Abstract
Differentiating 3T3-L1 cells exhibit a dramatic increase in the rate of insulin-stimulated glucose transport during their conversion from proliferating fibroblasts to nonproliferating adipocytes. On day 3 of 3T3-L1 cell differentiation, basal glucose transport and cell surface transferrin binding are markedly diminished. This occurs concomitant with the formation of a distinct insulin-responsive vesicular pool of intracellular glucose transporter 1 (GLUT1) and transferrin receptors as assessed by sucrose velocity gradients. The intracellular distribution of the insulin-responsive aminopeptidase is first readily detectable on day 3, and its gradient profile and response to insulin at this time are identical to that of GLUT1. With further time of differentiation, GLUT4 is expressed and targeted to the same insulin-responsive vesicles as the other three proteins. Our data are consistent with the notion that a distinct insulin-sensitive vesicular cargo compartment forms early during fat call differentiation and its formation precedes GLUT4 expression. The development of this compartment may result from the differentiation-dependent inhibition of constitutive GLUT1 and transferrin receptor trafficking such that there is a large increase in, or the new formation of, a population of postendosomal, insulin-responsive vesicles.
INTRODUCTION
The insulin-stimulated glucose transport that regulates postprandial blood glucose levels occurs principally as a result of the insulin-dependent translocation of glucose transporters from an intracellular storage pool to the cell surface (for review, see Kandror and Pilch, 1996a; Rea and James, 1997). The tissue-specific glucose transporter isoform glucose transporter 4 (GLUT4)1 (Kandror and Pilch, 1996a; Rea and James, 1997) is responsible for most of the transport function in fat and muscle, but the ubiquitous GLUT1 glucose transporter isoform is expressed to an appreciable extent in adipocytes, where it also shows insulin-dependent translocation to the cell surface (Zorzano et al., 1989; Holman et al., 1990). Despite extensive study (for review, see Kandror and Pilch, 1996a; Rea and James, 1997), much remains unknown about the cellular trafficking pathway of GLUT4, including its biochemical basis and relationship to other established cellular pathways of protein traffic and secretion. In particular, it is not yet clear whether the GLUT4-containing compartment represents a specialized secretory organelle, analogous to synaptic vesicles in the brain and unique to fat and muscle, or whether, during the process of differentiation, insulin sensitivity has been applied to a preexisting recycling pathway(s), such as endosome to cell surface recycling, which is present in nondifferentiated precursors.
Morphological studies of GLUT4 distribution in a variety of insulin-sensitive tissues have used immunogold electron microscopy to determine that intracellular GLUT4 is localized principally in small uniform vesicles near the cell surface, as well as in small tubulovesicular elements. These tissues include delipidated brown fat (Slot et al., 1991), white fat (Smith et al., 1991) and skeletal muscle (Wang et al., 1996; Ploug et al., 1998). We have also determined, on the basis of sucrose gradient sedimentation analysis, that GLUT4-containing vesicles from skeletal muscle and fat are mainly of a small uniform size (Kandror et al., 1995a). Morphologically, the GLUT4-containing vesicles resemble those of the endosomal pathway, such as is seen for the localization of the asialoglycoprotein receptor in liver (Geuze et al., 1982), a classical endosomal trafficking protein. However, there is mixed evidence for the colocalization of GLUT4 with endosomal markers present in fibroblasts and fat cells, such as the transferrin receptor (TfR).
In transfection studies involving ectopic expression of GLUT4 in 3T3-L1 fibroblasts, some colocalization of transfected GLUT4 with TfRs was observed (Hudson et al., 1992). On the other hand, transfected GLUT4 and endogenous TfRs segregate immediately upon endocytosis in Chinese hamster ovary (CHO) cells (Wei et al., 1998). In cultured murine fat cells, we (this paper) and others (Tanner and Lienhard, 1989) show that a portion of the TfR traffics very similarly to glucose transporters, whereas Martin et al. (1996) have found evidence for segregation of GLUT4 from TfRs in 3T3-L1 cells. Likewise, in rat fat cells studied by means of transporter-specific immunoadsorption, we have reported that 50% of the TfR colocalizes with GLUT4 (Kandror and Pilch, 1998). Malide et al. (1997a) do not observe this colocalization when using confocal microscopy procedures.
To gain further information concerning the nature of GLUT4 trafficking, we and others have determined the identity of a number of proteins colocalized in GLUT4-containing vesicles. As expected, proteins that are believed to constitute part of the membrane fusion machinery (Rothman and Söllner, 1997) required for vesicular trafficking are present in GLUT4 vesicles. These include members of the vesicle-associated membrane protein/cellubrevin family (Cain et al., 1992; Volchuk et al., 1995; Cheatham et al., 1996; Timmers et al., 1996) and secretory carrier-associated membrane proteins (Laurie et al., 1993; Thoidis et al., 1993). Two abundant GLUT4 vesicular proteins, visible by silver staining of immunoisolated membranes, have been identified by microsequencing and cloning. One is a novel aminopeptidase (insulin-responsive aminopeptidase [IRAP]) (Kandror et al., 1994; Keller et al., 1995), and the other is a type of sorting receptor, sortilin, of unknown physiological function (Lin et al., 1997; Morris et al., 1998) that is related to the receptor for mannose-6-phosphate. A small proportion (10–15%) of the latter protein (Kandror and Pilch, 1996b) is also colocalized with GLUT4 in the basal state in rat adipocytes, and this population appears to cycle to and from the cell surface along with GLUT4 (Kandror and Pilch, 1996b, 1998). A very important finding regarding the constituent vesicle proteins is that IRAP traffics in an insulin-sensitive manner indistinguishable from GLUT4, and this, along with the data for the three receptors noted above, suggests the possibility that the GLUT4-containing compartment has multiple cargo components, and that the presence or absence of one or more components may not be critical for its insulin-dependent response. To address this possibility, we examined the development of insulin-responsive vesicular trafficking as a function of 3T3-L1 fat cell differentiation in cell culture.
Green and Kehinde (1975) first demonstrated that confluent 3T3-L1 cells could be hormonally induced to differentiate into adipocytes over the course of 6–9 d. The differentiation process is characterized by cell rounding and accumulation of large lipid droplets and biochemically by the expression of various enzymes of lipid metabolism (for review, see Brun et al., 1996; Gregoire et al., 1998). Importantly, differentiation of 3T3-L1 cells also leads to a dramatic increase in the expression of insulin receptors (Reed et al., 1977; Rubin et al., 1978), GLUT4 (Garcia de Herreros and Birnbaum, 1989), and insulin receptor substrate 1 (IRS-1) (Rice and Garner, 1994), and the cells markedly increase their insulin-sensitive glucose uptake. In differentiated fat cells, insulin stimulates the rate of hexose transport 5- to 10-fold (see Figures 1 and 8), whereas their fibroblast precursors respond weakly (∼1.5-fold) (Resh, 1982; Garcia de Herreros and Birnbaum, 1989; Weiland et al., 1990; Yang et al., 1992). Although the proteins noted just above are necessary for the maximal insulin response, we demonstrate here that the onset of insulin-sensitive glucose uptake is a result of major changes, early in the fat cell differentiation program, in the physical and biochemical characteristics of intracellular vesicles containing glucose transporters, TfRs, and IRAP. Our data are consistent with the notion that the development of this compartment results from the expression of as yet unknown genes, and it precedes and may be independent of expression of its major cargo protein, GLUT4.
Figure 1.
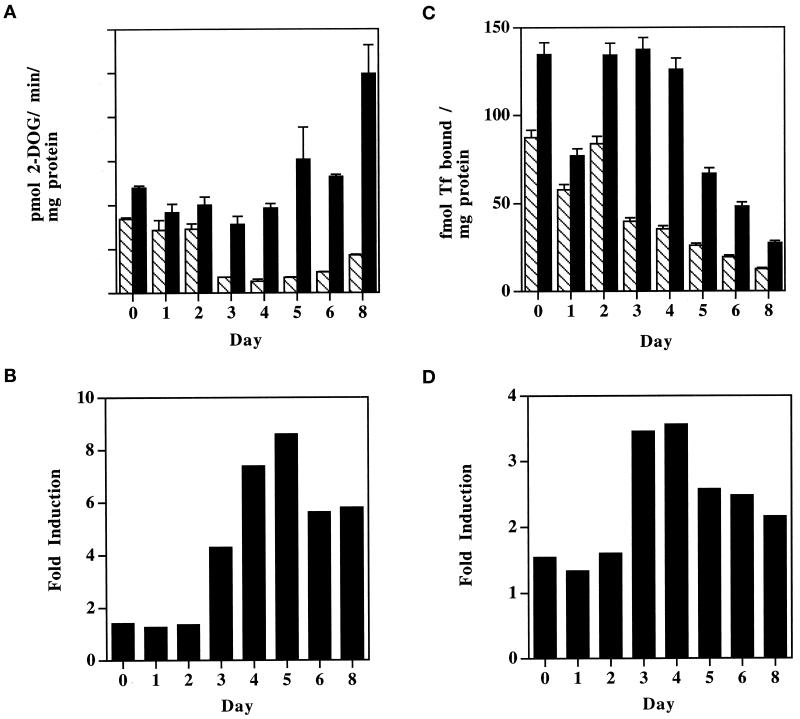
Insulin responsiveness of both 2-deoxyglucose uptake and cell surface transferrin binding by 3T3-L1 cells increases dramatically between days 2 and 3 of differentiation. On the indicated days after the induction of differentiation, cells were serum starved for 2 h before treatment with (black bars) or without (striped bars) 100 nM insulin for 15 min at 37°C. 3H-2-deoxyglucose (2-DOG) uptake (A) and cell surface 125I-transferrin (Tf) binding (C) were then determined as described in MATERIALS AND METHODS. Each bar and error bar represent the average and difference between duplicate determinations, respectively. The fold stimulation of 2-deoxyglucose uptake (B) and transferrin binding (D) was then calculated by dividing the average of the insulin-stimulated condition by that of the basal for each time point. These data are representative of three independent experiments.
Figure 8.
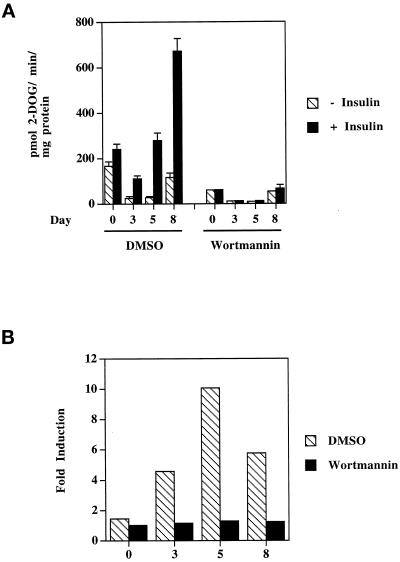
Wortmannin completely abolishes insulin stimulation of 2-deoxyglucose uptake by differentiating as well as by mature 3T3-L1 adipocytes. On the indicated days after the induction of differentiation, cells were serum starved for 2 h, treated or not with 1 μM wortmannin for 30 min, and stimulated or not with 100 nM insulin for 15 min at 37°C. 3H-2-Deoxyglucose uptake was then measured as described in MATERIALS AND METHODS. (A) Rates of 2-deoxyglucose (2-DOG) transport, with each bar and error bar representing the average and difference between duplicate determinations, respectively. (B) Fold stimulation, which was calculated by dividing the average value of the insulin-stimulated condition by that of the basal for each time point. These data are representative of three independent experiments.
MATERIALS AND METHODS
Materials
Dexamethasone, 3-isobutyl-1-methylxanthine, insulin, benzamidine, wortmannin, and digitonin were purchased from Sigma (St. Louis, MO). Aprotinin, leupeptin, pepstatin A, and PMSF were obtained from American Bioanalytical (Natick, MA). Fetal bovine and calf sera were purchased from Life Technologies (Gaithersburg, MD) and Dulbecco’s modified Eagle’s medium (DMEM) was from BioWhittaker (Walkersville, MD). 3H-2-Deoxyglucose and 125I-transferrin were purchased from New England Nuclear (Boston, MA).
Antibodies
In the present study, we used the monoclonal anti-GLUT4 antibody 1F8 (James et al., 1988), a goat polyclonal anti-GLUT4 antibody (a kind gift from Dr. Morris J. Birnbaum, Howard Hughes Medical Institute, University of Pennsylvania, Philadelphia, PA), a polyclonal antibody against GLUT1 (a kind gift from Dr. C. Carter-Su, University of Michigan, Ann Arbor, MI), monoclonal anti-TfR antibody H68 (Zymed Laboratories, South San Francisco, CA), monoclonal anti-caveolin-1 antibody (Transduction Laboratories, Lexington, KY), and anti-IRAP serum (Kandror and Pilch, 1994).
Cell Culture
Murine 3T3-L1 preadipocytes were cultured, maintained, and differentiated as described previously (Stephens et al., 1997). Briefly, cells were plated and grown for 2 d after confluence in DMEM supplemented with 10% calf serum. Differentiation was then induced (day 0) by changing the medium to DMEM containing 10% fetal bovine serum, 0.5 mM 3-isobutyl-1-methylxanthine, 1 μM dexamethasone, and 1.7 μM insulin. After 48 h, the differentiation medium was replaced with maintenance medium containing DMEM supplemented with 10% fetal bovine serum. The maintenance medium was changed every 48 h until the cells were used for experimentation.
3H-2-Deoxyglucose Uptake
This assay was performed in 3.5-cm dishes as previously described (Stephens et al., 1997). Briefly, the monolayers were washed twice with serum-free DMEM and deprived of serum for 2 h. Medium was replaced with DMEM containing either 100 nM insulin or carrier (1 mM HCl, 100× dilution), and cells were placed at 37°C for 15 min. Each well was then washed twice with 2 ml of Krebs-Ringer-HEPES (KRH) buffer (121 mM NaCl, 4.9 mM KCl, 1.2 mM MgS04, 0.33 mM CaCl2, 12 mM HEPES, pH 7.4) at 22°C. The assay was carried out in 1 ml KRH buffer per 3.5-cm well for 15 min at 22°C. The concentration of 2-deoxyglucose was 0.1 mM with 1 μCi of 3H-2-deoxyglucose/ml. The transport assay was terminated by aspirating the radioactive mixture and washing the monolayer three times with 2 ml of ice-cold KRH containing 25 mM d-glucose. Each monolayer was then solubilized in 1 ml of a buffered digitonin solution, and a 0.3-ml aliquot was removed for determination of radioactivity by liquid scintillation counting. Under these conditions, hexose uptake was linear for at least 30 min. Measurements were made in duplicate and corrected for specific activity and nonspecific diffusion (as determined in the presence of 5 μM cytochalasin B), which was <10% of the total uptake. The protein concentration was determined using the Bio-Rad (Hercules, CA) protein assay kit and was used to normalize counts.
When indicated, serum-starved cells were incubated for 30 min (37°C) in DMEM containing 1 μM wortmannin (Sigma) or carrier (DMSO; 1000× dilution). Wortmannin (or DMSO) was also included during incubation with insulin (or carrier).
Cell Surface 125I-Transferrin Binding
This assay was based on the method described previously (Tanner and Lienhard, 1987). At the indicated times, cell monolayers in 3.5-cm dishes were washed twice with serum-free DMEM and serum starved for 2 h. Cells were then washed with three 1-ml aliquots of Krebs-Ringer-phosphate (KRP; 12.5 mM HEPES, 120 mM NaCl, 6 mM KCl, 1.2 mM MgSO4, 1 mM CaCl2, 1 mM Na2HPO4, pH 7.4) buffer at 37°C followed by addition of 2 ml KRP containing either 100 nM insulin or carrier (1 mM HCl; 100× dilution). After a 15-min incubation at 37°C, dishes were placed on ice, and each well was washed immediately with three 1-ml aliquots of ice-cold KRP. Each monolayer was then incubated for 2 h at 4°C with 1 ml of 0.945 nM 125I-transferrin (65,000–80,000 cpm/well) in KRP containing 1 mg/ml BSA (Tanner and Lienhard, 1987). Unbound ligand was subsequently aspirated, and wells were washed (1 min/wash) with three 1-ml aliquots of ice-cold KRP. Each monolayer was then solubilized in 1 ml of 1 N NaOH, and the radioactivity was counted in an LKB (Piscataway, NJ) gamma counter. Nonspecific binding, determined by including 1 μM unlabeled diferric transferrin in the radioactive mixture, was subtracted from the total binding to determine receptor-specific binding. All data have been normalized to the protein concentration (Bio-Rad kit).
Preparation of Postnuclear Membranes
At the indicated times, 3T3-L1 cells grown in 10-cm dishes were rinsed with 37°C buffer A (250 mM sucrose, 20 mM HEPES, 1 mM EDTA, pH 7.4, 5 μM aprotinin, 10 μM leupeptin, 5 μM pepstatin, 5 mM benzamidine, and 1 mM PMSF) and then harvested in 2 ml of ice-cold buffer A. Cells were then homogenized using a Potter-Elvehjem Teflon pestle, and the homogenate was centrifuged for 20 min at 3000 × g. The membranes in the resulting supernatant were collected by centrifugation at 250,000 × g for 90 min and were resuspended in buffer A containing 1% SDS. Samples were stored at −80°C until ready to be analyzed. Protein content was determined using a bicinchoninic acid (BCA) kit (Pierce Chemical, Rockford, IL).
Subcellular Fractionation of 3T3-L1 Cells
Sixteen 10-cm dishes were used per condition. Before harvesting, cells were washed twice with 37°C serum-free DMEM and then serum starved for 2 h. Insulin (100 nM final concentration) or carrier (1 mM HCl, 100× dilution) was added for 30 min at 37°C. KCN was then added to a final concentration of 2 mM, and cells were left for 5 min at room temperature. Cells were washed twice with 37°C buffer A, harvested in ice-cold buffer A, and homogenized using a Potter-Elvehjem Teflon pestle. Total membranes were pelleted at 250,000 × g for 90 min, resuspended in buffer B (20 mM HEPES, 1 mM EDTA, pH 7.4) containing the same standard mixture of protease inhibitors present in buffer A, and fractionated by differential centrifugation into plasma membrane, heavy microsomes, light microsomes, and a nuclear and mitochondrial fraction as previously described (Stephens et al., 1997). These fractions were resuspended in buffer B, and protein content was determined using a BCA kit (Pierce).
Sedimentation of Light Microsomes in Sucrose Velocity Gradients
Light microsomes, resuspended in buffer B, were loaded onto a 4.6-ml 10–30% (wt/vol) continuous sucrose gradient and centrifuged at 48,000 rpm in a Beckman Instruments (Palo Alto, CA) SW-50.1 rotor for 55 min at 4°C. The sucrose gradients were prepared in a buffered solution composed of 20 mM HEPES, pH 7.4, 100 mM NaCl, and 1 mM EDTA. Membranes from the gradients were collected in 33–34 fractions starting from the bottom of the tubes. The protein profile was determined using the BCA kit (Pierce), and the linearity of the gradients was confirmed by measuring the refractive index of fractions. The position of the TfR, GLUT1, GLUT4, and IRAP was determined by Western blot analysis.
Immunoadsorption of GLUT4-containing Vesicles
Protein A–purified 1F8 antibody as well as nonspecific mouse immunoglobulin G (IgG, Sigma) were each coupled to acrylic beads (Reacti-Gel GF 2000, Pierce) at a concentration of 0.8–1.1 mg of antibody/ml of resin according to the manufacturer’s instructions. Before use, the antibody-coupled beads were saturated with 1% BSA in PBS for 1 h at room temperature, followed by three washes in cold (4°C) PBS. Light microsomes (in PBS, 200 μg) from basal and insulin-treated 3T3-L1 adipocytes were incubated with 5, 10, 20, and 40 μl of 1F8-coupled beads or with 40 ml of nonspecific antibody-coupled beads overnight at 4°C with mixing. The beads were washed three times with cold PBS, and the adsorbed material was subsequently eluted with 1% Triton X-100 in PBS and nonreducing Laemmli sample buffer. Before the second elution, the beads were washed three times with 1% Triton X-100 in cold PBS.
Gel Electrophoresis and Immunoblotting
Proteins were separated in SDS-polyacrylamide (acrylamide from National Diagnostics, Atlanta, GA) gels as described by Laemmli (1970) and transferred to a polyvinylidene difluoride membrane (Bio-Rad) in 25 mM Tris and 192 mM glycine. After transfer, the membrane was blocked with 10% nonfat dry milk in PBS for 1 h at room temperature. After incubation with the primary antibodies specified above, HRP-conjugated secondary antibodies (Sigma) and an enhanced chemiluminescent substrate kit (Amersham, Buckinghamshire, England) were used for detection.
Oil Red O Staining
Oil Red O staining was performed following the procedure described by Green and Kehinde (1975) with minor modifications. Briefly, cells were washed twice with PBS and fixed with 10% formaldehyde in PBS for 15 min. Cells were then stored at room temperature in distilled water containing 0.02% sodium azide until ready to be stained. Staining was carried out for 1 h in freshly diluted Oil Red O solution (six parts Oil Red O stock solution and four parts water; Oil Red O stock solution is 0.5% Oil Red O in isopropanol). This stain was then removed, and the cells were washed five times with water. The cells were then photographed using phase-contrast microscopy.
Quantification of Proteins after Western Blotting
Autoradiographs were scanned in a computing densitometer (Molecular Dynamics, Sunnyvale, CA) and graphed as arbitrary units.
RESULTS
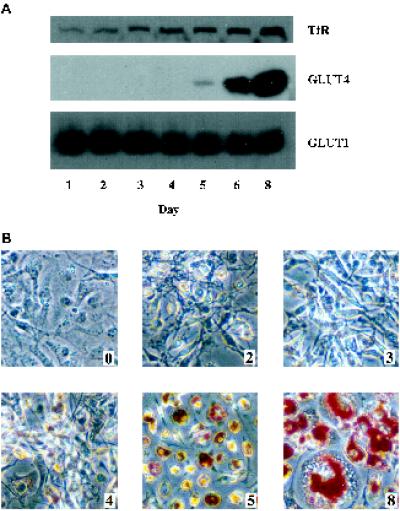
Basal and insulin-treated 3T3-L1 cells were assayed for 3H-2-deoxyglucose uptake (Figure 1A) and the number of cell surface TfRs was determined by equilibrium binding of 125I-transferrin (Figure 1C). On day 3 of differentiation, we see a dramatic decrease in basal glucose transport and transferrin binding, which accounts for the sudden increase in insulin responsiveness (Figure 1, B and D) at this time. The magnitude of insulin-stimulated 2-deoxyglucose transport is essentially constant between days 0 and 4 but increases from day 5 onward approximately proportionally to GLUT4 protein expression (Figure 2A). The number of cell surface TfRs in the insulin-stimulated state is also relatively constant between days 0 and 4 of the differentiation program. The decreased level of transferrin binding on day 1 is a variable result, which happens to be present in the representative experiment shown. Interestingly, there is a gradual decrease in cell surface TfRs after day 5, despite an increasing level of expression (Figure 2A). The accumulation of lipid droplets that occurs during the differentiation of 3T3-L1 cells into adipocytes is demonstrated in Figure 2B by Oil Red O staining of cells on the indicated days after the induction of differentiation. Significant cell rounding and lipid accumulation are first evident on day 5, when GLUT4 expression is first noted, and increase substantially thereafter, in correlation with GLUT4 expression (Figure 2A).
Figure 2.
Oil Red O staining of 3T3-L1 cells during differentiation and the time course of GLUT1, GLUT4, and TfR expression. (A) On the indicated days after the induction of differentiation, postnuclear membranes were prepared from 3T3-L1 cells as described in MATERIALS AND METHODS. Equal amounts of protein were electrophoresed and Western blotted for the indicated proteins. HRP-conjugated secondary antibodies and a chemiluminescent substrate kit were used for detection. These data are representative of three independent experiments. (B) On the indicated days after the induction of differentiation, cells were fixed and then stained with Oil Red O as described in MATERIALS AND METHODS. Shown are micrographs taken using phase contrast.
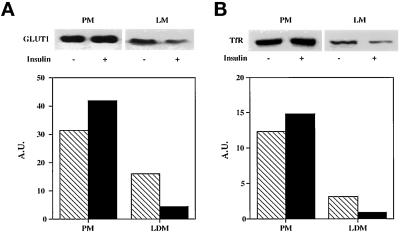
Most importantly, on day 3 when the cells first become highly insulin-responsive, GLUT4 is not yet expressed, and the cells show a fibroblastic morphology with minimal lipid accumulation (Figure 2B). This leaves GLUT1, whose expression is relatively constant during differentiation (Figure 2A), as the likely mediator of facilitative glucose transport. Subcellular fractionation of basal and insulin-treated 3T3-L1 cells on day 3 reveals insulin-dependent translocation of GLUT1 (Figure 3A) and TfR (Figure 3B) before GLUT4 expression, suggesting that this is the mechanism underlying insulin-stimulated 2-deoxyglucose uptake and cell surface transferrin binding at that time. The results shown in Figure 3 are reminiscent of previous studies showing GLUT4 (Clancy and Czech, 1990; Stephens et al., 1997), GLUT1 (Chakrabarti et al., 1994), and TfR (Tanner and Lienhard, 1989) translocation in differentiated 3T3-L1 cells. The insulin-sensitive depletion of GLUT1 and TfR from the intracellular pool is clearly evident in Figure 3, concomitant with their increase in the crude plasma membrane fraction, although this increase is not striking, as was previously noted for GLUT4 (Clancy and Czech, 1990; Stephens et al., 1997), GLUT1 (Chakrabarti et al., 1994), and the TfR (Tanner and Lienhard, 1989).
Figure 3.
Insulin stimulates translocation of GLUT1 and TfR from an intracellular pool to the cell surface on day 3 of 3T3-L1 differentiation. Seventy-two hours after the induction of differentiation, 3T3-L1 cells were serum starved for 2 h and treated with (black bars) or without (hatched bars) 100 nM insulin for 30 min. Subcellular fractions were prepared as described in MATERIALS AND METHODS. Equal protein from the indicated fractions was electrophoresed and Western blotted for GLUT1 (A) and TfR (B). HRP-conjugated secondary antibodies and chemiluminescence were used for detection of the blots shown in the upper panels. The data were scanned using a computing densitometer and displayed as arbitrary units (A.U.) in the lower panels. PM, plasma membranes; LM, light microsomes. Shown are representative blots from three independent experiments.
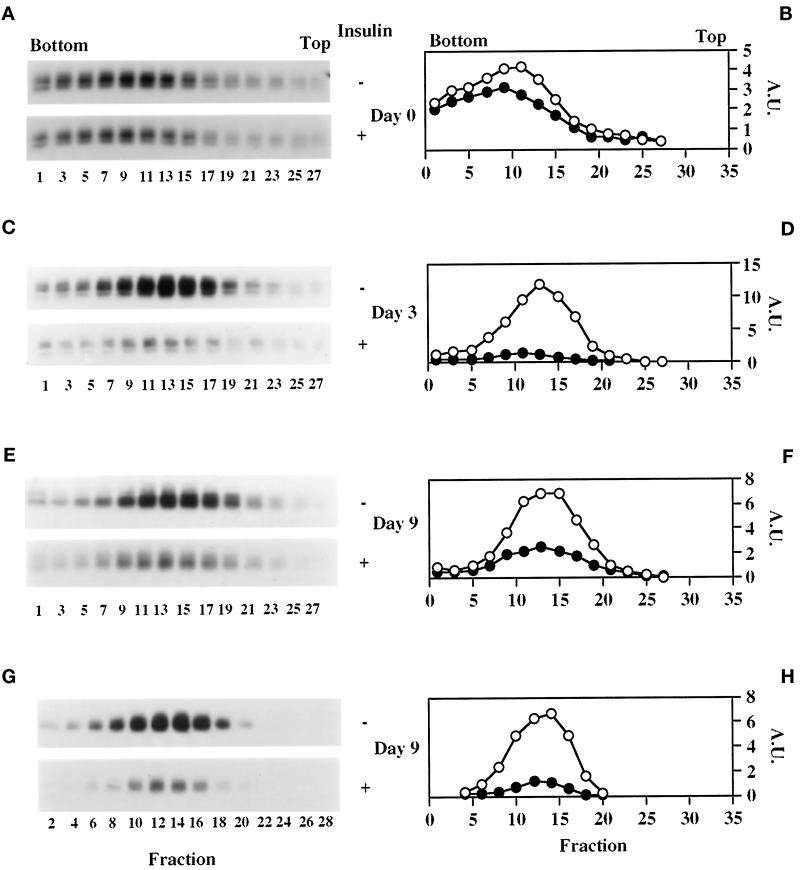
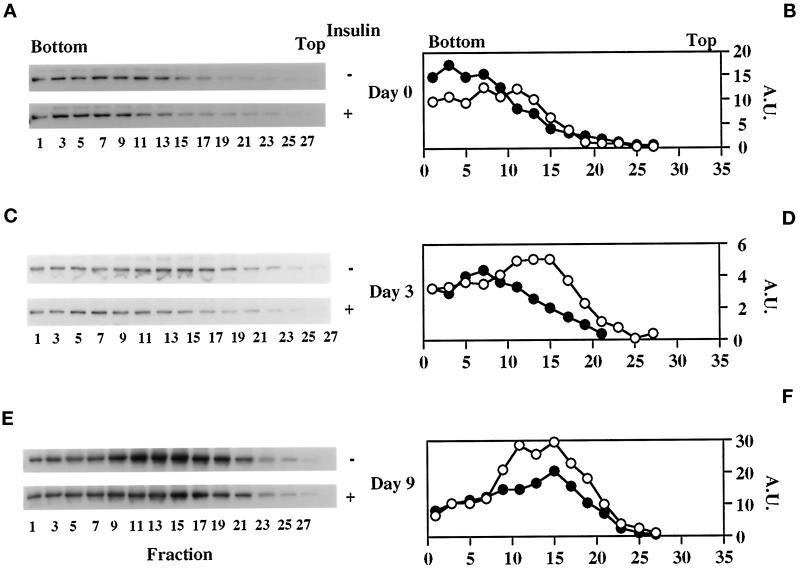
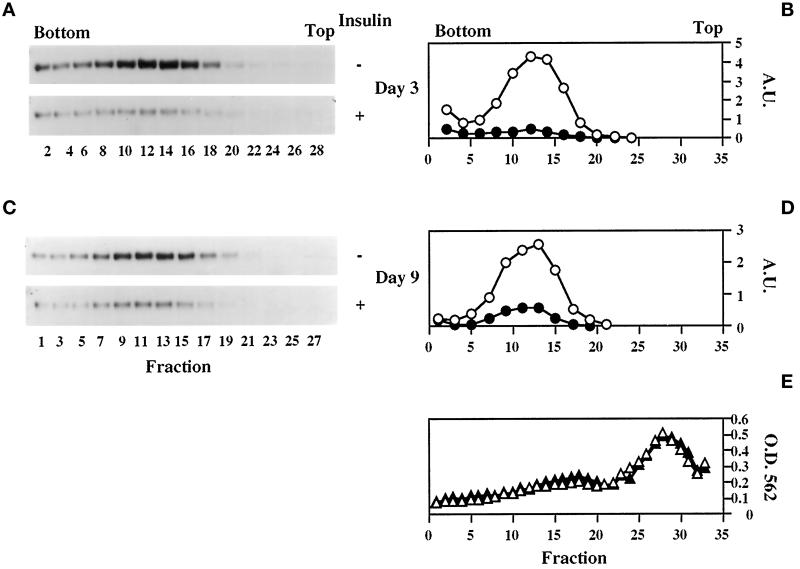
The acquisition of insulin-responsive 2-deoxyglucose transport and transferrin binding between days 2 and 3 is characterized by a coordinate decrease in both parameters under basal conditions (Figure 1, A and C) despite steady GLUT1 and increasing TfR expression (Figure 2A). This suggests that intracellular sequestration of these proteins is taking place during 3T3-L1 differentiation as has previously been demonstrated for GLUT1 (Yang et al., 1992) and, more recently, for IRAP (Ross et al., 1998). These previous studies, however, focused solely on cell surface versus total protein. Therefore, to characterize the nature of the GLUT1-, TfR-, and IRAP-containing intracellular membranes, light microsomes from basal and insulin-treated 3T3-L1 cells at different stages of differentiation were sedimented in continuous sucrose velocity gradients. As shown in Figure 4, intracellular GLUT1 shifts from a broad distribution on day 0 to a narrow and distinct distribution on day 3 that is identical to that on day 9. This narrowing of GLUT1 distribution is indicative of the formation of a relatively uniform vesicular compartment, and acute insulin treatment causes marked depletion of GLUT1 from this compartment, indicative of its translocation to the cell surface, on days 3 (Figure 4, C and D) and 9 (Figure 4, E and F), but not on day 0 (Figure 4, A and B). These data are entirely consistent with the 2-deoxyglucose uptake results (Figure 1, A and B). The distribution of GLUT4 in these gradients on day 9 (Figure 4, G and H) completely overlaps with that of GLUT1, with insulin stimulating translocation of both transporters, as has previously been demonstrated in rat (Zorzano et al., 1989; Kandror et al., 1995a) and 3T3-L1 (Calderhead et al., 1990; Yang and Holman, 1993) adipocytes. The overall distribution of the TfR also shifts to a lower-sedimenting peak during the differentiation process (Figure 5). However, the distribution of the TfR in differentiated cells (day 9; Figure 5, E and F) is still broader than that of GLUT1 and GLUT4. In agreement with the transferrin binding data (Figure 1, C and D), insulin does not markedly stimulate TfR translocation on day 0 (Figure 5, A and B) but does so on days 3 and 9 (Figure 5, C–F). Importantly, only the fractions that also contain GLUT1 and GLUT4 (fractions 9–19) exhibit an insulin-elicited decrease in TfRs. This result is consistent with previous studies, which have revealed only partial colocalization of TfRs with GLUT4 (Tanner and Lienhard, 1989; Martin et al., 1996; Kandror and Pilch, 1998; also see Figure 7). The distribution of IRAP (Figure 6) on days 3 and 9 totally overlaps with that of GLUT1 and GLUT4 (day 9), and insulin treatment also depletes IRAP from intracellular membranes. Interestingly, IRAP is not detectable in these gradients on day 0, most likely because of its low level of expression at that time (Ross et al., 1996; El-Jack et al., 1999) and because of its subcellular distribution at that time when a greater proportion is found at the cell surface than in fully differentiated adipocytes (Ross et al., 1998). Figure 6E shows a representative profile of the total protein in these gradients, which does not change to any significant degree during differentiation or upon insulin treatment.
Figure 4.
By day 3, GLUT1 resides in a distinct and fully insulin-responsive compartment, which cosediments with GLUT4-containing vesicles from differentiated cells. On days 0 (A and B), 3 (C and D), and 9 (E-H), 3T3-L1 cells were serum starved for 2 h before treatment with or without 100 nM insulin for 30 min. Light microsomes (1.5–2 mg) were then isolated and sedimented in a 4.6-ml 10–30% sucrose gradient as described in MATERIALS AND METHODS. The panels on the left show the Western blots of odd- and even-numbered gradient fractions, which were immunoblotted for GLUT1 and GLUT4, respectively. Detection was with HRP-conjugated secondary antibodies and chemiluminescence. These data were quantified by densitometry and are graphically displayed (circles) in the panels on the right as arbitrary units (A.U.). Open and closed symbols, basal and insulin-stimulated conditions, respectively. A representative profile of the total protein in these gradients is shown in Figure 6E. These data are representative of three independent experiments.
Figure 5.
During the course of adipocyte differentiation, the distribution of intracellular TfR in sucrose velocity gradients shifts as insulin responsiveness is acquired. Various conditions are as in Figure 4, except that odd-numbered fractions were blotted for TfR (A–F).
Figure 7.
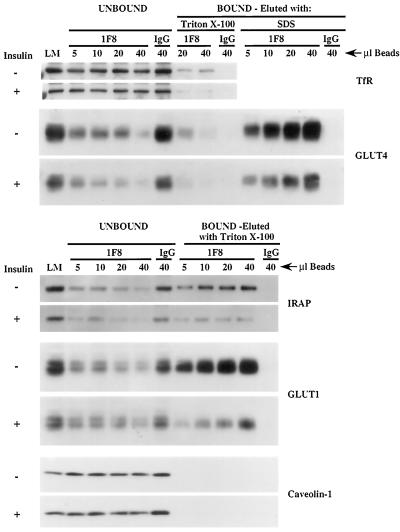
Anti-GLUT4 antibody immunoadsorbs most of GLUT4, GLUT1, and IRAP and a small proportion of TfR from light microsomes of 3T3-L1 adipocytes. The indicated volumes (microliters) of 1F8 antibody- or nonspecific IgG-coupled beads were incubated with 200 μg of light microsomes (LM) from insulin-treated and untreated 3T3-L1 adipocytes (day 9), washed, and eluted sequentially with 1% Triton X-100 in PBS followed by SDS-containing Laemmli sample buffer (nonreducing) as described in MATERIALS AND METHODS. Equal proportions of light microsomes (LM), supernatant (unbound), and bound protein were electrophoresed in 10% acrylamide gels and immunoblotted for the indicated proteins. The goat polyclonal anti-GLUT4 antibody was used for Western blotting. Detection was with HRP-conjugated secondary antibodies and chemiluminescence. These data are representative of three independent experiments. No bands specific for GLUT1, IRAP, and TfR were detectable upon elution by SDS (data not shown).
Figure 6.
IRAP has identical sedimentation distribution as GLUT1 and GLUT4 and is insulin responsive by day 3 of differentiation. Various conditions are as described in Figure 4, except that even- and odd-numbered fractions were immunoblotted for IRAP on days 3 (A and B) and 9 (C and D), respectively. Also shown (E) is a representative profile (O.D. 562) of the total protein (triangles) in these gradients (Figures 4–6), which does not change during differentiation or upon insulin treatment.
In Figure 7, we show a titration experiment in which light microsomes from basal and insulin-treated 3T3-L1 adipocytes were incubated with an increasing volume of anti-GLUT4 antibody coupled to acrylic beads. At the highest antibody amount (corresponding to 40 μl of beads), >90% of GLUT4, IRAP, and GLUT1 are adsorbed by the beads and are recovered after elution. IRAP and GLUT1 are not directly bound to antibody and can be eluted with Triton X-100, whereas SDS is required to elute GLUT4. The fact that similar proportions of total IRAP, GLUT1, and GLUT4 are bound at each bead amount tested suggests that these proteins are extensively colocalized in the same membrane vesicles obtained from both control and insulin-treated cells. On the other hand, only 10–20% of the TfR is bound to the beads and eluted with Triton X-100. The small degree of colocalization of TfRs with GLUT4 is consistent with Figure 5 and with previous studies (Martin et al., 1996; Kandror and Pilch, 1998). The colocalization of GLUT4 and GLUT1 is also in agreement with the observation that 3T3-L1 cells, unlike rat adipocytes (Zorzano et al., 1989), minimally segregate GLUT1 and GLUT4 (Calderhead et al., 1990). Caveolin-1, which we have previously shown to be excluded from GLUT4 vesicles in rat adipocytes (Kandror et al., 1995b), is not brought down with anti-GLUT4 antibody, indicating that adsorption of the other proteins is indeed specific, a result also supported by the use of an IgG control, which adsorbs no proteins.
In both native rat and 3T3-L1 adipocytes, the specific phosphatidylinositol-3-kinase (PI3-kinase) inhibitor wortmannin completely inhibits insulin-stimulated GLUT4 translocation and 2-deoxyglucose uptake (Clarke et al., 1994; Okada et al., 1994). Wortmannin has also been shown to inhibit insulin stimulation of GLUT1 (Clarke et al., 1994) and TfR (Shepherd et al., 1995) translocation in fully differentiated 3T3-L1 adipocytes. In Figure 8, we show that wortmannin strongly inhibits basal and insulin-dependent glucose transport (Figure 8A) and completely abolishes the stimulatory effect of insulin on this process (Figure 8B) in 3T3-L1 cells on days 3 and 5 of differentiation as well as in differentiated cells (day 9), indicating that insulin-sensitive glucose transport before GLUT4 expression (day 3) also requires PI3-kinase. This also suggests that the biochemical pathway(s) leading to insulin-stimulated vesicular trafficking is fully operative on day 3 of the differentiation program.
DISCUSSION
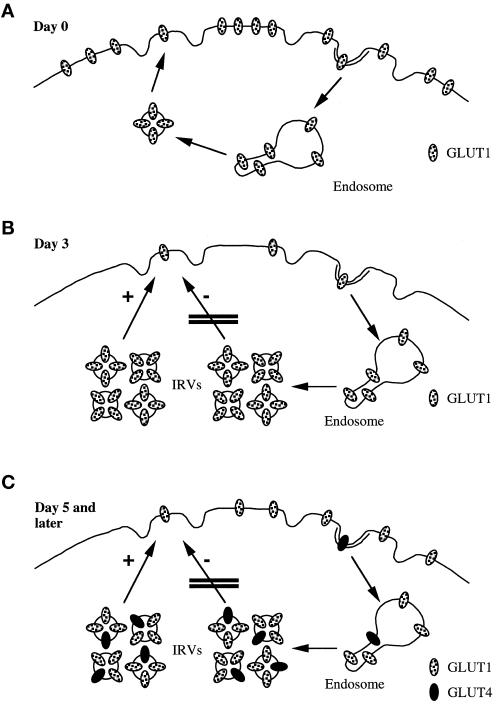
As noted in INTRODUCTION, there have been numerous studies published that followed the development of insulin-stimulated glucose transport during the differentiation of 3T3-L1 cells, including several that have addressed the subcellular distribution of glucose transporters (Weiland et al., 1990; Yang et al., 1992) and IRAP (Ross et al., 1998). However, none of these characterized the properties of the intracellular, transporter-containing vesicles during this process as we have done in the present manuscript. Our results show that the sedimentational properties of GLUT1-containing vesicles on day 3 of differentiation are identical to those of GLUT4 when the cells are fully differentiated (Figure 4). Indeed, an immunoadsorption experiment (Figure 7) shows that both transporter isoforms are present in the same vesicles in mature fat cells. Our results are consistent with the notion that an insulin-regulatable vesicular cargo compartment (insulin-responsive vesicles [IRVs]; Yeh et al., 1995) forms on day 3 of fat cell differentiation when the relatively broad distribution of GLUT1 and TfRs in sucrose gradients narrows considerably (Figures 4 and 5). At this time, there is submaximal expression of IRAP and no GLUT4 expression, but as their expression increases, they are targeted to these same IRVs (Figure 7). In Figure 9, we show a model depicting the development of IRVs during fat cell differentiation that is consistent with our present data and other data cited below. On day 0, GLUT1, along with IRAP and the TfR, which are not illustrated for the sake of clarity, traffics in a constitutive manner, and the population of postendosomal vesicles is small and/or very transient, as has been previously reported (Ghosh and Maxfield, 1995). On day 3, we postulate that new gene expression leads, in the basal state, to blocked movement of this postendosomal IRV compartment to the cell surface, and thus, this compartment becomes quantitatively predominant, and the exocytic step becomes rate limiting (see below). The narrowing of intracellular GLUT1 and TfR distributions in sucrose gradients shown in Figures 4 and 5 are indications of the buildup of the IRV pool.
Figure 9.
Model depicting formation of IRVs during adipocyte differentiation. Depicted are the three major pools of glucose transporters as described (Holman et al., 1994; Yeh et al., 1995). The IRV pool builds up as a result of its inhibited (−) movement to the cell surface on day 3 (B) of the differentiation program, and insulin signaling (+) relieves this inhibition. On day 5 and later, GLUT4 is expressed and targeted to these vesicles. The relative amount of the two transporter isoforms (∼3 to 1, GLUT1 to GLUT4), expected on day 9 and shown in C, is according to the method of Calderhead et al. (1990), and other vesicular proteins are not depicted for the sake of clarity.
Our data are consistent with kinetic analyses of GLUT4 trafficking in rat adipocytes and in 3T3-L1 cultured adipocytes (Holman et al., 1994; Yeh et al., 1995) that have led to a model describing three major GLUT4-containing compartments, a plasma membrane pool, an endosomal pool, and a postendosomal or IRV pool (Yeh et al., 1995). This model is supported by confocal microscopy analysis of rat adipocytes (Malide and Cushman, 1997) and by electron microscopy data from fat and skeletal muscle that show the most abundant GLUT4-containing structures to be small vesicles and short tubules of similar size (Slot et al., 1991; Smith et al., 1991; Wang et al., 1996; Ploug et al., 1998), by our sedimentational analysis (Kandror et al., 1995a), and by electron microscopy after vesicle isolation (James et al., 1987; Kandror et al., 1995a). Kinetic studies have indicated that insulin’s effect is predominantly to stimulate the exocytic movement of the IRV pool to the plasma membrane (Yang and Holman, 1993; Satoh et al., 1993), although some effect of insulin to inhibit the endocytosis of GLUT4 may also occur (Jhun et al., 1992; Czech and Buxton, 1993).
The model we propose in Figure 9 considers the IRV as an insulin-sensitive cargo compartment whose contents can be varied depending on the differentiation state of the fat cell, as depicted in the diagram comparing day 3 with day 5 and later. The fact that there is no change in the sedimentation coefficient of the vesicles in comparing day 3 and day 9 (Figures 4–6) supports this notion, as does additional data we have obtained. Overexpression of GLUT4 in fat cells from transgenic mice results in GLUT4-containing vesicles indistinguishable from their normal counterparts in sedimentation behavior, insulin responsiveness, and protein content other than GLUT4 (Tozzo et al., 1996). More recently, we have obtained vesicles from denervated rat skeletal muscle, in which GLUT4 expression is dramatically reduced (Coderre et al., 1992), the opposite expression level of the transgenic study, and again, the resultant vesicles show reduced GLUT4 content, but they sediment at the same rate as those from normal muscle and show a normal profile of other vesicle proteins such as IRAP and the receptors for transferrin and mannose-6-phosphate (Zhou et al., 1998) Thus, we think that the GLUT4-containing vesicular compartment represents an insulin-regulatable, postendosomal cargo vesicle whose behavior is not dependent on the presence of the major cargo proteins.
Whether all cargo proteins undergo a complete trafficking cycle through identical compartments is not clear. Recent studies of GLUT4 trafficking in transfected CHO cells (Wei et al., 1998), compartment ablation studies in 3T3-L1 fat cells (Martin et al., 1996), and confocal microscopy studies of rat adipocytes (Malide et al., 1997a) suggest that GLUT4 segregates to a substantial degree from TfRs. Therefore, the trafficking of GLUT4 and TfRs may be quite different and involve different postendosomal compartments. Indeed, we see that most (∼90%; see Figure 7) of the TfRs are excluded from GLUT4-containing vesicles, and that the distribution of TfRs in sucrose gradients is also much broader than that of glucose transporters, indicating their presence in a larger number of membrane compartments. On the other hand, and unlike the results of Malide et al. (1997a), in rat fat cells, we find that ∼50% of the TfRs colocalize with GLUT4 in the basal state, and importantly, they remain colocalized with GLUT4 after cellular insulin exposure, as determined by cell surface biotinylation and vesicle immunoadsorption upon endocytosis (Kandror and Pilch, 1998). GLUT4-transfected CHO cells segregate transporter from TfR upon endocytosis (Wei et al., 1998), and these proteins appear to have even less overlap than in the present study. The basis for these discrepancies may be cell type dependent variations in trafficking or methodological, and further investigation of these issues is warranted.
However, compartment ablation using transferrin-HRP conjugates to destroy TfR-containing compartments (Martin et al., 1996) used the same 3T3-L1 cell line as we do, as well as similar immunoadsorption protocols. Because the ablation protocol destroyed all the TfRs but only 40% of the GLUT4, the authors concluded that the latter was segregated into a unique compartment. We think that interpretation of these data also depends on the relative amounts of glucose transporters and TfRs, which were previously calculated such that one glucose transporter vesicle in three would have a TfR (Tanner and Lienhard, 1989). Thus, the results obtained by Martin et al. (1996) are what one might expect from the ratios of the two proteins, and not necessarily because of unique compartments.
In any case, further experiments are necessary for complete resolution of this issue. Our results point out a possible direction in this regard. We show here that the development of insulin-dependent vesicular traffic occurs abruptly during the course of fat cell differentiation, almost certainly as a result of the expression of one or more presently unknown genes. It remains undetermined as to what these genes are. Because we show the translocation process to be wortmannin inhibitable on day 3, we do not think the known components of the insulin signaling pathway, namely the insulin receptor, IRS-1, and PI3-kinase, are directly involved in the acquisition of hormone-sensitive vesicular trafficking. Others have also ruled out insulin receptor expression in this regard (Rubin et al., 1978; Resh, 1982), and we see no differences in IRS-1 (data not shown) and PI3-kinase expression (El-Jack et al., 1999) between days 2 and 3 of 3T3-L1 cell differentiation. The fact that GLUT4 transfection in various fibroblastic cells never results in an insulin response (Haney et al., 1991; Hudson et al., 1992; Kotliar and Pilch, 1992) also supports the notion that certain genes are missing in this latter context. Thus, we are attempting to identify the gene or genes responsible, and when we do, it will be possible to test the hypothesis that we can reconstitute, by transfection, insulin-sensitive vesicular trafficking under various conditions, including in the presence or absence of GLUT4 and IRAP expression, the two GLUT4 vesicle-specific proteins that, so far, exhibit identical behavior in mature adipocytes (Kandror and Pilch, 1994; Malide et al., 1997b).
ACKNOWLEDGMENTS
We thank Dr. Tanya Kupriyanova and Gino Vallega for providing the antibody-coupled beads, Deepanwita Prusty for help in photographing cells, and Dr. Ron Morrison for advice on culturing 3T3-L1 preadipocytes. We are grateful to Min Zhou for helpful advice and discussions. This work was supported by National Institutes of Health grants DK-30425 (to P.F.P.) and DK-52057 (to K.V.K.), Juvenile Diabetes Foundation grant 197029 (to K.V.K.), a research grant from the American Diabetes Association (to K.V.K.), and a medical student research fellowship from the American Heart Association (to A.K.E.-J.).
Abbreviations used:
- CHO
Chinese hamster ovary
- DMEM
Dulbecco’s modified Eagle’s medium
- GLUT
glucose transporter
- Ig
immunoglobulin
- IRAP
insulin-responsive aminopeptidase
- IRS
insulin receptor substrate
- IRV
insulin-responsive vesicle
- KRP
Krebs-Ringer-phosphate
- PI3-kinase
phosphatidylinositol-3-kinase
- TfR
transferrin receptor
REFERENCES
- Brun RP, Kim JB, Hu E, Altiok S, Spiegelman BM. Adipocyte differentiation: a transcriptional regulatory cascade. Curr Opin Cell Biol. 1996;8:826–832. doi: 10.1016/s0955-0674(96)80084-6. [DOI] [PubMed] [Google Scholar]
- Cain CC, Trimble WS, Lienhard GE. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J Biol Chem. 1992;267:11681–11684. [PubMed] [Google Scholar]
- Calderhead DM, Kitagawa K, Tanner LI, Holman GD, Lienhard GE. Insulin regulation of the two glucose transporters in 3T3–L1 adipocytes. J Biol Chem. 1990;265:13801–13808. [PubMed] [Google Scholar]
- Chakrabarti R, Buxton J, Joly M, Corvera S. Insulin-sensitive association of GLUT-4 with endocytic clathrin-coated vesicles revealed with the use of brefeldin A. J Biol Chem. 1994;269:7926–7933. [PubMed] [Google Scholar]
- Cheatham B, Volchuk A, Kahn CR, Wang L, Rhodes CJ, Klip A. Insulin-stimulated translocation of GLUT4 glucose transporters requires SNARE-complex proteins. Proc Natl Acad Sci USA. 1996;93:15169–15173. doi: 10.1073/pnas.93.26.15169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy BM, Czech MP. Hexose transport stimulation and membrane redistribution of glucose transporter isoforms in response to cholera toxin, dibutyryl cyclic AMP, and insulin in 3T3–L1 adipocytes. J Biol Chem. 1990;265:12434–12443. [PubMed] [Google Scholar]
- Clarke JF, Young PW, Yonezawa K, Kasuga M, Holman GD. Inhibition of the translocation of GLUT1 and GLUT4 in 3T3–L1 cells by the phosphatidylinositol 3-kinase inhibitor, wortmannin. Biochem J. 1994;300:631–635. doi: 10.1042/bj3000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre L, Monfar MM, Chen KS, Heydrick SJ, Kurowski TG, Ruderman NB, Pilch PF. Alteration in the expression of GLUT-1 and GLUT-4 protein and mRNA levels in denervated rat muscles. Endocrinology. 1992;131:1821–1825. doi: 10.1210/endo.131.4.1396328. [DOI] [PubMed] [Google Scholar]
- Czech MP, Buxton JM. Insulin action on the internalization of the GLUT4 glucose transporter in isolated rat adipocytes. J Biol Chem. 1993;268:9187–9190. [PubMed] [Google Scholar]
- El-Jack AK, Hamm JK, Pilch PF, Farmer SR. Reconstitution of insulin-sensitive glucose transport in fibroblasts requires expression of both PPARγ and C/EBPα. J Biol Chem. 1999;274:7946–7951. doi: 10.1074/jbc.274.12.7946. [DOI] [PubMed] [Google Scholar]
- Garcia de Herreros A, Birnbaum MJ. The acquisition of increased insulin-responsive hexose transport in 3T3–L1 adipocytes correlates with expression of a novel transporter gene. J Biol Chem. 1989;264:19994–19999. [PubMed] [Google Scholar]
- Geuze HJ, Slot JW, Strous GJ, Lodish HF, Schwartz AL. Immunocytochemical localization of the receptor for asialoglycoprotein in rat liver cells. J Cell Biol. 1982;92:865–870. doi: 10.1083/jcb.92.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh RN, Maxfield FR. Evidence for nonvectorial, retrograde transferrin trafficking in the early endosomes of HEp2 cells. J Cell Biol. 1995;128:549–561. doi: 10.1083/jcb.128.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- Haney PM, Slot JW, Piper RC, James DE, Mueckler M. Intracellular targeting of the insulin-regulatable glucose transporter (GLUT4) is isoform specific and independent of cell type. J Cell Biol. 1991;114:689–699. doi: 10.1083/jcb.114.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman GD, Kozka IJ, Clark AE, Flower CJ, Saltis J, Habberfield AD, Simpson IA, Cushman SW. Cell surface labeling of glucose transporter isoform GLUT4 by bis-mannose photolabel. Correlation with stimulation of glucose transport in rat adipose cells by insulin and phorbol ester. J Biol Chem. 1990;265:18172–18179. [PubMed] [Google Scholar]
- Holman GD, Lo Leggio L, Cushman SW. Insulin-stimulated GLUT4 glucose transporter recycling. A problem in membrane protein subcellular trafficking through multiple pools. J Biol Chem. 1994;269:17516–17524. [PubMed] [Google Scholar]
- Hudson AW, Ruiz M, Birnbaum MJ. Isoform-specific subcellular targeting of glucose transporters in mouse fibroblasts. J Cell Biol. 1992;116:785–797. doi: 10.1083/jcb.116.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DE, Brown R, Navarro J, Pilch PF. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988;333:183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- James DE, Lederman L, Pilch PF. Purification of insulin-dependent exocytic vesicles containing the glucose transporter. J Biol Chem. 1987;262:11817–11824. [PubMed] [Google Scholar]
- Jhun BH, Rampal AL, Liu H, Lachaal M, Jung CY. Effects of insulin on steady state kinetics of GLUT4 subcellular distribution in rat adipocytes. Evidence of constitutive GLUT4 recycling. J Biol Chem. 1992;267:17710–17715. [PubMed] [Google Scholar]
- Kandror KV, Coderre L, Pushkin AV, Pilch PF. Comparison of glucose-transporter-containing vesicles from rat fat and muscle tissues: Evidence for a unique endosomal compartment. Biochem J. 1995a;307:383–390. doi: 10.1042/bj3070383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandror KV, Pilch PF. gp160, a tissue-specific marker for insulin-activated glucose transport. Proc Natl Acad Sci USA. 1994;91:8017–8021. doi: 10.1073/pnas.91.17.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandror KV, Pilch PF. Compartmentalization of protein traffic in insulin-sensitive cells. Am J Physiol. 1996a;271:E1–E14. doi: 10.1152/ajpendo.1996.271.1.E1. [DOI] [PubMed] [Google Scholar]
- Kandror KV, Pilch PF. The insulin-like growth factor II/mannose 6-phosphate receptor utilizes the same membrane compartments as GLUT4 for insulin-dependent trafficking to and from the rat adipocyte cell surface. J Biol Chem. 1996b;271:21703–21708. doi: 10.1074/jbc.271.36.21703. [DOI] [PubMed] [Google Scholar]
- Kandror KV, Pilch PF. Multiple endosomal pathways in rat adipose cells. Biochem J. 1998;331:829–835. doi: 10.1042/bj3310829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandror KV, Stephens JM, Pilch PF. Expression and compartmentalization of caveolin in adipose cells: coordinate regulation with and structural segregation from GLUT4. J Cell Biol. 1995b;129:999–1006. doi: 10.1083/jcb.129.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandror KV, Yu L, Pilch PF. The major protein of GLUT4-containing vesicles, gp160, has aminopeptidase activity. J Biol Chem. 1994;269:30777–30780. [PubMed] [Google Scholar]
- Keller SR, Scott HM, Mastick CC, Aebersold R, Lienhard GE. Cloning and characterization of a novel insulin-regulated membrane aminopeptidase from GLUT4 vesicles. J Biol Chem. 1995;270:23612–23618. doi: 10.1074/jbc.270.40.23612. [DOI] [PubMed] [Google Scholar]
- Kotliar N, Pilch PF. Expression of the glucose transporter isoform GLUT 4 is insufficient to confer insulin-regulatable hexose uptake to cultured muscle cells. Mol Endocrinol. 1992;6:337–345. doi: 10.1210/mend.6.3.1584210. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurie SM, Cain CC, Lienhard GE, Castle JD. The glucose transporter GLUT4 and secretory carrier membrane proteins (SCAMPs) colocalize in rat adipocytes and partially segregate during insulin stimulation. J Biol Chem. 1993;268:19110–19117. [PubMed] [Google Scholar]
- Lin B-Z, Pilch PF, Kandror KV. Sortilin is a major protein component of GLUT4-containing vesicles. J Biol Chem. 1997;272:24145–24147. doi: 10.1074/jbc.272.39.24145. [DOI] [PubMed] [Google Scholar]
- Malide D, Cushman SW. Morphological effects of wortmannin on the endosomal system and GLUT4-containing compartments in rat adipose cells. J Cell Sci. 1997;110:2795–2806. doi: 10.1242/jcs.110.22.2795. [DOI] [PubMed] [Google Scholar]
- Malide D, Dwyer NK, Blanchette-Mackie EJ, Cushman SW. Immunocytochemical evidence that GLUT4 resides in a specialized translocation postendosomal VAMP2-positive compartment in rat adipose cells in the absence of insulin. J Histochem Cytochem. 1997a;45:1083–1096. doi: 10.1177/002215549704500806. [DOI] [PubMed] [Google Scholar]
- Malide D, St-Denis JF, Keller SR, Cushman SW. Vp165 and GLUT4 share similar vesicle pools along their trafficking pathways in rat adipose cells. FEBS Lett. 1997b;409:461–468. doi: 10.1016/s0014-5793(97)00563-2. [DOI] [PubMed] [Google Scholar]
- Martin S, Tellam J, Livingstone C, Slot JW, Gould GW, James DE. The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J Cell Biol. 1996;134:625–635. doi: 10.1083/jcb.134.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NJ, Ross SA, Lane WS, Moestrup SK, Petersen CM, Keller SR, Lienhard GE. Sortilin is the major 110-kDa protein in GLUT4 vesicles from adipocytes. J Biol Chem. 1998;273:3582–3587. doi: 10.1074/jbc.273.6.3582. [DOI] [PubMed] [Google Scholar]
- Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- Ploug T, van Deurs B, Ai H, Cushman SW, Ralston E. Analysis of GLUT4 distribution in whole skeletal muscle fibers: identification of distinct storage compartments that are recruited by insulin and muscle contractions. J Cell Biol. 1998;142:1429–1446. doi: 10.1083/jcb.142.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea S, James DE. Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997;46:1667–1677. doi: 10.2337/diab.46.11.1667. [DOI] [PubMed] [Google Scholar]
- Reed BC, Kaufmann SH, Mackall JC, Student AK, Lane MD. Alterations in insulin binding accompanying differentiation of 3T3–L1 preadipocytes. Proc Natl Acad Sci USA. 1977;74:4876–4880. doi: 10.1073/pnas.74.11.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD. Development of insulin responsiveness of the glucose transporter and the (Na+,K+)-adenosine triphosphatase during in vitro adipocyte Differentiation. J Biol Chem. 1982;257:6978–6986. [PubMed] [Google Scholar]
- Rice KM, Garner CW. Correlation of the insulin receptor substrate-1 with insulin-responsive deoxyglucose transport in 3T3–L1 adipocytes. Biochem Biophys Res Commun, 1994;198:523–530. doi: 10.1006/bbrc.1994.1077. [DOI] [PubMed] [Google Scholar]
- Ross SA, Keller SR, Lienhard GE. Increased intracellular sequestration of the insulin-regulated aminopeptidase upon differentiation of 3T3–L1 cells. Biochem J. 1998;330:1003–1008. doi: 10.1042/bj3301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, Scott HM, Morris NJ, Leung WY, Mao F, Lienhard GE, Keller SR. Characterization of the insulin-regulated membrane aminopeptidase in 3T3–L1 adipocytes. J Biol Chem. 1996;271:3328–3332. doi: 10.1074/jbc.271.6.3328. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Söllner TH. Membrane trafficking—throttles and dampers: controlling the engine of membrane fusion. Science. 1997;276:1212–1213. doi: 10.1126/science.276.5316.1212. [DOI] [PubMed] [Google Scholar]
- Rubin CS, Hirsch A, Fung C, Rosen OM. Development of hormone receptors and hormonal responsiveness in vitro. J Biol Chem. 1978;253:7570–7578. [PubMed] [Google Scholar]
- Satoh S, Nishimura H, Clark AE, Kozka IJ, Vannucci SJ, Simpson IA, Quon MJ, Cushman SW, Holman GD. Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells. Evidence that exocytosis is a critical site of hormone action. J Biol Chem. 1993;268:17820–17829. [PubMed] [Google Scholar]
- Shepherd PR, Soos MA, Siddle K. Inhibitors of phosphoinositide 3-kinase block exocytosis but not endocytosis of transferrin receptors in 3T3–L1 adipocytes. Biochem Biophys Res Commun. 1995;211:535–539. doi: 10.1006/bbrc.1995.1846. [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Charron MJ, Shah N, Lodish HF, Jarett L. Immunoelectron microscopic demonstration of insulin-stimulated translocation of glucose transporters to the plasma membrane of isolated rat adipocytes and masking of the carboxy-terminal epitope of intracellular GLUT4. Proc Natl Acad Sci USA. 1991;88:6893–6897. doi: 10.1073/pnas.88.15.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-α-induced insulin resistance in 3T3–L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem. 1997;272:971–976. doi: 10.1074/jbc.272.2.971. [DOI] [PubMed] [Google Scholar]
- Tanner LI, Lienhard GE. Insulin elicits a redistribution of transferrin receptors in 3T3–L1 adipocytes through an increase in the rate constant for receptor externalization. J Biol Chem. 1987;262:8975–8980. [PubMed] [Google Scholar]
- Tanner LI, Lienhard GE. Localization of transferrin receptors and insulin-like growth factor II receptors in vesicles from 3T3–L1 adipocytes that contain intracellular glucose transporters. J Cell Biol. 1989;108:1537–1545. doi: 10.1083/jcb.108.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoidis G, Kotliar N, Pilch PF. Immunological analysis of GLUT4-enriched vesicles. Identification of novel proteins regulated by insulin and diabetes. J Biol Chem. 1993;268:11691–11696. [PubMed] [Google Scholar]
- Timmers KI, Clark AE, Omatsu-Kanbe M, Whiteheart SW, Bennett MK, Holman GD, Cushman SW. Identification of SNAP receptors in rat adipose cell membrane fractions and in SNARE complexes coimmunoprecipitated with epitope-tagged N-ethylmaleimide-sensitive fusion protein. Biochem J. 1996;320:429–436. doi: 10.1042/bj3200429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzo E, Kahn BB, Pilch PF, Kandror KV. GLUT4 is targeted to specific vesicles in adipocytes of transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem. 1996;271:10490–10494. doi: 10.1074/jbc.271.18.10490. [DOI] [PubMed] [Google Scholar]
- Volchuk A, Sargeant R, Sumitani S, Liu Z, He L, Klip A. Cellubrevin is a resident protein of insulin-sensitive GLUT4 glucose transporter vesicles in 3T3–L1 adipocytes. J Biol Chem. 1995;270:8233–8240. doi: 10.1074/jbc.270.14.8233. [DOI] [PubMed] [Google Scholar]
- Wang WC, Hansen PA, Marshall BA, Holloszy JO, Mueckler M. Insulin unmasks a COOH-terminal GLUT4 epitope and increases glucose transport across T-tubules in skeletal muscle. J Cell Biol. 1996;135:415–430. doi: 10.1083/jcb.135.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei ML, Bonzelius F, Scully RM, Kelly RB, Herman GA. GLUT4 and transferrin receptor are differentially sorted along the endocytic pathway in CHO cells. J Cell Biol. 1998;140:565–575. doi: 10.1083/jcb.140.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland M, Schurmann A, Schmidt WE, Joost HG. Development of the hormone-sensitive glucose transport activity in differentiating 3T3–L1 murine fibroblasts. Role of the two transporter species and their subcellular localization. Biochem J. 1990;270:331–336. doi: 10.1042/bj2700331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Clark AE, Kozka IJ, Cushman SW, Holman GD. Development of an intracellular pool of glucose transporters in 3T3–L1 cells. J Biol Chem. 1992;267:10393–10399. [PubMed] [Google Scholar]
- Yang J, Holman GD. Comparison of GLUT4 and GLUT1 subcellular trafficking in basal and insulin-stimulated 3T3–L1 cells. J Biol Chem. 1993;268:4600–4603. [PubMed] [Google Scholar]
- Yeh JI, Verhey KJ, Birnbaum MJ. Kinetic analysis of glucose transporter trafficking in fibroblasts and adipocytes. Biochemistry. 1995;34:15523–15531. doi: 10.1021/bi00047a018. [DOI] [PubMed] [Google Scholar]
- Zhou M, Sevilla L, Vallega G, Chen P, Palacin M, Zorzano A, Pilch PF, Kandror KV. Insulin-dependent protein trafficking in skeletal muscle cells. Am J Physiol. 1998;275:E187–E196. doi: 10.1152/ajpendo.1998.275.2.E187. [DOI] [PubMed] [Google Scholar]
- Zorzano A, Wilkinson W, Kotliar N, Thoidis G, Wadzinkski BE, Ruoho AE, Pilch PF. Insulin-regulated glucose uptake in rat adipocytes is mediated by two transporter isoforms present in at least two vesicle populations. J Biol Chem. 1989;264:12358–12363. [PubMed] [Google Scholar]