Abstract
SPARC (secreted protein acidic and rich in cysteine)/BM 40/osteonectin is a matricellular protein shown to function as a counteradhesive factor that induces cell rounding and as an inhibitor of cell proliferation. These activities have been defined in cell culture, in which interpretation has been complicated by the presence of endogenous SPARC. We therefore sought to determine whether cell shape and proliferation would be affected by the absence of SPARC. Mesangial cells, fibroblasts, and aortic smooth muscle cells were isolated from SPARC-null and age-matched, wild-type mice. In contrast to wild-type cells, SPARC-null mesangial cells exhibited a flat morphology and an altered actin cytoskeleton. In addition, vinculin-containing focal adhesions were distributed over the center of SPARC-null cells, whereas in wild-type cells, the number of focal adhesions was reduced, and these structures were restricted largely to the cell periphery. Although the SPARC-null fibroblasts did not display overt differences in cell morphology, the cells responded to exogenous recombinant SPARC by rounding up in a manner similar to that of wild-type fibroblasts. Thus, the expression of endogenous SPARC is not required for the response of cells to SPARC. Additionally, SPARC-null mesangial cells, fibroblasts, and smooth muscle cells proliferated faster than their respective wild-type counterparts. Null cells also showed a greater sensitivity to the inhibition of cell cycle progression by the addition of recombinant SPARC. The increased proliferation rate of SPARC-null cells appeared to be mediated, at least in part, by an increase in the cell cycle regulatory protein cyclin A. We conclude that the expression of SPARC influences the cellular architecture of mesangial cells and that SPARC plays a role in the regulation of cell cycle in mesangial cells, fibroblasts, and smooth muscle cells.
INTRODUCTION
SPARC (secreted protein acidic and rich in cysteine)/BM 40/osteonectin is a matricellular protein expressed by a wide variety of cell types throughout both invertebrate and vertebrate organisms. That SPARC is highly conserved from Caenorhabditis elegans to humans indicates a basic functional role in animal tissues (Lane and Sage, 1994). The highest level of SPARC expression in mammals is found in bone; however, SPARC is also associated with other remodeling tissues and sites of high cellular turnover, such as the epithelial lining of the gut, wound healing, angiogenesis, tumor invasion, and pathological fibroses. SPARC has a characteristic modular structure with domains that are functionally distinct (Lane and Sage, 1994). A Ca2+-binding EF hand in the C-terminal domain of the protein has been implicated in the interaction with cell surfaces as well as in the binding to various components of the ECM that include fibrillar collagens and collagen IV (Sage et al., 1989; Iruela-Arispe et al., 1996; Sasaki et al., 1997). SPARC interacts with various growth factors (e.g., PDGF and vascular endothelial growth factor) and diminishes their mitotic activity in culture (Raines et al., 1992; Kupprion et al., 1998). SPARC has also been shown to regulate the expression of certain proteins involved in matrix turnover. For example, its induction of plasminogen activator inhibitor (PAI)-1 in endothelial cells leads to a decrease in plasminogen activation (Hasselaar et al., 1991). Moreover, certain of the matrix metalloproteinases are induced by SPARC in synovial fibroblasts, independently of the effect of SPARC on cell shape (Tremble et al., 1993). The multitude of functions attributed to SPARC to date implicate this protein as a potential modulator of various integral cellular processes.
As mentioned above, many cells in culture will respond to exogenous SPARC with a change in cell shape that leads to cell rounding (Sage et al., 1989; Lane and Sage, 1990). In fact, SPARC mediates focal adhesion disassembly in bovine aortic endothelial cells, specifically through the Ca2+-binding EF-hand (Murphy-Ullrich et al., 1995). Additionally, an inhibition of proliferation is observed on the addition of SPARC to cells in culture. SPARC diminishes the mitotic activity of endothelial cells, mesangial cells, and smooth muscle cells, among others (Funk and Sage, 1991, 1993; Pichler et al., 1996). Although the effects of SPARC in cell culture are well documented, the primary functions of this protein in vivo remain to be determined.
Two separate colonies of SPARC-null mice have been generated recently (Gilmour et al., 1998; Norose et al., 1998). A primary phenotype of these mice is cataractogenesis at an early age (Norose et al., 1998; Gilmour et al., 1998; Bassuk et al., 1999). The lens epithelial cells are perturbed at the site of differentiation along the equatorial plane, and large vacuoles are formed either between or within the differentiating cells (Bassuk et al., 1999). Whether cataract formation results from a disturbance in the epithelial cell cycle, epithelial cell shape, or perhaps another mechanism is not known. Other tissues of the mouse seem to develop relatively normally in the absence of SPARC, although an exhaustive study has not yet been performed. In fact, recent evidence suggests a significant defect in bone (Delany et al., 1998), although a preliminary study reported grossly normal bone development in SPARC-null mice (Gilmour et al., 1998).
Given the established effects of SPARC on cultured cells, we sought to determine whether primary cells isolated from SPARC-null mice exhibited differences in cell shape or cell cycle relative to wild-type counterparts and whether these cells could respond to SPARC in the absence of its endogenous expression. We compared three cell types from SPARC-null adult mice: skin fibroblasts, kidney mesangial cells, and aortic smooth muscle cells. Although only one cell type differed morphologically from wild-type controls, all of the adult cells examined showed significantly enhanced rates of proliferation in the absence of SPARC expression. SPARC therefore appears to be an important modulator of cell cycle and most likely influences cell division in various tissues in the context of normal remodeling, wound repair, and/or pathogenesis.
MATERIALS AND METHODS
Materials
Cell culture media were purchased from Life Technologies-BRL (Gaithersburg, MD), insulin supplement for the mesangial cell cultures was purchased from Collaborative Biomedical Products (Bedford, MA), and BODIPY-conjugated phalloidin was purchased from Molecular Probes (Eugene, OR). The mouse monoclonal anti-vinculin antibody was purchased from Sigma (St. Louis, MO). The mouse monoclonal anti-SPARC immunoglobulin G was from Hematological Technologies (Essex Junction, VT). Recombinant SPARC (rSPARC) was generated in insect cells via the baculovirus expression system (see below). The anti-cyclin anti-bodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Methods
Primary Cell Isolation.
129/SvJ × C57/Bl 6 wild-type and SPARC-null mice were generated as described and raised in a specific pathogen-free facility (Norose et al., 1998). Confirmation of complete ablation of SPARC expression was shown by Northern blotting and immunoblotting (Norose et al., 1998). For the isolation of adult skin fibroblasts, mice between the ages of 3 and 7 mo were euthanized by cervical dislocation. The shaved skin was removed to a sterile flask containing 0.25% trypsin (Life Technologies) in dissection buffer (10 mM glucose, 3 mM KCl, 130 mM NaCl, 1 mM Na2HPO4·7H20, 30 mM HEPES, pH 7.4) and incubated overnight at 4°C, after which the epidermis was separated from the dermis. The dermis was subjected to further digestion with 0.25% collagenase (Worthington Enzymes, Freehold, NJ) in DMEM for 2–3 h at 37°C. The cells were collected by centrifugation and plated in DMEM supplemented with 10% FBS (Summit Biotechnology, Ft. Collins, CO) and 500 U/ml penicillin G/500 U/ml streptomycin sulfate or Molecular, Cellular, Developmental Biology (MCDB) 201 supplemented with 5% FBS. Four fibroblast preparations were performed from three to five mice of either wild-type or SPARC-null background, and all assays were performed before passage five.
Isolation of mesangial cells was based on a method in which the glomeruli were digested partially with collagenase (Francki et al., 1995). Briefly, the kidneys were removed and dissected free of the capsule and medulla. The cortex was homogenized with a scalpel, passed over successive sieves with meshes of 180–106 μm, and collected on a final sieve with a pore size of 45 μm. The tissue (containing the glomeruli) was collected, washed with PBS, and digested with 2.5% collagenase (Worthington type CLS IV, 184 U/ml) in PBS for 15–25 min at 37°C. The glomeruli were washed in growth media (DMEM, 55%; Ham’s F12, 20%; l-glutamine, 2 mM; trace elements, 1%; transferrin, 5 μg/ml; insulin, 125 U/ml; FBS, 20%; 500 U/ml penicillin G/500 U/ml streptomycin sulfate/2 μg/ml amphotericin B), seeded in culture flasks, and incubated at 37°C for 10–14 d. At this point, the cultures were examined morphologically for contaminating cell types. Nonmesangial cells were removed by scraping. Additionally, the cultures were examined by immunofluorescence for specific cell-marker proteins. In both wild-type and SPARC-null cultures, >98% of the cells were stained for myosin, α-smooth muscle actin, desmin, vimentin, and collagen type IV, but were nonreactive with antibodies against von Willebrand factor (endothelial cells) and cytokeratin 18 and 19 (glomerular epithelial cells). Seven mesangial preparations were isolated from three to five mice of either wild-type or SPARC-null background, and all assays were performed before passage eight.
For the isolation of aortic smooth muscle cells, aortas were removed and dissected away from fat and extraneous tissue in sterile, ice-cold, serum-free DMEM. Each aorta was incubated in dissecting media consisting of serum-free DMEM, 2 mg/ml BSA (Sigma), 1 mg/ml collagenase (Worthington type CLS IV, 178 U/ml), 0.5 mg/ml soybean trypsin inhibitor (Worthington), and 125 μg/ml elastase (Sigma) for 30 min at 37°C. After removal of the adventia, aortas were minced and shaken gently at 37°C for 2 h in dissecting media. The dissociated tissues were subsequently rinsed with 5 ml of FBS, centrifuged at 2000 × g, and resuspended and plated in growth media (DMEM, 10% FBS, 1% nonessential amino acids [Life Technologies], 1% glutamine, 500 U/ml penicillin G/500 U/ml streptomycin sulfate). Greater than 95% of the cells were stained for α-smooth muscle actin, desmin, and myosin heavy chain. All assays were performed with cells before passage four.
Immunofluorescence.
Both cell types were plated on sterile glass coverslips at ∼2–4 × 104 cells/ml. Before staining, the cells were fixed in 4% paraformaldehyde for 30 min. The cells were rendered permeable in blocking solution (1% normal goat serum [Sigma] in PBS with 0.5% Triton X-100 [Bio-Rad, Hercules, CA]) for 1 h. Incubations in primary antibody were performed in blocking solution for 1 h at room temperature, followed by three rinses in PBS. Appropriate secondary antibodies conjugated to rhodamine or fluorescein (Vector, Burlingame, CA) were incubated in blocking solution for 1 h followed by rinsing and subsequent incubation with BODIPY-conjugated phalloidin for 30 min. The coverslips were mounted in Prolong Anti-Fade (Molecular Probes) for viewing with a Nikon Microphot SA microscope equipped for epifluorescence. Fibroblasts treated with rSPARC were allowed to adhere and spread for 18 h before the addition of 30 μg/ml purified rSPARC (0.9 μM). The cells were fixed 18 h after initial exposure to the recombinant protein. Equal amounts of non-SPARC–containing column fractions added to parallel cultures had no discernible effect on cell shape.
Production of rSPARC.
Human rSPARC was expressed in insect cells by use of the baculovirus expression system and was collected in protein-free media (Bradshaw, Bassuk, and Sage, unpublished experiments). rSPARC was purified by anion-exchange chromatography on a Q Sepharose, Fastflow column (Pharmacia, Piscataway, NJ) with a 200–400 mM LiCl gradient. rSPARC, eluted as the prominent band, was identified by SDS-PAGE (Laemmli, 1970) followed by staining with Coomassie Brilliant Blue R (Sigma). Four separate antibodies against different domains of SPARC reacted with the rSPARC by immunoblot analysis. Baculovirus-expressed rSPARC has activity similar to Escherichia coli-expressed rSPARC and to SPARC synthesized by cultured mammalian cells, as measured in bovine aortic endothelial cells by rounding and inhibition of proliferation (Funk and Sage, 1991; Bassuk et al., 1996).
Proliferation and 3H-Thymidine Incorporation Assays.
Proliferation assays were initiated by plating cells at equal concentrations, in triplicate, in 24-well plates. Because the mesangial cells did not appear to adhere uniformly, an initial count was performed 2 h after plating to establish the number of cells attached at the onset of the experiment. All cell counts were performed by hemacytometer after 1) rinsing the well with PBS to remove nonadherent cells, 2) trypsin digestion to remove the cells from the plate, 3) centrifugation to concentrate the cells, and 4) resuspension in an equal volume of PBS. Proliferation assays were performed in the growth media specific for each cell type as described above.
For 3H-thymidine incorporation assays, all cell types were plated at equal densities in triplicate in 24-well plates and allowed to adhere overnight. In some cases, the cells were synchronized in minimal concentrations of FBS: from 10 to 2% for fibroblasts, and for mesangial cells by the replacement of growth media with resting media (MCDB 201, l-glutamine, insulin supplement, trace elements, and penicillin/streptomycin) for 24 h. The cells were stimulated by the addition of FBS and incubated with 2 μCi/ml 3H-thymidine (6.7 Ci/mmol; Amersham, Arlington Heights, IL) for either 18 or 4 h after the overnight stimulation. 3H-thymidine incorporation was measured by 1) rinsing the cells twice in cold PBS, 2) adding 10% trichloroacetic acid for 30 min at 4°C, 3) washing in cold 100% ethanol, 4) solubilizing in 0.1 N NaOH for 30 min at 65°C, and 5) measuring radioactivity in a scintillation counter. Incubation of synchronized mesangial cells with SPARC was begun at the time of stimulation with FBS, by the addition of 30 μg/ml (0.9 μM) rSPARC. As a control, equal volumes of non-SPARC–containing column fractions from the purification of rSPARC (vehicle) were added to control wells in parallel to the experimental wells. Incorporation of 3H-thymidine was measured as described above.
Immunoblot Analysis of Cell Cycle Proteins.
Mesangial cells were grown as described above. Equal concentrations of SPARC-null and wild-type cells were plated in 60 mm2 tissue culture dishes in resting media. The cells became quiescent over 48 h, after which they received 10 ng/ml PDGF (R&D Systems, Minneapolis, MN). Cell layers were removed at the indicated time points in RIPA buffer (1% NP-40, 0.5% Na deoxycholate, 0.1% SDS in PBS) with protease inhibitors (complete protease inhibitor mixture; Boehringer Mannheim, Indianapolis, IN). Total protein was measured by a bicinchoninic acid protein assay (Pierce, Rockfield, IL) according to the manufacturer’s instructions. For analysis of cell cycle proteins, 50 μg total proteins were separated by SDS-PAGE on a 10% gel under reducing conditions and transferred to a nitrocellulose membrane (Schleicher and Schuell, Keene, NH). The blots were blocked in 3% nonfat dry milk with 0.05% Tween-20 (Sigma) for 2 h. Primary antibody incubations were performed at room temperature for ∼1 h, followed by three consecutive washes in blocking solution and incubation with the appropriate secondary antibody conjugated to horseradish peroxidase for ∼1 h. Immunoreactive proteins were detected with enhanced chemiluminescence according to the manufacturer’s instructions (New England Nuclear, Boston MA). Equal loading of protein was confirmed by staining of the transferred gel with Coomassie blue.
RESULTS
SPARC Expression and Cell Shape
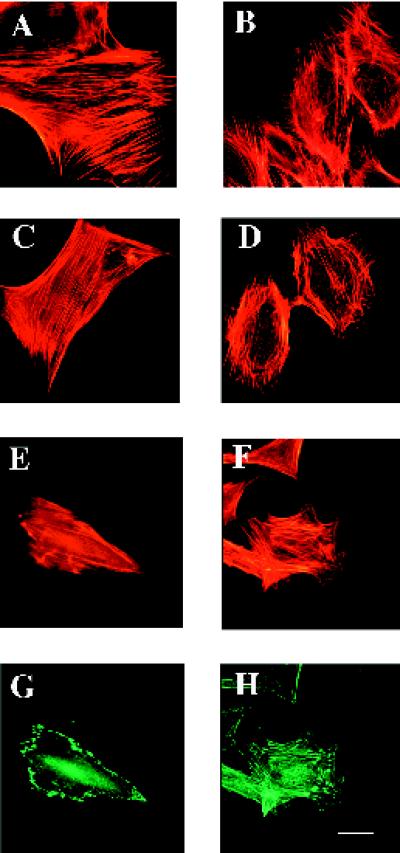
Because SPARC has been implicated in the regulation of cell shape, we asked first whether cells derived from a SPARC-null background were distinguishable morphologically from wild-type cells. We examined different cell types isolated from age-matched wild-type and SPARC-null animals. Although adult skin fibroblasts did not exhibit significant differences in cell shape, SPARC-null mesangial cells were morphologically distinct from their wild-type counterparts. Figure 1, A, C, and E, shows phalloidin staining of F-actin in wild-type cells, whereas B, D, and F show the actin profiles of the SPARC-null cells. In four separate cellular preparations from pools of at least five kidneys each, the SPARC-null mesangial cells exhibited consistently an altered morphology in comparison to wild-type cells. The SPARC-null cells were generally more spread and exhibited a greater degree of cell contact than was seen in the more elongated, wild-type cells. The actin cytoskeleton in the SPARC-null cells was altered in comparison to that of wild-type cells, with fewer actin cables spanning the length of the cell and more of the fibrillar structures concentrated at or near the periphery. In addition, the antivinculin immunoreactivity reflected fewer focal adhesions in the SPARC-null cells at the periphery and more in the central region of the cells, in comparison to the wild-type cells (Figure 1, G and H) (Otto, 1990). The increase in the amount and distribution of the focal adhesions was also reflected in the heightened resistance of the SPARC-null cells to trypsin digestion during routine culture.
Figure 1.
SPARC-null mesangial cells exhibit an altered morphology in comparison to wild-type cells. Wild-type (A, C, E, and G) and SPARC-null mesangial cells (B, D, F, and H) were grown on coverslips in growth media. (A–F) BODIPY-conjugated phalloidin was used to visualize the actin cytoskeleton. (G and H) An anti-vinculin antibody was used to detect focal contacts in the mesangial cells. Bar, 5 μm.
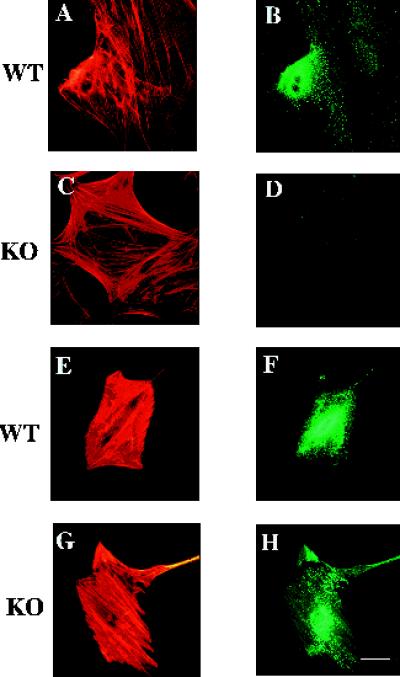
Although a difference in morphology was seen between the SPARC-null and wild-type mesangial cells, neither responded noticeably to exogenous SPARC with regard to changes in cell shape. Whereas the addition of SPARC to many cell types elicits a characteristic rounding effect, vascular smooth muscle cells, which appear to be highly similar to mesangial cells, do not undergo obvious alterations in shape when exposed to SPARC (Sage et al., 1989), consistent with our results. On the other hand, SPARC-null fibroblasts responded to rSPARC. As shown in Figure 2, both SPARC-null and wild-type fibroblasts rounded significantly in response to rSPARC (compare A and C with E and G, respectively). In addition, immunoreactivity for rSPARC was localized to discrete sites on the cell (Figure 2, F and H). Although it is difficult to determine, at this level of resolution, whether the rSPARC was internalized or merely cell surface-associated, it is clear that the response of fibroblasts to SPARC via changes in cell shape is not dependent on the expression of endogenous SPARC. In contrast to the mesangial cells, however, neither the SPARC-null fibroblasts nor the aortic smooth muscle cells exhibited any overt morphological differences in comparison to wild-type cells (Figure 2, and our unpublished results). Likewise, alterations in the cytoskeleton or in focal adhesions were not obvious in either the SPARC-null fibroblasts or the smooth muscle cells. Thus, we conclude that SPARC is important as a determinant of basal morphology in some cell types in culture (such as mesangial cells) but not in others; however, the property of exogenous SPARC to effect changes in cell shape is not dependent on endogenous expression of SPARC.
Figure 2.
SPARC-null and wild-type adult skin fibroblasts change shape in response to rSPARC. Wild-type (A, B, E and F) and SPARC-null fibroblasts (C, D, G and H) were cultured on glass coverslips in the absence (A–D) and presence of 0.9 μM rSPARC (E–H). (A, C, E, and G) BODIPY-conjugated phalloidin was used to visualize the actin cytoskeleton. (B, D, F, and H) Anti-SPARC antibody was used to detect SPARC immunoreactivity. Bar, 5 μm.
SPARC Expression and Cellular Proliferation
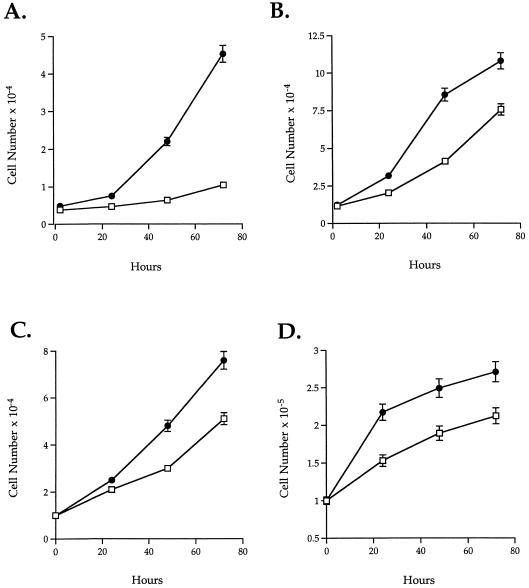
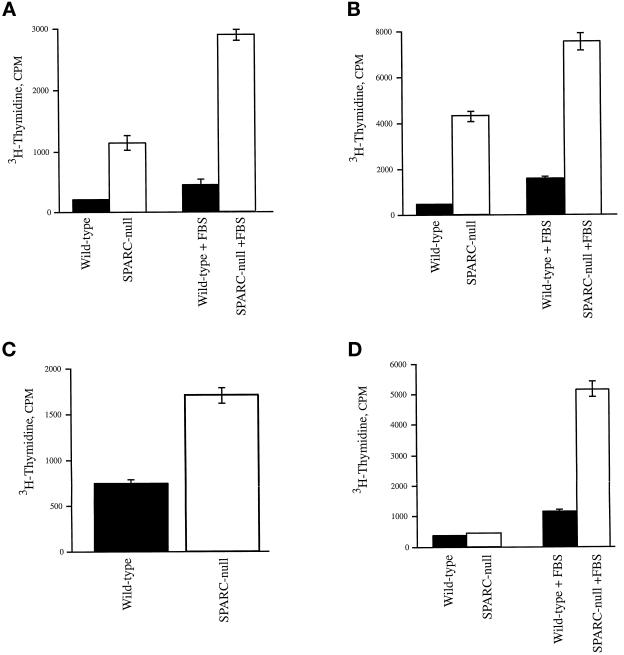
Another well-characterized property of SPARC is the regulation of cellular proliferation in various cultured cells (Lane and Sage, 1994). The addition of exogenous SPARC to both smooth muscle cells and fibroblasts results in an inhibition of proliferation (Funk and Sage, 1993). We therefore asked whether the lack of endogenous SPARC would affect the rate of proliferation of different cell types isolated from SPARC-null animals. Aortic smooth muscle cells, mesangial cells, and skin fibroblasts all exhibited an accelerated rate of proliferation relative to wild-type counterparts. We examined five separate mesangial and fibroblast preparations composed of three to five animals each, as well as one preparation of aortic smooth muscle cells from four animals. All preparations showed a notable difference in growth rates between wild-type and SPARC-null cells, although to different degrees. Representative data are shown in Figures 3 and 4. Two separate starting densities were tested for mesangial cells and fibroblasts in the proliferation assays shown in Figure 3. Both populations showed greater differences in rates between the wild-type and SPARC-null cells at lower starting densities (Figure 3, A and C). At higher densities, both cell types exhibited diminished growth rates as the culture surface area became limiting. In all the preparations tested, mesangial cells (Figure 3, C and D) showed consistently a greater disparity in growth rate between SPARC-null and wild-type cells, in comparison to fibroblasts. This result was also reflected in the 3H-thymidine incorporation assays shown in Figure 4. Although the SPARC-null fibroblasts usually incorporated twice as much 3H-thymidine over an 18 h period as was seen in their wild-type counterparts (Figure 4C), SPARC-null mesangial cells incorporated as much as 10 times over that of wild-type cells within the same period of time (Figure 4, A and B). Likewise, the aortic smooth muscle cells exhibited differences in proliferation rates (Figure 4D). SPARC-null smooth muscle cells showed slightly higher rates of 3H-thymidine incorporation in basal media; however, on stimulation with 10% FBS, SPARC-null smooth muscle cells showed an approximately fivefold increase in the rate of incorporation over that of wild-type cells (Figure 4D).
Figure 3.
Primary cells isolated from SPARC-null mice proliferate faster than wild-type counterparts. A and B represent mesangial cells (wild-type [□] and SPARC-null [●]) plated at equal densities (low, 5 × 102 cells/ml [A], and high, 1 × 103cells/ml [B]) in triplicate, in a 24-well plate. The mesangial cells were counted 2 h after plating to determine the number of attached cells at the onset of the experiment. No differences in initial plating efficiency were detected between the SPARC-null and the wild-type cells in either the fibroblast or the mesangial cell cultures. (C and D) Wild-type (□) and SPARC-null fibroblasts (●) were plated at equal concentrations in triplicate, in a 24-well plate. C shows an initial plating density of 1 × 103 cells/ml (low), whereas D shows a higher initial plating density of 1 × 104 cells/ml. All values have error bars that represent the SEM.
Figure 4.
SPARC-null primary cells incorporate higher levels of 3H-thymidine in comparison to wild-type cells. (A and B) Two separate preparations of wild-type (black bars) and SPARC-null mesangial cells (white bars) were assayed for incorporation of 3H-thymidine. Cells grown in resting media (see MATERIALS AND METHODS) for 24 h were treated with either 10% FBS in resting media or resting media alone for a further 18 h. 3H-Thymidine was added for the final 4 h of incubation. (C) Equal concentrations of wild-type (black bars) and SPARC-null fibroblasts (white bars) were plated in triplicate, in 24-well plates. The cells were grown in minimal media (1% FBS) for 24 h and subsequently stimulated with growth media (10% FBS) for 18 h. 3H-Thymidine was added for the final 4 h of incubation. (D) Wild-type (black bars) and SPARC-null aortic smooth muscle cells (white bars) were plated at equal concentrations in serum-free media for 48 h and either stimulated with 10% FBS or left in serum-free media for an additional 18 h, as described above for mesangial cells. 3H-Thymidine was added for the final 4 h of incubation. Error bars represent the SEM.
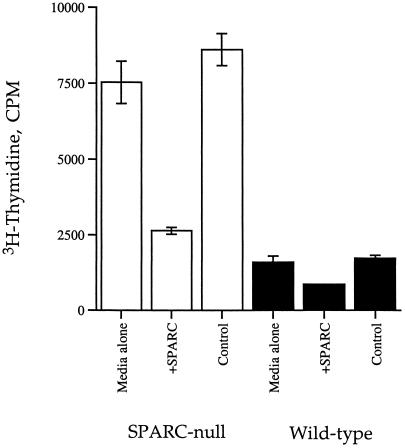
We attempted to restore the increased rate of proliferation to levels characteristic of wild-type cells. Accordingly, rSPARC was added to both SPARC-null and wild-type cells, and the rates of proliferation were monitored by incorporation of 3H-thymidine. As shown in Figure 5, 0.9 μM rSPARC inhibited the rate of proliferation of both SPARC-null and wild-type mesangial cells. The inhibition was greater for the SPARC-null (70% decrease, relative to control) versus the wild-type cells (50% of control), presumably because the endogenous expression of SPARC by the wild-type cells renders these cells less sensitive to added SPARC. Similar results were obtained for the skin fibroblasts (data not shown). Given that SPARC-null mesangial cells do not respond to rSPARC with a detectable change in cell shape, the effect of rSPARC on proliferation is probably not a result of cell rounding (see DISCUSSION for further comments on this point).
Figure 5.
rSPARC inhibits proliferation of SPARC-null cells to a greater degree than wild-type counterparts. Wild-type (black bars) and SPARC-null mesangial cells (white bars) were grown as described in Figure 4, except that 0.9 μM rSPARC was added at the time of stimulation. Equal volumes of buffer were added to control wells (see MATERIALS AND METHODS). Error bars represent the SEM.
Cell Cycle Control in a SPARC-null Background
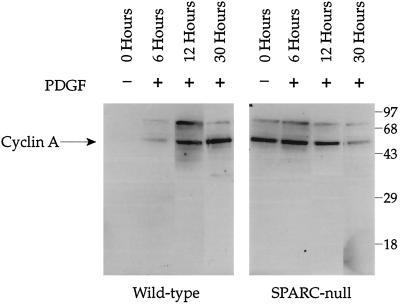
To investigate the role of SPARC in the regulation of proliferation, we prepared extracts of wild-type and SPARC-null mesangial cells and determined the levels of various proteins implicated in control of the cell cycle. Mesangial cells were synchronized in resting media for 48 h before stimulation with PDGF (10 ng/ml). Cell layers were analyzed for levels of cyclin A protein 6, 12, and 30 h after stimulation. SPARC-null mesangial cells showed significantly higher levels of cyclin A after 48 h of serum starvation (73 times more cyclin A signal associated with SPARC-null cells versus wild-type cells at 0 h). They also responded to PDGF more rapidly than wild-type cells, with a maximal response at 6 h (37 times higher cyclin A expression in SPARC-null cells than in wild-type cells at 6 h), compared with 30 h for the wild-type cells (wild-type cells showed three times as much cyclin A expression as the SPARC-null cells at 30 h). This result is consistent with the 3H-thymidine incorporation assays, which showed a higher level of basal proliferation for SPARC-null mesangial cells in resting media in comparison to wild-type cells (Figure 4, A and B). The decrease in cyclin A observed at 30 h was probably due to the confluent state attained by the SPARC-null mesangial cells. Preliminary experiments indicated an increase in the basal levels of cyclin D1 in the SPARC-null versus the wild-type cells; however, this result was not consistent among cell isolates (our unpublished results). We also did not observe consistent differences in the levels of p27 or retinoblastoma protein in SPARC-null versus wild-type cell extracts (our unpublished results). SPARC decreases cell proliferation through an inhibition in the mid–late G1 phase of the cell cycle (Sage and Funk, 1991, 1993). Because cyclin A is known to function over a similar temporal range, specifically at the G1/S boundary, an increase in cyclin A in SPARC-null cells is consistent with the increased rate of proliferation observed in Figures 3–6.
Figure 6.
Basal levels of cyclin A are increased in SPARC-null versus wild-type mesangial cells. Both cell types were plated at equal concentrations and grown in resting media for 48 h before the addition of 10 ng/ml PDGF. Cells were harvested in RIPA buffer with protease inhibitors at 6, 12, and 30 h. Equal amounts of protein were loaded in each lane, separated on a 10% SDS-PAGE gel under reducing conditions, transferred to nitrocellulose, and probed with an anti-cyclin A antibody. Immunoreactive bands were detected by enhanced chemiluminescence. Equal loading of protein was confirmed by staining of the transferred gel with Coomassie blue. Molecular weight markers (kilodaltons × 10−3) are indicated. The identity of the band at Mr 75,000 is unknown.
DISCUSSION
Although SPARC has been shown to be both an inhibitor of proliferation and a modulator of cell shape upon its addition to cells in vitro, these studies have been hampered by the high expression of endogenous SPARC that is characteristic of most cultured cells (Lane and Sage, 1994). The isolation of primary cells (with minimal subculture) from SPARC-null mice has allowed us to characterize the functions of SPARC more rigorously. We have found that skin fibroblasts were not affected morphologically by the absence of SPARC, whereas SPARC-null mesangial cells displayed significant differences in cell shape. In addition, all adult cell types examined to date, specifically skin fibroblasts, mesangial cells, and aortic smooth muscle cells, proliferated faster in the absence of endogenous SPARC. The increase observed in cell division in the SPARC-null cells was sensitive to the addition of rSPARC, which inhibited 3H-thymidine incorporation to a greater degree in SPARC-null versus wild-type cells.
When added to various cultured cells, SPARC induces cell rounding (Sage et al., 1989). We show here that SPARC will promote rounding in skin fibroblasts regardless of endogenous SPARC expression. Therefore, synthesis of SPARC is not required for a response to SPARC; however, skin fibroblasts isolated from SPARC-null animals did not display inherent differences in morphology in comparison to wild-type cells. Conversely, neither SPARC-null nor wild-type mesangial cells rounded significantly in response to rSPARC, although SPARC-null mesangial cells were morphologically distinct from wild-type cells. The addition of rSPARC to SPARC-null cells did not have overt effects on cell shape with regard to cytoskeletal reorganization or changes in the distribution of vinculin-positive focal adhesions; however, in contrast to our results with fibroblasts, rSPARC was not found to be associated with the surface of the SPARC-null mesangial cells, a result indicating that SPARC might need to be expressed from within the cell to influence cell shape in mesangial cells (Bradshaw, personal observation). The altered cytoskeleton and increased focal adhesions in the SPARC-null mesangial cells were reminiscent of cells isolated from mice with targeted deletions of other genes implicated in cell shape and adhesion. For example, fibroblasts from focal adhesion kinase-null backgrounds exhibited more focal adhesions and migrated faster than their wild-type counterparts (Ilic et al., 1995). Recently, Shp-2 tyrosine phosphatase-null fibroblasts have been described with increased focal adhesions and cell–cell contacts in comparison to wild-type cells and similar to SPARC-null mesangial cells (Yu et al., 1998). It is important to point out that both the focal adhesion kinase-null and Shp-2–null cells were primary embryo fibroblasts, and the effects of SPARC on morphology were seen to date only in mesangial cells; however, a possible component of the SPARC signal cascade in these cells could include one of these (or other) proteins involved in the regulation of cell adhesion.
SPARC inhibits proliferation in various cultured cell types and in response to a number of different mitogenic stimuli. For example, SPARC inhibited stimulation of cell division by endothelial cells in response to serum, basic fibroblast growth factor, PDGF, and vascular endothelial growth factor (Funk and Sage, 1991; Hasselaar and Sage, 1992; Raines et al., 1992; Kupprion et al., 1998). Although SPARC binds directly to PDGF and vascular endothelial growth factor and thereby inhibits interaction of these factors with their receptors, no direct interaction of SPARC with basic fibroblast growth factor has been shown. Therefore, SPARC might regulate mitogenic stimulation by at least two different mechanisms: inhibition of growth factor binding to cognate receptor, or interference of a signaling pathway through a specific interaction at the cell surface, or both. Because a receptor/binding partner for SPARC has not yet been identified, SPARC might alternatively act as an antagonist of a signaling pathway via its interference with, for example, an integrin-ligand interaction (Damsky and Werb, 1992).
The fact that three separate primary cell types from SPARC-null mice exhibited an accelerated rate of proliferation implies that SPARC participates in a specific pathway of cell cycle modulation. In fact, recent evidence shows that primary lens epithelial cells also proliferate faster in culture in comparison to wild-type counterparts, a property that could contribute to cataract formation in vivo (Yan, Clark, and Sage, unpublished observations). It is possible that the rounding response induced by SPARC also contributes to the inhibition of cell cycle progression, because it is known that most cells must attach and spread to initiate cell division; however, we do not believe that the effects of SPARC on cell shape are entirely or even substantially responsible for growth inhibition, because mesangial cells exhibited a diminished level of DNA synthesis but did not round significantly in response to rSPARC. In addition, recent data from endothelial cell cultures showed that cell rounding induced by SPARC was sensitive to tyrosine kinase inhibitors, whereas the inhibition of proliferation was not affected by these reagents (Motamed and Sage, 1998). In balance, these two activities of SPARC appear to use distinct pathways.
To characterize the effects of SPARC on proliferation, we investigated different known cell cycle regulatory proteins in the SPARC-null cells. Human vascular smooth muscle cells treated with SPARC or peptides from its C-terminal Ca2+-binding domain exhibited decreased levels of cyclin A (Motamed, personal communication). Consistent with these data, we observed a significant increase in the basal levels of cyclin A in SPARC-null mesangial cells (73 times over that of wild-type). Interestingly, we also found a significant decrease in the levels of TGF-β in SPARC-null compared with wild-type mesangial cells (Francki et al., 1998). SPARC has been shown to stimulate production of TGF-β in rat mesangial cells in vitro and in vivo (Bassuk, Pichler, Rothmier, Pippen, Gordon, Meek, Bradshaw, Lombardi, Strandjord, Reed, Sage, Couser, and Johnson, unpublished observations). TGF-β is known to inhibit cell cycle progression specifically during the G1 phase (Shankland, 1996; Alexandrow and Moses, 1997; Ko et al., 1998), and SPARC has previously been reported to arrest cells at a similar point in the cell cycle (Funk and Sage, 1991). Therefore, a decrease in the level of TGF-β expression could account, at least in part, for the accelerated rates of proliferation exhibited by SPARC-null mesangial cells. That no significant differences in TGF-β expression were detected in skin fibroblasts, however, indicates that the lack of TGF-β expression is unlikely to be the sole reason for the differences in proliferation rates that we saw in all the cell types. We also cannot rule out the possibility that another growth factor(s) may contribute to the effect of SPARC on proliferation.
The fact that the SPARC-null animals are born without overt abnormalities indicates that SPARC is not required for the normal development of most tissues. Because we have not detected differences in basal proliferation rates of embryonic fibroblasts (Bradshaw, unpublished observations), the differences we observed in cultured adult cells are likely to be more representative of a function for SPARC under pathological conditions. For example, in glomerulosclerosis, TGF-β is thought to play a major role in the expansion of ECM components (including collagen type I), which leads to a decline in kidney function. SPARC-null mesangial cells express significantly less collagen type I as well as less TGF-β, relative to wild-type cells (Francki et al., 1998). Moreover, ovarian epithelial cells transfected with SPARC cDNA showed reduced growth rates in culture and decreased formation of tumors in nude mice (Mok et al., 1996). SPARC therefore could titrate or augment the response of various cell types to the plethora of factors released during injury or disease. Along these lines it will be interesting to induce pathological models of injury in SPARC-null mice to determine the responses of specific tissues in the absence of this protein.
ACKNOWLEDGMENTS
We thank Juliet G. Carbon for excellent technical assistance and our colleagues J. Bassuk, S. Funk, M. Gooden, D. Graves, M. Reed, R. Vernon, and Q. Yan for helpful discussions and suggestions. This work was supported by National Institute of Health grants GM-40711, HL-18645, and DK-47459, with additional funding from the Seattle Diabetes Research Council, and by National Institutes of Health grant GM-18705 to A.D.B. Additional support was provided by National Institutes of Health training grant DK-07467 to A.D.B. and by Fr 1223/1-1 from the Deutsche Forschungsgemeinschaft to A.F.
REFERENCES
- Alexandrow MG, Moses HL. Kips off to myc: implications for TGF-β signaling. J Cell Biochem. 1997;66:427–432. [PubMed] [Google Scholar]
- Bassuk JA, Baneyx F, Vernon RB, Funk SE, Sage EH. Expression of biologically active human SPARC in Escherichia coli. Arch Biochem Biophys. 1996;325:8–19. doi: 10.1006/abbi.1996.0002. [DOI] [PubMed] [Google Scholar]
- Bassuk JA, Birkebak T, Rothmier JD, Clark JM, Bradshaw A, Muchowski PJ, Howe CC, Clark JI, Sage EH. Disruption of the Sparc locus in mice alters the differentiation of lenticular epithelial cells and leads to cataract formation. Exp Eye Res. 1999;68:321–331. doi: 10.1006/exer.1998.0608. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Werb Z. Signal transduction by integrin receptors for extracellular matrix: cooperative processing of extracellular matrix information. Curr Opin Cell Biol. 1992;4:772–781. doi: 10.1016/0955-0674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- Delany A, Amling M, Priemel M, Delling G, Howe C, Baron R, Canalis E. Osteonectin null mice develop severe osteopenia. Bone. 1998;23:199S. [Google Scholar]
- Francki, A., Bradshaw, A.D., Bassuk, J.A., Carbon, J.G., Howe, C., and Sage, E.H. (1998). SPARC regulates collagen type I and TGF-β1 expression in mouse mesangial cells. Mol. Biol. Cell 9(suppl), 167a (abstract).
- Francki A, Uciechowski P, Floege J, Von der Ohe J, Resch K, Radeke HH. Autocrine growth regulation of human glomerular mesangial cells is primarily mediated by basic fibroblast growth factor. Am J Pathol. 1995;147:1372–1382. [PMC free article] [PubMed] [Google Scholar]
- Funk SE, Sage EH. The Ca2+-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc Natl Acad Sci USA. 1991;88:2648–2652. doi: 10.1073/pnas.88.7.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk SE, Sage EH. Differential effects of SPARC and cationic SPARC peptides on DNA synthesis by endothelial cells and fibroblasts. J Cell Physiol. 1993;154:53–63. doi: 10.1002/jcp.1041540108. [DOI] [PubMed] [Google Scholar]
- Gilmour DT, Lyon GJ, Carlton MBL, Sanes JR, Cunningham JM, Anderson JR, Hogan BLM, Evans MJ, Colledge WH. Mice deficient for the secreted glycoprotein SPARC/osteonectin/BM40 develop normally but show severe age-onset cataract formation and disruption of the lens. EMBO J. 1998;17:1860–1870. doi: 10.1093/emboj/17.7.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselaar P, Loskutoff DJ, Sawdey M, Sage EH. SPARC induces the expression of type 1 plasminogen activator inhibitor in cultured bovine aortic endothelial cells. J Biol Chem. 1991;266:13178–13184. [PubMed] [Google Scholar]
- Hasselaar P, Sage EH. SPARC antagonizes the effect of basic fibroblast growth factor on the migration of bovine aortic endothelial cells. J Cell Biochem. 1992;49:272–283. doi: 10.1002/jcb.240490310. [DOI] [PubMed] [Google Scholar]
- Ilic D, et al. Reduced motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe LM, Vernon RB, Wu H, Jaenisch R, Sage EH. Type I collagen-deficient mov-13 mice do not retain SPARC in the extracellular matrix: implications for fibroblast function. Dev Dyn. 1996;207:171–183. doi: 10.1002/(SICI)1097-0177(199610)207:2<171::AID-AJA5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Ko TC, Yu W, Sakai T, Sheng H, Shao J, Beauchamp RD, Thompson EA. TGF-β1 effects on proliferation of rat intestinal epithelial cells are due to inhibition of cyclin D1 expression. Oncogene. 1998;16:3445–3454. doi: 10.1038/sj.onc.1201902. [DOI] [PubMed] [Google Scholar]
- Kupprion C, Motamed K, Sage EH. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem. 1998;273:29635–29640. doi: 10.1074/jbc.273.45.29635. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane TF, Sage EH. Functional mapping of SPARC: peptides from two distinct Ca++-binding sites modulate cell shape. J Cell Biol. 1990;111:3065–3076. doi: 10.1083/jcb.111.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994;8:163–173. [PubMed] [Google Scholar]
- Mok SC, Chan WY, Wong KK, Muto MG, Berkowitz RS. SPARC, an extracellular matrix protein with tumor-suppressing activity in human ovarian epithelial cells. Oncogene. 1996;12:1895–1901. [PubMed] [Google Scholar]
- Motamed K, Sage EH. SPARC inhibits endothelial cell adhesion but not proliferation through a tyrosine phosphorylation-dependent pathway. J Cell Biochem. 1998;70:543–552. doi: 10.1002/(sici)1097-4644(19980915)70:4<543::aid-jcb10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Lane TF, Pallero MA, Sage EH. SPARC mediates focal adhesion disassembly in endothelial cells through a follistatin-like region and the Ca2+-binding EF-hand. J Cell Biochem. 1995;57:341–350. doi: 10.1002/jcb.240570218. [DOI] [PubMed] [Google Scholar]
- Norose K, Clark JI, Syed NA, Basu A, Heberkaty ES, Sage EH, Howe CC. SPARC deficiency leads to early onset cataractogenesis. Invest Ophthal Vis Sci. 1998;39:2674–2680. [PubMed] [Google Scholar]
- Otto JJ. Vinculin. Cell Motil Cytoskeleton. 1990;16:1–6. doi: 10.1002/cm.970160102. [DOI] [PubMed] [Google Scholar]
- Pichler RH, et al. SPARC is expressed by mesangial cells in experimental mesangial proliferative nephritis and inhibits platelet-derived-growth-factor-mediated mesangial cell proliferation in vitro. Am J Pathol. 1996;148:1153–1167. [PMC free article] [PubMed] [Google Scholar]
- Raines EW, Lane TF, Iruela-Arispe ML, Ross R, Sage EH. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci USA. 1992;89:1281–1285. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage H, Vernon RB, Funk SE, Everitt EA, Angello J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca2+-dependent binding to the extracellular matrix. J Cell Biol. 1989;109:341–356. doi: 10.1083/jcb.109.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Göhring W, Mann K, Maurer P, Hohenester E, Knauper V, Murphy G, Timpl R. Limited cleavage of extracellular matrix protein BM-40 by matrix metalloproteinases increases its affinity for collagens. J Biol Chem. 1997;272:9237–9243. doi: 10.1074/jbc.272.14.9237. [DOI] [PubMed] [Google Scholar]
- Shankland SJ. Cell-cycle control and renal disease. Kidney Intl. 1996;52:294–308. doi: 10.1038/ki.1997.335. [DOI] [PubMed] [Google Scholar]
- Tremble PM, Lane TF, Sage EH, Werb Z. SPARC, a secreted protein associated with morphogenesis and tissue remodeling, induces expression of metalloproteinases in fibroblasts through a novel extracellular matrix-dependent pathway. J Cell Biol. 1993;121:1433–1444. doi: 10.1083/jcb.121.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D-H, Qu C-K, Henegariu O, Lu X, Feng G-S. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]