Abstract
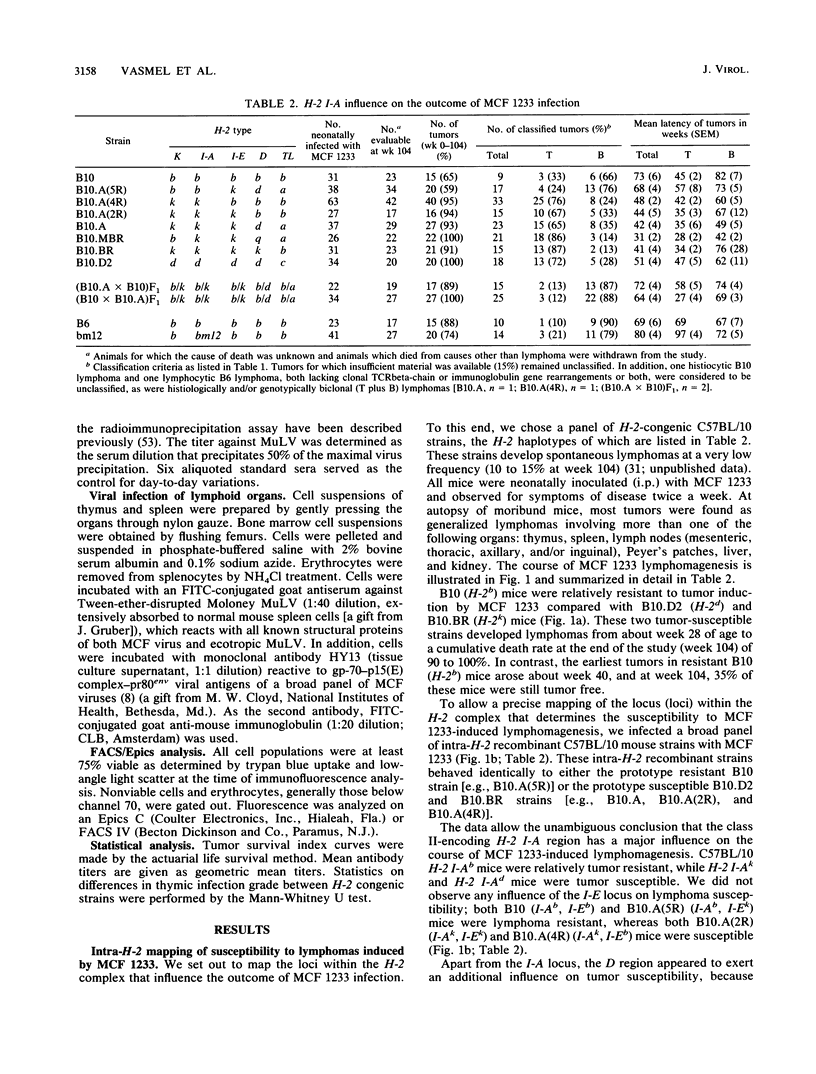
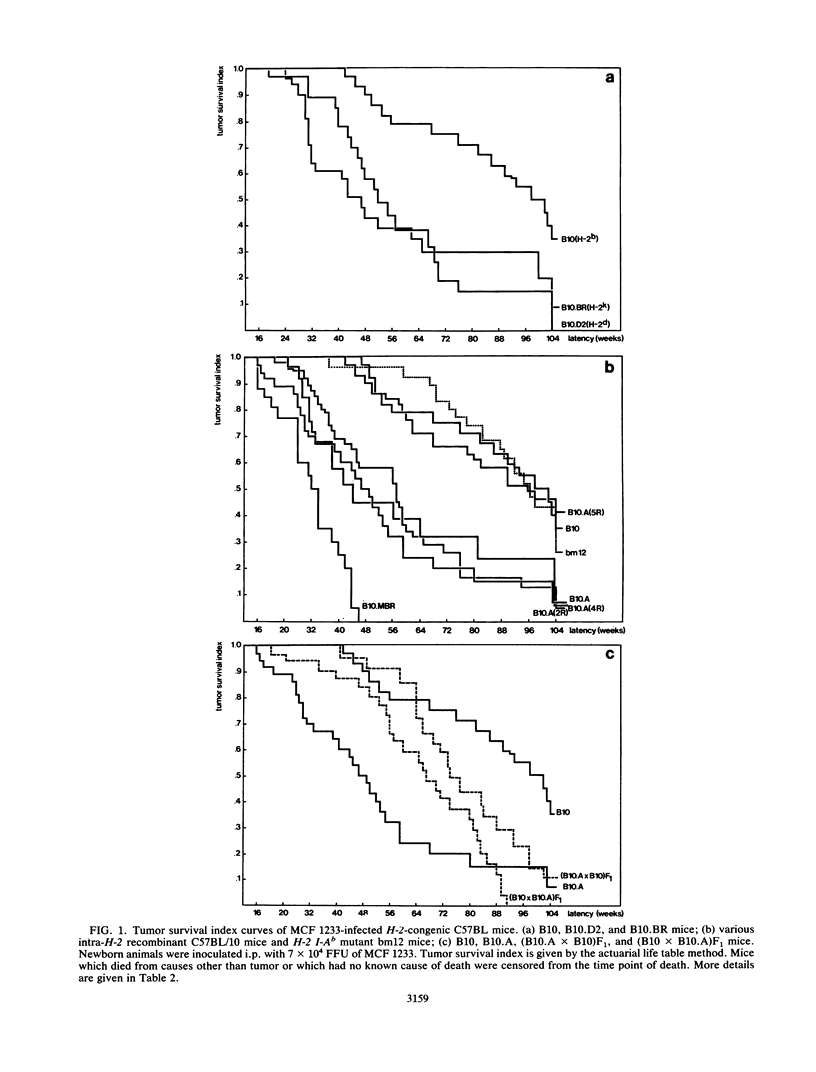
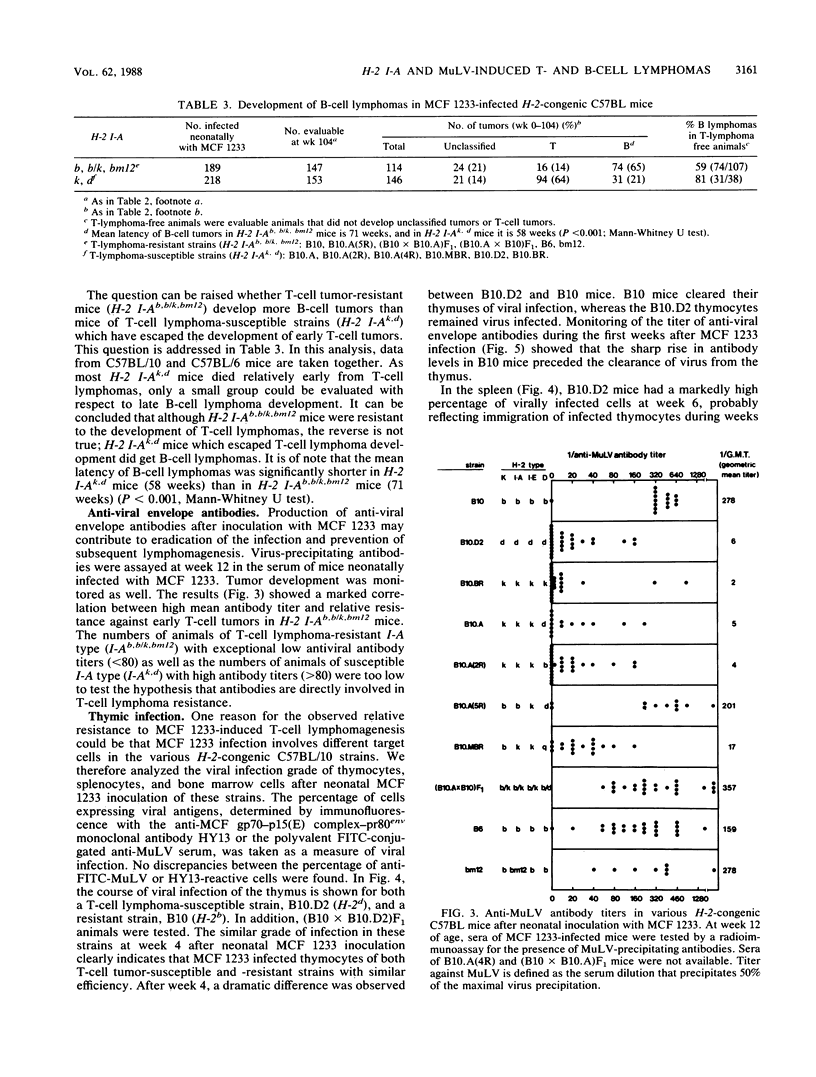
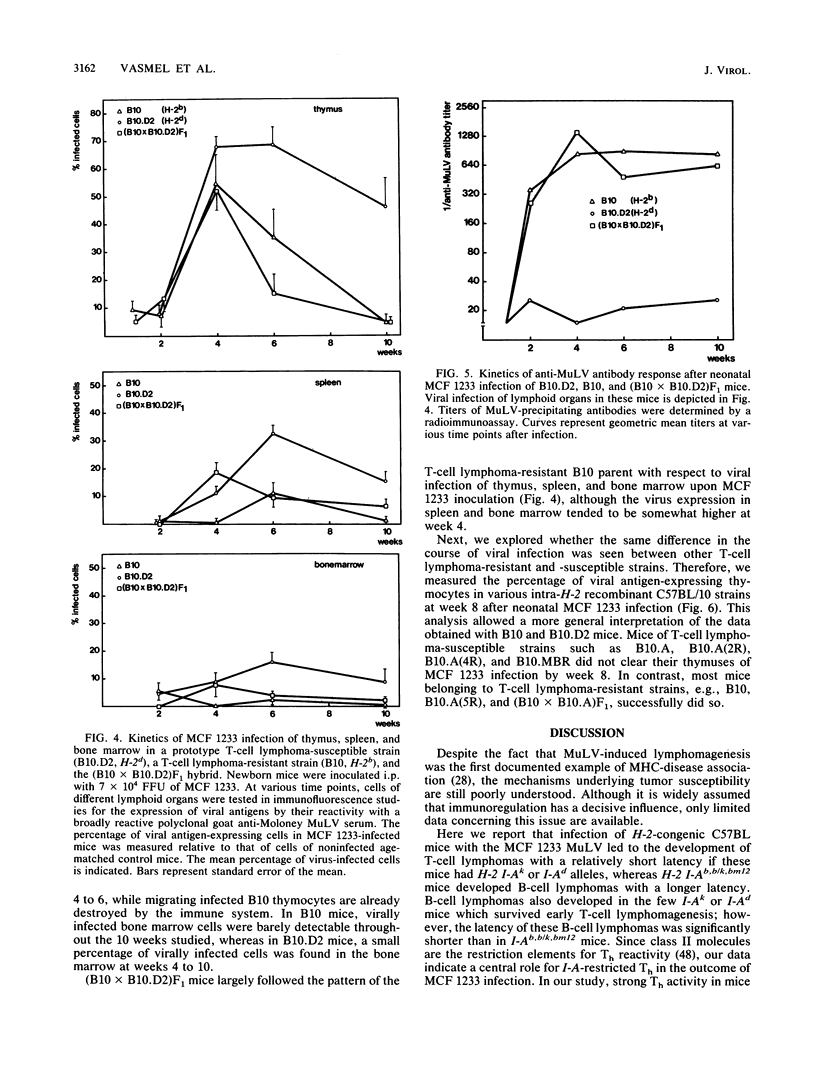
We studied the relative importance of class I and class II major histocompatibility complex (MHC) immunoregulation in the control of T- and B-cell lymphomas induced by murine leukemia virus. Previously, we have described a mink cell focus-inducing (MCF) murine leukemia virus, MCF 1233, which induces not only lymphoblastic T-cell lymphomas but also follicle center cell or lymphoblastic B-cell lymphomas. We now report that the outcome of neonatal infection with MCF 1233 in H-2-congenic C57BL/10 and C57BL/6 mice is decisively influenced by the H-2 I-A locus. A total of 64% of H-2 I-Ak, d mice [B10.BR, B10.D2, B10.A(2R), B10.A(4R), and B10.MBR] developed T-cell lymphomas after MCF 1233 infection (mean latency, 37 weeks). In contrast, H-2 I-Ab [B10, B10.A(5R), B6], H-2 I-Ab/k [(B10.A x B10)F1 and (B10 x B10.A)F1], and H-2 I-Abm12 (bm12) mice were resistant against T-cell lymphomagenesis, but 65% of these H-2 I-Ab, b/k, bm12 animals developed B-cell lymphomas (mean latency, 71 weeks). Animals of T-cell lymphoma-susceptible strains that escaped from T-cell lymphomagenesis developed B-cell lymphomas with similar frequency as animals of T-cell lymphoma-resistant strains, but with a shorter latency. H-2 class II-determined regulation of antiviral immunity was reflected in the presence of high titers of antiviral envelope antibodies in T-cell lymphoma-resistant B-cell lymphoma-susceptible H-2 I-Ab, b/k, bm12 mice, whereas in T-cell lymphoma-susceptible H-2 I-Ak,d mice no antiviral antibodies were found. At week 4 after neonatal MCF 1233 infection, a high percentage of thymocytes were virally infected in both T-cell lymphoma-susceptible and -resistant mice. However, T-cell lymphoma-resistant animals cleared the thymic infection between weeks 4 and 10 of age, coinciding with a sharp rise in serum levels of antiviral antibodies. We conclude that the pleiotropic effects of MCF 1233 infection in H-2-congenic mice result from MHC class II I-A-determined T-cell response differences.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Boog C. J., Boes J., Melief C. J. Stimulation with dendritic cells decreases or obviates the CD4+ helper cell requirement in cytotoxic T lymphocyte responses. Eur J Immunol. 1988 Feb;18(2):219–223. doi: 10.1002/eji.1830180206. [DOI] [PubMed] [Google Scholar]
- Britt W. J., Chesebro B. H-2D control of recovery from Friend virus leukemia: H-2D region influences the kinetics of the T lymphocyte response to Friend virus. J Exp Med. 1983 Jun 1;157(6):1736–1745. doi: 10.1084/jem.157.6.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Holmes K. L., Hügin A., Frederickson T. N., Morse H. C., 3rd Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 1987 Jul 2;328(6125):77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Warnke R., Sklar J. Monoclonality of lymphoproliferative lesions in cardiac-transplant recipients. Clonal analysis based on immunoglobulin-gene rearrangements. N Engl J Med. 1984 Feb 23;310(8):477–482. doi: 10.1056/NEJM198402233100801. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W. Characterization of target cells for MCF viruses in AKR mice. Cell. 1983 Jan;32(1):217–225. doi: 10.1016/0092-8674(83)90512-3. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Chesebro B., Portis J. L., Weir M. MCF-specific murine monoclonal antibodies made against AKR-247 MCF virus recognize a unique determinant associated with the gp70-p-15(E) complex. J Virol. 1982 Mar;41(3):1112–1117. doi: 10.1128/jvi.41.3.1112-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Cuypers H. T., Selten G., Quint W., Zijlstra M., Maandag E. R., Boelens W., van Wezenbeek P., Melief C., Berns A. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984 May;37(1):141–150. doi: 10.1016/0092-8674(84)90309-x. [DOI] [PubMed] [Google Scholar]
- Debré P., Boyer B., Gisselbrecht S., Bismuth A., Lévy J. P. Genetic control of sensitivity to Moloney leukemia virus in mice. III. The three H-2 linked Rmv genes are immune response genes controlling the antiviral antibody response. Eur J Immunol. 1980 Dec;10(12):914–918. doi: 10.1002/eji.1830101205. [DOI] [PubMed] [Google Scholar]
- Doherty P. C. T cells and viral infections. Br Med Bull. 1985 Jan;41(1):7–14. doi: 10.1093/oxfordjournals.bmb.a072028. [DOI] [PubMed] [Google Scholar]
- Farber E. The multistep nature of cancer development. Cancer Res. 1984 Oct;44(10):4217–4223. [PubMed] [Google Scholar]
- Fredrickson T. N., Langdon W. Y., Hoffman P. M., Hartley J. W., Morse H. C., 3rd Histologic and cell surface antigen studies of hematopoietic tumors induced by Cas-Br-M murine leukemia virus. J Natl Cancer Inst. 1984 Feb;72(2):447–454. [PubMed] [Google Scholar]
- Fredrickson T. N., Morse H. C., 3rd, Rowe W. P. Spontaneous tumors of NFS mice congenic for ecotropic murine leukemia virus induction loci. J Natl Cancer Inst. 1984 Aug;73(2):521–524. doi: 10.1093/jnci/73.2.521. [DOI] [PubMed] [Google Scholar]
- Fredrickson T. N., Morse H. C., 3rd, Yetter R. A., Rowe W. P., Hartley J. W., Pattengale P. K. Multiparameter analyses of spontaneous nonthymic lymphomas occurring in NFS/N mice congenic for ecotropic murine leukemia viruses. Am J Pathol. 1985 Nov;121(2):349–360. [PMC free article] [PubMed] [Google Scholar]
- Gleichmann E., Gleichmann H., Wilke W. Autoimmunization and lymphomagenesis in parent to F1 combinations differing at the major histocompatibility complex: model for spontaneous disease caused by altered self-antigens? Transplant Rev. 1976;31:156–224. doi: 10.1111/j.1600-065x.1976.tb01454.x. [DOI] [PubMed] [Google Scholar]
- Green W. R. Genetic control of the induction of cytolytic T lymphocyte responses to AKR/Gross viral leukemias. I. H-2-encoded dominant gene control. J Immunol. 1984 May;132(5):2658–2664. [PubMed] [Google Scholar]
- Ihle J. N., Domotor J. J., Jr, Bengali K. M. Characterization of the type and group specificities of the immune response in mice to murine leukemia viruses. J Virol. 1976 Apr;18(1):124–131. doi: 10.1128/jvi.18.1.124-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Lazar B. Natural immunity in mice to the envelope glycoprotein of endogenous ecotropic type C viruses: neutralization of virus infectivity. J Virol. 1977 Mar;21(3):974–980. doi: 10.1128/jvi.21.3.974-980.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Accessory cell-T lymphocyte interactions. Antigen-dependent and -independent clustering. J Exp Med. 1986 Feb 1;163(2):247–261. doi: 10.1084/jem.163.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaak D. D., Asjö B., Hoover E. A., Cerny J. Phenotypic heterogeneity of leukemias associated with Friend MuLV infection: studies on T-cell lymphomas and null cell leukemias in euthymic and thymus-deficient mice. Leuk Res. 1984;8(4):617–627. doi: 10.1016/0145-2126(84)90010-9. [DOI] [PubMed] [Google Scholar]
- Klein J., Juretic A., Baxevanis C. N., Nagy Z. A. The traditional and a new version of the mouse H-2 complex. Nature. 1981 Jun 11;291(5815):455–460. doi: 10.1038/291455a0. [DOI] [PubMed] [Google Scholar]
- LILLY F., BOYSE E. A., OLD L. J. GENETIC BASIS OF SUSCEPTIBILITY TO VIRAL LEUKAEMOGENESIS. Lancet. 1964 Dec 5;2(7371):1207–1209. doi: 10.1016/s0140-6736(64)91043-8. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Lombardi L., Newcomb E. W., Dalla-Favera R. Pathogenesis of Burkitt lymphoma: expression of an activated c-myc oncogene causes the tumorigenic conversion of EBV-infected human B lymphoblasts. Cell. 1987 Apr 24;49(2):161–170. doi: 10.1016/0092-8674(87)90556-3. [DOI] [PubMed] [Google Scholar]
- Melief C. J., Vlug A., de Goede R. E., de Bruyne C., Barendsen W., de Greeve P. Naturally occurring leukemia viruses in H-2 congenic C57BL mice. I. High lymphoma incidence following milk-borne transmission of virus. J Natl Cancer Inst. 1980 May;64(5):1179–1189. [PubMed] [Google Scholar]
- Meruelo D. H-2D control of leukaemia susceptibility: mechanism and implications. J Immunogenet. 1980 Feb;7(1):81–90. doi: 10.1111/j.1744-313x.1980.tb00710.x. [DOI] [PubMed] [Google Scholar]
- Montagnier L., Gruest J., Chamaret S., Dauguet C., Axler C., Guétard D., Nugeyre M. T., Barré-Sinoussi F., Chermann J. C., Brunet J. B. Adaptation of lymphadenopathy associated virus (LAV) to replication in EBV-transformed B lymphoblastoid cell lines. Science. 1984 Jul 6;225(4657):63–66. doi: 10.1126/science.6328661. [DOI] [PubMed] [Google Scholar]
- Mucenski M. L., Taylor B. A., Copeland N. G., Jenkins N. A. Characterization of somatically acquired ecotropic and mink cell focus-forming viruses in lymphomas of AKXD recombinant inbred mice. J Virol. 1987 Sep;61(9):2929–2933. doi: 10.1128/jvi.61.9.2929-2933.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorloos A. A., van Beek A. A., Melief C. J. Cryopreservation of cells for immunological typing of non-Hodgkin's lymphomas. Cancer Res. 1980 Aug;40(8 Pt 1):2890–2894. [PubMed] [Google Scholar]
- O'Donnell P. V., Fleissner E., Lonial H., Koehne C. F., Reicin A. Early clonality and high-frequency proviral integration into the c-myc locus in AKR leukemias. J Virol. 1985 Aug;55(2):500–503. doi: 10.1128/jvi.55.2.500-503.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. V., Woller R., Chu A. Stages in development of mink cell focus-inducing (MCF) virus-accelerated leukemia in AKR mice. J Exp Med. 1984 Sep 1;160(3):914–934. doi: 10.1084/jem.160.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J., Stockert E. Immunogenetics of cell surface antigens of mouse leukemia. Annu Rev Genet. 1977;11:127–160. doi: 10.1146/annurev.ge.11.120177.001015. [DOI] [PubMed] [Google Scholar]
- Pals S. T., Zijstra M., Radaszkiewicz T., Quint W., Cuypers H. T., Schoenmakers H. J., Melief C. J., Berns A., Gleichmann E. Immunologic induction of malignant lymphoma: graft-vs-host reaction-induced B cell lymphomas contain integrations of predominantly ecotropic murine leukemia proviruses. J Immunol. 1986 Jan;136(1):331–339. [PubMed] [Google Scholar]
- Pattengale P. K., Taylor C. R. Experimental models of lymphoproliferative disease. The mouse as a model for human non-Hodgkin's lymphomas and related leukemias. Am J Pathol. 1983 Nov;113(2):237–265. [PMC free article] [PubMed] [Google Scholar]
- Peled A., Haran-Ghera N. High incidence of B cell lymphomas derived from thymectomized AKR mice expressing TL.4 antigen. J Exp Med. 1985 Sep 1;162(3):1081–1086. doi: 10.1084/jem.162.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci P. G., Knowles D. M., 2nd, Arlin Z. A., Wieczorek R., Luciw P., Dina D., Basilico C., Dalla-Favera R. Multiple monoclonal B cell expansions and c-myc oncogene rearrangements in acquired immune deficiency syndrome-related lymphoproliferative disorders. Implications for lymphomagenesis. J Exp Med. 1986 Dec 1;164(6):2049–2060. doi: 10.1084/jem.164.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn I. Depressed immunity and the development of cancer. Clin Exp Immunol. 1981 Dec;46(3):459–474. [PMC free article] [PubMed] [Google Scholar]
- Pierres A., Naquet P., Van Agthoven A., Bekkhoucha F., Denizot F., Mishal Z., Schmitt-Verhulst A. M., Pierres M. A rat anti-mouse T4 monoclonal antibody (H129.19) inhibits the proliferation of Ia-reactive T cell clones and delineates two phenotypically distinct (T4+, Lyt-2,3-, and T4-, Lyt-2,3+) subsets among anti-Ia cytolytic T cell clones. J Immunol. 1984 Jun;132(6):2775–2782. [PubMed] [Google Scholar]
- Reinisch C. L., Sing A. P., Bacon E. R., Corley R. B., Gershon R. K. Regulation of B cell lymphomagenesis by a malignant Qal+ inducer T cell clone. J Exp Med. 1984 Mar 1;159(3):906–920. doi: 10.1084/jem.159.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R. The pathogenesis of Abelson virus lymphomas of the mouse. Biochim Biophys Acta. 1982 Jun 28;651(4):213–244. doi: 10.1016/0304-419x(82)90013-0. [DOI] [PubMed] [Google Scholar]
- Sato H., Boyse E. A., Aoki T., Iritani C., Old L. J. Leukemia-associated transplantation antigens related to murine leukemia virus. The X.1 system: immune response controlled by a locus linked to H-2. J Exp Med. 1973 Sep 1;138(3):593–606. doi: 10.1084/jem.138.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selten G., Cuypers H. T., Zijlstra M., Melief C., Berns A. Involvement of c-myc in MuLV-induced T cell lymphomas in mice: frequency and mechanisms of activation. EMBO J. 1984 Dec 20;3(13):3215–3222. doi: 10.1002/j.1460-2075.1984.tb02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel H. J., Schwarz H., Fischinger P., Bolognesi D., Schäfer W. Role of antibodies to murine leukemia virus p15E transmembrane protein in immunotherapy against AKR leukemia: a model for studies in human acquired immunodeficiency syndrome. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5893–5897. doi: 10.1073/pnas.84.16.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Molecular complexity of leukocyte surface glycoproteins related to the macrophage differentiation antigen Mac-1. J Exp Med. 1981 Nov 1;154(5):1517–1524. doi: 10.1084/jem.154.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasmel W. L., Radaszkiewicz T., Miltenburg A. M., Zijlstra M., Melief C. J. Refinement and precision in the classification of murine lymphomas by genotyping with immunoglobulin and T cell receptor probes. Leukemia. 1987 Mar;1(3):155–162. [PubMed] [Google Scholar]
- Vlug A., Melief C. J., de Bruyne C., Schoenmakers H., Molenaar J. L. Naturally occurring leukemia viruses in H-2 congenic C57BL mice. II. Antibody response to viral envelope antigens. J Natl Cancer Inst. 1980 May;64(5):1191–1198. [PubMed] [Google Scholar]
- Vlug A., Schoenmakers H. J., Melief C. J. Genes of the H-2 complex regulate the antibody response to murine leukemia virus. J Immunol. 1981 Jun;126(6):2355–2360. [PubMed] [Google Scholar]
- Vlug A., Zijlstra M., de Goede R. E., Hesselink W. G., Schoenmakers H. J., Melief C. J. H-2 control of the cytotoxic antibody response against a newly defined MuLV-related cell-surface antigen: G(B10.A). Int J Cancer. 1983 May 15;31(5):617–626. doi: 10.1002/ijc.2910310514. [DOI] [PubMed] [Google Scholar]
- Weinhold K. J., Hüper G., Matthews T. J., Fischinger P. J., Ihle J. N., Schwarz H., Thiel H. J., Schäfer W., Bolognesi D. P. Properties of mouse leukemia viruses. XIX. Effective antibody therapy of AKR leukemia occurs independently of virus neutralization and produces long-term changes in the virus status of the thymus. Virology. 1984 May;135(1):105–117. doi: 10.1016/0042-6822(84)90121-1. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Melief C. J. Virology, genetics and immunology of murine lymphomagenesis. Biochim Biophys Acta. 1986 Oct 28;865(2):197–231. doi: 10.1016/0304-419x(86)90028-4. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Quint W., Cuypers T., Radaszkiewicz T., Schoenmakers H., de Goede R., Melief C. Ecotropic and mink cell focus-forming murine leukemia viruses integrate in mouse T, B, and non-T/non-B cell lymphoma DNA. J Virol. 1986 Mar;57(3):1037–1047. doi: 10.1128/jvi.57.3.1037-1047.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Schoenmakers H. J., Melief C. J. Differences in oncogenicity among murine leukemia virus isolates are not correlated with the magnitude of H-2 regulated anti-viral envelope antibody responses. Leuk Res. 1986;10(9):1121–1129. doi: 10.1016/0145-2126(86)90057-3. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., de Goede R. E., Schoenmakers H. J., Schinkel A. H., Hesselink W. G., Portis J. L., Melief C. J. Naturally occurring leukemia viruses in H-2 congenic C57BL mice. III. Characterization of C-type viruses isolated from lymphomas induced by milk transmission of B-ecotropic virus. Virology. 1983 Feb;125(1):47–63. doi: 10.1016/0042-6822(83)90062-4. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., de Goede R. E., Schoenmakers H., Radaszkiewicz T., Melief C. J. Ecotropic and dualtropic mink cell focus-inducing murine leukemia viruses can induce a wide spectrum of H-2 controlled lymphoma types. Virology. 1984 Oct 30;138(2):198–211. doi: 10.1016/0042-6822(84)90345-3. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]



