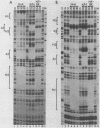
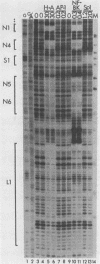
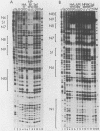
Abstract
The human papovavirus BK has a noncoding regulatory region located between the divergently transcribed early and late coding regions. Many strains of BK virus (BKV) have direct DNA sequence repeats in the regulatory region, although the number and extent of these repeats varies widely between independent isolates. Until recently, little was known about the individual functional elements within the BKV regulatory region, and the biological significance of the variable repeat structure has been unclear. To characterize the interaction between sequences in the BKV regulatory region and host cell transcription factors, we have carried out DNase I footprinting and competitive binding experiments on three strains of BKV, including one strain that does not contain direct sequence repeats. We have used relatively crude fractions from HeLa cell nuclear extracts, as well as DNA affinity-purified preparations of proteins. Our results demonstrate that BK(Dunlop), BK(WW), and BK(MM) each contain multiple binding sites for a factor, NF-BK, that is a member of the nuclear factor 1 family of transcription factors. We predict the presence of three to eight binding sites for NF-BK in the other strains of BKV for which a DNA sequence is available. This suggests that the binding of this protein is likely to be required for biological activity of the virus. In addition to NF-BK sites, BK(WW) and BK(MM) each contain a single binding site for transcription factor Sp1, and BK(Dunlop) contains two binding sites for transcription factor AP-1. The AP-1 sites in BK(Dunlop) span the junction of adjacent direct repeats, suggesting that repeat formation may be an important mechanism for de novo formation of binding sites not present in a parental strain.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Ayer D. E., Dynan W. S. Simian virus 40 major late promoter: a novel tripartite structure that includes intragenic sequences. Mol Cell Biol. 1988 May;8(5):2021–2033. doi: 10.1128/mcb.8.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Blaese R. M., Strober W., Brown R. S., Waldmann T. A. The Wiskott-Aldrich syndrome. A disorder with a possible defect in antigen processing or recognition. Lancet. 1968 May 18;1(7551):1056–1061. doi: 10.1016/s0140-6736(68)91411-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buetti E., Kühnel B. Distinct sequence elements involved in the glucocorticoid regulation of the mouse mammary tumor virus promoter identified by linker scanning mutagenesis. J Mol Biol. 1986 Aug 5;190(3):379–389. doi: 10.1016/0022-2836(86)90009-4. [DOI] [PubMed] [Google Scholar]
- Caputo A., Corallini A., Grossi M. P., Carrà L., Balboni P. G., Negrini M., Milanesi G., Federspil G., Barbanti-Brodano G. Episomal DNA of a BK virus variant in a human insulinoma. J Med Virol. 1983;12(1):37–49. doi: 10.1002/jmv.1890120105. [DOI] [PubMed] [Google Scholar]
- Coleman D. V., Wolfendale M. R., Daniel R. A., Dhanjal N. K., Gardner S. D., Gibson P. E., Field A. M. A prospective study of human polyomavirus infection in pregnancy. J Infect Dis. 1980 Jul;142(1):1–8. doi: 10.1093/infdis/142.1.1. [DOI] [PubMed] [Google Scholar]
- Davison B. L., Leighton T., Rabinowitz J. C. Purification of Bacillus subtilis RNA polymerase with heparin-agarose. In vitro transcription of phi 29 DNA. J Biol Chem. 1979 Sep 25;254(18):9220–9226. [PubMed] [Google Scholar]
- Deyerle K. L., Cassill J. A., Subramani S. Analysis of the early regulatory region of the human papovavirus BK. Virology. 1987 May;158(1):181–193. doi: 10.1016/0042-6822(87)90252-2. [DOI] [PubMed] [Google Scholar]
- Deyerle K. L., Subramani S. Linker scan analysis of the early regulatory region of human papovavirus BK. J Virol. 1988 Sep;62(9):3378–3387. doi: 10.1128/jvi.62.9.3378-3387.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley J. F., Stillman B. Purification of a cellular, double-stranded DNA-binding protein required for initiation of adenovirus DNA replication by using a rapid filter-binding assay. Mol Cell Biol. 1986 May;6(5):1363–1373. doi: 10.1128/mcb.6.5.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn A., Bollekens J., Staub A., Benoist C., Mathis D. A multiplicity of CCAAT box-binding proteins. Cell. 1987 Sep 11;50(6):863–872. doi: 10.1016/0092-8674(87)90513-7. [DOI] [PubMed] [Google Scholar]
- Dougherty R. M., DiStefano H. S. Isolation and characterization of a papovavirus from human urine. Proc Soc Exp Biol Med. 1974 Jun;146(2):481–487. doi: 10.3181/00379727-146-38131. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Fromm M., Berg P. Simian virus 40 early- and late-region promoter functions are enhanced by the 72-base-pair repeat inserted at distant locations and inverted orientations. Mol Cell Biol. 1983 Jun;3(6):991–999. doi: 10.1128/mcb.3.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Piatak M., Mertz J. E., Weissman S. M., Lebowitz P. Altered utilization of splice sites and 5' termini in late RNAs produced by leader region mutants of simian virus 40. J Virol. 1982 Nov;44(2):610–624. doi: 10.1128/jvi.44.2.610-624.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P. E., Gardner S. D. Strain differences and some serological observations on several isolates of human polyomaviruses. Prog Clin Biol Res. 1983;105:119–132. [PubMed] [Google Scholar]
- Goudsmit J., Baak M. L., Sleterus K. W., Van der Noordaa J. Human papovavirus isolated from urine of a child with acute tonsillitis. Br Med J (Clin Res Ed) 1981 Nov 21;283(6303):1363–1364. doi: 10.1136/bmj.283.6303.1363-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Herr W., Clarke J. The SV40 enhancer is composed of multiple functional elements that can compensate for one another. Cell. 1986 May 9;45(3):461–470. doi: 10.1016/0092-8674(86)90332-6. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Khoury G., Byrne J. C., Takemoto K. K., Martin M. A. Physical map of the BK virus genome. J Virol. 1975 Oct;16(4):959–973. doi: 10.1128/jvi.16.4.959-973.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P. M., Mullarkey M. F., Takemoto K. K., Martin M. A. Characterization of human papovavirus BK DNA. J Virol. 1975 Jan;15(1):173–181. doi: 10.1128/jvi.15.1.173-181.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Tjian R. Sp1 binds to promoter sequences and activates herpes simplex virus 'immediate-early' gene transcription in vitro. Nature. 1985 Sep 12;317(6033):179–182. doi: 10.1038/317179a0. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Kühnel B., Buetti E., Diggelmann H. Functional analysis of the glucocorticoid regulatory elements present in the mouse mammary tumor virus long terminal repeat. A synthetic distal binding site can replace the proximal binding domain. J Mol Biol. 1986 Aug 5;190(3):367–378. doi: 10.1016/0022-2836(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Lecatsas G., van Wyk J. A. DNA viruses in urine after renal transplantation. S Afr Med J. 1978 May 20;53(20):787–788. [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Leegwater P. A., van Driel W., van der Vliet P. C. Recognition site of nuclear factor I, a sequence-specific DNA-binding protein from HeLa cells that stimulates adenovirus DNA replication. EMBO J. 1985 Jun;4(6):1515–1521. doi: 10.1002/j.1460-2075.1985.tb03811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegwater P. A., van der Vliet P. C., Rupp R. A., Nowock J., Sippel A. E. Functional homology between the sequence-specific DNA-binding proteins nuclear factor I from HeLa cells and the TGGCA protein from chicken liver. EMBO J. 1986 Feb;5(2):381–386. doi: 10.1002/j.1460-2075.1986.tb04223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miksicek R., Borgmeyer U., Nowock J. Interaction of the TGGCA-binding protein with upstream sequences is required for efficient transcription of mouse mammary tumor virus. EMBO J. 1987 May;6(5):1355–1360. doi: 10.1002/j.1460-2075.1987.tb02375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Moreau P., Hen R., Wasylyk B., Everett R., Gaub M. P., Chambon P. The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic Acids Res. 1981 Nov 25;9(22):6047–6068. doi: 10.1093/nar/9.22.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowock J., Borgmeyer U., Püschel A. W., Rupp R. A., Sippel A. E. The TGGCA protein binds to the MMTV-LTR, the adenovirus origin of replication, and the BK virus enhancer. Nucleic Acids Res. 1985 Mar 25;13(6):2045–2061. doi: 10.1093/nar/13.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowock J., Sippel A. E. Specific protein-DNA interaction at four sites flanking the chicken lysozyme gene. Cell. 1982 Sep;30(2):607–615. doi: 10.1016/0092-8674(82)90257-4. [DOI] [PubMed] [Google Scholar]
- Nyborg J. K., Dynan W. S., Chen I. S., Wachsman W. Binding of host-cell factors to DNA sequences in the long terminal repeat of human T-cell leukemia virus type I: implications for viral gene expression. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1457–1461. doi: 10.1073/pnas.85.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnani M., Negrini M., Reschiglian P., Corallini A., Balboni P. G., Scherneck S., Macino G., Milanesi G., Barbanti-Brodano G. Molecular and biological properties of BK virus-IR, a BK virus variant isolated from a human tumor. J Virol. 1986 Aug;59(2):500–505. doi: 10.1128/jvi.59.2.500-505.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pater M. M., Pater A., di Mayorca G. Genome analysis of MG virus, a human papovavirus. J Virol. 1981 Sep;39(3):968–972. doi: 10.1128/jvi.39.3.968-972.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauw W., Choufoer J. Isolation of a variant of BK virus with altered restriction endonuclease pattern. Arch Virol. 1978;57(1):35–42. doi: 10.1007/BF01315635. [DOI] [PubMed] [Google Scholar]
- Piatak M., Ghosh P. K., Norkin L. C., Weissman S. M. Sequences locating the 5' ends of the major simian virus 40 late mRNA forms. J Virol. 1983 Nov;48(2):503–520. doi: 10.1128/jvi.48.2.503-520.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatak M., Subramanian K. N., Roy P., Weissman S. M. Late messenger RNA production by viable simian virus 40 mutants with deletions in the leader region. J Mol Biol. 1981 Dec 15;153(3):589–618. doi: 10.1016/0022-2836(81)90409-5. [DOI] [PubMed] [Google Scholar]
- Rosenthal N., Kress M., Gruss P., Khoury G. BK viral enhancer element and a human cellular homolog. Science. 1983 Nov 18;222(4625):749–755. doi: 10.1126/science.6314501. [DOI] [PubMed] [Google Scholar]
- Rubinstein R., Pare N., Harley E. H. Structure and function of the transcriptional control region of nonpassaged BK virus. J Virol. 1987 May;61(5):1747–1750. doi: 10.1128/jvi.61.5.1747-1750.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Somasekhar M. B., Mertz J. E. Sequences involved in determining the locations of the 5' ends of the late RNAs of simian virus 40. J Virol. 1985 Dec;56(3):1002–1013. doi: 10.1128/jvi.56.3.1002-1013.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Rabson A. S., Mullarkey M. F., Blaese R. M., Garon C. F., Nelson D. Isolation of papovavirus from brain tumor and urine of a patient with Wiskott-Aldrich syndrome. J Natl Cancer Inst. 1974 Nov;53(5):1205–1207. doi: 10.1093/jnci/53.5.1205. [DOI] [PubMed] [Google Scholar]
- Villarreal L. P., White R. T., Berg P. Mutational alterations within the simian virus 40 leader segment generate altered 16S and 19S mRNA's. J Virol. 1979 Jan;29(1):209–219. doi: 10.1128/jvi.29.1.209-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Kotake S., Nozawa A., Muto T., Uchida S. Tumorigenicity of human BK papovavirus plaque isolates, wild-type and plaque morphology mutant, in hamsters. Int J Cancer. 1982 May 15;29(5):583–586. doi: 10.1002/ijc.2910290515. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K. Change of DNA near the origin of replication enhances the transforming capacity of human papovavirus BK. J Virol. 1982 Jun;42(3):978–985. doi: 10.1128/jvi.42.3.978-985.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K. Decreasing the number of 68-base-pair tandem repeats in the BK virus transcriptional control region reduces plaque size and enhances transforming capacity. J Virol. 1985 Sep;55(3):823–825. doi: 10.1128/jvi.55.3.823-825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K. Evolutionary changes of transcriptional control region in a minute-plaque viable deletion mutant of BK virus. J Virol. 1986 Aug;59(2):260–266. doi: 10.1128/jvi.59.2.260-266.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K., Nozawa A., Yuasa Y., Uchida S. Viable deletion mutant of human papovavirus BK that induces insulinomas in hamsters. J Virol. 1979 Dec;32(3):934–942. doi: 10.1128/jvi.32.3.934-942.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., de Villiers J., Schaffner W. An SV40 "enhancer trap" incorporates exogenous enhancers or generates enhancers from its own sequences. Cell. 1984 Apr;36(4):983–992. doi: 10.1016/0092-8674(84)90048-5. [DOI] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Wildeman A. G., Zenke M., Schatz C., Wintzerith M., Grundström T., Matthes H., Takahashi K., Chambon P. Specific protein binding to the simian virus 40 enhancer in vitro. Mol Cell Biol. 1986 Jun;6(6):2098–2105. doi: 10.1128/mcb.6.6.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. J., Bernhardt G., Major E. O., di Mayorca G. Comparison of the serology, transforming ability, and polypeptide composition of human papovaviruses isolated from urine. J Virol. 1976 Mar;17(3):762–775. doi: 10.1128/jvi.17.3.762-775.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J. H., Davidson I., Macchi M., Rosales R., Vigneron M., Staub A., Chambon P. In vitro binding of several cell-specific and ubiquitous nuclear proteins to the GT-I motif of the SV40 enhancer. Genes Dev. 1987 Oct;1(8):794–807. doi: 10.1101/gad.1.8.794. [DOI] [PubMed] [Google Scholar]
- Yang R. C., Wu R. BK virus DNA: complete nucleotide sequence of a human tumor virus. Science. 1979 Oct 26;206(4417):456–462. doi: 10.1126/science.228391. [DOI] [PubMed] [Google Scholar]
- de Vries E., van Driel W., van den Heuvel S. J., van der Vliet P. C. Contactpoint analysis of the HeLa nuclear factor I recognition site reveals symmetrical binding at one side of the DNA helix. EMBO J. 1987 Jan;6(1):161–168. doi: 10.1002/j.1460-2075.1987.tb04734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Schegget J., Sol C. J., Baan E. W., van der Noordaa J., van Ormondt H. Naturally occurring BK virus variants (JL and Dik) with deletions in the putative early enhancer-promoter sequences. J Virol. 1985 Jan;53(1):302–305. doi: 10.1128/jvi.53.1.302-305.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]