Abstract
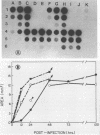
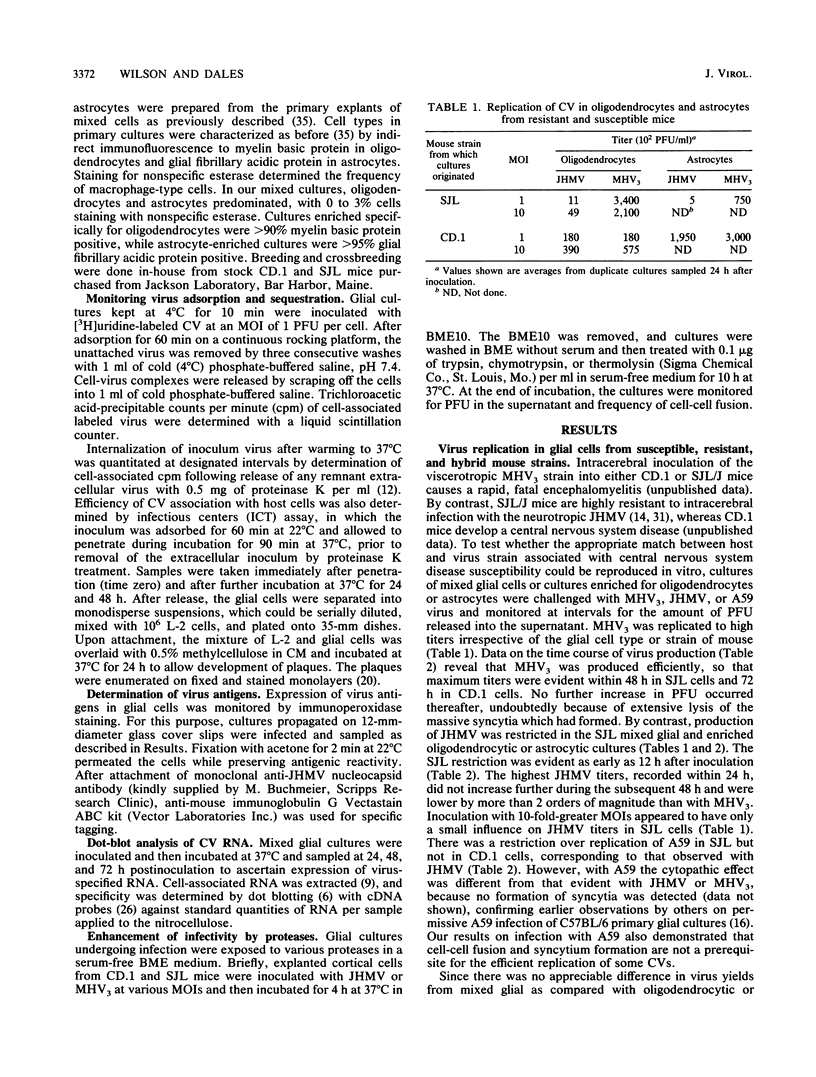
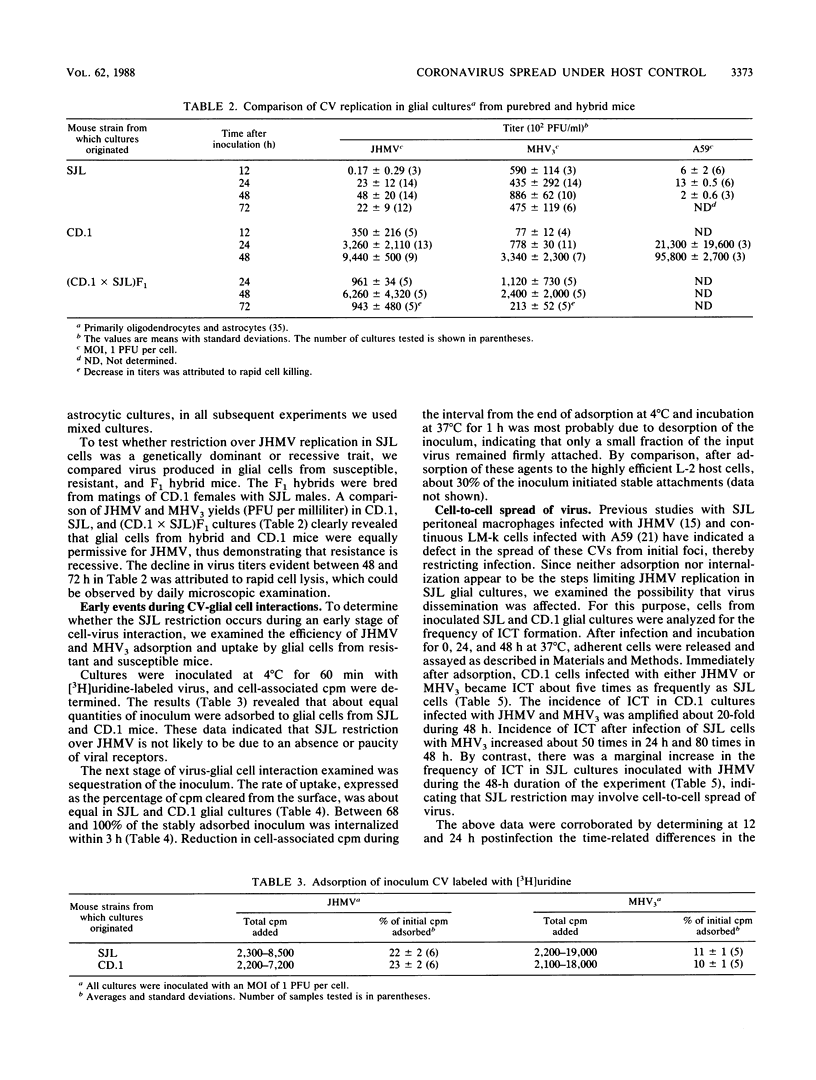
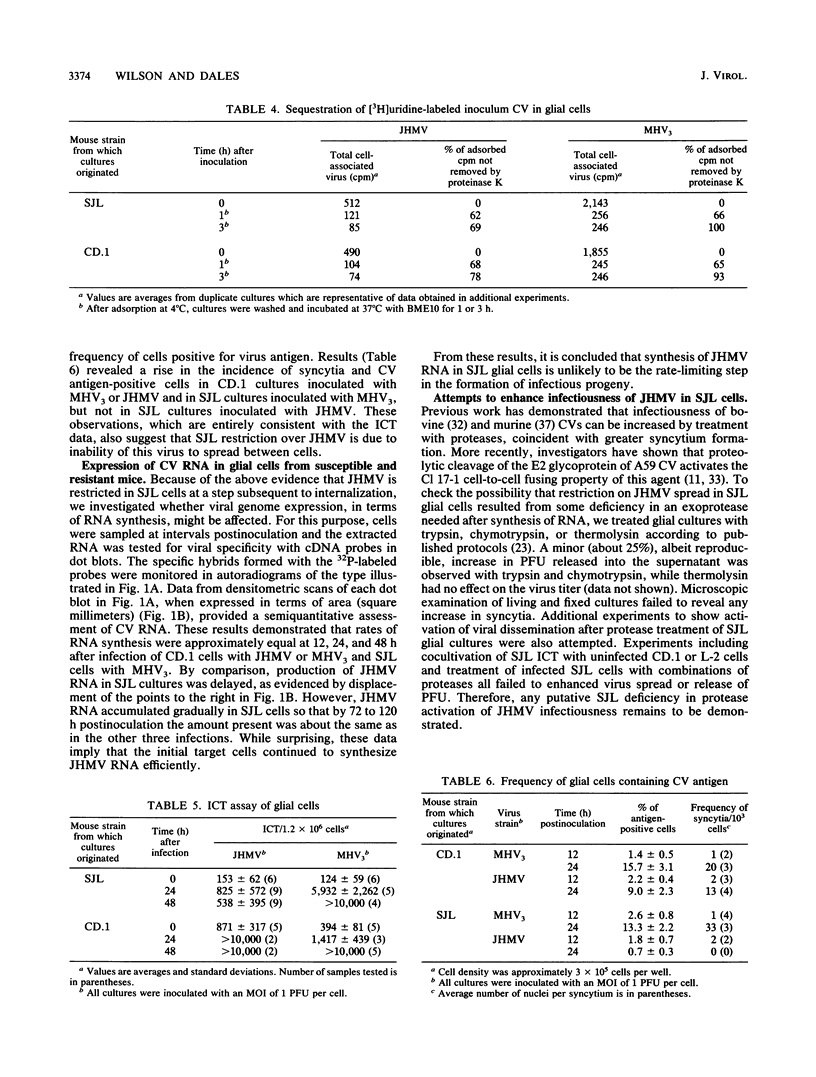
Resistance or susceptibility of various mouse strains to central nervous system disease caused by different strains of coronavirus is well known. Data from the present study draw attention to an additional, genetically determined mechanism controlling CV infections. The resistance to A59 and JHM virus (JHMV) associated with SJL mice was maintained in explanted glial cultures which, by contrast, fully supported a productive infection by the serorelated mouse hepatitis virus type 3. A comparative analysis of the infectious process in glial cell explants from SJL and CD.1 mice helped to define the stage at which restriction is manifested. Cultures of oligodendrocytes and astrocytes from these strains of mice were challenged with JHMV or mouse hepatitis virus type 3, and cell-virus interactions were monitored, including adsorption, uptake of inoculum, transcription, and cell-to-cell dissemination. The sequence of early events from adsorption to genome activation occurred with about equal efficiency with both viruses and genetically different cells, indicating that SJL resistance is not due to any deficiency in specific receptors or penetration of the inoculum or general expression of viral functions. However, intercellular spread of the infection was restricted in SJL glial cells owing to an as yet undefined component. Since cells from (SJL x CD.1)F1 mice were fully susceptible to JHMV, resistance to virus spread must be due to a deficiency in some factor, perhaps a proteolytic activity necessary for dissemination.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beushausen S., Dales S. In vivo and in vitro models of demyelinating disease, XXI: relationship between differentiation of rat oligodendrocytes and control of JHMV replication. Adv Exp Med Biol. 1987;218:239–254. doi: 10.1007/978-1-4684-1280-2_29. [DOI] [PubMed] [Google Scholar]
- Beushausen S., Dales S. In vivo and in vitro models of demyelinating disease. XI. Tropism and differentiation regulate the infectious process of coronaviruses in primary explants of the rat CNS. Virology. 1985 Feb;141(1):89–101. doi: 10.1016/0042-6822(85)90185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beushausen S., Narindrasorasak S., Sanwal B. D., Dales S. In vivo and in vitro models of demyelinating disease: activation of the adenylate cyclase system influences JHM virus expression in explanted rat oligodendrocytes. J Virol. 1987 Dec;61(12):3795–3803. doi: 10.1128/jvi.61.12.3795-3803.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. F., Weismiller D. G., Holmes K. V. Genetic resistance to mouse hepatitis virus correlates with absence of virus-binding activity on target tissues. J Virol. 1987 Jan;61(1):185–189. doi: 10.1128/jvi.61.1.185-189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Lewicki H. A., Talbot P. J., Knobler R. L. Murine hepatitis virus-4 (strain JHM)-induced neurologic disease is modulated in vivo by monoclonal antibody. Virology. 1984 Jan 30;132(2):261–270. doi: 10.1016/0042-6822(84)90033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheley S., Anderson R., Cupples M. J., Chan E. C., Morris V. L. Intracellular murine hepatitis virus-specific RNAs contain common sequences. Virology. 1981 Jul 30;112(2):596–604. doi: 10.1016/0042-6822(81)90305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A. R., Knobler R. L., Powell H., Buchmeier M. J. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoprotein responsible for attachment and cell--cell fusion. Virology. 1982 Jun;119(2):358–371. doi: 10.1016/0042-6822(82)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A. R., Tunison L. A., Knobler R. L. Mouse hepatitis virus type 4 infection of primary glial cultures from genetically susceptible and resistant mice. Infect Immun. 1983 Jun;40(3):1192–1197. doi: 10.1128/iai.40.3.1192-1197.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter-Mackie M., Adler R., Wilson G., Dales S. In vivo and in vitro models of demyelinating diseases. XII. Persistence and expression of corona JHM virus functions in RN2-2 Schwannoma cells during latency. Virus Res. 1985 Oct;3(3):245–261. doi: 10.1016/0168-1702(85)90049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel R. G., Lampert P. W., Talbot P. J., Buchmeier M. J. Site-specific alteration of murine hepatitis virus type 4 peplomer glycoprotein E2 results in reduced neurovirulence. J Virol. 1986 Aug;59(2):463–471. doi: 10.1128/jvi.59.2.463-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frana M. F., Behnke J. N., Sturman L. S., Holmes K. V. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: host-dependent differences in proteolytic cleavage and cell fusion. J Virol. 1985 Dec;56(3):912–920. doi: 10.1128/jvi.56.3.912-920.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Knobler R. L., Haspel M. V., Oldstone M. B. Mouse hepatitis virus type 4 (JHM strains). induced fatal central nervous system disease. I. genetic control and murine neuron as the susceptible site of disease. J Exp Med. 1981 Apr 1;153(4):832–843. doi: 10.1084/jem.153.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobler R. L., Tunison L. A., Oldstone M. B. Host genetic control of mouse hepatitis virus type 4 (JHM strain) replication. I. Restriction of virus amplification and spread in macrophages from resistant mice. J Gen Virol. 1984 Sep;65(Pt 9):1543–1548. doi: 10.1099/0022-1317-65-9-1543. [DOI] [PubMed] [Google Scholar]
- Lavi E., Suzumura A., Hirayama M., Highkin M. K., Dambach D. M., Silberberg D. H., Weiss S. R. Coronavirus mouse hepatitis virus (MHV)-A59 causes a persistent, productive infection in primary glial cell cultures. Microb Pathog. 1987 Aug;3(2):79–86. doi: 10.1016/0882-4010(87)90066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Le Prevost C., Levy-Leblond E., Virelizier J. L., Dupuy J. M. Immunopathology of mouse hepatitis virus type 3 infection. Role of humoral and cell-mediated immunity in resistance mechanisms. J Immunol. 1975 Jan;114(1 Pt 1):221–225. [PubMed] [Google Scholar]
- Lucas A., Flintoff W., Anderson R., Percy D., Coulter M., Dales S. In vivo and in vitro models of demyelinating diseases: tropism of the JHM strain of murine hepatitis virus for cells of glial origin. Cell. 1977 Oct;12(2):553–560. doi: 10.1016/0092-8674(77)90131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy-Leblond E., Oth D., Dupuy J. M. Genetic study of mouse sensitivity to MHV3 infection: influence of the H-2 complex. J Immunol. 1979 Apr;122(4):1359–1362. [PubMed] [Google Scholar]
- Mizzen L., Cheley S., Rao M., Wolf R., Anderson R. Fusion resistance and decreased infectability as major host cell determinants of coronavirus persistence. Virology. 1983 Jul 30;128(2):407–417. doi: 10.1016/0042-6822(83)90266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Protease activation mutants of sendai virus. Activation of biological properties by specific proteases. Virology. 1976 Jan;69(1):265–277. doi: 10.1016/0042-6822(76)90213-0. [DOI] [PubMed] [Google Scholar]
- Sorensen O., Coulter-Mackie M. B., Puchalski S., Dales S. In vivo and in vitro models of demyelinating disease. IX. Progression of JHM virus infection in the central nervous system of the rat during overt and asymptomatic phases. Virology. 1984 Sep;137(2):347–357. doi: 10.1016/0042-6822(84)90227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen O., Dugre R., Percy D., Dales S. In vivo and in vitro models of demyelinating disease: endogenous factors influencing demyelinating disease caused by mouse hepatitis virus in rats and mice. Infect Immun. 1982 Sep;37(3):1248–1260. doi: 10.1128/iai.37.3.1248-1260.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen O., Perry D., Dales S. In vivo and in vitro models of demyelinating diseases. III. JHM virus infection of rats. Arch Neurol. 1980 Aug;37(8):478–484. doi: 10.1001/archneur.1980.00500570026003. [DOI] [PubMed] [Google Scholar]
- Sorensen O., Saravani A., Dales S. In vivo and in vitro models of demyelinating disease, XXIII: Infection by JHM virus of athymic rats. Adv Exp Med Biol. 1987;218:383–390. doi: 10.1007/978-1-4684-1280-2_47. [DOI] [PubMed] [Google Scholar]
- Sorensen O., Saravani A., Dales S. In vivo and in vitro models of demyelinating disease. XVII. The infectious process in athymic rats inoculated with JHM virus. Microb Pathog. 1987 Feb;2(2):79–90. doi: 10.1016/0882-4010(87)90100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Frelinger J. A. Macrophages and resistance to JHM virus CNS infection. Adv Exp Med Biol. 1981;142:387–398. doi: 10.1007/978-1-4757-0456-3_32. [DOI] [PubMed] [Google Scholar]
- Storz J., Rott R., Kaluza G. Enhancement of plaque formation and cell fusion of an enteropathogenic coronavirus by trypsin treatment. Infect Immun. 1981 Mar;31(3):1214–1222. doi: 10.1128/iai.31.3.1214-1222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Ricard C. S., Holmes K. V. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: activation of cell-fusing activity of virions by trypsin and separation of two different 90K cleavage fragments. J Virol. 1985 Dec;56(3):904–911. doi: 10.1128/jvi.56.3.904-911.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P. J., Salmi A. A., Knobler R. L., Buchmeier M. J. Topographical mapping of epitopes on the glycoproteins of murine hepatitis virus-4 (strain JHM): correlation with biological activities. Virology. 1984 Jan 30;132(2):250–260. doi: 10.1016/0042-6822(84)90032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Beushausen S., Dales S. In vivo and in vitro models of demyelinating diseases. XV. Differentiation influences the regulation of coronavirus infection in primary explants of mouse CNS. Virology. 1986 Jun;151(2):253–264. doi: 10.1016/0042-6822(86)90047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Dales S. In vivo and in vitro models of demyelinating diseases, XX: Replication of coronaviruses in primary neural cultures from genetically resistant and susceptible mice. Adv Exp Med Biol. 1987;218:223–230. doi: 10.1007/978-1-4684-1280-2_27. [DOI] [PubMed] [Google Scholar]
- Yoshikura H., Tejima S. Role of protease in mouse hepatitis virus-induced cell fusion. Studies with a cold-sensitive mutant isolated from a persistent infection. Virology. 1981 Sep;113(2):503–511. doi: 10.1016/0042-6822(81)90178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]