Abstract
Rho1p is a yeast homolog of mammalian RhoA small GTP-binding protein. Rho1p is localized at the growth sites and required for bud formation. We have recently shown that Bni1p is a potential target of Rho1p and that Bni1p regulates reorganization of the actin cytoskeleton through interactions with profilin, an actin monomer-binding protein. Using the yeast two-hybrid screening system, we cloned a gene encoding a protein that interacted with Bni1p. This protein, Spa2p, was known to be localized at the bud tip and to be implicated in the establishment of cell polarity. The C-terminal 254 amino acid region of Spa2p, Spa2p(1213–1466), directly bound to a 162-amino acid region of Bni1p, Bni1p(826–987). Genetic analyses revealed that both the bni1 and spa2 mutations showed synthetic lethal interactions with mutations in the genes encoding components of the Pkc1p-mitogen-activated protein kinase pathway, in which Pkc1p is another target of Rho1p. Immunofluorescence microscopic analysis showed that Bni1p was localized at the bud tip in wild-type cells. However, in the spa2 mutant, Bni1p was not localized at the bud tip and instead localized diffusely in the cytoplasm. A mutant Bni1p, which lacked the Rho1p-binding region, also failed to be localized at the bud tip. These results indicate that both Rho1p and Spa2p are involved in the localization of Bni1p at the growth sites where Rho1p regulates reorganization of the actin cytoskeleton through Bni1p.
INTRODUCTION
Reorganization of the actin cytoskeleton plays an essential role in various cell functions. Many actin-binding proteins have been isolated and characterized, but it has not been thoroughly understood how reorganization of the actin cytoskeleton is regulated by these proteins in response to a variety of stimuli. The Rho family belongs to the small G protein superfamily and consists of the Rho, Rac, and Cdc42 subfamilies (Hall, 1994; Takai et al., 1995). Recent studies have revealed that the Rho family members are key regulators for reorganization of the actin cytoskeleton. The Rho family members have been shown to regulate various cell functions, such as cell shape change, formation of stress fibers and focal adhesions, cell motility, membrane ruffling, cytokinesis, cell aggregation, and smooth muscle contraction (Hall, 1994; Takai et al., 1995). They have two interconvertible forms: guanosine diphosphate (GDP)-bound inactive and guanosine triphosphate (GTP)-bound active forms. The GTP-bound form interacts with its specific target(s) and performs its cell functions. Many potential targets of the Rho family members have been identified, but it has not yet been fully clarified how they regulate reorganization of the actin cytoskeleton through these targets (Machesky and Hall, 1996).
Cells of the budding yeast Saccharomyces cerevisiae grow by budding for cell division, and the actin cytoskeleton plays a pivotal role in the budding and cytokinesis processes (Drubin, 1991). Cortical actin patches are clustered at the growth sites, including the site of bud emergence in unbudded cells and the bud tip and the cytokinesis site in budded cells, whereas actin fibers are generally oriented along the long axes of the mother–bud pairs (Adams and Pringle, 1984). S. cerevisiae possesses Rho family genes, including RHO1 (Madaule et al., 1987), RHO2 (Madaule et al., 1987), RHO3 (Matsui and Toh-e, 1992), RHO4 (Matsui and Toh-e, 1992), and CDC42 (Adams et al., 1990; Johnson and Pringle, 1990). RHO1 is a homolog of the mammalian RhoA gene and rho1 mutants are deficient in the budding process (Yamochi et al., 1994). Consistent with this, immunofluorescence microscopic study indicates that Rho1p is localized at the growth sites, including the presumptive budding site, the bud tip, and the cytokinesis site (Yamochi et al., 1994). These results suggest that Rho1p regulates the processes of bud formation through reorganization of the actin cytoskeleton. Concerning the upstream regulators of Rho1p, we have identified and characterized Rdi1p and Rom1p/Rom2p as a GDP dissociation inhibitor (Masuda et al., 1994) and GDP/GTP exchange proteins (Ozaki et al., 1996), respectively. Rom7p/Bem4p is a novel Rho1p-interacting protein, although its role in the regulation or function of Rho1p remains to be clarified (Hirano et al., 1996; Mack et al., 1996). On the other hand, concerning the downstream targets of Rho1p, it has been shown that one target is Pkc1p, a homolog of mammalian protein kinase C (Nonaka et al., 1995; Kamada et al., 1996), which regulates cell wall integrity through activation of the mitogen-activated protein (MAP) kinase cascade (Levin and Errede, 1995). It has also been shown that another target of Rho1p is 1,3-β-glucan synthase (glucan synthase) (Drgonováet al., 1996; Qadota et al., 1996), which is involved in cell wall synthesis.
In addition, we have demonstrated that Bni1p (bud neck involved) is a third potential target of Rho1p that is involved in reorganization of the actin cytoskeleton (Kohno et al., 1996). BNI1 (Jansen et al., 1996; Zahner et al., 1996) and related genes in other organisms, including diaphanous (Castrillon and Wasserman, 1994) and cappuccino (Emmons et al., 1995) in Drosophila, figA (Marhoul and Adams, 1995) and sepA (Harris et al., 1997) in Aspergillus, and fus1 (Petersen et al., 1995) and cdc12 (Chang et al., 1997) in Schizosaccharomyces pombe, have been shown to be involved in cytokinesis, establishment of cell polarity, or normal cell morphology (Frazier and Field, 1997). These proteins share conserved domains named FH1 and FH2 (formin homology) (Castrillon and Wasserman, 1994). We have also shown that Bni1p and Bnr1p, the product of the related gene BNR1 (BNI1 related), regulate reorganization of the actin cytoskeleton by interacting with profilin at their proline-rich FH1 domains (Imamura et al., 1997). Profilin is an actin monomer-binding protein and is implicated in polymerization of actin (Sohn and Goldschmidt-Clermont, 1994). Recently, it has been shown that Bni1p also interacts with Cdc42p and that this Cdc42p-Bni1p system is involved in the morphological change during mating (Evangelista et al., 1997). It has also been shown that Bni1p interacts with Rho3p and Rho4p, in addition to Rho1p and Cdc42p (Evangelista et al., 1997; Imamura et al., unpublished observations), although the physiological significance of these interactions remain to be clarified. The Rho1p-Bni1p system has been shown to be conserved in mammalian cells and to regulate reorganization of the actin cytoskeleton (Watanabe et al., 1997).
The sites for reorganization of the actin cytoskeleton are spatially regulated not only during the budding process in budding yeast, but also in a variety of cell functions, including membrane ruffling, cell locomotion, cytokinesis, and establishment of cell polarity. Bni1p has been shown to be localized at the growth sites as is Rho1p (Jansen et al., 1996). Therefore, an important issue to be addressed is how Rho1p and Bni1p are localized at the growth sites.
In this study, we have attempted to identify a protein involved in the localization of Bni1p at the growth sites. We have isolated Spa2p as a Bni1p-interacting protein by the yeast two-hybrid method. SPA2 encodes a 163-kDa, nonessential protein with predicted coiled-coil domains that is localized at the sites of polarized growth during both budding and mating (Snyder, 1989; Gehrung and Snyder, 1990). SPA2 is involved in morphogenesis in S. cerevisiae (Gehrung and Snyder, 1990), but its function remains obscure. We show here that one function of Spa2p is to localize Bni1p at the bud site and that the Rho family members are also involved in this Spa2p-dependent localization of Bni1p.
MATERIALS AND METHODS
Strains, Media, and Yeast Transformations
Yeast strains used in this study are listed in Table 1. Yeast strains were grown on rich medium that contained 2% Bacto-peptone (Difco Laboratories, Detroit, MI), 1% Bacto-yeast extract (Difco), 0.04% adenine sulfate, 0.02% uracil, and 2% glucose (YPDAU). Yeast transformations were performed by the lithium acetate method (Gietz et al., 1992). Transformants were selected on SD medium, which contained 2% glucose and 0.7% yeast nitrogen base without amino acids (Difco). SG medium contained 3% galactose, 0.2% sucrose, and 0.7% yeast nitrogen base without amino acids. SD or SG medium was supplemented with amino acids or bases when required. Standard yeast genetic manipulations were performed as described (Sherman et al., 1986). Escherichia coli strain DH5α was used for construction and propagation of plasmids.
Table 1.
Yeast strains used in this study
| Strain | Genotype |
|---|---|
| OHNY1 | MATa ura3 leu2 trp1 his3 ade2 (Nonaka et al., 1995) |
| OHNY2 | MATα ura3 leu2 trp1 his3 ade2 (Nonaka et al., 1995) |
| HNY78 | MATa ura3 leu2 trp1 his3 ade2 rho1::RhoA-URA3 (Nonaka et al., 1995) |
| TFY7 | MATα ura3 leu2 trp1 his3 ade2 pkc1::LEU2 (Kohno et al., 1996) |
| KFY3 | MATα ura3 leu2 trp1 his3 ade2 bck1::LEU2 |
| MFY2 | MATα ura3 leu2 trp1 his3 ade2 mpk1::TRP1 |
| BTY1 | MATa ura3 leu2 trp1 his3 ade2 bni1::HIS3-1 (Kohno et al., 1996) |
| BTY3 | MATa ura3 leu2 trp1 his3 ade2 bni1::HIS3-2 |
| TYSH1 | MATa ura3 leu2 trp1 his3 ade2 spa2::HIS3 |
| TYSH6 | MATα ura3 leu2 trp1 his3 ade2 spa2::HIS3 |
| DMYSU6 | MATα ura3 leu2 trp1 his3 ade2 spa2::URA3 bck1::LEU2 |
| KFY5 | MATα ura3 leu2 trp1 his3 ade2 bni1::HIS3-1 bck1::LEU2 |
| NTFSY1 | MATα ura3 leu2 trp1 his3 ade2 bni1::HIS3-2 spa2::URA3 |
| L40 | MATa trp1 leu2 his3 LYS2::lexA-HIS3 URA3::lexA-lacZ (Vojtek et al., 1993) |
Except for L40, the strains used in this study are isogenic.
Molecular Biological Techniques
Standard molecular biological techniques were used for construction of plasmids, DNA sequencing, and PCR (Sambrook et al., 1989). Plasmids used in this study are listed in Table 2. DNA sequences were determined using ALFred DNA sequencer (Pharmacia Biotech, Uppsala, Sweden) and PCR was performed using the GeneAmp PCR System 2400 (Perkin Elmer-Cetus, Norwalk, CT).
Table 2.
Plasmids used in this study
| Plasmid | Characteristicsa |
|---|---|
| pBTM116-HA | DBDLexA,bTRP1, 2 μm (Imamura et al., 1997). |
| pBTM116-HA-BNI1 (490-1954) | DBDLexA-BNI1 (490-1954), TRP1, 2 μm; made by inserting the 4.4-kb BamHI–SmaI fragment from pACTII-HK-BNI1 (490-1954) (Kohno et al., 1996) into the BamHI–SmaI site of pBTM116-HA. |
| pBTM116-HA-BNI1 (490-1750) | DBDLexA-BNI1 (490-1750), TRP1, 2 μm; made by deleting the 0.6-kb SacII–SmaI fragment from pBTM116-HA-BNI1 (490-1954). |
| pBTM116-HA-BNI1 (490-1415) | DBDLexA-BNI1 (490-1415), TRP1, 2 μm; made by deleting the 1.6-kb PstI–SmaI fragment from pBTM116-HA-BNI1 (490-1954). |
| pBTM116-HA-BNI1 (490-1226) | DBDLexA-BNI1 (490-1226), TRP1, 2 μm; made by inserting the 2.2-kb BamHI–BglII fragment from pACTII-HK-BNI1 (490-1954) (Kohno et al., 1996) into the BamHI site of pBTM116-HA. |
| pBTM116-HA-BNI1 (490-987) | DBDLexA-BNI1 (490-987), TRP1, 2 μm; made by deleting the 2.9-kb SalI–SmaI fragment from pBTM116-HA-BNI1 (490-1954). |
| pBTM116-HA-BNI1 (490-824) | DBDLexA-BNI1 (490-824), TRP1, 2 μm; made by deleting the 3.4-kb XhoI–SmaI fragment from pACTII-HK-BNI1 (490-1954) to generate pACTII-HK-BNI1 (490-824) and subsequently inserting the 1.0-kb BamHI-BglII fragment from pACTII-HK-BNI1 (490-824) into the BamHI site of pBTM116-HA. |
| pBTM116-HA-BNI1 (490-725) | DBDLexA-BNI1 (490-725), TRP1, 2 μm; made by deleting the 3.7-kb BanII–SmaI fragment from pACTII-HK-BNI1 (490-1954) to generate pACTII-HK-BNI1 (490-725) and subsequently inserting the 0.7-kb BamHI–BglII fragment from pACTII-HK-BNI1 (490-725) into the BamHI site of pBTM116-HA. |
| pBTM116-HA-BNI1 (601-987) | DBDLexA-BNI1 (601-987), TRP1, 2 μm; made by inserting the 1.15-kb BamHI-SalI PCR fragment encoding amino acid positions from 601 to 987 of Bni1p into the BamHI-SalI site of pBTM116-HA. The 1.15-kb BNI1 DNA fragment was amplified by PCR using the upstream primer 1,5′-GGCCGGATCCATTGAGTTATATGACAATAATAAACTTG, and the downstream primer 2,5′-GGCCGTCGACATTAAAGTCAATACCAAATCCTTTGTATTTTG. |
| pBTM116-HA-BNI1 (727-987) | DBDLexA-BNI1 (727-987), TRP1, 2 μm; made by inserting the 0.78-kb BamHI–SalI PCR fragment encoding amino acid positions from 727 to 987 of Bni1p into the BamHI–SalI site of pBTM116-HA. The 0.78-kb BNI1 DNA fragment was amplified by PCR using the upstream primer 3,5′-GGCCGGATCCCTAACGAAGAAATTGGAAGAAATTCAGGC, and the downstream primer 2,5′-GGCCGTCGACATTAAAGTCAATACCAAATCCTTTGTATTTTG. |
| pBTM116-HA-BNI1 (826-987) | DBDLexA-BNI1 (826-987), TRP1, 2 μm; made by inserting the 0.49-kb BamHI-SalI PCR fragment encoding amino acid positions from 826 to 987 of Bni1p into the BamHI–SalI site of pBTM116-HA. The 0.49-kb BNI1 DNA fragment was amplified by PCR using the upstream primer 4,5′-GGCCGGATCCAGCCTAAATTCTTCAGAGAAAGCGAATATC, and the downstream primer 2,5′-GGCCGTCGACATTAAAGTCAATACCAAATCCTTTGTATTTTG. |
| pBTM116-HA-SPA2 (1213-1466) | DBDLexA-SPA2 (1213-1466), TRP1, 2 μm; made by inserting the 0.76-kb BamHI-SmaI PCR fragment encoding amino acid positions from 1213 to 1466 of Spa2p into the BamHI-SmaI site of pBTM116-HA. The 0.76-kb SPA2 DNA fragment was amplified by PCR using the upstream primer 5,5′-GGCCGGATCCGAAAATTCACAGGTAAAAAAGACGAGCCACTC, and the downstream primer 6,5′-GGCCCCCGGGTTACTTCAACTTCGAATTCAAATAATTTATTTC. |
| pACT-SPA2 (1213-1466) | ADGAL4,bLEU2, 2 μm; isolated in this study from a yeast cDNA library provided by S. Elledge. |
| pACTII-HK | ADGAL4, LEU2, 2 μm (Ozaki et al., 1996). |
| pACTII-HK-SPA2 (1213-1466) | ADGAL4-SPA2 (1213-1466), LEU2, 2 μm; made by inserting the 0.76-kb BamHI–SmaI fragment from pBTM116-HA-SPA2 (1213-1466) into the BamHI–SmaI site of pACTII-HK. |
| pACTII-HK-BNI1 (1-1954) | ADGAL4-BNI1 (1-1954), LEU2, 2 μm (Kohno et al., 1996). |
| pACTII-HK-BNI1 (1-1750) | ADGAL4-BNI1 (1-1750), LEU2, 2 μm; made by deleting the 0.6-kb SacII–SmaI fragment from pACTII-HK-BNI1 (1-1954). |
| pACTII-HK-BNI1 (1-1327) | ADGAL4-BNI1 (1-1327), LEU2, 2 μm; made by deleting the 1.9-kb NaeI–SmaI fragment from pACTII-HK-BNI1 (1-1954). |
| pACTII-HK-BNI1 (1-824) | ADGAL4-BNI1 (1-824), LEU2, 2 μm; made by deleting the 3.4-kb XhoI–SmaI fragment from pACTII-HK-BNI1 (1-1954). |
| pACTII-HK-BNI1 (1-725) | ADGAL4-BNI1 (1-725), LEU2, 2 μm; made by deleting the 3.7-kb BanII–SmaI fragment from pACTII-HK-BNI1 (1-1954). |
| pUC18-SPA2-1 | SPA2; made by inserting the 4.4-kb PCR fragment of SPA2 open reading frame into the BamHI–HincII site of pUC18. The 4.4-kb SPA2 DNA fragment was amplified by PCR using the upstream primer 7,5′-GGCCGGATCCATGGGTACGTCAAGCGAGGTTTCTCTCGCAC, and the downstream primer 6,5′-GGCCCCCGGGTTACTTCAACTTCGAATTCAAATAATTTATTTC. |
| pUC18-SPA2-2 | SPA2; made by inserting the 5.7-kb fragment of SPA2, including the 0.57-kb upstream and 0.77-kb downstream noncoding regions of SPA2, into the SalI-BamHI site of pUC18. The 5.7-kb SPA2 DNA fragment was amplified by PCR using the upstream primer 8,5′-GGCCG TCGACGACACTGCTATATCCTTCGACTACGTAACC, and the downstream primer 9,5′-GGCCGGATCCTCGACAGAAAAACTTTAAGCTTACAATGGC. |
| pUC19-BNI1 | BNI1; made by inserting the 6.5-kb BamHI-SmaI BNI1 fragment from pBS-BNI1 (Kohno et al., 1996) into the BamHI-SmaI site of pUC19. |
| pUC18-spa2::HIS3 | spa2::HIS3, a derivative of pUC18-SPA2-1; made by replacing the 2.0-kb MunI-HincII internal fragment of SPA2, corresponding to amino acid positions from 352 to 1033 of Spa2p, with the 1.8-kb HIS3 fragment. |
| pUC18-spa2::URA3 | spa2::URA3, a derivative of pUC18-SPA2-2; made by replacing the 4.4-kb EcoRV-XbaI internal fragment of SPA2 corresponding to amino acid positions from 15 to 1466 of Spa2p with the 1.2-kb URA3 fragment. |
| pBS-bni1::HIS3 | bnil1::HIS3-1 (Kohno et al., 1996). |
| pUC19-bni1::HIS3-2 | bni1::HIS3-2, a derivative of pUC19-BNI1; made by replacing the 3.1-kb MunI–BsaAI internal fragment of BNI1 corresponding to amino acid positions from 678 to 1715 of Bni1p with the 1.8-kb HIS3 fragment. |
| pRS314 | TRP1, CEN6 (Sikorski and Hieter, 1989). |
| pRS314-GAL1 | TRP1, CEN6; made by inserting the 0.74-kb ScaI–HindIII fragment, containing the GAL1 promoter, a multicloning site, and the TDH1 terminator, into the SpeI (filled-in)–HindIII site of pRS314. |
| pRS314-GAL1-myc | TRP1, CEN6; made by inserting the synthetic oligonucleotide encoding three copies of the myc epitope, EQKLISEEDL, which is derived from the human c-myc protein, into the EcoRI–PstI site in the multicloning site of pRS314-GAL1. |
| pRS314-GAL1-myc-BNI1 | TRP1, CEN6, GAL1-myc-BNI1; made by inserting the 5.9-kb BamHI–SmaI fragment from pACTII-HK-BNI1 (1-1954) containing the BNI1 open reading frame (Kohno et al., 1996) into the BamHI–SmaI site of the multicloning site of pRS314-GAL1-myc, to place BNI1 downstream of the three myc epitopes. |
| pRS314-GAL1-myc-BNI1 (490-1954) | TRP1, CEN6, GAL1-myc-BNI1 (490-1954); made by inserting the 4.4-kb BamHI-SmaI fragment from pACTII-HK-BNI1 (490-1954) (Kohno et al., 1996) into the BamHI-SmaI site of pRS314-GAL1-myc. |
| pMAL-c2-SPA2 (1213-1466) | MBP-SPA2 (1213-1466); made by inserting the 0.76-kb BamHI–BglII DNA fragment of SPA2 from pACTII-HK-SPA2 (1213-1466) into the BamHI site of pMAL-c2 (New England BioLabs). |
| pGEX-4T-2-HA | GST-HA (Imamura et al., 1997). |
| pGEX-4T-2-HA-BNI1 (826-987) | GST-HA-BNI1 (826-987); made by inserting the 0.49-kb BamHI-SalI DNA fragment of BNI1 from pBTM116-HA-BNI1 (826-987) into the BamHI–SalI site of pGEX-4T-2-HA. |
Underlined sequences are portions of the BNI1 or SPA2 gene.
DBDLexA and ADGAL4 are the DNA-binding domain of LexA and the transcriptional activation domain of GAL4, respectively.
Screening for a Bni1p-interacting Protein by the Yeast Two-Hybrid Method
Strain L40 carrying pBTM116-HA-BNI1(490–1954) was transformed with a yeast cDNA library made in pACT (kindly provided by Stephen J. Elledge). Transformants were screened for growth on SD plates lacking tryptophan, leucine, and histidine but containing 2 mM 3-amino-1,2,4-triazole, which is a specific inhibitor of His3p. His+ colonies were then placed on a nitrocellulose filter and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside for β-galactosidase activity as described (Vojtek et al., 1993). From the His+ and lacZ+ positive clones obtained with this screening, library plasmids were recovered through E. coli transformation. The recovered plasmids were transformed again into L40 containing pBTM116-HA-BNI1(490–1954) to select clones that reproducibly conferred the His+ and lacZ+ phenotypes. The nucleotide sequences of the insert DNAs of selected clones were determined. For quantitative assay of β-galactosidase activity, cells of each transformant were cultured in SD-Trp-Leu medium, and the β-galactosidase activity was measured according to the O-nitrophenyl-β-d-galactopyranoside assay method as described (Guarente, 1983).
Disruption of SPA2 and BNI1
SPA2 was disrupted as follows. Plasmids pUC18-spa2::HIS3 and pUC18-spa2::URA3 were cut with PvuII, and each of the digested DNAs was introduced into OHNY1 and OHNY2. Genomic DNA was isolated from each transformant, and the proper disruption of SPA2 was verified by PCR (our unpublished observations). BNI1 was disrupted as follows. pUC19-bni1::HIS3–2 was cut with PvuII and the digested DNA was introduced into OHNY1. The proper disruption of BNI1 was verified by PCR (our unpublished observations). The other bni1 disruption mutation, which was described previously (Kohno et al., 1996), is referred to as bni1::HIS3–1 in this study. These spa2 and bni1 mutant strains were used for further genetic studies.
Cytological Techniques
Actin and DNA were stained with rhodamine-phalloidin (Molecular Probes, Eugene, OR) and DAPI (Sigma Chemical, St. Louis, MO), respectively, as described (Yamochi et al., 1994). Immunofluorescence microscopy was performed as described (Yamochi et al., 1994) using the 9E10 anti-myc monoclonal antibody (Evan et al., 1985). Stained cells were observed with a Zeiss Axiophot microscope (Carl Zeiss, Oberkochen, Germany) and photographed with a peltier cooling 3CCD color camera (C5810–01; Hamamatsu Photonics KK., Hamamatsu, Japan).
Materials and Chemicals for Biochemical Assays
Recombinant Spa2p(1213–1466) was purified from overexpressing E. coli DH5α, containing plasmid pMAL-c2-SPA2(1213–1466), as a maltose-binding protein (MBP) fusion protein using an amylose resin column (New England BioLabs, Beverly, MA) as described (Guan et al., 1987). Recombinant Bni1p(826–987) was purified from overexpressing E. coli DH5α, containing plasmid pGEX-4T-2-HA-BNI1(826–987), as a GST fusion protein using a glutathione Sepharose 4B column (Pharmacia P-L Biochemicals, Milwaukee, WI) as described (Kikuchi et al., 1992).
Assay for the Binding of Recombinant Spa2p(1213–1466) with Bni1p(826–987)
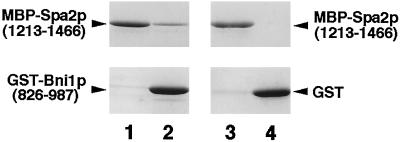
Purified MBP-Spa2p(1213–1466) (60 pmol) or MBP (90 pmol) in 100 μl of Buffer A (25 mM Tris-HCl [pH 7.5], 0.5 mM EDTA, 1 mM DTT, and 0.1% Nonidet P-40) was incubated with glutathione Sepharose 4B beads (20 μl) that were prebound to GST-Bni1p(826–987) (48 pmol) or GST (72 pmol) and incubated for 30 min at 4°C. The mixtures were then briefly centrifuged and 90 μl of each supernatant were saved. The beads were then washed three times with 1 ml of Buffer A. The proteins bound to the beads were eluted with 60 μl of Laemmli’s buffer (Laemmli, 1970). The supernatant and the eluate were subjected to SDS-PAGE, followed by protein staining with Coomassie brilliant blue.
RESULTS
Identification of Spa2p as a Bni1p-interacting Protein by the Yeast Two-Hybrid Screening Method
To search for genes that encode Bni1p-interacting proteins, the yeast two-hybrid method was used. A truncated Bni1p, Bni1p(490–1954), was used as a bait in this screening. Among 2 × 106 total transformants screened, 62 positive clones (His+ and lacZ+) were identified, and the library plasmids were recovered from these clones. Among these 62 plasmids, 30 clones were found to confer the His+ and lacZ+ phenotypes on L40 containing pBTM116-HA-BNI1(490–1954). DNA sequencing of the insert DNAs of these clones revealed that one clone encoded amino acid positions from 1213 to 1466 of Spa2p, a protein of 1,466 amino acids (Gehrung and Snyder, 1990). Since Spa2p, like Bni1p, had been implicated in polarized cell growth (Snyder, 1989), we investigated the physiological significance of these Bni1p–Spa2p interactions.
Bni1p consists of at least three domains, the Rho1p-binding, FH1, and FH2 domains. The Spa2-binding region of Bni1p was delimited by the yeast two-hybrid method (Figure 1). Various truncated fragments of BNI1 were cloned into a two-hybrid vector, and it was found that Spa2p(1213–1466) interacted with a region of amino acid positions from 826 to 987 of Bni1p. This unique region (Spa2p-binding domain) is located between the Rho1p-binding and FH1 domains. The Spa2p-binding domain is not present in Bnr1p and, consistent with this, Bnr1p did not bind to Spa2p (1213–1466) in the yeast two-hybrid method (our unpublished observations).
Figure 1.
Bni1p–Spa2p interactions in the yeast two-hybrid method. Plasmids encoding ADGAL4 and DBDLexA fused to truncated Bni1ps were transformed into strain L40 expressing DBDLexA-Spa2p(1213–1466) and ADGAL4-Spa2p(1213–1466), respectively. Bni1ps-Spa2p(1213–1466) interactions were examined in each transformant by the qualitative and quantitative assay methods for β-galactosidase activity. Closed bars, blue colony color; open bars, white colony color. The values (Miller units) are the averages of β-galactosidase activities for three transformants. Each measured value was within 50% of the average. FH1 and FH2 are formin homology domains 1 and 2, respectively. (A) Interactions of Bni1ps with DBDLexA-Spa2p(1213–1466). (B) Interactions of Bni1ps with ADGAL4-Spa2p(1213–1466).
Direct Interaction of Spa2p with Bni1p
To examine whether Spa2p directly interacts with Bni1p, Spa2p (1213–1466) and Bni1p(826–987) were fused to MBP and GST, respectively, and these fusion proteins were expressed in E. coli and purified. MBP-Spa2p(1213–1466) bound to GST-Bni1p(826–987) but not to GST (Figure 2). MBP did not bind to GST-Bni1p(826–987) (our unpublished observations). This result indicates that Spa2p(1213–1466) directly interacts with Bni1p(826–987).
Figure 2.
Direct Spa2p-Bni1p interaction. MBP-Spa2p(1213–1466) was incubated with either GST-Bni1p(826–987) or GST, coupled to glutathione Sepharose 4B beads. The beads were sedimented, the supernatants were saved, and the proteins bound to the beads were eluted. Comparable aliquots of the supernatants and the eluates were subjected to SDS-PAGE, followed by protein staining with Coomassie brilliant blue. Lanes 1 and 3, the supernatants; lanes 2 and 4, the eluates.
Genetic Interaction of SPA2 and BNI1 with the PKC1-MAP Kinase Pathway
The physiological significance of the physical interaction of Spa2p with Bni1p was examined genetically. We have previously shown that a bni1 mutation is synthetically lethal with both the rho1::RhoA mutation, in which RHO1 is replaced by the mammalian RhoA gene, and the mutation in PKC1, another target of Rho1p (Kohno et al., 1996). We tested whether the spa2 mutation is also synthetically lethal with the rho1::RhoA and pkc1 mutations. The spa2 mutant, TYSH6, was crossed with the rho1::RhoA mutant, HNY78, and it was found that the spa2 rho1::RhoA mutant was synthetically lethal (Table 3). The spa2 mutation was also synthetically lethal with the pkc1 mutation (Table 4). It has previously been shown that the spa2 mutation is synthetically lethal with the bck1/slk1 (MAP kinase kinase kinase) and slt2/mpk1 (MAP kinase) mutations (Costigan et al., 1992; Cid et al., 1995). We tested whether the bni1 mutation is also synthetically lethal with the bck1/slk1 and slt2/mpk1 mutations. The bni1 mutant, BTY1, was crossed with the bck1 mutant, KFY3, and it was found that the bni1 bck1 mutant was synthetically lethal (Table 5). The bni1 mutation was also synthetically lethal with the mpk1 mutation (Table 6). Therefore, both the bni1 and spa2 mutations show synthetic lethal interaction with the mutations of genes encoding components of the PKC1-MAP kinase pathway.
Table 3.
Synthetic lethality between spa2 and rho1::RhoA mutationsa
| Genotype | Viable | Inviableb |
|---|---|---|
| spa2 rho1::RhoA | 0 | 52 |
| spa2 RHO1 | 46 | 2 |
| SPA2 rho1::RhoA | 42 | 6 |
| SPA2 RHO1 | 52 | 0 |
TYSH6 (spa2::HIS3) was crossed with HNY78 (rho1::RhoA-URA3) and the resulting diploid was subjected to tetrad analysis.
Genotypes of the inviable segregants were inferred from those of the viable segregants.
Table 4.
Synthetic lethality between spa2 and pkc1 mutationsa
| Genotype | Viable | Inviableb |
|---|---|---|
| spa2 pkc1 | 0 | 45 |
| spa2 PKC1 | 41 | 0 |
| SPA2 pkc1 | 37 | 4 |
| SPA2 PKC1 | 44 | 1 |
TYSH1 (spa2::HIS3) was crossed with TFY7 (pkc1::LEU2), and the resulting diploid was subjected to tetrad analysis.
Genotypes of the inviable segregants were inferred from those of the viable segregants.
Table 5.
Synthetic lethality between bni1 and bck1 mutationsa
| Genotype | Viable | Inviableb |
|---|---|---|
| bni1 bck1 | 0 | 42 |
| bni1 BCK1 | 51 | 1 |
| BNI1 bck1 | 52 | 0 |
| BNI1 BCK1 | 40 | 2 |
BTY1 (bni1::HIS3-1) was crossed with KFY3 (bck1::LEU2) and the resulting diploid was subjected to tetrad analysis.
Genotypes of the inviable segregants were inferred from those of the viable segregants.
Table 6.
Synthetic lethality between bni1 and mpk1 mutationsa
| Genotype | Viable | Inviableb |
|---|---|---|
| bni1 mpk1 | 0 | 36 |
| bni1 MPK1 | 48 | 4 |
| BNI1 mpk1 | 47 | 5 |
| BNI1 MPK1 | 35 | 1 |
BTY1 (bni1::HIS3-1) was crossed with MFY2 (mpk1::TRP1) and the resulting diploid was subjected to tetrad analysis.
Genotypes of the inviable segregants were inferred from those of the viable segregants.
Recently, a gene named SPH1 (SPA2 homolog) has been shown to be required for bipolar budding and shmoo formation as SPA2 does (Arkowitz and Lowe, 1997). We examined whether the sph1 mutation is synthetically lethal with the RhoA and pkc1 mutations. The sph1 mutation was not synthetically lethal with the RhoA and pkc1 mutations (our unpublished observations). These results suggest that SPH1 does not genetically interact with BNI1. Consistent with this, SphIp did not interact with Bni1p in the yeast two-hybrid method (our unpublished observations). SPH1 appears to function in a signaling pathway different from that of SPA2 and BNI1.
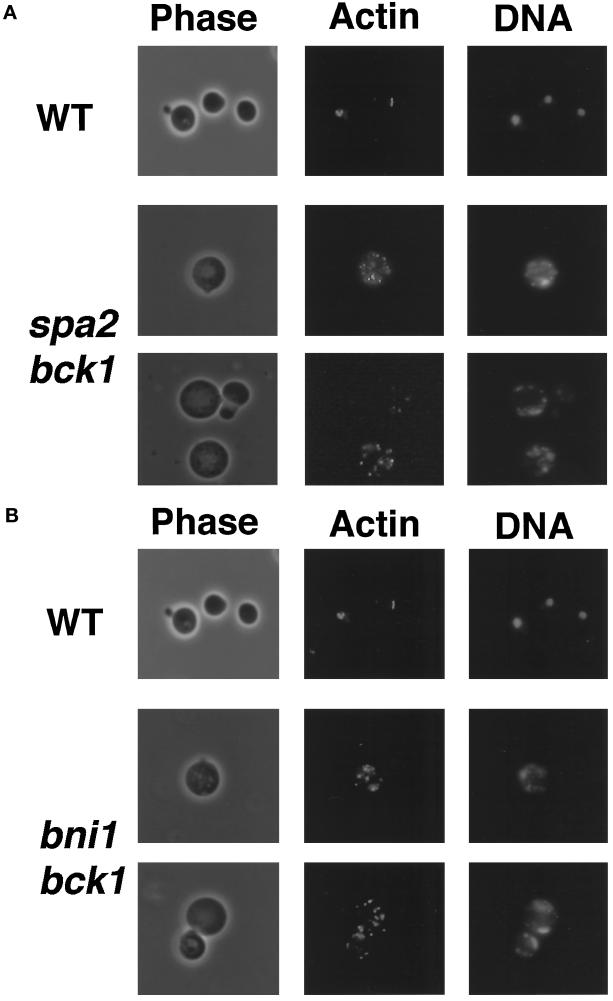
We have shown that the bni1 rho1::RhoA mutant shows abnormal morphology and distribution of cortical actin patches (Kohno et al., 1996). It was found that the spa2 bck1 and bni1 bck1 mutants grew in the presence of 1 M sorbitol in the medium (our unpublished observations). Cells of the spa2 bck1 and bni1 bck1 mutants were grown in YPDAU medium containing 1 M sorbitol, transferred to YPDAU medium, and incubated for 10 h. The growth-arrested cells were subsequently observed under a microscope. These cells showed similar phenotypes, including large and round morphology and abnormal distribution of cortical actin patches (Figure 3).
Figure 3.
Cell morphology of the spa2 bck1 and bni1 bck1 mutants. Cells of haploid strains, OHNY2 (WT), DMYSU6 (spa2 bck1), and KFY5 (bni1 bck1), were incubated at 24°C in YPDAU medium for 10 h, fixed, and stained with rhodamine-phalloidin and DAPI for actin and DNA, respectively. Cells were subsequently subjected to microscopic observation. All fields were photographed at the same magnification. (A) The spa2 bck1 mutant. (B) The bni1 bck1 mutant.
Our results indicate that SPA2 and BNI1 function in the same signaling pathway. To confirm this, we constructed a spa2 bni1 double mutant, which was found to be normal in growth and morphology, like each single mutant (our unpublished observations). We concluded that the interaction of Spa2p with Bni1p is physiologically significant.
Involvement of Spa2p and the Rho Family Members in the Localization of Bni1p
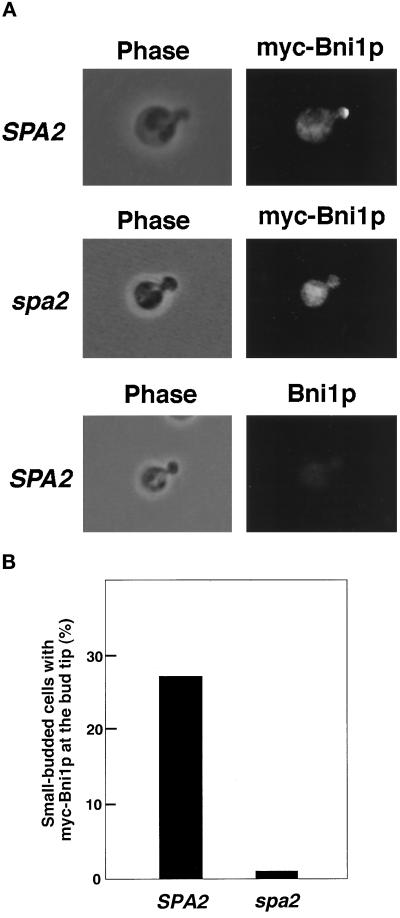
To explore the functional significance of the Spa2p–Bni1p interactions, we examined whether Spa2p is involved in the localization of Bni1p. Both Spa2p (Snyder, 1989) and Bni1p (Jansen et al., 1996) have been shown to be localized at the growth sites such as the bud tip. A myc-tagged BNI1 gene was expressed under the control of the GAL1 promoter in the bni1 and bni1 spa2 mutants. It has been shown that the overexpression of BNI1 has little effect on cell growth and morphology (Evangelista et al., 1997). This myc-BNI1 gene suppressed the lethality of the bni1 bck1 mutant, indicating that the addition of the myc tag does not impair the function of Bni1p (our unpublished observations). Indirect immunofluorescence microscopic analysis indicated that myc-Bni1p was localized at the bud tip in the bni1 mutant (Figure 4). However, in the bni1 spa2 mutant, myc-Bni1p was not localized at the bud tip and instead localized diffusely in the cytoplasm. Western blot analysis indicated that the expression level of myc-Bni1p in the bni1 mutant was similar to that in the bni1 spa2 mutant (our unpublished observations). In the bni1 mutant, myc-Bni1p was localized at the bud neck in large-budded cells (our unpublished observations). However, we could not examine whether SPA2 is required for the localization of myc-Bni1p at the bud neck, since cells with myc-Bni1p at the bud neck were too rare to be analyzed quantitatively. We also examined whether BNI1 is involved in the localization of Spa2p, but myc-Spa2p was localized at the bud tip in the bni1 mutant as in the wild-type strain (our unpublished observations). These results indicate that Spa2p is involved in the localization of Bni1p at the bud tip and that the phenotypes of the spa2 mutant may be due, at least in part, to mislocalization of Bni1p.
Figure 4.
Localization of Bni1p in the spa2 mutant. Cells of strains, BTY3 (SPA2) expressing myc-Bni1p, NTFSY1 (spa2) expressing myc-Bni1p, and OHNY1 (SPA2) expressing Bni1p, were incubated in SG-Trp medium for 8 h at 24°C, stained with the anti-myc monoclonal antibody, and subsequently subjected to immunofluorescence microscopic observation. (A) Localization of myc-Bni1p. All fields were photographed at the same magnification. (B) Percentages of small-budded cells with myc-Bni1p at the bud tip. More than 1000 cells with a small bud were observed for each determination.
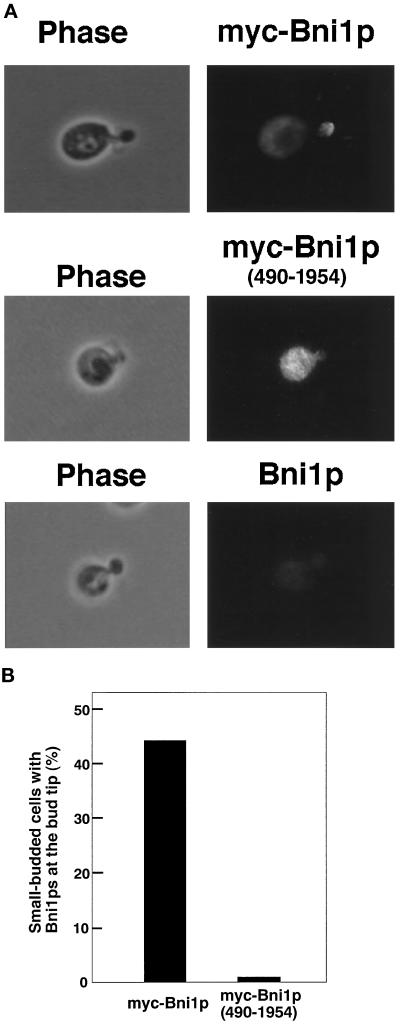
It has been shown that the GTP-bound forms of Rho1p (Kohno et al., 1996), Cdc42p, Rho3p, and Rho4p (Evangelista et al., 1997; our unpublished observations), bind to the N-terminal region of Bni1p. Thus, we examined whether the Rho family members are also involved in the localization of Bni1p. For this purpose, it was preferable to examine the mutants defective in the Rho family members for the localization of Bni1p. However, simultaneous mutations in these genes would result in lethality due to multiple deficiencies in the signaling pathways regulated by the Rho family members. Therefore, the localization of Bni1p(490–1954), which lacked the Rho1p-binding region, was studied. myc-Bni1p(490–1954) was expressed under the control of the GAL1 promoter in the ni1 mutant and stained with the anti-myc monoclonal antibody. Constitutive expression of a Bni1p lacking the N-terminal region causes aberrant cell morphology (Evangelista et al., 1997). However, the expression of myc-Bni1p (490–1954), which was induced by incubating cells in a galactose-containing medium for 8 h at 24°C, did not affect the proportion of budded cells and the sizes of the buds (our unpublished observations). myc-Bni1p(490–1954) was not localized at the bud tip in the bni1 mutants (Figure 5). myc-Bni1p(490–1954) was also not localized at the bud tip in the bni1 spa2 mutant (our unpublished observations). Western blot analysis indicated that the expression level of myc-Bni1p(490–1954) was similar to that of myc-Bni1p (our unpublished observations). These results indicate that the Rho1p-binding region of Bni1p is required for localization of Bni1p at the bud tip. The Rho family members as well as Spa2p are involved in the localization of Bni1p at the bud tip.
Figure 5.
Localization of Bni1p(490–1954), a Bni1p lacking the Rho1p-binding region. Cells of strains, BTY3 expressing myc-Bni1p or myc-Bni1p(490–1954) or OHNY1 expressing Bni1p, were incubated in SG-Trp medium for 8 h at 24°C, stained with the anti-myc monoclonal antibody, and subsequently subjected to immunofluorescence microscopic observation. (A) Localization of myc-Bni1p or myc-Bni1p(490–1954). All fields were photographed at the same magnification. (B) Percentages of small-budded cells with myc-Bni1p or myc-Bni1p(490–1954) at the bud tip. More than 1000 cells with a small bud were observed for each determination.
DISCUSSION
In this work, we have demonstrated that Spa2p interacts at its C-terminal region with the amino acid region from 826 to 987 of Bni1p. Genetic results indicate that these Bni1p-Spa2p interactions are of physiological significance. Both bni1 and spa2 mutations show synthetic lethal interactions with mutations in the components of the PKC1-MAP kinase pathway. It should be noted that both Bni1p and Pkc1p, a most upstream component of the MAP kinase pathway, are targets of Rho1p (Nonaka et al., 1995; Kamada et al., 1996; Kohno et al., 1996). The bni1 and spa2 mutants are deficient in mating projection formation and default mating induced by mating pheromone in haploid cells (Gehrung and Snyder, 1990; Dorer et al., 1997; Evangelista et al., 1997). These two mutants are also deficient in bipolar-specific budding pattern in diploid cells (Zahner et al., 1996). Moreover, the bni1 and spa2 mutants display a cytokinesis defect, most evident in diploid cells (Snyder et al., 1991; Kohno et al., 1996). Recent studies indicate that these phenotypes of the bni1 and spa2 mutants are caused by deficiency in the control of the actin cytoskeleton: Bni1p interacts with profilin at its FH1 region (Evangelista et al., 1997; Imamura et al., 1997) and with Bud6p/Aip3p at its C-terminal region (Amberg et al., 1997; Evangelista et al., 1997). Bud6p is a novel actin-binding protein, and the phenotypes of the bud6 mutant are also similar to those of the bni1 and spa2 mutants.
Actin filaments, which regulate various cell functions, are believed to be linked to the plasma membrane through an anchoring protein system. The ezrin/radixin/moesin (ERM)-CD44 system is one such system in mammalian cells, and we have recently reported that the Rho subfamily members regulate reorganization of the actin cytoskeleton at least in part through the ERM-CD44 system (Takaishi et al., 1995; Hirao et al., 1996; Kotani et al., 1997; Takahashi et al., 1997). Since Rho1p and Bni1p are localized in the vicinity of the plasma membrane at the bud tip, where vigorous reorganization of the actin cytoskeleton occurs, the Rho1p-Bni1p system may play important roles in regulating reorganization of actin filaments and linking them to the plasma membrane. The result of indirect immunofluorescence microscopic analysis indicates that Spa2p is required to localize Bni1p at the bud tip. Therefore, Spa2p may be a component of a complex that anchors Bni1p to the plasma membrane. Recently, a protein named Pea2p has been shown to possess functions and localization similar to those of Spa2p (Valtz and Herskowitz, 1996). However, neither Spa2p nor Pea2p possesses a domain implicated in localization at the membrane, such as a transmembrane segment. It is thus important to identify the factor(s) that anchors the Bni1p-Spa2p complex to the plasma membrane. Very recently, a small region of Spa2p (amino acid positions from 397 to 549), which is different from the Bni1p-binding region, has been shown to be sufficient for localization at the bud tip (Arkowitz and Lowe, 1997). Spa2p may interact at this region with a membrane protein to anchor Bni1p to the plasma membrane.
Indirect immunofluorescence microscopic analysis suggested that the Rho family members are also required for the localization of Bni1p at the bud tip. Overexpression of Bni1p lacking the Rho1p-binding domain causes aberrant reorganization of the actin cytoskeleton (Evangelista et al., 1997), indicating that the interactions of the Rho family members with Bni1p are important for the localization and the function of Bni1p. One possibility for the function of the Rho family members is that the GTP-bound active forms of the Rho family members enable Bni1p to interact with Spa2p to link actin filaments to the plasma membrane. It would be interesting to examine whether the Rho family members affect the interactions of Bni1p with Spa2p. We have attempted to express and purify full-length Bni1p in yeast, insect, and mammalian cell systems. However, due to its insolubility and proteolytic degradation, we have not yet succeeded in purifying it.
The target of the Rho subfamily members that is involved in the regulation of the ERM-CD44 system has not yet been clarified. Recently, a protein related to Bni1p, mDia (a mammalian homolog of Diaphanous), has been shown to be a potential target of RhoA (Watanabe et al., 1997). mDia also interacts with profilin at its FH1 domain and is suggested to be involved in reorganization of the actin cytoskeleton. On the analogy of the Rho1p-Bni1p-Spa2p system in yeast, there may be a Spa2p-like protein in mammalian cells, and this protein may somehow link the RhoA-mDia system with the ERM-CD44 system. Thus, the Bni1p-Spa2p interactions revealed in this study may illuminate the mechanisms of the Rho family-regulated reorganization of the actin cytoskeleton.
ACKNOWLEDGMENTS
We thank Stephen J. Elledge for the yeast cDNA library for the two-hybrid screening. We also thank K. Irie and K. Matsumoto for the BCK1 disruption plasmid and an mpk1 mutant, RC17–4B. This investigation was supported by grants-in-aid for Scientific Research and for Cancer Research from the Ministry of Education, Science, Sports, and Culture, Japan (1996, 1997), by grants-in-aid for Abnormalities in Hormone Receptor Mechanisms and for Aging and Health from the Ministry of Health and Welfare, Japan (1996, 1997), and by grants from the Human Frontier Science Program (1996, 1997) and the Uehara Memorial Foundation (1996).
REFERENCES
- Adams AEM, Johnson DI, Longnecker RM, Sloat BF, Pringle JR. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol. 1990;111:131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AEM, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg DC, Zahner JE, Mulholland JW, Pringle JR, Botstein D. Aip3/Bud6, a yeast actin-interacting protein that is involved in morphogenesis and the selection of bipolar budding sites. Mol Biol Cell. 1997;8:729–753. doi: 10.1091/mbc.8.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkowitz RA, Lowe N. A small conserved domain in the yeast Spa2p is necessary and sufficient for its polarized localization. J Cell Biol. 1997;138:17–36. doi: 10.1083/jcb.138.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon DH, Wasserman SA. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development. 1994;120:3367–3377. doi: 10.1242/dev.120.12.3367. [DOI] [PubMed] [Google Scholar]
- Chang F, Drubin D, Nurse P. cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J Cell Biol. 1997;137:169–182. doi: 10.1083/jcb.137.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid VJ, Durán A, del Ray F, Snyder MP, Nombela C, Sánchez M. Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol Rev. 1995;59:345–386. doi: 10.1128/mr.59.3.345-386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan C, Gehrung S, Snyder M. A synthetic lethal screen identifies SLK1, a novel protein kinase homolog implicated in yeast cell morphogenesis and cell growth. Mol Cell Biol. 1992;12:1162–1178. doi: 10.1128/mcb.12.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorer R, Boone C, Kimbrough T, Kim J, Hartwell LH. Genetic analysis of default mating behavior in Saccharomyces cerevisiae. Genetics. 1997;146:39–55. doi: 10.1093/genetics/146.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drgonová J, Drgon T, Tanaka K, Kollár R, Chen G-C, Ford RA, Chan CSM, Takai Y, Cabib E. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science. 1996;272:277–279. doi: 10.1126/science.272.5259.277. [DOI] [PubMed] [Google Scholar]
- Drubin DG. Development of cell polarity in budding yeast. Cell. 1991;65:1093–1096. doi: 10.1016/0092-8674(91)90001-f. [DOI] [PubMed] [Google Scholar]
- Emmons S, Phan H, Calley J, Chen W, James B, Manseau L. Cappuccino, a Drosophila maternal effect gene required for polarity of the egg and embryo, is related to the vertebrate limb deformity locus. Genes Dev. 1995;9:2482–2494. doi: 10.1101/gad.9.20.2482. [DOI] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista M, Blundell K, Longtine MS, Chow CJ, Adames N, Pringle JR, Peter M, Boone C. Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Field CM. Actin cytoskeleton: are FH proteins local organizers? Curr Biol. 1997;7:R414–R417. doi: 10.1016/s0960-9822(06)00205-3. [DOI] [PubMed] [Google Scholar]
- Gehrung S, Snyder M. The SPA2 gene of Saccharomyces cerevisiae is important for pheromone-induced morphogenesis and efficient mating. J Cell Biol. 1990;111:1451–1464. doi: 10.1083/jcb.111.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz D, Jean AS, Woods RA, Schiestl RH. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan C, Li P, Riggs PD, Inoue H. Vectors that facilitate the expression and purification of foreign peptides in Escherichia coli by fusion to maltose-binding protein. Gene. 1987;67:21–30. doi: 10.1016/0378-1119(88)90004-2. [DOI] [PubMed] [Google Scholar]
- Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Harris SD, Hamer L, Sharpless KE, Hamer JE. The Aspergillus nidulans sepA gene encodes an FH1/2 protein involved in cytokinesis and the maintenance of cellular polarity. EMBO J. 1997;16:3474–3483. doi: 10.1093/emboj/16.12.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano H, et al. ROM7/BEM4 encodes a novel protein interacting with the Rho1p small GTP-binding protein in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4396–4403. doi: 10.1128/mcb.16.8.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, Takai Y, Tsukita S, Tsukita S. Regulation mechanism of ERM (Ezrin/Radixin/Moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura H, Tanaka K, Hihara T, Umikawa M, Kamei T, Takahashi K, Sasaki T, Takai Y. Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 1997;16:2745–2755. doi: 10.1093/emboj/16.10.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R-P, Dowzer C, Michaelis C, Galova M, Nasmyth K. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin Myo4p and other cytoplasmic proteins. Cell. 1996;84:687–697. doi: 10.1016/s0092-8674(00)81047-8. [DOI] [PubMed] [Google Scholar]
- Johnson DI, Pringle JR. Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J Cell Biol. 1990;111:143–152. doi: 10.1083/jcb.111.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Qadota H, Python CP, Anraku Y, Ohya Y, Levin DE. Activation of yeast protein kinase C by Rho1 GTPase. J Biol Chem. 1996;271:9193–9196. doi: 10.1074/jbc.271.16.9193. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Kuroda S, Sasaki T, Kotani K, Hirata K, Katayama M, Takai Y. Functional interactions of stimulatory and inhibitory GDP/GTP exchange proteins and their common substrate small GTP-binding protein. J Biol Chem. 1992;267:14611–14615. [PubMed] [Google Scholar]
- Kohno H, Tanaka K, Mino A, Umikawa M, Imamura H, Fujiwara T, Fujita Y, Hotta K, Qadota H, Watanabe T, Ohya Y, Takai Y. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP-binding protein in Saccharomyces cerevisiae. EMBO J. 1996;15:6060–6068. [PMC free article] [PubMed] [Google Scholar]
- Kotani H, Takaishi K, Sasaki T, Takai Y. Rho regulates association of both the ERM family and vinculin with the plasma membrane in MDCK cells. Oncogene. 1997;14:1705–1713. doi: 10.1038/sj.onc.1200998. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin DE, Errede B. The proliferation of MAP kinase signaling pathways in yeast. Curr Opin Cell Biol. 1995;7:197–202. doi: 10.1016/0955-0674(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Rho: a connection between membrane receptor signalling and the cytoskeleton. Trends Cell Biol. 1996;6:304–310. doi: 10.1016/0962-8924(96)10026-x. [DOI] [PubMed] [Google Scholar]
- Mack D, Nishimura K, Dennehey BK, Arbogast T, Parkinson J, Toh-e A, Pringle JR, Bender A, Matsui Y. Identification of the bud emergence gene BEM4 and its interactions with Rho-type GTPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4387–4395. doi: 10.1128/mcb.16.8.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaule P, Axel R, Myers AM. Characterization of two members of the rho gene family from the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1987;84:779–783. doi: 10.1073/pnas.84.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhoul JF, Adams TH. Identification of developmental regulatory genes in Aspergillus nidulans by overexpression. Genetics. 1995;139:537–547. doi: 10.1093/genetics/139.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Tanaka K, Nonaka H, Yamochi W, Maeda A, Takai Y. Molecular cloning and characterization of yeast rho GDP dissociation inhibitor. J Biol Chem. 1994;269:19713–19718. [PubMed] [Google Scholar]
- Matsui Y, Toh-e A. Yeast RHO3 and RHO4 ras superfamily genes are necessary for bud growth, and their defect is suppressed by a high dose of bud formation genes CDC42 and BEM1. Mol Cell Biol. 1992;12:5690–5699. doi: 10.1128/mcb.12.12.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka H, Tanaka K, Hirano H, Fujiwara T, Kohno H, Umikawa M, Mino A, Takai Y. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 1995;14:5931–5938. doi: 10.1002/j.1460-2075.1995.tb00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K, Tanaka K, Imamura H, Hihara T, Kameyama T, Nonaka H, Hirano H, Matsuura Y, Takai Y. Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 1996;15:2196–2207. [PMC free article] [PubMed] [Google Scholar]
- Petersen J, Weilguny D, Egel R, Nielsen O. Characterization of fus1 of Schizosaccharomyces pombe: a developmentally controlled function needed for conjugation. Mol Cell Biol. 1995;15:3697–3707. doi: 10.1128/mcb.15.7.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadota H, Python CP, Inoue SB, Arisawa M, Anraku Y, Zheng Y, Watanabe T, Levin DE, Ohya Y. Identification of yeast Rho1p GTPase as a regulatory subunit of 1, 3-β-glucan synthase. Science. 1996;272:279–281. doi: 10.1126/science.272.5259.279. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. The SPA2 protein of yeast localizes to sites of cell growth. J Cell Biol. 1989;108:1419–1429. doi: 10.1083/jcb.108.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M, Gehrung S, Page BD. Studies concerning the temporal and genetic control of cell polarity in Saccharomyces cerevisiae. J Cell Biol. 1991;114:515–532. doi: 10.1083/jcb.114.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn RH, Goldschmidt-Clermont PJ. Profilin: at the crossroads of signal transduction and the actin cytoskeleton. BioEssays. 1994;16:465–472. doi: 10.1002/bies.950160705. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, Tsukita S, Takai Y. Direct interaction of Rho GDI with Ezrin/Radixin/Moesin initiates the activation of Rho small G protein. J Biol Chem. 1997;272:23371–23375. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Tanaka K, Nakanishi H. Rho as a regulator of the cytoskeleton. Trends Biochem Sci. 1995;20:227–231. doi: 10.1016/s0968-0004(00)89022-2. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kameyama T, Tsukita S, Tsukita S, Takai Y. Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene. 1995;11:39–48. [PubMed] [Google Scholar]
- Valtz N, Herskowitz I. Pea2 protein of yeast is localized to sites of polarized growth and is required for efficient mating and bipolar budding. J Cell Biol. 1996;135:725–739. doi: 10.1083/jcb.135.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek AB, Hollenberg SM, Cooper JA. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM, Narumia S. p140mDia, a mammalian homolog of Drosophila diapha-nous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamochi W, Tanaka K, Nonaka H, Maeda A, Musha T, Takai Y. Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J Cell Biol. 1994;125:1077–1093. doi: 10.1083/jcb.125.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahner JE, Harkins HA, Pringle JR. Genetic analysis of the bipolar pattern of bud site selection in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:1857–1870. doi: 10.1128/mcb.16.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]