FIGURE 2.
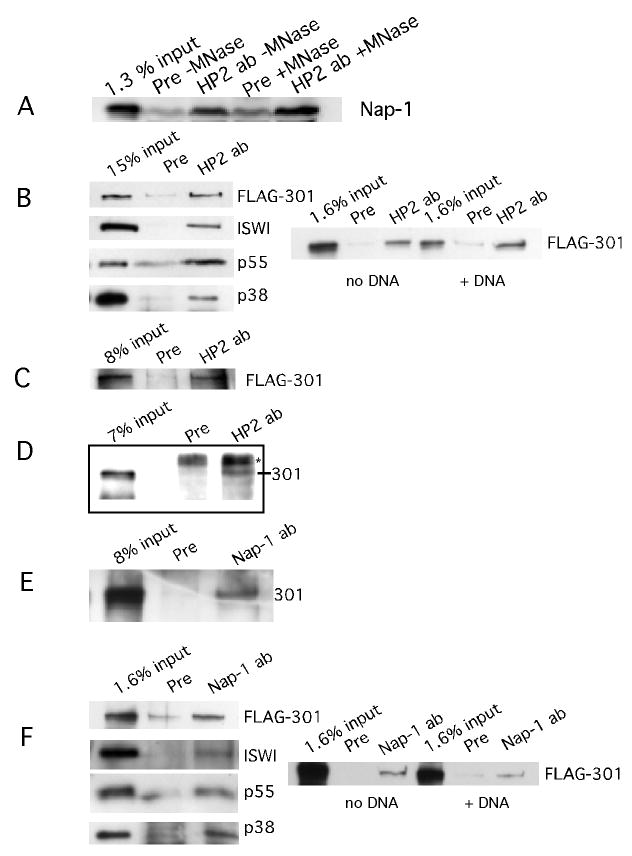
HP2 interacts with Nap-1 and NURF.
FIGURE 2A. HP2 interacts with Nap-1 in an embryo extract. HP2 antibodies or HP2 preimmune serum were added to Drosophila embryo extract and the immunoprecipitated material was tested for the presence of Nap-1 by western blot. The input is shown in the first lane; the following lanes indicate that in the presence of preimmune serum, no Nap-1 is pulled down while in the presence of HP2 antibodies, Nap-1 is immunoprecipitated. By comparison with the input sample, we estimate that 1-2% of the Nap-1 is immunoprecipitated. This percent is indicative of our immunoprecipitation efficiency throughout this paper. No loss of binding occurs between Nap-1 and HP2 upon digestion of the nuclear DNA to completion with micrococcal nuclease.
FIGURE 2B. HP2 interacts with the NURF complex; DNA does not mediate the interaction. HP2 rabbit antibodies were mixed with FLAG purified NURF complex plus HP2-S transcribed and translated in the rabbit reticulocyte lysate system. Preimmune serum was used as a control. As can be seen in the top panel, NURF301 is pulled down when HP2-specific antibodies are present, but not in the presence of the preimmune serum. The first lane indicates the input. The same is true for ISWI, p55, and p38, the other three components of NURF. To the right, it can be seen that upon the addition of 100-fold excess plasmid DNA to the binding reaction, HP2 antibodies are still able to pull down the NURF complex, demonstrating that the coprecipitation is not dependent on the binding of the individual proteins to DNA. The input into the reactions is shown; rabbit preimmune serum acts as a negative control.
FIGURE 2C. HP2 rabbit antibodies can pull down NURF301 from Sf9 cell extract. Sf9 extract from cells in which the NURF301 subunit was overproduced was incubated with HP2 antibodies, immunoprecipitated, the pellet run out on SDS-PAGE gels, and probed with antibodies against the FLAG tag. The first lane shows FLAG-tagged NURF301 in the load. HP2 antibodies pull down NURF301, whereas the preimmune serum does not, as shown in lanes 3 and 2.
FIGURE 2D. HP2 rabbit antibodies immunoprecipitate NURF from a Drosophila embryo extract. Embryo extract was prepared as in the chromatography experiments and immunoprecipitations performed as described for the Sf9 cell extract. The western blots were then probed with rabbit NURF301 antibodies. The first lane shows NURF 301 in the load and the following lanes show that NURF301 is precipitated with HP2 rabbit antibodies and not with HP2 preimmune serum. The * indicates a nonspecific product.
FIGURE 2E. Nap-1 antibodies can pull down NURF from an Sf9 extract in which NURF301 is overproduced. A preimmune serum was used as a control. The presence of NURF was assayed by probing the blot with FLAG M2 antibodies. In the first lane is the material added to the immunoprecipitation reactions. Lane 2 shows the results of the immunoprecipitation performed with preimmune serum. Lane 3 shows that Nap-1 antibodies can pull down NURF301, the unique subunit of NURF.
FIGURE 2F. Nap-1 interacts with the NURF complex; DNA does not mediate the interaction. Nap-1 antibodies were mixed with FLAG purified NURF complex plus Nap-1 transcribed and translated in the rabbit reticulocyte lysate system. Preimmune serum was used as a control. As can be seen in the top panel, NURF301 is pulled down when Nap-1-specific antibodies are present, but not in the presence of the preimmune serum. The first lane indicates the input. The same is true for ISWI, p55, and p38, the other three components of NURF. To the right, it can be seen that upon the addition of 100 fold excess plasmid DNA to the binding reaction, Nap-1 antibodies are still able to pull down the NURF complex. The input into the reactions is shown; rabbit preimmune serum acts as a negative control. Note that the right panel in Figure 2F was exposed longer than that in Figure 2B.
