocked by Hg2+ (Kushmerick et al., 1995; Varadaraj et al., 1999). AQP1 maximum expression occurs around P30 and continues throughout life. Lens transparency also reaches its peak at P30 and this event coincides with the maximum expression of AQP1 in the anterior and equatorial epithelial cells (Fig. 4, Fig 6). High membrane water permeability (Fig. 5A) in the epithelial cells around P30 suggests the total establishment of the lens microcirculatory system. Even though embryonic lens water permeability and transparency were not studied due to technical difficulties, from the data obtained (Fig. 4, Fig 5A–C, Fig 6), we infer that establishment of the microcirculatory system may begin around E17.5 with the expression of the more efficient AQP1 water channel.
In AQP0-deficient mice, the homozygous genotype develops a cataract before E17.5 (Shiels et al., 2001), whereas the heterozygous genotype initially develops mild fiber cell distortion, and later develops a cataract (~6 months). In the homozygous lens, the fiber cells are severely disoriented, indicating a structural role for AQP0. Heterozygous mouse lenses showed differences in fiber cell shape and suture formation (Al-Ghoul et al., 2003). This could be the result of weak cell-to-cell adhesion; mild fiber cell distortion begins before E17.5. The presence of about half the normal amount of AQP0 protein in the heterozygous lens appears to be sufficient to avoid degeneration of the lens during the first few months of life. However, with progression in the size of the lens, the quantity of AQP0 protein expressed appears to be insufficient for providing the necessary cell to cell adhesion. Moreover, the microcirculatory system becomes increasingly important for homeostasis of the central fiber cells. At this point, the presence of only half the normal amount of AQP0 and the consequent reduction in microcirculation appear to cause inadequacies in meeting the increasing demand for water transport, and the stress eventually manifests as a cataract.
The two aquaporins appear to share at least one vital function: namely, water transport in the microcirculatory system. The question is, why the expression of AQP0 in the embryonic lens begins before the complete differentiation of the primary fiber cells? The early expression may be needed to establish the highly ordered lens architecture. Therefore, initially AQP0 may function as an adhesion protein to glue the cells in close proximity in order to minimize the extracellular space between cells and to reduce light scattering (Dunia et al., 1987; Zampighi et al., 1989, 1992; Michea et al., 1994, 1995; Gonen et al., 2004a,b, 2005). Moreover, AQP0 needs to be synthesized and targeted to the plasma membrane before the maturation of the fiber cells, because at later stages the mature fiber cells lose their ability to synthesize AQP0.
Human AQP1 and AQP2 natural mutations resulted in recessive traits (Deen et al., 1994, 1995; Preston et al., 1994) whereas all of the thus far documented AQP0 mutations in human (Berry et al., 2000; Francis et al., 2000a,b; Geyer et al., 2006) and mice (Shiels and Griffin, 1993; Shiels and Bassnett, 1996; Shiels et al., 2000; Okamura et al., 2003) led to dominant lens cataract. Mutations in structural proteins cause dominant inherited traits (Francis et al., 2000a,b). This finding strengthens our notion that AQP0 primarily starts with a structural role for the embryonic lens, and later, as the lens grows bigger in size, participates in the microcirculatory system as a water transporter.
EXPERIMENTAL PROCEDURES
Collection of Mouse Tissues
Wild mice (FVB strain) were obtained from Charles River Inc. Female and male mice were paired around 6 p.m. The morning following the appearance of a vaginal plug was designated E0.5 (Embryonic stage 0.5). Pregnant mice were sacrificed by intraperitoneal injection of pentobarbital (720–800 mg/kg body weight) at different time periods of gestation. The American Association for Accreditation of Laboratory Animal Care (AAALAC) approved these procedures. At stages E9.0 through E12.0, entire embryos were collected surgically; at stages E12.5–E15.0, head regions were collected. Eyes were collected at stages E16–20. Lenses were collected from P0.5–P20 (P = Post-natal day), weaned young ones (P21–30), and adult mice. Lenses were dissected and anterior epithelial cells, equatorial epithelial cells, and fiber cells were separated as described by Varadaraj et al. (1999). Physiological saline solution (in mM: NaCl 150, KCl 4.7, MgCl2 1, CaCl2 1, glucose 5, HEPES 5, pH 7.5) was used during sample collection.
RT-PCR
Total RNA for AQP0 RT-PCR studies was extracted from mouse embryos, embryonic head, embryonic eye, whole lens, lens anterior epithelial cells, and lens fiber cells at various stages using RNA STAT-60 (TEL-TEST Inc., Friendswood, TX), following the manufacturer’s instructions. Total RNA for AQP1 RT-PCR studies was extracted only from embryonic lenses E17.0 and older, due to technical difficulties in separating the lens from other eye tissues where AQP1 expression starts earlier than in the lens. Mouse-specific AQP0 and AQP1 primer pairs were synthesized. Primers for AQP0: sense (5′ atg tgg gaa ctt cgg tct gc) and antisense (5′ aca ggg cct gag tct tca gtt cg). Primers for AQP1: sense (5′ atg gcc agt gaa atc aag aag) and antisense (5′ ttt ggg ctt cat ctc cac cct gga g). Total RNA extracted from different embryonic, postnatal, and adult stages served as templates for the respective single tube RT-PCR (GIBCO-BRL) that was carried out using a Gradient Thermal Cycler (Stratagene) as described by Varadaraj and Skinner (1994a) and Kumari et al. (2000, 2001). RT-PCR products were fractionated on agarose gels, stained with ethidium bromide, viewed over UV, and images were digitized using Kodak Image Station and Kodak 1D V. 3.5.3 software. We used cloned cDNA as a positive template to amplify mouse AQP0. We have also performed a negative control reaction by adding RNase I to the epithelial cell or fiber cell total RNA, before the RT-PCR reaction. Total RNA was extracted from each sample, and each time point was repeated at least 4 times from different litters born to different parents.
Immunohistochemistry
Lens immunocytochemical experiments were performed as described by Varadaraj and Skinner (1994b) and Varadaraj et al. (1999, 2005) with slight modifications for the current study. Briefly, tissues were fixed, cryo-protected, and cryosectioned at 10–16-µm thickness using a cryomicrotome (Leica). Embryonic lenses are very delicate; hence, great care was taken to safeguard the morphology of the cryosections. We used cryosections because antibody binding is better than in paraffin sections. Sections were immunostained with polyclonal rabbit antibody raised against human AQP0 or AQP1 (Chemicon, Alpha Diagnostic International or Abcam, Inc.), mounted in anti-fade Vectamount (Vector Labs) containing nuclear stain DAPI and viewed. Optimized Z-sectional digital images were acquired with a Zeiss Axiovert 200 inverted microscope equipped with AxioCam and Zeiss AxioVision 4.1 software (Carl Zeiss MicroImaging, Inc.), and processed using Adobe Photoshop 7.0. Figures show representative fields from multiple experiments.
Western Blotting
Mouse lens membrane proteins were used for Western blotting to confirm the expression of the selected aquaporins, namely, AQP0, AQP1, AQP3, AQP4, or AQP5. Lens membranes were prepared as described previously by Bok et al. (1982) and Konig and Zampighi (1995). The membrane pellets were homogenized in 50–100 µl of protein extraction buffer and sonicated for 2 min. The lysate was centrifuged at 12,000g for 20 min, and the supernatant, mixed with NuPAGE LDS sample buffer, was fractionated using NuPAGE Novex Bis-Tris gel with MOPS-SDS running buffer (Invitrogen). Western blotting was performed as described by Varadaraj et al. (1996, 1997). Briefly, proteins from the denaturing gels were transferred to PVDF nylon membranes and specific antibodies raised against AQP0 and AQP1 (Chemicon, Alpha Diagnostic International or Abcam, Inc.) were used to detect the expression of AQP0 in the fiber cells and AQP1 in the epithelial cells.
Water Permeability
Water transport studies were conducted in postnatal and adult lenses; embryonic lenses were not subjected to such studies due to technical difficulties. Individual lens epithelial cells from the anterior pole and the equatorial regions were isolated; lens fiber cell membrane vesicles were prepared as described by Varadaraj et al. (1999), with slight modifications. In brief, lens capsules were separated from the posterior of the lens and partially cut towards the equator to form four flaps. A small circle was cut on the anterior pole with a radius approximately one-fifth the distance from the pole to the equator; a small ring of the equatorial region was also cut. From these two isolated anterior and equatorial regions of the capsule, individual epithelial cells were separated using collagenase. Cells from the anterior pole were 10–15 µm in diameter and those from the equatorial region were of three different size groups. The equatorial cells were further selected during the water permeability studies, using a micrometer attached to the microscope. Based on their size and AQP1 expression, the selected cells were slightly bigger (16–25 µm) than the anterior epithelial cells (cuboidal ~10–15 µm) and smaller than the differentiating epithelial cells (elongated >26 µm). Water permeability measurements of individual fiber cell membrane vesicles and anterior and equatorial epithelial cells were performed as described previously by Varadaraj et al. (1999, 2005).
Lens Transparency
Postnatal and adult mouse lenses were investigated for their relative lens transparency. Lenses from P0.5, P2, P6.5, P15, P30, and 4-month-old mouse were dissected as described previously, photographed digitally under comparable conditions, and analyzed using Sigma Scan software. The digitized images consisted of pixels of different grey values. Since we have used dark field illumination, transparent lenses appeared dark and the less transparent ones appeared white. About 75% of the lens diameter was evaluated to exclude the circular reflexes of the illumination device near the lens equator. Zero intensity denoted 100% transparency; in other words, the greater the intensity, the lower the lens transparency. The transparency index of each lens was calculated as intensity per pixel (Transparency Index = total intensity/number of pixels) and was low in clear lens and high in less transparent lens.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: EY06391; Grant sponsor: Alcon Research Ltd.; Grant number: 39733.
ABBREVIATIONS
- AQP0
Aquaporin 0
- MIP
Major Intrinsic Protein of Lens
- AQP1
Aquaporin 1
REFERENCES
- Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S. Aquaporin water channels: from atomic structure to clinical medicine. J Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghoul KJ, Kirk T, Kuszak AJ, Zoltoski RK, Shiels A, Kuszak JR. Lens structure in MIP-deficient mice. Anat Rec. 2003;273A:714–730. doi: 10.1002/ar.a.10080. [DOI] [PubMed] [Google Scholar]
- Ball LE, Little M, Nowak MW, Garland DL, Crouch RK, Schey KL. Water permeability of C-terminally truncated aquaporin 0 (AQP0 1-243) observed in the aging human lens. Invest Ophthalmol Vis Sci. 2003;44:4820–4828. doi: 10.1167/iovs.02-1317. [DOI] [PubMed] [Google Scholar]
- Bard JBL, Kaufman MH, Dubreuil C, Brune RM, Burger A, Baldock RA, Davidson DR. An internet-accessible database of mouse developmental anatomy based on a systematic nomenclature. Mech Dev. 1998;74:111–120. doi: 10.1016/s0925-4773(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Berry V, Francis P, Kaushal S, Moore A, Bhattacharya S. Missense mutations in MIP underlie autosomal dominant ’polymorphic’ and lamellar cataracts linked to 12q. Nat Genet. 2000;25:15–17. doi: 10.1038/75538. [DOI] [PubMed] [Google Scholar]
- Bok D, Dockstader J, Horwitz J. Immuno-cytochemical localization of the lens main intrinsic polypeptide (MIP26) in communicating junctions. J Cell Biol. 1982;92:213–220. doi: 10.1083/jcb.92.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco L, Filoni S, Cioni C. Lens formation from cornea in the presence of the old lens in larval Xenopus laevis. J Exp Zool. 1980;213:9–14. doi: 10.1002/jez.1402130103. [DOI] [PubMed] [Google Scholar]
- Bosco L, Valle C, Willems D. In-vivo and in-vitro experimental-analysis of lens regeneration in larval Xenopus laevis. Dev Growth Differ. 1993;35:257–270. doi: 10.1111/j.1440-169X.1993.00257.x. [DOI] [PubMed] [Google Scholar]
- Chandy G, Zampighi GA, Kreman M, Hall JE. Comparison of the water transporting properties of MIP and AQP1. J Membr Biol. 1997;159:29–39. doi: 10.1007/s002329900266. [DOI] [PubMed] [Google Scholar]
- Chepelinsky AB. The ocular lens fiber membrane specific protein MIP/aquaporin. J Exp Zool. 2003;300A:41–46. doi: 10.1002/jez.a.10307. [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Lens development - fiber elongation and lens orientation. Science. 1963;142:1489–1490. doi: 10.1126/science.142.3598.1489. [DOI] [PubMed] [Google Scholar]
- Deen PMT, Verdijk MAJ, Knoers N, Wieringa B, Monnens LAH, Vanos CH, Vanoost BA. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- Deen PMT, Croes H, Vanaubel R, Ginsel LA, Vanos CH. Water channels encoded by mutant aquaporin-2 genes in nephrogenic diabetes-insipidus are impaired in their cellular routing. J Clin Invest. 1995;95:2291–2296. doi: 10.1172/JCI117920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunia I, Manenti S, Rousselet A, Benedetti EL. Electron-microscopic observations of reconstituted proteoliposomes with the purified major intrinsic membrane-protein of eye lens fibers. J Cell Biol. 1987;105:1679–1689. doi: 10.1083/jcb.105.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber SC, Robinson ML, Makarenkova HP, Lang RA. Bmp signaling is required for development of primary lens fiber cells. Development. 2002;129:3727–3737. doi: 10.1242/dev.129.15.3727. [DOI] [PubMed] [Google Scholar]
- Fan JG, Fariss RN, Purkiss AG, Slingsby C, Sandilands A, Quinlan R, Wistow G, Chepelinsky AB. Specific interaction between lens MIP/Aquaporin-0 and two members of the gamma-crystallin family. Mol Vision. 2005;11:76–87. [PubMed] [Google Scholar]
- Filoni S, Bosco L, Paglioni N, Cioni C. Lens formation from pericorneal epidermis in the presence of the old lens in larval Xenopus laevis. J Exp Zool. 1980;211:303–309. doi: 10.1002/jez.1402130103. [DOI] [PubMed] [Google Scholar]
- Filoni S, Bernardini S, Cannata SM, Dalessio A. Lens regeneration in larval Xenopus laevis: Experimental analysis of the decline in the regenerative capacity during development. Dev Biol. 1997;187:13–24. doi: 10.1006/dbio.1997.8598. [DOI] [PubMed] [Google Scholar]
- Francis P, Berry V, Bhattacharya S, Moore A. Congenital progressive polymorphic cataract caused by a mutation in the major intrinsic protein of the lens, MIP (AQP0) Br J Ophthalmol. 2000a;84:1376–1379. doi: 10.1136/bjo.84.12.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis P, Chung JJ, Yasui M, Berry V, Moore A, Wyatt MK, Wistow G, Bhattacharya SS, Agre P. Functional impairment of lens aquaporin in two families with dominantly inherited cataracts. Hum Mol Genet. 2000b;9:2329–2334. doi: 10.1093/oxfordjournals.hmg.a018925. [DOI] [PubMed] [Google Scholar]
- Freeman G. Lens regeneration from cornea in Xenopus laevis. J Exp Zool. 1963;154:39–66. doi: 10.1002/jez.1401540105. [DOI] [PubMed] [Google Scholar]
- Geyer DD, Spence MA, Johannes M, Flodman P, Clancy KP, Berry R, Sparkes RS, Jonsen MD, Isenberg SJ, Bateman JB. Novel single-base deletional mutation in major intrinsic protein (MIP) in autosomal dominant cataract. Am J Ophthalmol. 2006;141:761–763. doi: 10.1016/j.ajo.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen T, Cheng Y, Kistler J, Walz T. Aquaporin-0 membrane junctions form upon proteolytic cleavage. J Mol Biol. 2004a;342:1337–1345. doi: 10.1016/j.jmb.2004.07.076. [DOI] [PubMed] [Google Scholar]
- Gonen T, Sliz P, Kistler J, Cheng YF, Walz T. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature. 2004b;429:193–197. doi: 10.1038/nature02503. [DOI] [PubMed] [Google Scholar]
- Gonen T, Cheng YF, Sliz P, Hiroaki Y, Fujiyoshi Y, Harrison SC, Walz T. Lipid-protein interactions in double-layered two-dimensional AQPO crystals. Nature. 2005;438:633–638. doi: 10.1038/nature04321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S, Zeuthen T, La Cour M, Ottersen OP, Agre P, Nielsen S. Aquaporins in complex tissues: distribution of aquaporins 1–5 in human and rat eye. Am J Physiol. 1998;274:C1332–C1345. doi: 10.1152/ajpcell.1998.274.5.C1332. [DOI] [PubMed] [Google Scholar]
- Huebert RC, Splinter PL, Garcia F, Marinelli RA, LaRusso NF. Expression and localization of aquaporin water channels in rat hepatocytes. J Biol Chem. 2002;277:22710–22717. doi: 10.1074/jbc.M202394200. [DOI] [PubMed] [Google Scholar]
- Konig N, Zampighi GA. Purification of bovine lens cell-to-cell channels composed of connexin44 and connexin50. J Cell Science. 1995;108:3091–3098. doi: 10.1242/jcs.108.9.3091. [DOI] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K, Valiunas V, Ramanan SV, Christensen EA, Beyer EC, Brink PR. Functional expression and biophysical properties of polymorphic variants of the human gap junction protein connexin37. Biochem Biophys Res Commun. 2000;274:216–224. doi: 10.1006/bbrc.2000.3054. [DOI] [PubMed] [Google Scholar]
- Kumari SS, Varadaraj K, Valiunas V, Brink PR. Site-directed mutations in the transmembrane domain M3 of human connexin37 alter channel conductance and gating. Biochem Biophys Res Commun. 2001;280:440–447. doi: 10.1006/bbrc.2000.4121. [DOI] [PubMed] [Google Scholar]
- Kushmerick C, Rice SJ, Baldo GJ, Haspel HC, Mathias RT. Ion, water and neutral solute transport in Xenopus oocytes expressing frog lens MIP. Exp Eye Res. 1995;61:351–362. doi: 10.1016/s0014-4835(05)80129-0. [DOI] [PubMed] [Google Scholar]
- Kushmerick C, Varadaraj K, Mathias RT. Effects of lens major intrinsic protein on glycerol permeability and metabolism. J Membr Biol. 1998;161:9–19. doi: 10.1007/s002329900310. [DOI] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem. 1998;273:4296–4309. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Rae JL. The lens: local transport and global transparency. Exp Eye Res. 2004;78:689–698. doi: 10.1016/j.exer.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Phys Rev. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- Michea LF, Andrinolo D, Ceppi H, Lagos N. Biochemical-evidence for adhesion-promoting role of major intrinsic protein isolated from both normal and cataractous human lenses. Exp Eye Res. 1995;61:293–301. doi: 10.1016/s0014-4835(05)80124-1. [DOI] [PubMed] [Google Scholar]
- Michea LF, Delafuente M, Lagos N. Lens major intrinsic protein (MIP) promotes adhesion when reconstituted into large unilamellar liposomes. Biochemistry. 1994;33:7663–7669. doi: 10.1021/bi00190a021. [DOI] [PubMed] [Google Scholar]
- Moon C, Williams JB, Preston GM, Copeland NG, Gilbert DJ, Nathans D, Jenkins NA, Agre P. The mouse aquaporin-1 gene. Genomics. 1995;30:354–357. doi: 10.1006/geno.1995.0029. [DOI] [PubMed] [Google Scholar]
- Mulders SM, Preston GM, Deen PMT, Guggino WB, Vanos CH, Agre P. Water channel properties of major intrinsic protein of lens. J Biol Chem. 1995;270:9010–9016. doi: 10.1074/jbc.270.15.9010. [DOI] [PubMed] [Google Scholar]
- Nejsum LN, Kwon TH, Jensen UB, Fumagalli O, Frokiaer J, Krane CM, Menon AG, King LS, Agre PC, Nielsen S. Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc Natl Acad Sci USA. 2002;99:511–516. doi: 10.1073/pnas.012588099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth-Cahalan KL, Hall JE. pH and calcium regulate the water permeability of aquaporin 0. J Biol Chem. 2000;275:6777–6782. doi: 10.1074/jbc.275.10.6777. [DOI] [PubMed] [Google Scholar]
- Nemeth-Cahalan KL, Kalman K, Hall JE. Molecular basis of pH and Ca2+ regulation of aquaporin water permeability. J Gen Physiol. 2004;123:573–580. doi: 10.1085/jgp.200308990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura T, Miyoshi I, Takahashi K, Mototani Y, Ishigaki S, Kon Y, Kasai N. Bilateral congenital cataracts result from a gain-of-function mutation in the gene for aquaporin-0 in mice. Genomics. 2003;81:361–368. doi: 10.1016/s0888-7543(03)00029-6. [DOI] [PubMed] [Google Scholar]
- Patil RV, Saito I, Yang X, Wax MB. Expression of aquaporins in the rat ocular tissue. Exp Eye Res. 1997;64:203–209. doi: 10.1006/exer.1996.0196. [DOI] [PubMed] [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red-cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Preston GM, Smith BL, Zeidel ML, Moulds JJ, Agre P. Mutations in aquaporin-1 in phenotypically normal humans without functional chip water channels. Science. 1994;265:1585–1587. doi: 10.1126/science.7521540. [DOI] [PubMed] [Google Scholar]
- Reeve JG, Wild AE. Lens regeneration from cornea of larval Xenopus laevis in the presence of the lens. J Embryol Exp Morphol. 1978;48:205–214. [PubMed] [Google Scholar]
- Rose KML, Gourdie RG, Prescott AR, Quinlan RA, Crouch RK, Schey KL. The C terminus of lens aquaporin 0 interacts with the cytoskeletal proteins filensin and CP49. Invest Ophthalmol Vis Sci. 2006;47:1562–1570. doi: 10.1167/iovs.05-1313. [DOI] [PubMed] [Google Scholar]
- Schey KL, Little M, Fowler JG, Crouch RK. Characterization of human lens major intrinsic protein structure. Invest Ophthalmol Vis Sci. 2000;41:175–182. [PubMed] [Google Scholar]
- Shiels A, Bassnett S. Mutations in the founder of the MIP gene family underlie cataract development in the mouse. Nat Genet. 1996;12:212–215. doi: 10.1038/ng0296-212. [DOI] [PubMed] [Google Scholar]
- Shiels A, Griffin CS. Aberrant expression of the gene for lens major intrinsic protein in the cat mouse. Curr Eye Res. 1993;12:913–921. doi: 10.3109/02713689309020398. [DOI] [PubMed] [Google Scholar]
- Shiels A, MacKay D, Bassnett S, Al-Ghoul K, Kuszak J. Disruption of lens fiber cell architecture in mice expressing a chimeric AQP0-LTR protein. FASEB J. 2000;14:2207–2212. doi: 10.1096/fj.99-1071com. [DOI] [PubMed] [Google Scholar]
- Shiels A, Bassnett S, Varadaraj K, Mathias R, Al-Ghoul K, Kuszak J, Donoviel D, Lilleberg S, Friedrich G, Zambrowicz B. Optical dysfunction of the crystalline lens in aquaporin-0-deficient mice. Physiol Genomics. 2001;7:179–186. doi: 10.1152/physiolgenomics.00078.2001. [DOI] [PubMed] [Google Scholar]
- Tan FG, Donovan AK, Ledee DR, Zelenka PS, Fariss RN, Chepelinsky AB. gamma E-crystallin recruitment to the plasma membrane by specific interaction between lens MIP/aquaporin-0 and gamma E-crystallin. Invest Ophthalmol Vis Sci. 2004;45:863–871. doi: 10.1167/iovs.03-0708. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Skinner DM. Denaturants or cosolvents improve the specificity of PCR amplification of a G+C-rich DNA using genetically-engineered DNA-polymerases. Gene. 1994a;140:1–5. doi: 10.1016/0378-1119(94)90723-4. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Skinner DM. Cytoplasmic localization of transcripts of a complex G+C-rich crab satellite DNA. Chromosoma. 1994b;103:423–431. doi: 10.1007/BF00362287. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kumari SS, Skinner DM. Actin-encoding cDNAs and gene expression during the intermolt cycle of the Bermuda land crab Gecarcinus lateralis. Gene. 1996;171:177–184. doi: 10.1016/0378-1119(96)00018-2. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kumari SS, Skinner DM. Molecular characterization of four members of the alpha-tubulin gene family of the Bermuda land crab Gecarcinus lateralis. J Exp Zool. 1997;278:63–77. [PubMed] [Google Scholar]
- Varadaraj K, Kushmerick C, Baldo GJ, Bassnett S, Shiels A, Mathias RT. The role of MIP in lens fiber cell membrane transport. J Membr Biol. 1999;170:191–203. doi: 10.1007/s002329900549. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Kumari S, Shiels A, Mathias RT. Regulation of aquaporin water permeability in the lens. Invest Ophthalmol Vis Sci. 2005;46:1393–1402. doi: 10.1167/iovs.04-1217. [DOI] [PubMed] [Google Scholar]
- Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci. 2005;118:3225–3232. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- Yancey SB, Koh K, Chung J, Revel JP. Expression of the gene for main intrinsic polypeptide (MIP) - separate spatial distributions of MIP and beta-crystallin gene transcripts in rat lens development. J Cell Biol. 1988;106:705–714. doi: 10.1083/jcb.106.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XS, Jiang JX. Interaction of major intrinsic protein (aquaporin-0) with fiber connexins in lens development. J Cell Sci. 2004;117:871–880. doi: 10.1242/jcs.00945. [DOI] [PubMed] [Google Scholar]
- Yu XS, Yin XY, Lafer EM, Jiang JX. Development regulation of the direct interaction between the intracellular loop of connexin 45.6 and the C terminus of major intrinsic protein (aquaporin-0) J Biol Chem. 2005;280:22081–22090. doi: 10.1074/jbc.M414377200. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Hall JE, Ehring GR, Simon SA. The structural organization and protein-composition of lens fiber junctions. J Cell Biol. 1989;108:2255–2275. doi: 10.1083/jcb.108.6.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi GA, Simon SA, Hall JE. The specialized junctions of the lens. Int Rev Cytol. 1992;136:185–225. doi: 10.1016/s0074-7696(08)62053-7. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Eskandari S, Hall JE, Zampighi L, Kreman M. Micro-domains of AQPO in lens equatorial fibers. Exp Eye Res. 2002;75:505–519. doi: 10.1006/exer.2002.2041. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Kreman M, Lanzavecchia S, Turk E, Eskandari S, Zarnpighi L, Wright EM. Structure of functional single AQP0channels in phospholipid membranes. J Mol Biol. 2003;325:201–210. doi: 10.1016/s0022-2836(02)01200-7. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chen T, Church R. Temporal expression of three mouse lens fiber cell membrane protein genes during early development. Mol Vision. 2002;8:143–148. [PubMed] [Google Scholar]