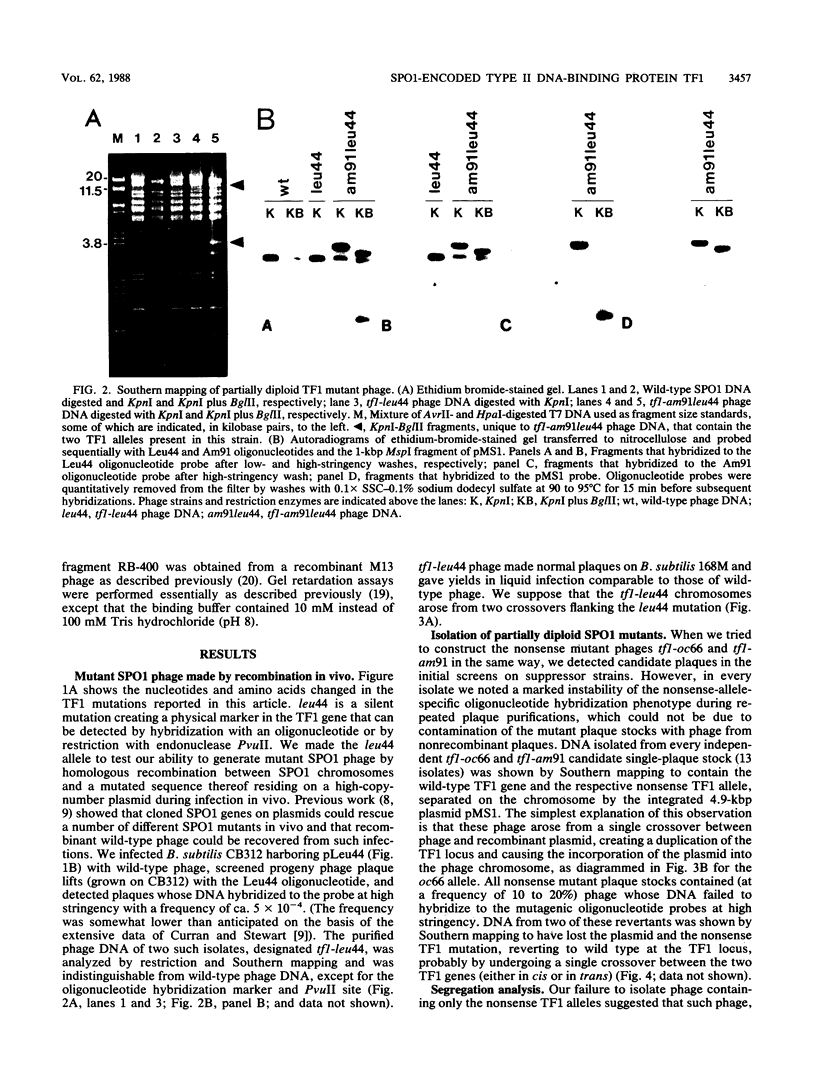
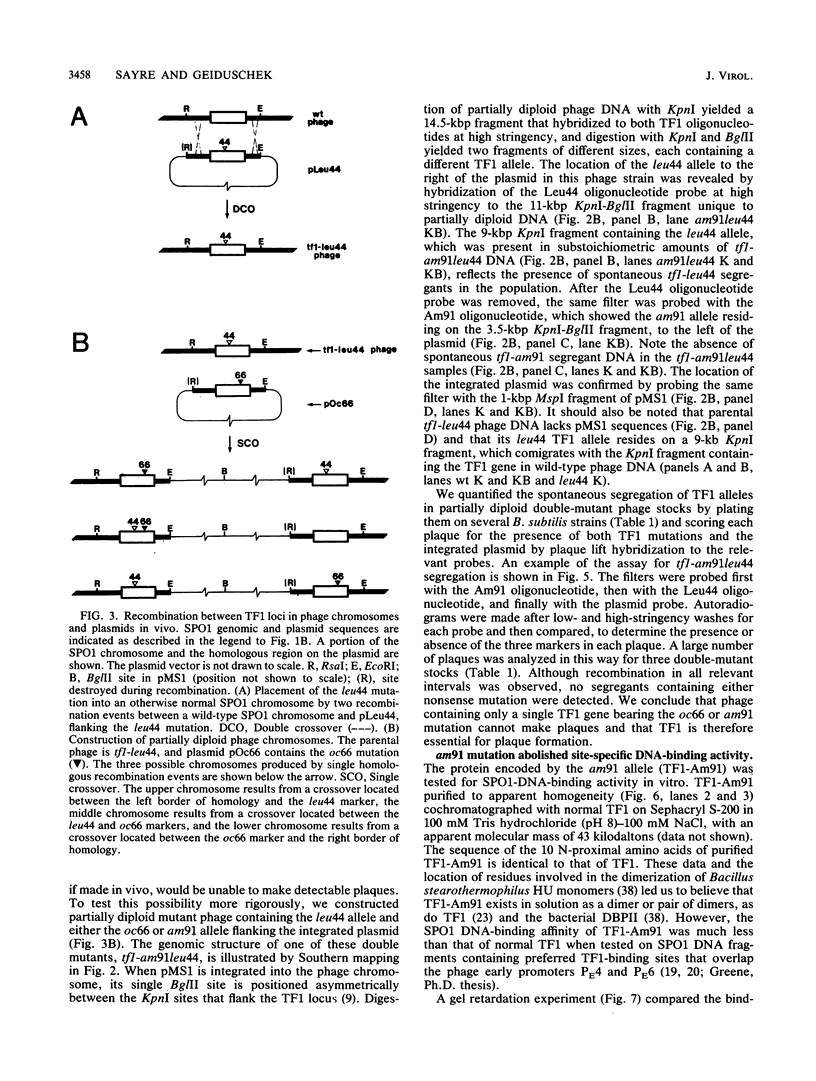
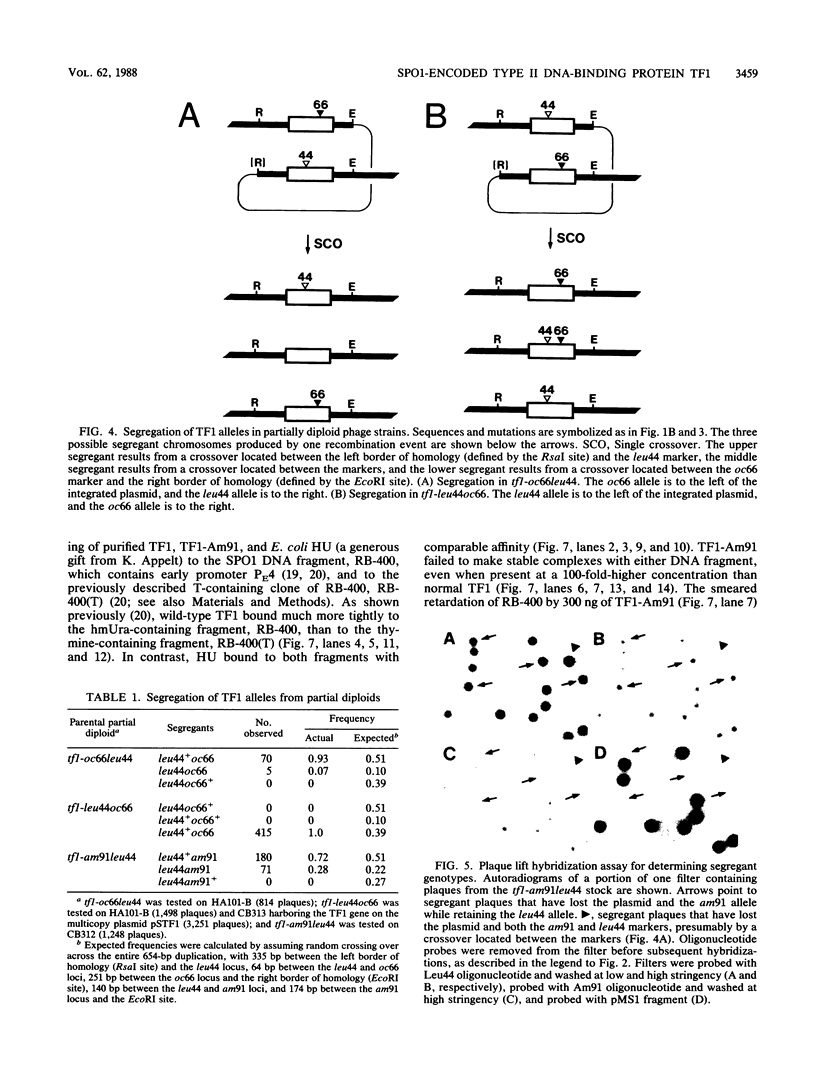
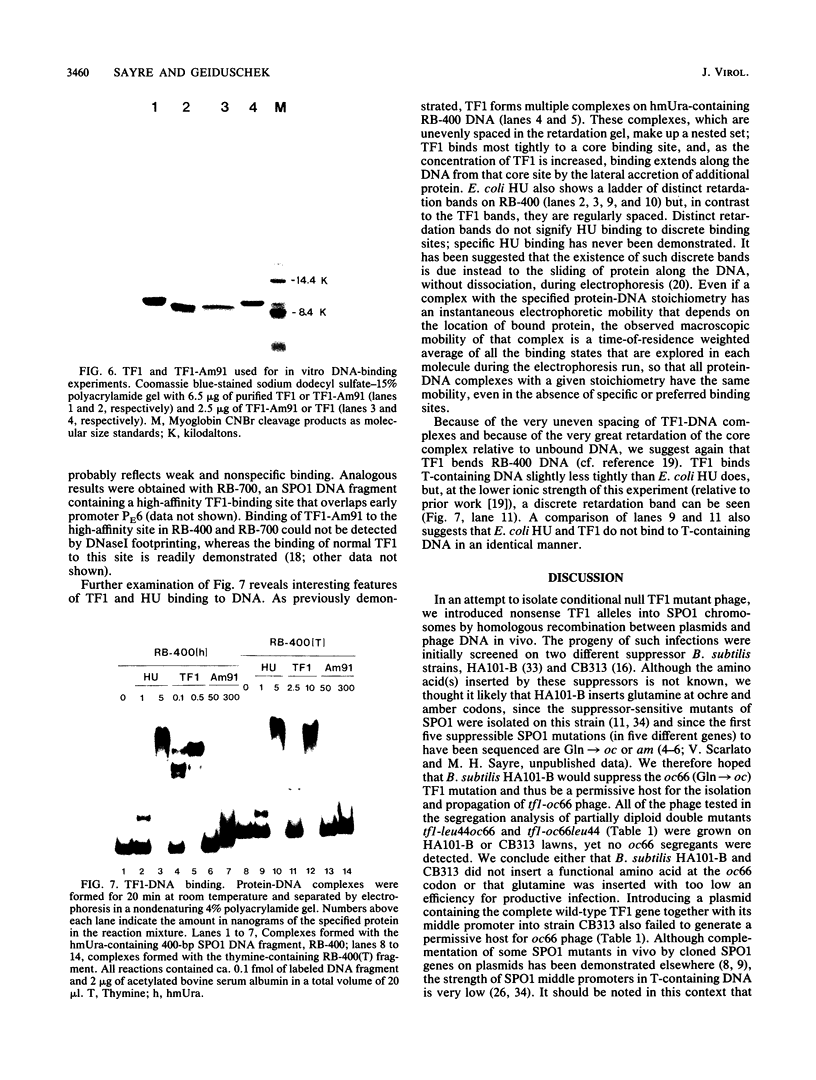
Abstract
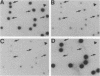
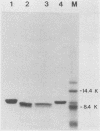
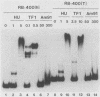
The lytic Bacillus subtilis bacteriophage SPO1 encodes an abundant, 99-amino-acid type II DNA-binding protein, transcription factor 1 (TF1). TF1 is special in this family of procaryotic chromatin-forming proteins in its preference for hydroxymethyluracil-containing DNA, such as SPO1 DNA, and in binding with high affinity to specific sites in the SPO1 chromosome. We constructed recessive null alleles of the TF1 gene and introduced them into SPO1 chromosomes. Segregation analysis with partially diploid phage heterozygous for TF1 showed that phage bearing only these null alleles was inviable. Deletion of the nine C-proximal amino acids of TF1 prohibited phage multiplication in vivo and abolished its site-specific DNA-binding activity in vitro.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Broyles S. S., Pettijohn D. E. Interaction of the Escherichia coli HU protein with DNA. Evidence for formation of nucleosome-like structures with altered DNA helical pitch. J Mol Biol. 1986 Jan 5;187(1):47–60. doi: 10.1016/0022-2836(86)90405-5. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Costanzo M., Brzustowicz L., Hannett N., Pero J. Bacteriophage SPO1 genes 33 and 34. Location and primary structure of genes encoding regulatory subunits of Bacillus subtilis RNA polymerase. J Mol Biol. 1984 Dec 15;180(3):533–547. doi: 10.1016/0022-2836(84)90026-3. [DOI] [PubMed] [Google Scholar]
- Costanzo M., Hannett N., Brzustowicz L., Pero J. Bacteriophage SPO1 gene 27: location and nucleotide sequence. J Virol. 1983 Nov;48(2):555–560. doi: 10.1128/jvi.48.2.555-560.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M., Pero J. Structure of a Bacillus subtilis bacteriophage SPO1 gene encoding RNA polymerase sigma factor. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1236–1240. doi: 10.1073/pnas.80.5.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N. L., Nash H. A. E. coli integration host factor binds to specific sites in DNA. Cell. 1984 Dec;39(3 Pt 2):707–716. doi: 10.1016/0092-8674(84)90478-1. [DOI] [PubMed] [Google Scholar]
- Curran J. F., Stewart C. R. Cloning and mapping of the SPO1 genome. Virology. 1985 Apr 15;142(1):78–97. doi: 10.1016/0042-6822(85)90424-6. [DOI] [PubMed] [Google Scholar]
- Curran J. F., Stewart C. R. Recombination and expression of a cloned fragment of the DNA of Bacillus subtilis bacteriophage SP01. Virology. 1982 Jul 30;120(2):307–317. doi: 10.1016/0042-6822(82)90032-0. [DOI] [PubMed] [Google Scholar]
- Flamm E. L., Weisberg R. A. Primary structure of the hip gene of Escherichia coli and of its product, the beta subunit of integration host factor. J Mol Biol. 1985 May 25;183(2):117–128. doi: 10.1016/0022-2836(85)90206-2. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriophage SPO1 development: six classes of SPO1 RNA. J Mol Biol. 1971 Apr 28;57(2):279–297. doi: 10.1016/0022-2836(71)90346-9. [DOI] [PubMed] [Google Scholar]
- Geider K., Hoffmann-Berling H. Proteins controlling the helical structure of DNA. Annu Rev Biochem. 1981;50:233–260. doi: 10.1146/annurev.bi.50.070181.001313. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Armelin M. C., Petrusek R., Bread C., Duffy J. J., Johnson G. Effects of the transciption inhibitory protein, TF1, on phage SP01 promoter complex formation and stability. J Mol Biol. 1977 Dec 25;117(4):825–842. doi: 10.1016/s0022-2836(77)80001-6. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P. Suppressor system in Bacillus subtilis 168. J Bacteriol. 1969 Mar;97(3):1397–1402. doi: 10.1128/jb.97.3.1397-1402.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J. R., Brennan S. M., Andrew D. J., Thompson C. C., Richards S. H., Heinrikson R. L., Geiduschek E. P. Sequence of the bacteriophage SP01 gene coding for transcription factor 1, a viral homologue of the bacterial type II DNA-binding proteins. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7031–7035. doi: 10.1073/pnas.81.22.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J. R., Geiduschek E. P. Site-specific DNA binding by the bacteriophage SP01-encoded type II DNA-binding protein. EMBO J. 1985 May;4(5):1345–1349. doi: 10.1002/j.1460-2075.1985.tb03783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J. R., Morrissey L. M., Foster L. M., Geiduschek E. P. DNA binding by the bacteriophage SPO1-encoded type II DNA-binding protein, transcription factor 1. Formation of nested complexes at a selective binding site. J Biol Chem. 1986 Sep 25;261(27):12820–12827. [PubMed] [Google Scholar]
- Greene J. R., Morrissey L. M., Geiduschek E. P. DNA binding by the bacteriophage SPO1-encoded type II DNA-binding protein, transcription factor 1. Site-specific binding requires 5-hydroxymethyluracil-containing DNA. J Biol Chem. 1986 Sep 25;261(27):12828–12833. [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. G., Geiduschek E. P. Purification of the bacteriophage SP01 transcription factor 1. J Biol Chem. 1972 Jun 10;247(11):3571–3578. [PubMed] [Google Scholar]
- Johnson G. G., Geiduschek E. P. Specificity of the weak binding between the phage SPO1 transcription-inhibitory protein, TF1, and SPO1 DNA. Biochemistry. 1977 Apr 5;16(7):1473–1485. doi: 10.1021/bi00626a036. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee G., Hannett N. M., Korman A., Pero J. Transcription of cloned DNA from Bacillus subtilis phage SP01. Requirement for hydroxymethyluracil-containing DNA by phage-modified RNA polymerase. J Mol Biol. 1980 May 25;139(3):407–422. doi: 10.1016/0022-2836(80)90138-2. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- Marqusee S., Baldwin R. L. Helix stabilization by Glu-...Lys+ salt bridges in short peptides of de novo design. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8898–8902. doi: 10.1073/pnas.84.24.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- McKenzie T., Hoshino T., Tanaka T., Sueoka N. The nucleotide sequence of pUB110: some salient features in relation to replication and its regulation. Plasmid. 1986 Mar;15(2):93–103. doi: 10.1016/0147-619x(86)90046-6. [DOI] [PubMed] [Google Scholar]
- Okubo S., Yanagida T., Fujita D. J., Olsson-Wilhelm B. M. The genetics of bacteriophage SPO1. Biken J. 1972 Jun;15(2):81–97. [PubMed] [Google Scholar]
- Okubo S., Yanagida T. Isolation of a suppressor mutant in Bacillus subtilis. J Bacteriol. 1968 Mar;95(3):1187–1188. doi: 10.1128/jb.95.3.1187-1188.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo J. M., Greene J. R., Richards S. H., Geiduschek E. P. The phage SPO1-specific RNA polymerase, E.gp28, recognizes its cognate promoters in thymine-containing DNA. Virology. 1986 Aug;153(1):46–52. doi: 10.1016/0042-6822(86)90006-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tanaka I., Appelt K., Dijk J., White S. W., Wilson K. S. 3-A resolution structure of a protein with histone-like properties in prokaryotes. Nature. 1984 Aug 2;310(5976):376–381. doi: 10.1038/310376a0. [DOI] [PubMed] [Google Scholar]
- Wilson D. L., Geiduschek E. P. A template-selective inhibitor of in vitro transcription. Proc Natl Acad Sci U S A. 1969 Feb;62(2):514–520. doi: 10.1073/pnas.62.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]