Abstract
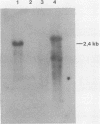
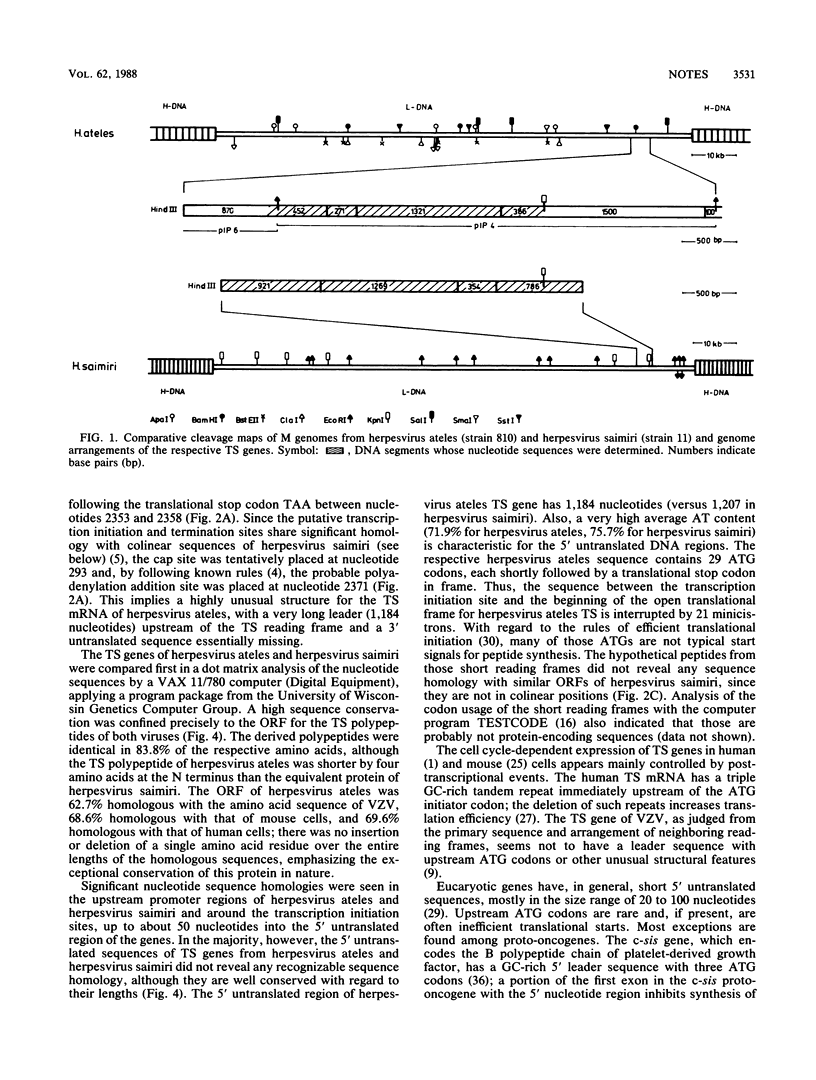
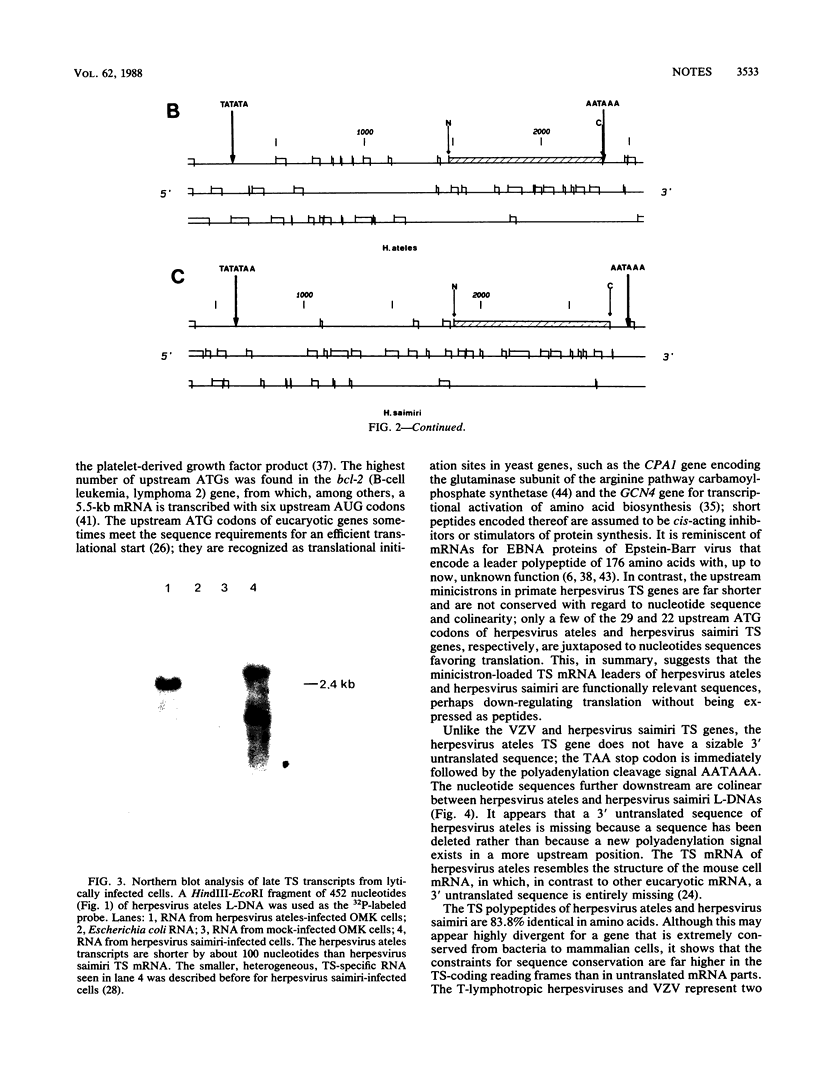
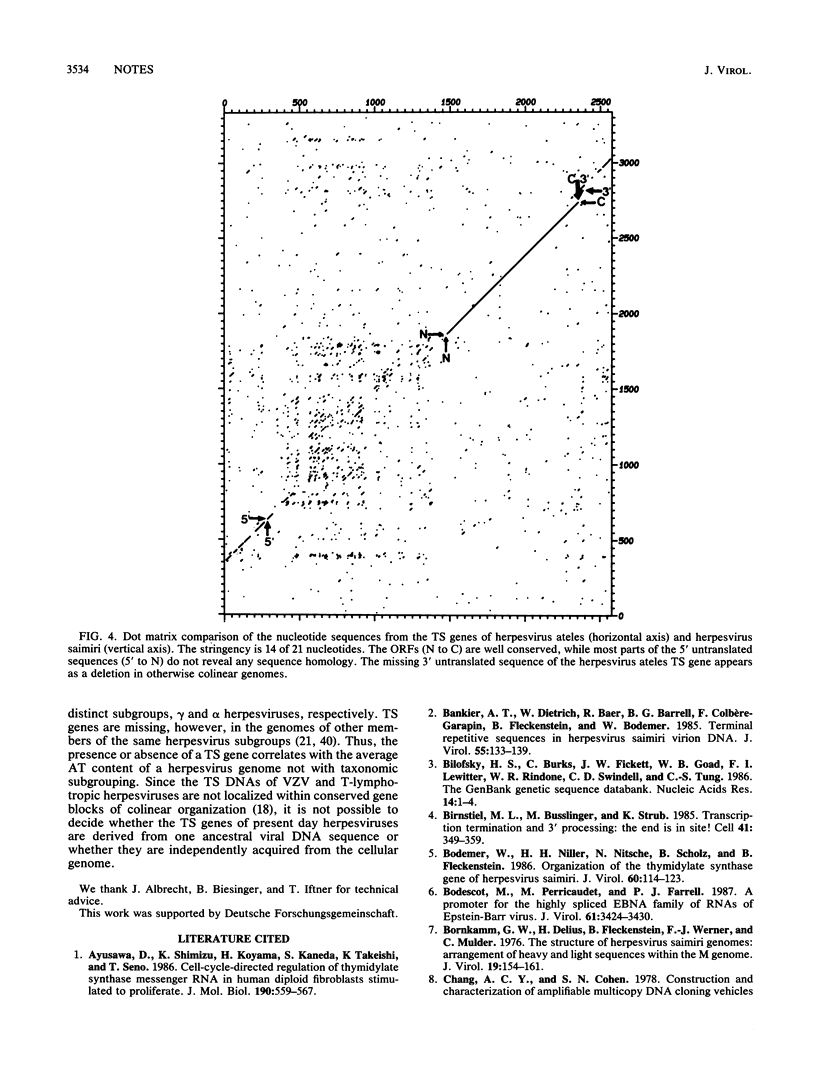
The putative thymidylate synthase (TS) gene of herpesvirus ateles, a T-lymphotropic tumor virus of New World primates, has a single large open reading frame encoding a polypeptide of 32.9 kilodaltons. The gene is transcribed into an unspliced 2.4-kilobase mRNA that is abundantly expressed late in virus replication. The AT-rich 5' untranslated leader sequence of TS mRNA in herpesvirus ateles-infected cells is remarkable in length (1,184 nucleotides), containing 29 minicistrons; this may indicate a role in translation regulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Shimizu K., Koyama H., Kaneda S., Takeishi K., Seno T. Cell-cycle-directed regulation of thymidylate synthase messenger RNA in human diploid fibroblasts stimulated to proliferate. J Mol Biol. 1986 Aug 20;190(4):559–567. doi: 10.1016/0022-2836(86)90241-x. [DOI] [PubMed] [Google Scholar]
- Bankier A. T., Dietrich W., Baer R., Barrell B. G., Colbère-Garapin F., Fleckenstein B., Bodemer W. Terminal repetitive sequences in herpesvirus saimiri virion DNA. J Virol. 1985 Jul;55(1):133–139. doi: 10.1128/jvi.55.1.133-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilofsky H. S., Burks C., Fickett J. W., Goad W. B., Lewitter F. I., Rindone W. P., Swindell C. D., Tung C. S. The GenBank genetic sequence databank. Nucleic Acids Res. 1986 Jan 10;14(1):1–4. doi: 10.1093/nar/14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Bodemer W., Niller H. H., Nitsche N., Scholz B., Fleckenstein B. Organization of the thymidylate synthase gene of herpesvirus saimiri. J Virol. 1986 Oct;60(1):114–123. doi: 10.1128/jvi.60.1.114-123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodescot M., Perricaudet M., Farrell P. J. A promoter for the highly spliced EBNA family of RNAs of Epstein-Barr virus. J Virol. 1987 Nov;61(11):3424–3430. doi: 10.1128/jvi.61.11.3424-3430.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkamm G. W., Delius H., Fleckenstein B., Werner F. J., Mulder C. Structure of Herpesvirus saimiri genomes: arrangement of heavy and light sequences in the M genome. J Virol. 1976 Jul;19(1):154–161. doi: 10.1128/jvi.19.1.154-161.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk L. A., Nigida S. M., Deinhardt F., Wolfe L. G., Cooper R. W., Hernandez-Camacho J. I. Herpesvirus ateles: properties of an oncogenic herpesvirus isolated from circulating lymphocytes of spider monkeys (Ateles sp.). Int J Cancer. 1974 Oct 15;14(4):473–482. doi: 10.1002/ijc.2910140407. [DOI] [PubMed] [Google Scholar]
- Falk L., Johnson D., Deinhardt F. Transformation of marmoset lymphocytes in vitro with Herpesvirus ateles. Int J Cancer. 1978 May 15;21(5):652–657. doi: 10.1002/ijc.2910210517. [DOI] [PubMed] [Google Scholar]
- Falk L., Wright J., Wolfe L., Deinhardt F. Herpesvirus ateles: transformation in vitro of marmoset splenic lymphocytes. Int J Cancer. 1974 Aug 15;14(2):244–251. doi: 10.1002/ijc.2910140213. [DOI] [PubMed] [Google Scholar]
- Fickett J. W. Recognition of protein coding regions in DNA sequences. Nucleic Acids Res. 1982 Sep 11;10(17):5303–5318. doi: 10.1093/nar/10.17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Mulder C., Werner F. J., Daniel M. D., Falk L. A., Delius H. Herpesvirus ateles DNA and its homology with Herpesvirus saimiri nucleic acid. J Virol. 1978 Jan;25(1):361–373. doi: 10.1128/jvi.25.1.361-373.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gompels U. A., Craxton M. A., Honess R. W. Conservation of gene organization in the lymphotropic herpesviruses herpesvirus Saimiri and Epstein-Barr virus. J Virol. 1988 Mar;62(3):757–767. doi: 10.1128/jvi.62.3.757-767.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Bodemer W., Cameron K. R., Niller H. H., Fleckenstein B., Randall R. E. The A+T-rich genome of Herpesvirus saimiri contains a highly conserved gene for thymidylate synthase. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3604–3608. doi: 10.1073/pnas.83.11.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W. Herpes simplex and 'the herpes complex': diverse observations and a unifying hypothesis. The eighth Fleming lecture. J Gen Virol. 1984 Dec;65(Pt 12):2077–2107. doi: 10.1099/0022-1317-65-12-2077. [DOI] [PubMed] [Google Scholar]
- Hunt R. D., Meléndez L. V., García F. G., Trum B. F. Pathologic features of Herpesvirus ateles lymphoma in cotton-topped marmosets (Saguinus oedipus). J Natl Cancer Inst. 1972 Dec;49(6):1631–1639. doi: 10.1093/jnci/49.6.1631. [DOI] [PubMed] [Google Scholar]
- Jahn G., Knust E., Schmolla H., Sarre T., Nelson J. A., McDougall J. K., Fleckenstein B. Predominant immediate-early transcripts of human cytomegalovirus AD 169. J Virol. 1984 Feb;49(2):363–370. doi: 10.1128/jvi.49.2.363-370.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenh C. H., Deng T. L., Li D. W., DeWille J., Johnson L. F. Mouse thymidylate synthase messenger RNA lacks a 3' untranslated region. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8482–8486. doi: 10.1073/pnas.83.22.8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenh C. H., Geyer P. K., Johnson L. F. Control of thymidylate synthase mRNA content and gene transcription in an overproducing mouse cell line. Mol Cell Biol. 1985 Oct;5(10):2527–2532. doi: 10.1128/mcb.5.10.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen H., Schümperli D., Rosenberg M. Affecting gene expression by altering the length and sequence of the 5' leader. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7698–7702. doi: 10.1073/pnas.81.24.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda S., Takeishi K., Ayusawa D., Shimizu K., Seno T., Altman S. Role in translation of a triple tandemly repeated sequence in the 5'-untranslated region of human thymidylate synthase mRNA. Nucleic Acids Res. 1987 Feb 11;15(3):1259–1270. doi: 10.1093/nar/15.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E., Dietrich W., Fleckenstein B., Bodemer W. Virus-specific transcription in a Herpesvirus saimiri-transformed lymphoid tumor cell line. J Virol. 1983 Nov;48(2):377–383. doi: 10.1128/jvi.48.2.377-383.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez L. V., Hunt R. D., King N. W., Barahona H. H., Daniel M. D., Fraser C. E., Garcia F. G. Herpesvirus ateles, a new lymphoma virus of monkeys. Nat New Biol. 1972 Feb 9;235(58):182–184. doi: 10.1038/newbio235182b0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meléndez L. V., Castellanos H., Barahona H. H., Daniel M. D., Hunt R. D., Fraser C. E., García F. G., King N. W. Two new herpesviruses from spider monkeys (Ateles geoffroyi). J Natl Cancer Inst. 1972 Jul;49(1):233–238. [PubMed] [Google Scholar]
- Mueller P. P., Hinnebusch A. G. Multiple upstream AUG codons mediate translational control of GCN4. Cell. 1986 Apr 25;45(2):201–207. doi: 10.1016/0092-8674(86)90384-3. [DOI] [PubMed] [Google Scholar]
- Rao C. D., Igarashi H., Chiu I. M., Robbins K. C., Aaronson S. A. Structure and sequence of the human c-sis/platelet-derived growth factor 2 (SIS/PDGF2) transcriptional unit. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2392–2396. doi: 10.1073/pnas.83.8.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Thielan B., Collins T. Sequences of the 5' portion of the human c-sis gene: characterization of the transcriptional promoter and regulation of expression of the protein product by 5' untranslated mRNA sequences. Nucleic Acids Res. 1987 Aug 11;15(15):6017–6036. doi: 10.1093/nar/15.15.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Hummel M., Braun D., Birkenbach M., Kieff E. Nucleotide sequences of mRNAs encoding Epstein-Barr virus nuclear proteins: a probable transcriptional initiation site. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5096–5100. doi: 10.1073/pnas.83.14.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Honess R. W., Taylor L., Morran J., Davison A. J. Varicella-zoster virus specifies a thymidylate synthetase. J Gen Virol. 1987 May;68(Pt 5):1449–1455. doi: 10.1099/0022-1317-68-5-1449. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wang F., Petti L., Braun D., Seung S., Kieff E. A bicistronic Epstein-Barr virus mRNA encodes two nuclear proteins in latently infected, growth-transformed lymphocytes. J Virol. 1987 Apr;61(4):945–954. doi: 10.1128/jvi.61.4.945-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M., Feller A., Messenguy F., Piérard A. The leader peptide of yeast gene CPA1 is essential for the translational repression of its expression. Cell. 1987 Jun 19;49(6):805–813. doi: 10.1016/0092-8674(87)90618-0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]