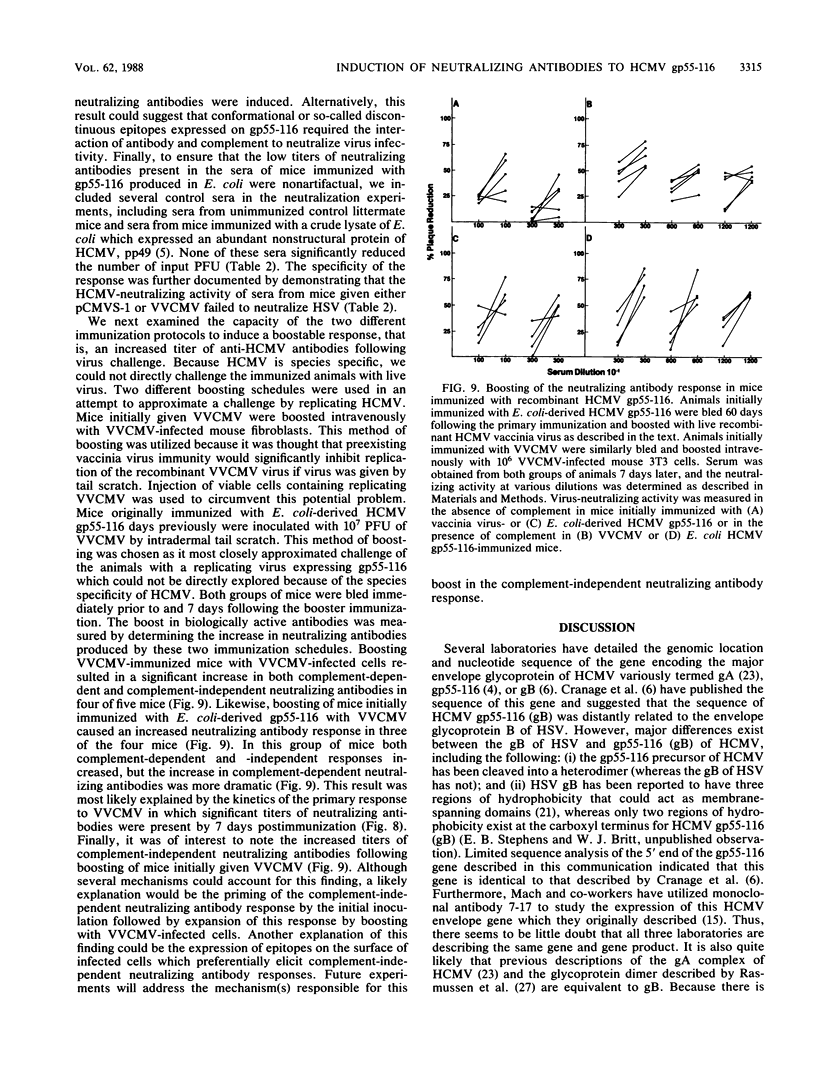
Abstract
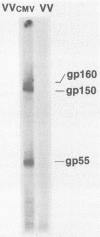
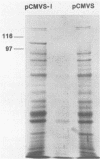
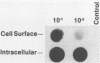
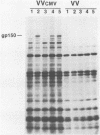
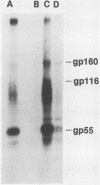
The human cytomegalovirus (HCMV) envelope glycoprotein complex gp55-116 was expressed in both Escherichia coli and cells infected with a recombinant vaccinia virus. E. coli produced a single protein of Mr 100,000 which approximated the size of the nonglycosylated gp55-116 precursor found in HCMV-infected cells. Cells infected with the recombinant vaccinia virus contained three intracellular forms of Mr 160,000, 150,000, and 55,000 which were detected by a monoclonal antibody reactive with gp55. Comparison of the immunological properties of these recombinant proteins indicated that several of the HCMV gp55-116 monoclonal antibodies and sera from patients infected with HCMV reacted with the vaccinia virus-derived proteins whereas a more restricted group of monoclonal antibodies recognized the E. coli-produced protein. Immunization of mice with either E. coli or vaccinia virus recombinant HCMV gp55-116 resulted in production of virus-neutralizing antibodies. In contrast to the almost exclusive production of complement-dependent neutralizing antibodies following immunization with recombinant vaccinia virus, the E. coli-derived protein induced complement-independent neutralizing antibodies.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britt W. J., Auger D. Identification of a 65 000 dalton virion envelope protein of human cytomegalovirus. Virus Res. 1985 Dec;4(1):31–36. doi: 10.1016/0168-1702(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Britt W. J., Auger D. Synthesis and processing of the envelope gp55-116 complex of human cytomegalovirus. J Virol. 1986 Apr;58(1):185–191. doi: 10.1128/jvi.58.1.185-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology. 1984 Jun;135(2):369–378. doi: 10.1016/0042-6822(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Cranage M. P., Kouzarides T., Bankier A. T., Satchwell S., Weston K., Tomlinson P., Barrell B., Hart H., Bell S. E., Minson A. C. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986 Nov;5(11):3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix R. D., Pereira L., Baringer J. R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981 Oct;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Morrison R. P., Wehrly K., Nishio J., Chesebro B. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science. 1986 Nov 7;234(4777):728–731. doi: 10.1126/science.3490689. [DOI] [PubMed] [Google Scholar]
- Elango N., Prince G. A., Murphy B. R., Venkatesan S., Chanock R. M., Moss B. Resistance to human respiratory syncytial virus (RSV) infection induced by immunization of cotton rats with a recombinant vaccinia virus expressing the RSV G glycoprotein. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1906–1910. doi: 10.1073/pnas.83.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M., Ooka T., Matsuo T., Kieff E. Epstein-Barr virus glycoprotein homologous to herpes simplex virus gB. J Virol. 1987 Feb;61(2):499–508. doi: 10.1128/jvi.61.2.499-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönczöl E., Hudecz F., Ianacone J., Dietzschold B., Starr S., Plotkin S. A. Immune responses to isolated human cytomegalovirus envelope proteins. J Virol. 1986 May;58(2):661–664. doi: 10.1128/jvi.58.2.661-664.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes K., Alford C., Britt W. Antibody response to virus-encoded proteins after cytomegalovirus mononucleosis. J Infect Dis. 1987 Oct;156(4):615–621. doi: 10.1093/infdis/156.4.615. [DOI] [PubMed] [Google Scholar]
- Kari B., Lussenhop N., Goertz R., Wabuke-Bunoti M., Radeke R., Gehrz R. Characterization of monoclonal antibodies reactive to several biochemically distinct human cytomegalovirus glycoprotein complexes. J Virol. 1986 Nov;60(2):345–352. doi: 10.1128/jvi.60.2.345-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law K. M., Wilton-Smith P., Farrar G. H. A murine monoclonal antibody recognising a single glycoprotein within a human cytomegalovirus virion envelope glycoprotein complex. J Med Virol. 1985 Nov;17(3):255–266. doi: 10.1002/jmv.1890170307. [DOI] [PubMed] [Google Scholar]
- Mach M., Utz U., Fleckenstein B. Mapping of the major glycoprotein gene of human cytomegalovirus. J Gen Virol. 1986 Jul;67(Pt 7):1461–1467. doi: 10.1099/0022-1317-67-7-1461. [DOI] [PubMed] [Google Scholar]
- Mackett M., Yilma T., Rose J. K., Moss B. Vaccinia virus recombinants: expression of VSV genes and protective immunization of mice and cattle. Science. 1985 Jan 25;227(4685):433–435. doi: 10.1126/science.2981435. [DOI] [PubMed] [Google Scholar]
- Meyers J. D., Flournoy N., Thomas E. D. Nonbacterial pneumonia after allogeneic marrow transplantation: a review of ten years' experience. Rev Infect Dis. 1982 Nov-Dec;4(6):1119–1132. doi: 10.1093/clinids/4.6.1119. [DOI] [PubMed] [Google Scholar]
- Pellett P. E., Biggin M. D., Barrell B., Roizman B. Epstein-Barr virus genome may encode a protein showing significant amino acid and predicted secondary structure homology with glycoprotein B of herpes simplex virus 1. J Virol. 1985 Dec;56(3):807–813. doi: 10.1128/jvi.56.3.807-813.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett P. E., Kousoulas K. G., Pereira L., Roizman B. Anatomy of the herpes simplex virus 1 strain F glycoprotein B gene: primary sequence and predicted protein structure of the wild type and of monoclonal antibody-resistant mutants. J Virol. 1985 Jan;53(1):243–253. doi: 10.1128/jvi.53.1.243-253.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Hoffman M., Cremer N. Electrophoretic analysis of polypeptides immune precipitated from cytomegalovirus-infected cell extracts by human sera. Infect Immun. 1982 Jun;36(3):933–942. doi: 10.1128/iai.36.3.933-942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Hoffman M., Tatsuno M., Dondero D. Polymorphism of human cytomegalovirus glycoproteins characterized by monoclonal antibodies. Virology. 1984 Nov;139(1):73–86. doi: 10.1016/0042-6822(84)90331-3. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Matthews T. J., Robey W. G., Lynn D. L., Robert-Guroff M., Mueller W. T., Langlois A. J., Ghrayeb J., Petteway S. R., Jr, Weinhold K. J. HTLV-III/LAV-neutralizing antibodies to an E. coli-produced fragment of the virus envelope. Science. 1986 Dec 12;234(4782):1392–1395. doi: 10.1126/science.2431482. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Masur H., Rook A. H., Armstrong G., Frederick W. R., Epstein J., Manischewitz J. F., Macher A. M., Jackson L., Ames J. Herpesvirus infections in the acquired immune deficiency syndrome. JAMA. 1984 Jul 6;252(1):72–77. [PubMed] [Google Scholar]
- Rasmussen L. E., Nelson R. M., Kelsall D. C., Merigan T. C. Murine monoclonal antibody to a single protein neutralizes the infectivity of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Feb;81(3):876–880. doi: 10.1073/pnas.81.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L., Mullenax J., Nelson R., Merigan T. C. Viral polypeptides detected by a complement-dependent neutralizing murine monoclonal antibody to human cytomegalovirus. J Virol. 1985 Aug;55(2):274–280. doi: 10.1128/jvi.55.2.274-280.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Shafferman A. Conditional expression of the vesicular stomatitis virus glycoprotein gene in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6670–6674. doi: 10.1073/pnas.78.11.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Moss B. Infectious poxvirus vectors have capacity for at least 25 000 base pairs of foreign DNA. Gene. 1983 Nov;25(1):21–28. doi: 10.1016/0378-1119(83)90163-4. [DOI] [PubMed] [Google Scholar]
- Stanberry L. R., Bernstein D. I., Burke R. L., Pachl C., Myers M. G. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J Infect Dis. 1987 May;155(5):914–920. doi: 10.1093/infdis/155.5.914. [DOI] [PubMed] [Google Scholar]
- Stinski M. F. Human cytomegalovirus: glycoproteins associated with virions and dense bodies. J Virol. 1976 Aug;19(2):594–609. doi: 10.1128/jvi.19.2.594-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Weis J. H., Salstrom J. S., Enquist L. W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982 Oct 22;218(4570):381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]