Abstract
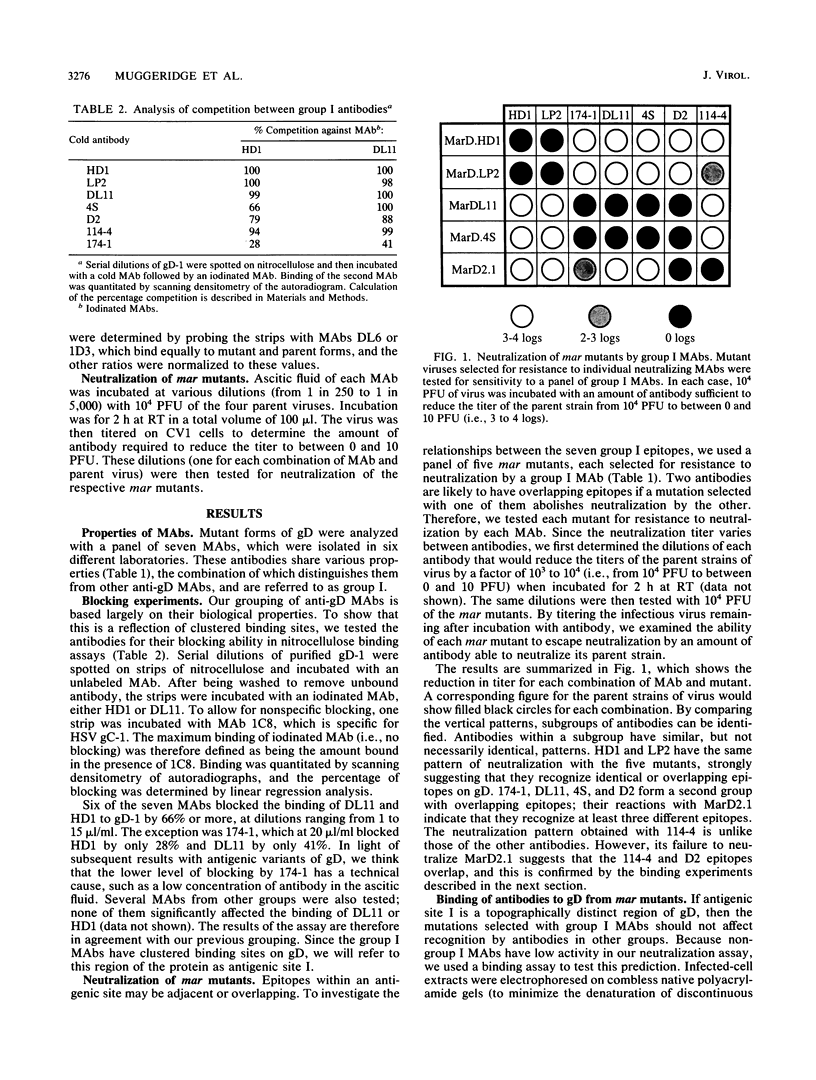
Herpes simplex virus glycoprotein D is a component of the virion envelope and appears to be involved in attachment, penetration, and cell fusion. Monoclonal antibodies against this protein can be arranged in groups on the basis of a number of biological and biochemical properties. Group I antibodies are type common, have high complement-independent neutralization titers, and recognize discontinuous (conformational) epitopes; they are currently being used in several laboratories to study the functions of glycoprotein D. We have used a panel of neutralization-resistant mutants to examine the relationships between these antibodies in detail. We found that they can be divided into two subgroups, Ia and Ib, such that mutations selected with Ia antibodies have little or no effect on binding and neutralization by Ib antibodies and vice versa. In addition, Ia antibodies are able to bind deletion and truncation mutants of glycoprotein D that Ib antibodies do not recognize, suggesting that their epitopes are physically distinct. However, with one exception, Ia and Ib antibodies block each other strongly in binding assays with purified glycoprotein D, whereas antibodies from other groups have no effect. We have therefore defined the sum of the Ia and Ib epitopes as antigenic site 1.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman P. W., Gregory T., Crase D., Lasky L. A. Protection from genital herpes simplex virus type 2 infection by vaccination with cloned type 1 glycoprotein D. Science. 1985 Mar 22;227(4693):1490–1492. doi: 10.1126/science.2983428. [DOI] [PubMed] [Google Scholar]
- Caton A. J., Brownlee G. G., Yewdell J. W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982 Dec;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Chan W. L. Protective immunization of mice with specific HSV-1 glycoproteins. Immunology. 1983 Jun;49(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Dietzschold B., Ponce de Leon M., Long D., Golub E., Varrichio A., Pereira L., Eisenberg R. J. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984 Jan;49(1):102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Isola V. J., Kuhns J., Berman P. W., Eisenberg R. J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing ("native" gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986 Oct;60(1):157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Wilcox W. C., Sodora D. L., Long D., Levin J. Z., Eisenberg R. J. Expression of herpes simplex virus type 1 glycoprotein D deletion mutants in mammalian cells. J Virol. 1988 Jun;62(6):1932–1940. doi: 10.1128/jvi.62.6.1932-1940.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer K. J., Mackett M., Wohlenberg C., Notkins A. L., Moss B. Vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D prevents latent herpes in mice. Science. 1985 May 10;228(4700):737–740. doi: 10.1126/science.2986288. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Eisenberg R. J., Ponce de Leon M., Golub E., Hudecz F., Varrichio A., Cohen G. H. Fine structure analysis of type-specific and type-common antigenic sites of herpes simplex virus glycoprotein D. J Virol. 1984 Nov;52(2):431–435. doi: 10.1128/jvi.52.2.431-435.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix R. D., Pereira L., Baringer J. R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981 Oct;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Cerini C. P., Heilman C. J., Joseph A. D., Dietzschold B., Golub E., Long D., Ponce de Leon M., Cohen G. H. Synthetic glycoprotein D-related peptides protect mice against herpes simplex virus challenge. J Virol. 1985 Dec;56(3):1014–1017. doi: 10.1128/jvi.56.3.1014-1017.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Pereira L., Hampar B., Zweig M., Cohen G. H. Effect of monoclonal antibodies on limited proteolysis of native glycoprotein gD of herpes simplex virus type 1. J Virol. 1982 Feb;41(2):478–488. doi: 10.1128/jvi.41.2.478-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Ponce de Leon M., Matthews J. T., Spear P. G., Gibson M. G., Lasky L. A., Berman P., Golub E., Cohen G. H. Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J Virol. 1985 Feb;53(2):634–644. doi: 10.1128/jvi.53.2.634-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Pereira L., Long D., Cohen G. H. Purification of glycoprotein gD of herpes simplex virus types 1 and 2 by use of monoclonal antibody. J Virol. 1982 Mar;41(3):1099–1104. doi: 10.1128/jvi.41.3.1099-1104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard W., Webster R. G. Antigenic drift in influenza A viruses. I. Selection and characterization of antigenic variants of A/PR/8/34 (HON1) influenza virus with monoclonal antibodies. J Exp Med. 1978 Aug 1;148(2):383–392. doi: 10.1084/jem.148.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard W., Yewdell J., Frankel M. E., Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981 Apr 23;290(5808):713–717. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber-Katz E., Hollosi M., Dietzschold B., Hudecz F., Fasman G. D. The T cell response to the glycoprotein D of the herpes simplex virus: the significance of antigen conformation. J Immunol. 1985 Aug;135(2):1385–1390. [PubMed] [Google Scholar]
- Highlander S. L., Sutherland S. L., Gage P. J., Johnson D. C., Levine M., Glorioso J. C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987 Nov;61(11):3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Marlin S. D., Levine M., Glorioso J. Antigenic variants of herpes simplex virus selected with glycoprotein-specific monoclonal antibodies. J Virol. 1983 Feb;45(2):672–682. doi: 10.1128/jvi.45.2.672-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümel G., Kaerner H. C., Levine M., Schröder C. H., Glorioso J. C. Passive immune protection by herpes simplex virus-specific monoclonal antibodies and monoclonal antibody-resistant mutants altered in pathogenicity. J Virol. 1985 Dec;56(3):930–937. doi: 10.1128/jvi.56.3.930-937.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D., Madara T. J., Ponce de Leon M., Cohen G. H., Montgomery P. C., Eisenberg R. J. Glycoprotein D protects mice against lethal challenge with herpes simplex virus types 1 and 2. Infect Immun. 1984 Feb;43(2):761–764. doi: 10.1128/iai.43.2.761-764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Highlander S. L., Holland T. C., Levine M., Glorioso J. C. Antigenic variation (mar mutations) in herpes simplex virus glycoprotein B can induce temperature-dependent alterations in gB processing and virus production. J Virol. 1986 Jul;59(1):142–153. doi: 10.1128/jvi.59.1.142-153.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin S. D., Holland T. C., Levine M., Glorioso J. C. Epitopes of herpes simplex virus type 1 glycoprotein gC are clustered in two distinct antigenic sites. J Virol. 1985 Jan;53(1):128–136. doi: 10.1128/jvi.53.1.128-136.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Moss B., Berman P. W., Laskey L. A., Rouse B. T. Mechanisms of antiviral immunity induced by a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: cytotoxic T cells. J Virol. 1987 Mar;61(3):726–734. doi: 10.1128/jvi.61.3.726-734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson A. C., Hodgman T. C., Digard P., Hancock D. C., Bell S. E., Buckmaster E. A. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J Gen Virol. 1986 Jun;67(Pt 6):1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- Noble A. G., Lee G. T., Sprague R., Parish M. L., Spear P. G. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology. 1983 Aug;129(1):218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]
- Norrild B., Bjerrum O. J., Ludwig H., Vestergaard B. F. Analysis of herpes simplex virus type 1 antigens exposed on the surface of infected tissue culture cells. Virology. 1978 Jun 15;87(2):307–316. doi: 10.1016/0042-6822(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R., Samsonoff C., Mercer S., Panicali D. Construction of live vaccines using genetically engineered poxviruses: biological activity of vaccinia virus recombinants expressing the hepatitis B virus surface antigen and the herpes simplex virus glycoprotein D. Proc Natl Acad Sci U S A. 1984 Jan;81(1):193–197. doi: 10.1073/pnas.81.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Parish M. L., Noble A. G., Spear P. G. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J Virol. 1985 Aug;55(2):483–488. doi: 10.1128/jvi.55.2.483-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter S. D., Zweig M., Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981 Dec;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley M. L., Hoxie J. A., Friedman H. M. Herpes simplex virus type 1 infection of endothelial, epithelial, and fibroblast cells induces a receptor for C3b. J Immunol. 1985 Apr;134(4):2673–2678. [PubMed] [Google Scholar]
- Stannard L. M., Fuller A. O., Spear P. G. Herpes simplex virus glycoproteins associated with different morphological entities projecting from the virion envelope. J Gen Virol. 1987 Mar;68(Pt 3):715–725. doi: 10.1099/0022-1317-68-3-715. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Lasky L. A., Moss B. Herpes simplex virus (HSV)-specific human T-cell clones recognize HSV glycoprotein D expressed by a recombinant vaccinia virus. J Virol. 1986 Aug;59(2):506–509. doi: 10.1128/jvi.59.2.506-509.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]






