Abstract
Rubella virus E1 glycoprotein normally complexes with E2 in the endoplasmic reticulum (ER) to form a heterodimer that is transported to and retained in the Golgi complex. In a previous study, we showed that in the absence of E2, unassembled E1 subunits accumulate in a tubular pre-Golgi compartment whose morphology and biochemical properties are distinct from both rough ER and Golgi. We hypothesized that this compartment corresponds to hypertrophied ER exit sites that have expanded in response to overexpression of E1. In the present study we constructed BHK cells stably expressing E1 protein containing a cytoplasmically disposed epitope and isolated the pre-Golgi compartment from these cells by cell fractionation and immunoisolation. Double label indirect immunofluorescence in cells and immunoblotting of immunoisolated tubular networks revealed that proteins involved in formation of ER-derived transport vesicles, namely p58/ERGIC 53, Sec23p, and Sec13p, were concentrated in the E1-containing pre-Golgi compartment. Furthermore, budding structures were evident in these membrane profiles, and a highly abundant but unknown 65-kDa protein was also present. By comparison, marker proteins of the rough ER, Golgi, and COPI vesicles were not enriched in these membranes. These results demonstrate that the composition of the tubular networks corresponds to that expected of ER exit sites. Accordingly, we propose the name SEREC (smooth ER exit compartment) for this structure.
INTRODUCTION
The endoplasmic reticulum (ER)1 is the largest endomembrane system within eukaryotic cells and performs a wide variety of functions including calcium uptake and release, lipid and protein synthesis, protein translocation, folding, glycosylation, concentration, and export to the Golgi complex (for review see Rose and Doms, 1988; Hurtley and Helenius, 1989; Sitia and Meldolesi, 1992). Classically, the ER has been recognized to be composed of three morphologically distinct subcompartments, rough ER (RER), smooth ER, and the nuclear envelope. Recently, however, it has been suggested that the ER may be further divided into specialized subdomains that are distinct in terms of their protein constituents and/or morphological appearance (Sitia and Meldolesi, 1992; Nishikawa et al., 1994).
Subdomains of the ER thought to be involved in protein concentration and export can be distinguished on the basis of morphology and/or the presence of marker proteins. In cells engaged in secretion of large amounts of proteins, transitional elements of the ER (Palade, 1975) can be identified by their characteristic part-rough/part-smooth appearance. This region of the ER is involved in the formation of transport vesicles that ferry cargo to the Golgi complex and can be further subdivided into two subdomains: 1) a domain that sequesters Sec23p, a protein component of COPII vesicles (Barlowe et al., 1994) (classical transitional elements), and 2) a region enriched in components of COPI vesicles (coatomer-rich ER) (Orci et al., 1991, 1994). Presumably, the Sec23p-rich region of the ER is involved in generation of anterograde vesicles containing cargo destined for the Golgi and beyond, whereas coatomer-rich ER may be involved in retrograde transport from the Golgi. Isolated ER membranes from Saccharomyces cerevisiae have been shown to produce both COPI and COPII vesicles in vitro (Bednarek et al., 1995), so it is also possible that the coatomer-rich ER is involved in anterograde transport.
We have previously demonstrated that unassembled rubella virus (RV) E1 subunits accumulate in a tubular pre-Golgi compartment, which is a subdomain of the ER and is in continuity with RER cisternae (Hobman et al., 1992). This compartment was not affected by pharmacological reagents that disrupt the structure of RER and Golgi, and indirect immunofluorescence indicated that a number of ER proteins were excluded from the E1-containing tubules despite their continuity with the RER. We also demonstrated that an itinerant membrane protein, vesicular stomatitis virus (VSV) G protein, was able to enter and exit these tubular networks (Hobman et al., 1992, 1993). Based on these findings we postulated that expression of unassembled E1 subunits causes the expansion of transitional elements or ER exit sites. In the present study, we have further characterized this pre-Golgi compartment using a combination of indirect immunofluorescence, subcellular fractionation, and immunoisolation. The results from this study provide additional evidence to support our hypothesis that the E1-containing tubular networks correspond to hypertrophied ER exit sites.
MATERIALS AND METHODS
Reagents
Reagents and supplies were from the following sources: Protein A-Sepharose was purchased from Pharmacia (Alameda, CA). Fibronectin, SDS, dialyzed FBS, and BSA were purchased from Sigma Chemical (St. Louis, MO). Promix [35S]methionine/cysteine (1000 Ci/mmol) and [14C]-labeled protein standards were purchased from Amersham (Arlington Heights, IL). Texas Red-conjugated goat anti-mouse IgG and FITC-conjugated donkey anti-rabbit IgG (each double-labeling grade) were purchased from Jackson ImmnunoResearch Laboratories (West Grove, PA). FITC-conjugated goat anti-human IgG was purchased from Zymed (South San Francisco, CA). Secondary antibodies, goat anti-mouse IgG, and goat anti-rabbit IgG conjugated to HRP were obtained from Bio-Rad (Richmond, CA). Lipofectin, DMEM (high glucose), Optimem serum-free media, FBS, and α-MEM without nucleosides were obtained from Life Technologies (Gaithersburg, MD). MEM lacking cysteine/methionine was purchased from ICN Biomedicals (Irvine, CA). DOSPER transfection reagent, Pefabloc, and Pwo polymerase were purchased from Boehringer Mannheim (Laval, Quebec, Canada). Rabbit antiserum to p58 and α-mannosidase II (Man II) have been described previously (Saraste and Svensson, 1991; Velasco et al., 1993). A mouse hybridoma against p58, 7DB2, was developed by injecting mice with p58 purified from rat pancreas (Hendricks and Farquhar, unpublished data). Rabbit antiserum to the cytoplasmic (CT) domain of VSV G protein was a gift from Dr. Carolyn Machamer (Johns Hopkins University, Baltimore, MD). Anti-ERGIC 53 monoclonal antibody and rabbit anti-p63 (Schweizer et al., 1988, 1993a) were provided by Dr. Hans-Peter Hauri University of Basel (Basel, Switzerland). M3A5, a mouse monoclonal that recognizes β-COP (Allan and Kreis, 1986), was obtained from Dr. Thomas Kreis, (University of Geneva, Geneva, Switzerland). Rabbit anti-ERp 72 serum was provided by Dr. Peter Kim (Harvard Medical School, Boston, MA). Rabbit anti-calnexin antibodies were obtained from Drs. John Bergeron (McGill University, Montreal, Quebec, Canada), David Williams (University of Toronto, Toronto, Ontario, Canada), or were purchased from Stressgen (Victoria, British Columbia, Canada). Rabbit antibodies to Sec23p and Sec13p were kindly provided by Drs. Randy Schekman (University of California, Berkeley, CA) and Chris Kaiser (Massachusetts Institute of Technology, Cambridge, MA), respectively. Rabbit antiserum to the β-subunit of glucosidase II (Arendt and Ostergaard, 1997) was a gift from Dr. Hanne Ostergaard (Department of Medical Microbiology and Immunology, University of Alberta, Alberta, Canada). Antibodies to calreticulin, BiP, protein disulfide isomerase, and GRP94 were also from Stressgen. Vero cells were obtained from Dr. Steve Rice (Biochemistry Department, University of Alberta). BHK cells were obtained from Dr. Shirley Gillam, British Columbia Children’s Research Centre (Vancouver, British Columbia, Canada).
Rabbit antiserum to the CT domain of rubella E1 was produced by immunizing rabbits with a synthetic peptide NH2-KGLYYLRGAIAPR-COOH coupled to rabbit serum albumin with glutaraldehyde. All injections and bleeds were done by Chemicon (Temecula, CA).
cDNA Constructs
Construction of the E1 cDNA construct has been described previously (Hobman et al., 1988). The E1-GTMCT construct was prepared by replacing the 600-base pair (bp) BamHI fragment that encodes the carboxy-terminal 175 amino acids of E1, from pCMV5-E1 with the 600-bp BamHI fragment from pCMV5- E2E1-GTMCT (Hobman et al., 1995). E1-GCT was constructed by PCR using the antisense primer 5′-CGCAAG CTT ACT TTC CAA GTC GGT TCA TCT CTA TGT CTG TGC GCG GTG CTA TAG CGC C-3′. This primer introduces the carboxy-terminal 10 amino acids and a stop codon from VSV G protein at the end of the E1 CT domain. All E1 cDNAs were subcloned into the mammalian cell expression vectors pCMV5 (Andersson et al., 1989) and pNUT (Palmiter et al., 1987) for expression in CHO and BHK cells, respectively.
PCRs
Pwo polymerase was used in PCRs according to the manufacturer’s instructions to introduce the epitope recognized by P5D4 into the rubella E1 cDNA. Generally, 20–30 cycles were used for each reaction to minimize the chances of introducing second-site mutations. All products were verified by DNA sequencing.
Cell Culture and Transfection
CHODG44 cells were cultured and stably transfected as described (Hobman et al., 1992). BHK cells were cultured in DMEM hi-glucose, 10% FBS, antibiotics, 15 mM HEPES. BHK cells were transfected with pNUT-E1 cDNAs, and stable transformants were selected with 500 μM methotrexate. Stably transfected cells were named according to the recombinant protein they were expressing. For example, BHK-E1-GCT cells express rubella E1 fused to the carboxy-terminal 10 amino acids of VSV G (see Figure 1). For transient expression, Vero cells cultured in DMEM hi-glucose containing 5% FBS were transfected using DOSPER (Boehringer Mannheim) according to manufacturer’s instructions.
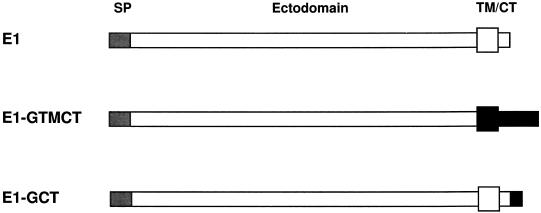
Figure 1.
Epitope-tagged E1 constructs. The protein E1-GTMCT contains the VSV G TM and CT domains in place of the corresponding E1 domains, whereas E1-GCT has the C-terminal 10 amino acids from VSV G fused to the end of the entire E1 molecule. N-terminal signal peptides (SP) are indicated as hatched boxes. C-terminal TM and CT domains (TM/CT) are represented by white (E1) or black (VSV G) regions.
Metabolic Labeling and Radioimmunoprecipitation
Confluent 35-mm dishes of cells were washed once with PBS and incubated in MEM minus cysteine and methionine with 5% dialyzed FBS for 15 min at 37°C. Cells were labeled for 10–15 min with 150 μCi [35S]Promix in 250 μl of the same media followed by chase periods in growth media containing 25× excess methionine and cysteine. Radiolabeled cells were washed three times with ice-cold PBS and lysed on ice in 500 μl of 1% NP-40, 150 mM NaCl, 50 mM Tris-HCl, pH 8.0, containing 100 μg/ml of the protease inhibitor Pefabloc. Lysates were centrifuged at 14,000 × g for 5 min at 4°C before immunoprecipitation with human anti-RV serum and protein A-Sepharose. Immune complexes were washed three times with RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 50 mM Tris-HCl, pH 8.0), and once with water. Endo H digestions were performed as described previously (Hobman et al., 1992). Samples were boiled in 2× SDS-gel sample buffer for 5 min before loading onto gels.
SDS-PAGE and Autoradiography
Proteins were separated on 10% polyacrylamide gels before fixation in isopropanol-water-acetic acid (25:65:10) for 30 min. Gels were then soaked in 1.0 M sodium salicylate/0.01% 2-mercaptoethanol for 20 min before drying and exposure to Kodak XAR film at −80°C.
Immunoisolated membranes were solubilized in nonreducing SDS-sample buffer to minimize extraction of primary antibody from the beads. After removal of the beads, eluates were first reduced by addition of 2-mercaptoethanol and subjected to SDS-PAGE on 10% gels, followed by staining with Coomassie R-250.
Immunoblotting
Proteins were transferred from 10% polyacrylamide gels to polyvinylidene difluoride (PVDF) membranes using a semidry transfer apparatus (Tyler Instruments, Edmonton, Alberta, Canada) according to the manufacturer’s instructions. Membranes were blocked in Tris-buffered saline, 0.05% Tween 20 containing 4% skim milk. Primary and secondary antibody incubations were done in the same solution. Membranes were washed three to four times (10 min each) after each antibody in Tris-buffered saline, 0.05% Tween 20. Blots were then developed using ECL reagents from Amersham Canada (Oakville, Ontario, Canada) and exposed to Fuji RX film (Fuji Photo Film, Tokyo, Japan). Protein bands were quantitated using a Bio-Rad densitometer.
Immunofluorescence Microscopy
Cells were cultured on 12-mm glass coverslips, fixed with methanol at minus 20°C, and processed for indirect immunofluorescence as described (Hobman et al., 1992).
Subcellular Fractionation and Immunoisolation
BHK-E1GCT cells were grown to confluency in 1600-cm2 roller bottles. Cells were washed once with PBS and detached with trypsin-EDTA followed by washes once each with PBS/5% calf serum and 0.25 M sucrose. Typically, 2–3 ml of packed cells were obtained from four roller bottles. Washed cells were resuspended in four to five volumes of 0.25 M sucrose, 0.5 mM MgCl2 containing Pefabloc and homogenized by passage 5 to 10 times through a ball-bearing homogenizer (Balch et al., 1984), 0.012 inch clearance. Homogenates were diluted with an equal volume of 0.25 M sucrose and centrifuged twice at 1000 × g/4°C for 10 min to obtain a postnuclear supernatant (PNS). A procedure adapted from Bergstrand and Dallner (1969) was used to separate smooth membranes from rough microsomes. The PNS was adjusted to 15 mM CsCl to cause aggregation of rough membranes before loading onto 3.5 ml of 1.3 M sucrose/15 mM CsCl in SW41 tubes. Samples were centrifuged at 41,000 rpm for 3 h at 4°C. Aggregated rough microsomes sedimented through the 1.3 M sucrose layer and pelleted at the bottom of the tubes whereas smooth membranes collected on top of the 1.3 M sucrose layer. Smooth microsomes were used as starting material for immunoisolation since this is where the majority of E1 or E1-GCT was found (see Figure 6). Typically, 12–18 mg of smooth microsomes were used for each immunoisolation.
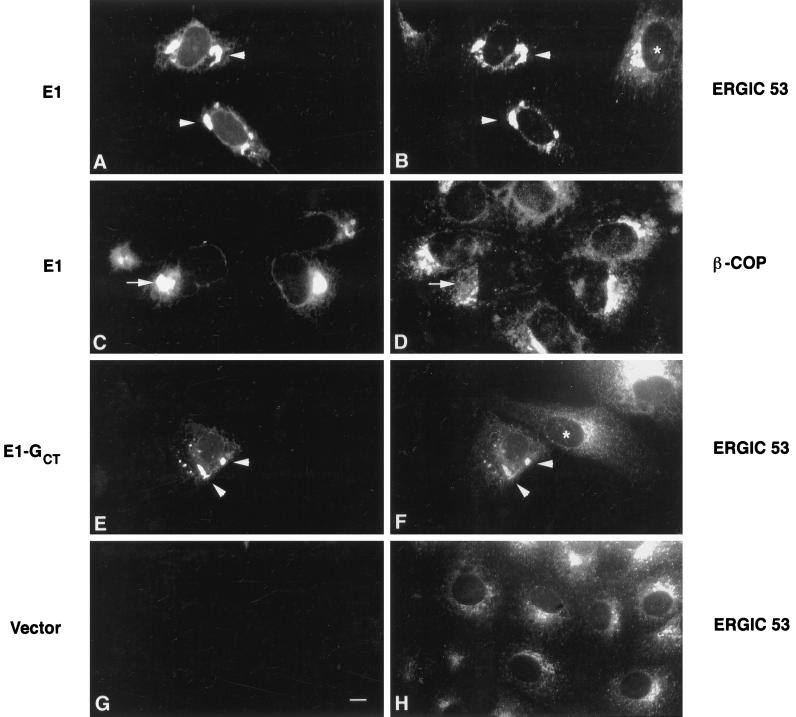
Figure 6.
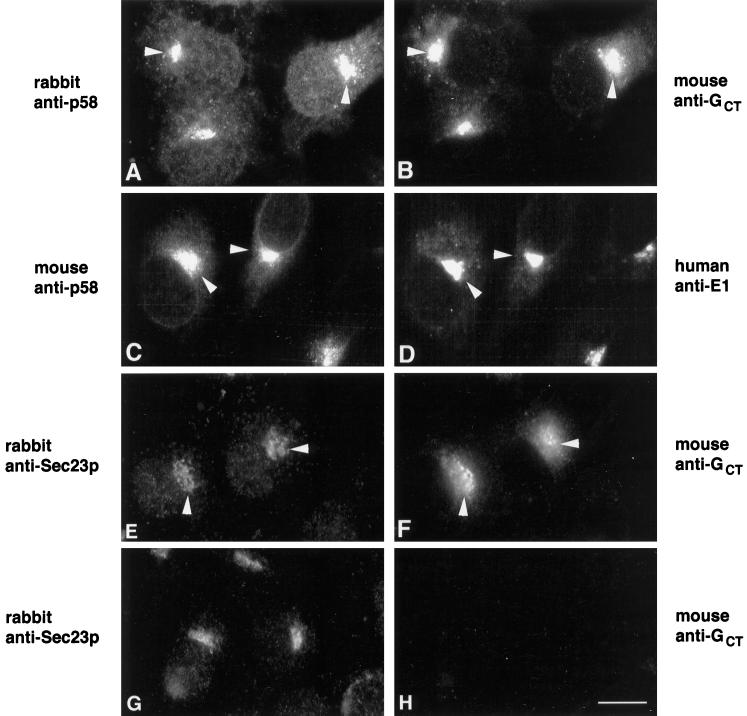
ERGIC 53, but not β-COP, colocalizes with E1 and E1-GCT in transiently transected Vero cells. Vero cells were transiently transfected with expression plasmids encoding rubella E1 or E1-GCT and processed for indirect immunofluorescence after 40 h as described in Figure 3. E1 and E1-GCT were visualized using human anti-E1 serum and FITC-goat anti-human IgG (panels A, C, E, and G). Monoclonal antibodies to ERGIC 53 and β-COP and Texas Red-goat anti-mouse IgG were used in panels B, D, F, and H as indicated. In cells expressing E1 and E1-GCT, the majority of ERGIC 53 was present in the tubular pre-Golgi compartment (A, B, E, and F, arrowheads), whereas in untransfected cells it was concentrated in a perinuclear vesicular pattern in the Golgi region (B, F, and H, asterisks). In contrast, staining for E1 and β-COP (C and D, arrows) or E1-GCT and β-COP (our unpublished observations) did not overlap significantly. In panel G, cells were transfected with vector alone to show that the human anti-E1 serum did not cross-react with endogenous ERGIC 53. Bar, 10 μm.
M500 Dynabeads (Dynal, Lake Success, NY) covalently coated with goat anti-mouse IgG1 (Fc-specific) were incubated with P5D4 IgG (2 μg IgG/mg beads) in immunoisolation buffer (PBS, 5% FBS, 2 mM EDTA) for 2–4 h at 4°C on a rotating device. The beads were immobilized on a magnetic rack, and the IgG solution was removed and replaced with immunoisolation buffer for a total of four washes. E1-GCT–containing membranes were extracted from smooth microsomes with P5D4-coated magnetic beads by gentle rotation at 4°C for 8–16 h. Beads were washed four to five times (15 min each) with immunoisolation buffer before processing for EM. If samples were to be used for gel electrophoresis followed by immunoblotting or Coomassie blue staining, three PBS washes were used to wash away the FBS.
Protein Determination
Protein microassays were performed using a modified protocol derived from Winterborne (1993). Cell fractions solublized in SDS sample buffer were spotted onto 1-cm square Whatman 3 MM paper and stained for 1 h with 0.04% Coomassie blue R250 in 25% ethanol/12% acetic acid. Samples were destained with 10% ethanol, 5% acetic acid before drying. The samples were then eluted with 1 ml of 1 M potassium acetate/70% ethanol for 1 h and the absorbance at 590 nm was determined. BSA was used as the protein standard.
Electron Microscopy
Cells grown on 12-mm coverslips were fixed in 1.5% glutaraldehyde/0.1 M cacodylate (pH 7.4) containing 5% sucrose for 1 h at room temperature, followed by three washes (5 min each) in cacodylate buffer. Samples were then postfixed with 1% OsO4/0.05 M potassium ferricyanide/0.1 M phosphate buffer (pH 7.4) for 1 h on ice followed by three water washes (5 min each). Cells were dehydrated in a graded series of ethanol and embedded in Epon. Sections were cut parallel to the coverslips and stained with 2% uranyl acetate and lead citrate.
Immunoisolated tubular networks still attached to magnetic beads were fixed in 3% glutaraldehyde/0.1 M cacodylate (pH 7.4) for 1 h at room temperature followed by three washes (5 min each) in cacodylate buffer. Samples were postfixed with OsO4 as described above and stained en bloc with 2% uranyl acetate, dehydrated in ethanol, and embedded in epon. Where indicated, rabbit anti-GCT and Protein A gold (5 nm) were used to detect E1-GCT in isolated membranes. Samples were examined with a Philips CM-10 or 410 electron microscope (Philips Technologies, Cheshire, CT).
RESULTS
Replacement of the E1 Transmembrane (TM) and CT Domains Does Not Affect Localization to the Tubular Networks
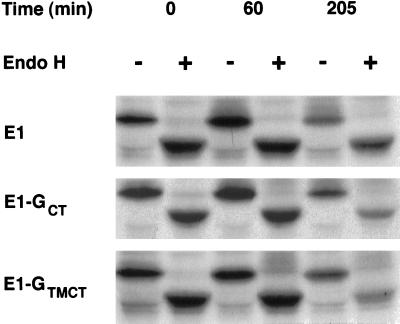
As a first step in establishing the function of the E1-containing membranes, we set out to isolate them from stably transfected CHO cells expressing RV E1 (CHO-E1) (Hobman et al., 1992) by subcellular fractionation and immunoisolation on magnetic beads (Howell et al., 1994). Preliminary fractionation experiments indicated that the bulk of E1 could be recovered in crude smooth membrane preparations (our unpublished observations). The smooth membrane fractions were largely depleted of RER markers and therefore represented a suitable starting material for purification of the E1-containing compartment by immunoadsorption with antibodies directed to the CT domain of E1. Initially, we raised a rabbit polyclonal serum against the 13-amino acid CT domain of E1 to be used for immunoisolation; however, it could not be used due to inactivation during affinity purification (our unpublished observations). To circumvent this problem, we constructed E1 variants that contained an epitope from the CT domain of VSV G protein, which is recognized by the monoclonal antibody P5D4. The resulting two constructs, E1-GTMCT and E1-GCT, contain the ectodomain of E1 fused inframe to the TM and CT domains of VSV G or the entire E1 fused to the last 10 amino acids of the VSV G CT domain, respectively (Figure 1). To verify that these E1 chimeras were localized to the same pre-Golgi compartment as E1, CHO cells stably expressing these proteins were analyzed by indirect immunofluorescence and biosynthetic labeling. The resultant constructs, E1-GCT and E1-GTMCT, behaved similarly to E1: they were localized to tubular membranous structures situated in the juxtanuclear region (our unpublished observations) and remained largely sensitive to endo H after 3.5 h chase (Figure 2). These results indicate that the TM/CT domains of E1 are not required for its segregation into the tubular networks and that altering these domains does not result in transport to the Golgi complex.
Figure 2.
E1-GTMCT and E1-GCT are retained in a pre-Golgi compartment. Stably transfected CHO cells expressing E1, E1-GCT, or E1-GTMCT were pulse labeled for 15 min with 35S-methionine/cysteine and then incubated for 0, 60, or 205 min in media containing excess methionine and cysteine. The cells were lysed and immunoprecipitated with human antirubella virus serum and protein A-Sepharose. The immune complexes were denatured, incubated at 37°C with or without endo H for 16 h, and then separated by SDS-PAGE on 10% gels followed by fluorography. Similar to E1, the bulk of E1-GCT and E1-GTMCT remains sensitive to endo H after 205 min.
Construction of Stably Transfected BHK Cells Expressing E1-GCT
Previously we showed by indirect immunofluorescence that Golgi markers such as Man II and RER membrane proteins such as calnexin were absent from the tubular networks in CHODG44 cells (Hobman et al., 1992). We could not determine whether p58, a marker of the ER-Golgi intermediate compartment (ERGIC) (Saraste et al., 1987), was present, because staining for p58 was very weak in CHODG44 cells. To circumvent this problem we expressed E1-GCT in BHK cells, which gave a stronger p58 signal by indirect immunofluorescence.
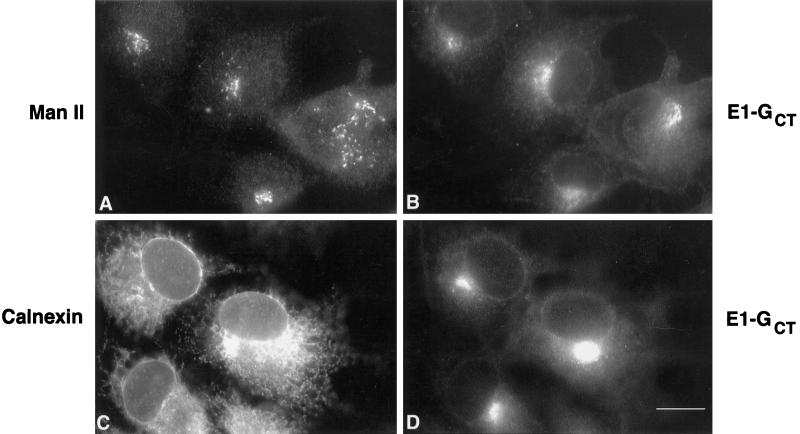
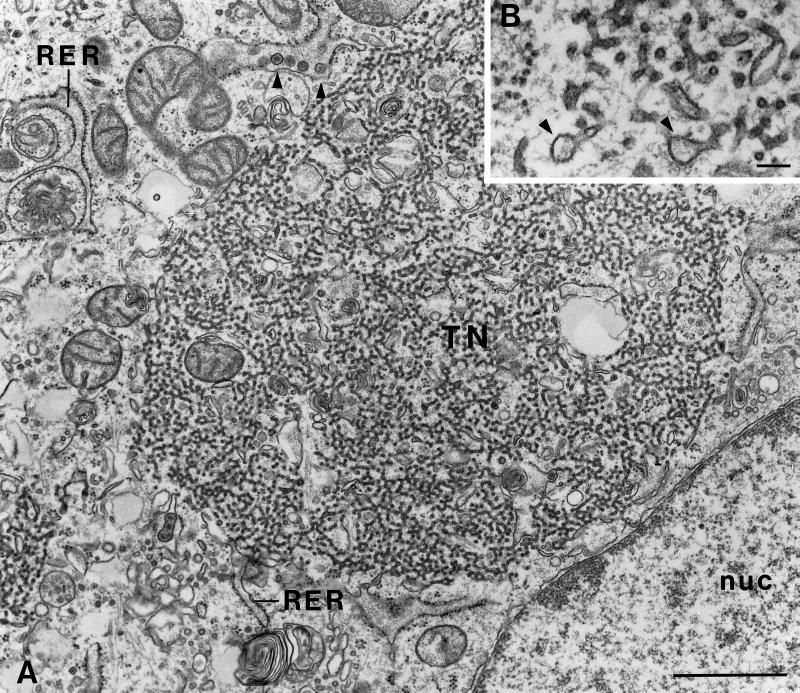
To establish that BHK cells behaved the same as CHO cells we examined stably transfected BHK cells expressing E1-GCT by immunofluorescence after staining with P5D4 and found that, as in the case of CHO cells, E1-GCT was confined mainly to structures of variable size located in the juxtanuclear region whose distribution was distinct from Golgi (Man II) (Figure 3, A and B) and ER (calnexin) markers (Figure 3, C and D). Electron microscopy (EM) verified that BHK-E1-GCT cells contain tubular networks comparable to those in CHO-E1 cells (Figure 4, A and B). As with many cultured cell lines, BHK cells contain a latent R-type retrovirus (Urade et al., 1993), but the virus particles were present only in the RER and were usually absent from the tubular networks in BHK cells (Figure 4A, arrowheads). Based on these findings and the fact that we could obtain significantly more cell mass from the BHK-E1-GCT cells, immunoisolation was performed using BHK-E1-GCT cells.
Figure 3.
E1-GCT does not colocalize with Golgi or RER markers in BHK cells. Stably transfected BHK cells expressing E1-GCT grown on 12-mm coverslips were fixed and permeabilized with methanol at −20°C before processing for double label indirect immunofluorescence. Golgi and RER membranes were labeled, respectively, with rabbit anti-mannosidase II (Man II) and anticalnexin in panels A and C, respectively. E1-GCT was stained in panels B and D using a monoclonal antibody, P5D4, directed against the VSV G CT domain. Secondary antibodies were FITC-donkey anti-rabbit IgG and Texas Red-goat anti-mouse IgG. Bar, 10 μm.
Figure 4.
BHK-E1-GCT cells contain elaborate arrays of tubular networks. BHK-E1-GCT cells grown on 12-mm coverslips were processed for EM as described above. (A) A nest of smooth tubular membranes (TN) can be seen in the juxtanuclear region. The diameter of the tubules is ∼25–30 nm. The type R virus particles, which measure approximately 110 nm in diameter, have a round nucleoid with radial spokes and were most often observed in distended cisterna of the RER (arrowheads). Bar, 1 μm. nuc, nucleus. (B) Distended membrane profiles that may correspond to budding structures can be seen at the ends of tubules (arrowheads) at higher magnification. Bar, 0.1 μm.
The ERGIC Marker p58/ERGIC 53 and the Transitional ER Marker Sec23p Overlap with E1-GCT in the Juxtanuclear Region
Our earlier work indicating that a cargo protein, VSV G, is able to enter and exit the E1-containing tubular networks (Hobman et al., 1992, 1993) suggested that proteins involved in the selection and transport of cargo might be concentrated in this compartment. To find out if this is the case, we determined the distribution in BHK E1-GCT cells of Sec23p, a component of COPII vesicles that mediates cargo export from the ER (Barlowe et al., 1994), and p58, a mannose lectin that continually cycles between the ER and Golgi complex via ERGIC (Saraste and Svensson, 1991). Sec23p is concentrated in the transitional ER from which COPII vesicles bud (Orci et al., 1994). We found by indirect immunofluorescence that Sec23p and E1-GCT were concentrated in very similar, but not identical, juxtanuclear profiles (Figure 5, E and F, arrowheads). Considerable overlap was also observed between E1-GCT and p58 (Figure 5, A–D), but as with Sec23p, the distributions of E1-GCT and p58 were not identical. This was not unexpected since under these conditions, E1 is not transported beyond the tubular networks, whereas Sec23p and p58 are incorporated into ER-derived vesicles. In transiently transfected Vero cells, the overlap between E1-GCT or E1 and the primate p58 homolog, ERGIC-53, was even more pronounced (Figure 6 A, B, E, and F, arrowheads). By contrast, in nontransfected cells, the bulk of the ERGIC-53 was restricted to the Golgi region (Figure 6, B and F, asterisks). β-COP, a COPI-specific vesicle protein, did not colocalize with E1-GCT in Vero cells (Figure 6, C and D). Taken together, these results demonstrate that proteins involved in the formation of anterograde transport vesicles are concentrated at or near the site of E1 arrest.
Figure 5.
E1-GCT colocalizes with proteins involved in generation of ER-derived transport vesicles. BHK-E1-GCT cells were fixed with methanol as described above and incubated with rabbit (A) and mouse (C) antibodies directed against the ERGIC marker p58 or rabbit anti-Sec23p (E). E1-GCT was detected using P5D4 (B), human anti-E1 (D), or rabbit anti-GCT (F). The distribution of Sec23p (G) and mouse anti-GCT (H) in untransfected BHK cells are also shown. Secondary antibodies were FITC-donkey anti-rabbit IgG, FITC-goat anti-human IgG, and Texas Red-goat anti-mouse IgG. Overlap between p58 and Sec23p with E1-GCT in the juxtanuclear region is indicated by arrowheads. Bar, 10 μm.
Immunoisolation of E1-containing Membranes
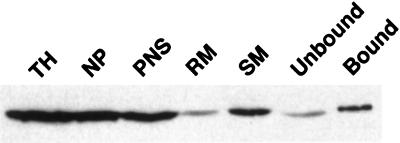
Our next step was to obtain fractions enriched in E1-containing membranes. The strategy used was to prepare a PNS from BHK E1-GCT cells, separate it into rough and smooth membrane fractions, and use the latter as starting material for immunoisolation on magnetic beads coated with a monoclonal antibody, P5D4, that recognizes the CT domain of VSV G. We found that ∼50% of the E1-GCT pelleted with the 1000 × g nuclear pellet (Figure 7), which is not surprising in view of the large size (up to 2 μM) of many of the membrane aggregates. The majority of the remaining E1-GCT sedimented with the smooth microsome fraction with very little found in the rough microsomes. Examination of smooth microsome fractions by EM confirmed the presence of largely intact masses of E1-containing membranes (our unpublished observations), confirming that smooth microsomes represent a suitable starting material for immunoisolation.
Figure 7.
Immunoisolation of E1-GCT containing membranes. BHK-E1-GCT cells were homogenized and separated into total homogenate (TH), postnuclear supernatant (PNS), nuclear pellet (NP), smooth membranes (SM), rough membranes (RM), and immunoisolate fractions (bound) prepared using P5D4-coated magnetic beads. Equivalent volumes normalized to starting material for each fraction were electrophoresed through 10% polyacrylamide gels followed by transfer to PVDF membranes. E1-GCT was detected by probing membranes with rabbit antibody directed against the CT domain of VSV G followed by goat anti-rabbit IgG-HRP and ECL detection. The smooth membrane fraction was used as the starting material for immunoisolation. Most of the E1-GCT was recovered in the bound fraction; however, a significant portion did not bind to the beads (unbound).
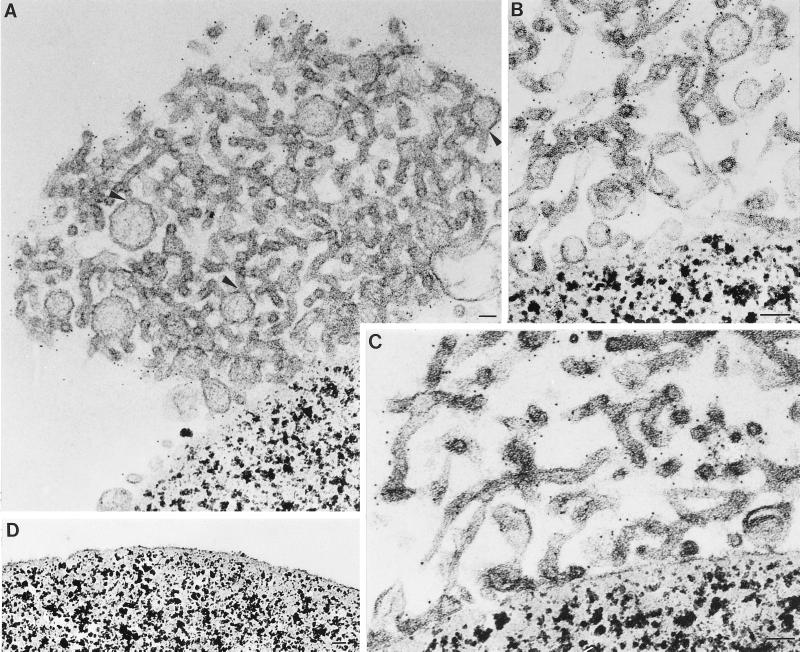
Magnetic beads were coated with either Mab P5D4 (recognizes the endodomain of VSV G) or Mab BW8G65 (recognizes the ectodomain of VSV G). The beads were then incubated with smooth microsome fractions and processed for western blot analysis and EM. The majority of E1-GCT was recovered on the P5D4-coated beads (Figure 7, bound fraction). Analysis of the beads by EM revealed that large aggregates of the tubular, smooth membranes were bound to the surface of the P5D4-coated beads (Figure 8, A–C). By contrast, beads coated with BW8G65 did not contain bound tubular networks (Figure 8D). The bound tubular membranes were identical to those seen in situ in cells overexpressing E1 or E1-GT (Figure 4, A and B). The presence of E1-GCT in the immunoisolated tubules was confirmed by immunogold labeling with a rabbit antibody directed against the VSV G CT domain (Figure 8, A–C).
Figure 8.
Isolated tubular networks contain vesicles and budding structures. (A–C) Immunoisolated tubular networks isolated from BHK-E1GCT cells were processed for EM while still attached to P5D4-coated magnetic beads. Vesicles and budding structures that are associated with the tubular nests are evident in panels A and B. In panel A, regions of apparent continuity between budding structures and the tubules are indicated by arrowheads. Samples were incubated with a rabbit antibody directed against the VSV G epitope on E1-GCT followed by 5 nm gold coupled to protein A. Labeling is confined to the cytoplasmic surface of the tubules. (D) Beads coated with an irrelevant monoclonal antibody (BW8G65) were incubated with smooth membrane fractions prepared from BHK-E1-GCT cells and prepared for EM as described above. No smooth membranes are seen attached to the beads. Bar, 50 nm in all panels.
Among the isolated tubular networks were what appeared to be budding structures protruding from the ends of the tubules (Figure 8A, arrowheads). The buds were delimited by membranes that were typically in continuity with the tubular membranes. Similar budding structures were sometimes seen in situ (Figure 4B, arrowheads). The nature and function of these structures are presently unclear, but they resemble COPII vesicles in size and appearance. Some of the budding profiles shown in Figure 8A are ∼100 nm in diameter, a size that is larger than normal COPII vesicles. The origin of these structures is not known; however, since they were not prevalent among the tubular networks in situ (Figure 8, A and B), it seems likely that they are derived from smaller budding profiles that have become swollen during immunoisolation or during preparation for EM.
E1-GCT–Containing Membranes Are Enriched in ERGIC 53/58, Sec23p, and Sec13p but Not in RER, Golgi, or COPI Vesicle Markers
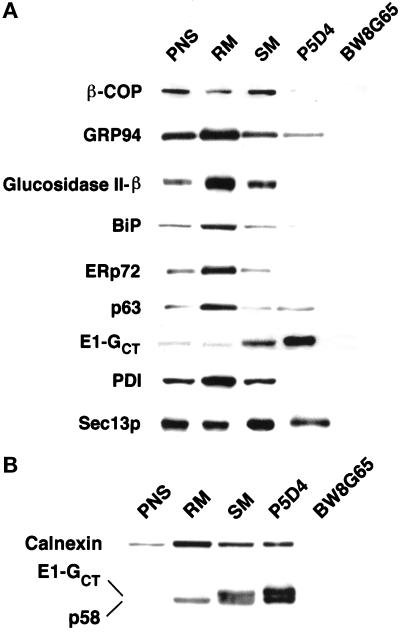
Next we analyzed isolated tubular networks by immunoblotting with a panel of antibodies to resident ER, ERGIC, and Golgi proteins and to proteins involved in generation of anterograde and retrograde transport vesicles. The protein concentration of each fraction was measured, and equal amounts of protein were separated by SDS-PAGE, transferred to PVDF, and probed with various antibodies. To enable accurate quantitation, only one-fifth the amount of protein in the immunoisolates was loaded since the E1-GCT signal was so strong in the P5D4 fraction. The blots were scanned using a laser densitometer, and our analysis indicated that the P5D4-immunoisolated fractions were enriched >50 fold for E1-GCT compared with the PNS (Figure 9, A and B). Mab BW8G65, specific for the ectodomain of VSV G protein, did not bind to the E1-GCT-containing membranes (Figure 9, A and B). The ER markers GRP94, glucosidase II β-subunit, BiP, ERp72, p63, calnexin, and protein disulfide isomerase were concentrated in the rough microsome fraction with smaller amounts in the smooth microsome fraction (Figure 9, A and B). This corroborates our previous finding by immunofluorescence that soluble ER proteins are not concentrated in the tubular networks (Hobman et al., 1992). A significant amount of GRP94, a KDEL-bearing protein, was detected in the P5D4 immunoisolates, but unlike E1-GCT, it was not enriched compared with the smooth microsome starting material. Results with calnexin were variable. In the experiment shown in Figure 9B, significant quantities of calnexin copurified with E1-GCT in the tubular networks, whereas in other fractionations, little or no detectable calnexin was present (our unpublished observations). The latter was more consistent with our indirect immunofluorescence finding, which indicated that calnexin was not concentrated in the E1-GCT-containing pre-Golgi structures (Figure 3, C and D). Moreover, we were unable to detect interactions between E1-GCT and calnexin using a coimmunoprecipitation protocol (Hammond and Helenius, 1994) optimized to preserve interactions between calnexin and nascent proteins (our unpublished observations). Finally, a small amount of p63, an ER membrane protein of unknown function (Schweizer et al., 1993a), copurified with E1-GCT but was not significantly enriched compared with E1-GCT (Figure 9A). The significance of the presence of calnexin and p63 in the immunoisolated fractions is unclear, but we cannot rule out the possibility that small amounts of RER membranes were present.
Figure 9.
Immunoblotting of P5D4-immunoisolated membranes with anti-ER, Golgi and ERGIC markers. BHK-E1-GCT cells were homogenized and separated into postnuclear supernatant (PNS), rough microsomes (RM), smooth membranes (SM), P5D4-immunoisolates (P5D4), and immunoisolates obtained using B8G65, a mAb of the same isotype as P5D4, that recognizes the ectodomain of VSV-G. Immunoisolates of PNS, RM, and SM (15 μg each) and P5D4 and BW8G65 (3 μg each) were separated on 10% gels before transfer to PVDF. Blots were probed with rabbit anti-VSV G CT serum (to detect E1-GCT) and various antibodies to marker proteins for ER, Golgi, and ERGIC followed by goat anti-rabbit and anti-mouse IgG conjugated to HRP. Blots were then subjected to ECL development. (A) Soluble RER proteins and the ERGIC/Golgi marker β-COP are largely depleted or absent from the P5D4 immunoisolates. In contrast, E1-GCT and Sec13p are enriched. (B) The ERGIC marker p58 is highly enriched in the E1-GCT–containing membranes.
The COPI vesicle coat proteins β-COP (Figure 9A) and γ-COP (our unpublished observations) were most concentrated in the smooth microsome fraction but were depleted in P5D4 immunoisolates (Figure 9A). By contrast, p58/ERGIC 53 was highly enriched in the immunoisolates (Figure 9B). Sec13p, a cytosolic protein that is recruited to regions of the ER involved in vesicle formation (Shaywitz et al., 1995; Bannykh et al., 1996; Tang et al., 1997), was also abundant in immunoisolates but was not enriched to the same extent as p58/ERGIC 53 (Figure 9, A and B). These results, together with our immunofluorescence findings (Figures 5 and 6), clearly demonstrate that two proteins involved in cargo export from the ER, p58/ERGIC 53 and Sec23p, are concentrated at the site of E1/E1-GCT arrest. Collectively, our data indicate that the tubular networks have the properties expected of hypertrophied ER exit sites.
Resident Proteins of the E1-GCT–Containing Membranes
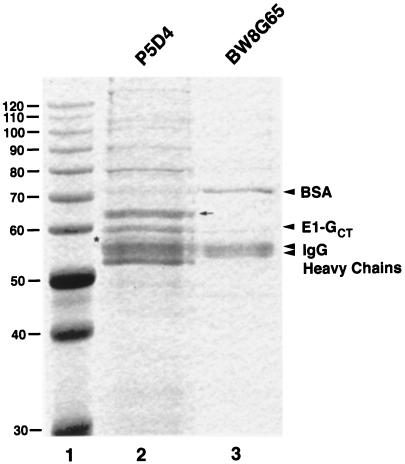
Immunoisolates prepared using either P5D4- or BW8G65-coated magnetic beads were separated by SDS-PAGE and stained with Coomassie R-250 to determine what other proteins might be enriched in this compartment. The most abundant proteins in P5D4-bound membranes were IgG heavy chains from P5D4 and goat anti-mouse IgG (primary antibody), E1-GCT, and a 65-kDa protein of unknown identity (Figure 10, lane 2). The abundance of this protein is consistent with the possibility that it represents a structural component of the tubular networks. The protein was isolated for microsequencing, and the sequences of two internal peptides were obtained. A search of the nonredundant protein and nucleic acid databases with these sequences did not produce any matches, suggesting that this protein is novel. Less abundant proteins with apparent molecular masses of ∼58, 80, 90, 100, 105, and >130 kDa were also evident (Figure 10, lane 2). Presumably, the ∼58-kDa protein located between E1GCT and the IgG heavy chain bands is the ERGIC marker protein p58 (Figure 10, lane 2, arrow). The 80-kDa band, which appears as a doublet, was also subjected to microsequencing, and two sequences were obtained. One of the peptides was highly homologous to BiP, whereas the other peptide was unique.
Figure 10.
Composition of E1-GCT–containing membranes. BHK-E1-GCT cells were homogenized and immunoisolates were prepared using P5D4 or BW8G65 as described above. Samples were subjected to SDS-PAGE on 10% gels and stained with Coomassie R-250. Protein molecular mass standards (kDa) are shown in lane 1. Only the portion of the gel above 30 kDa is shown, as only the IgG light bands were discernable in this region. The positions of known proteins are indicated. IgG Hc, IgG heavy chains. The IgG Hc region in both lanes is a doublet caused by the presence of secondary antibodies (P5D4 in lane 2, BW8G65 in lane 3) and primary antibody, goat anti-mouse IgG1, Fc-specific (both lanes). The P5D4 IgG Hc is the lowest band in the IgG Hc region, whereas the BW8G65 Hc migrates just below the goat anti-mouse IgG Hc. P5D4 immunoisolates contain a prominent 65-kDa protein (lane 2, arrow) of unknown identity that is not found in the nonspecific immunoisolate (lane 3). The asterisk in lane 2 denotes a 58-kDa protein that is thought to be p58/ERGIC53.
DISCUSSION
The ER has been appropriately described as a “dynamic patchwork of specialized subregions” (Sitia and Meldolesi, 1992). In this paper we extended our earlier studies (Hobman et al., 1992) characterizing a novel subdomain of the ER defined by the presence of transport-arrested rubella E1. This membrane compartment does not contain ribosomes, and unlike “classical” ER, is not cisternal but is composed of networks of narrow tubules, ∼30 nm in diameter, that are in direct continuity with the RER (Hobman et al., 1992). The degree of development of the tubular network is proportional to the level of expression of the E1 ectodomain in transfected cells.
We initially hypothesized that these tubules may correspond to ER exit sites based on the following observations: 1) an itinerant protein (VSV G), was able to enter and then rapidly exit this compartment en route to the Golgi complex and cell surface (Hobman et al., 1992); and 2) shortly after release of a reversible transport block, numerous VSV G-containing vesicles were observed in the immediate vicinity of the E1-containing membranes (Hobman et al., 1993). In this study we provide direct evidence for this hypothesis by showing that Sec23p, a protein required for generation of ER transport vesicles, is concentrated in or near the tubular networks as there is significant colocalization between E1-GCT and Sec23p by indirect immunofluorescence in transfected BHK cells. Sec23p is a soluble guanosine triphosphatase-activating protein that binds to the guanosine triphosphate (GTP)-binding protein Sar1p on the cytosolic surface of the ER (Yoshihisa et al., 1993; Kuehn and Schekman, 1997). Sec23p, together with Sar1p, Sec24p, Sec13p, and Sec31p, assembles into a complex that coats ER transport vesicles (COPII). We were not able to colocalize Sec23p to the tubular networks by immuno-EM due to poor reactivity of the antibody under our conditions. Sec13p was also enriched in this compartment, although not to the same extent as E1-GCT. Since the immunoisolations were not done in the presence of GTPγS, it is possible that the Sec13p recovered in the immunoisolates is under-represented due to loss of coats during the immunoisolation. The same reservation applies to potential loss of COPI components during immunoisolation. We also tested for the presence of other proteins involved in biogenesis of ER transport vesicles, i.e., Sec31p, Sec12p, and Sar1p, by immunoblotting of purified tubules and indirect immunofluorescence, but the results were inconclusive due to poor immunoreactivity in BHK cells or high background staining.
The most abundant known protein excluding E1-GCT in the tubular networks is p58/ERGIC 53, a mannose lectin (Arar et al., 1995) that is incorporated into ER-derived transport vesicles and is essential for ER to Golgi trafficking (Rowe et al., 1996; Tisdale et al., 1997). Exactly how p58/ERGIC 53 participates in ER-to-Golgi trafficking is unknown, but it has been suggested that it may serve as a sorting receptor facilitating anterograde transport by actively recruiting newly synthesized glycoproteins into COPII-coated vesicles during budding from the ER (Farquhar and Hauri, 1997) or, alternatively, that it may function as a late (post-ER) chaperone downstream from BiP and calnexin (Hauri and Schweizer, 1997; Scales et al., 1997). Our finding that p58 is concentrated in the tubular membranes fits with our earlier hypothesis that E1 accumulates at ER exit sites. We are currently in the process of testing whether E1 interacts with p58.
Proliferation of ER-derived tubular networks is not unique to transfected cells expressing viral membrane proteins. Several conditions that impair transport of proteins between the ER and Golgi complex were also shown to induce the formation of expanded tubular membranes at the ER/Golgi interface. For example, disruption of the Golgi complex by adding high concentrations of cytosol and an ATP-regenerating system to permeabilized NRK cells resulted in the development of networks of convoluted tubules connected to the RER that are morphologically similar to the E1-containing structures (Hidalgo et al., 1993). Similarly, Raposo et al. (1995) recently demonstrated that unassembled major histocompatibility complex (MHC) class I heavy chains accumulate in an expanded network of tubular and fenestrated membranes that resemble the E1-containing tubules and contain ERGIC 53, but not protein disulfide isomerase. Interestingly, these investigators found that ubiquitin and ubiquitin-conjugating enzymes were present in the MHC class I-containing compartment, suggesting that it may be involved in translocation across the RER and degradation (Raposo et al., 1995). Finally, tubulovesicular profiles very similar in appearance to the E1-induced compartment have been described in enlarged neurites and presynaptic terminals in frontal biopsies obtained from Alzheimer’s patients (Richard et al., 1989). Whether or not the expansion of this ER subcompartment has any role in pathogenesis of Alzheimer’s disease has not been determined.
Our results provide direct evidence that the site of E1 arrest corresponds to amplified ER exit sites rather than a degradative compartment for the following reasons: 1) Cytosolic (Sec 23p, Sec 13p) and membrane (p58/ERGIC 53) proteins involved in biogenesis of COPII vesicles are concentrated at this site. 2) Itinerant proteins can enter and exit this compartment en route to the Golgi complex. 3) Proteins involved in ER degradation (ERp 72) (Urade et al., 1993) are absent. 4) Many ER proteins are excluded from this site. 5) 60-to 70-nm vesicle and budding profiles were observed within the tubular nests. Based on these findings, we propose the name SEREC (smooth ER exit compartment) for these membranes.
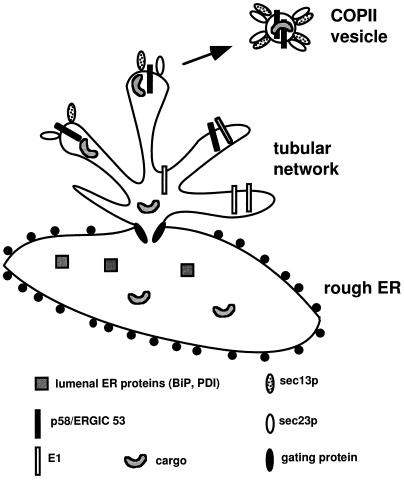
Our data conform best to the “privileged site” model (Kuehn and Schekman, 1997) for generation of ER-derived transport vesicles. This model predicts that there are specialized regions of the ER or “hot-spots” where cargo and proteins involved in vesicle biogenesis congregate. Bannykh et al. (1996) recently reported finding such regions in the ER of mammalian cells. According to this model, “gating” proteins are postulated to direct TM cargo proteins to privileged sites in the membrane where budding occurs (Figure 11). The gating proteins are also postulated to sieve soluble cargo protein into the budding sites while restricting most resident ER proteins. The gating proteins would be expected to be fairly abundant in these regions of the ER. Possible candidates for such a function could be the 65- and/or 80-kDa proteins found in the immunoisolated tubular networks. If our prediction is correct, E1-GCT is able to enter the “privileged sites” but is somehow prevented from entering vesicles. Further characterization of the resident protein SEREC may provide further insight into how the ER sorts and concentrates proteins for export into vesicles.
Figure 11.
Model showing arrangement of tubular networks in relation to RER. The model was adapted from the “priviledged site budding” model by Kuehn and Schekman (1997). Expression of E1 causes the proliferation of a smooth subdomain of the ER from which COPII vesicles are derived. Access of most lumenal ER and membrane proteins into the tubular networks is limited by gating proteins. The concentration of these proteins is highest in the RER denoted by the presence of ribosomes (black circles). Only cargo and proteins that have satisfied the requirements of the ER quality control system are allowed access into this region. Proteins involved in biogenesis of COPII transport vesicles accumulate in these smooth regions of the ER. Although E1 is allowed access into the smooth membranes, it is somehow prevented from entering the transport vesicles. Newly formed transport vesicles are also seen in the vicinity of these membrane nests.
ACKNOWLEDGMENTS
This paper is dedicated to the memory of Luann Woodward. We thank Drs. Carolyn Machamer, Hans-Peter Hauri, Peter Kim, John Bergeron, David Williams, Chris Kaiser, Hanne Ostergaard, Thomas Kreis, Jaako Saraste, and Randy Schekman for their generous gifts of antibodies and Steve Rice and Shirley Gillam for cell lines. We also thank Dr. Bruce Stevenson for critical reading of the manuscript and Mike McCaffery for help with figures. This work was supported by grants from the Medical Research Council of Canada and the Alberta Heritage Foundation for Medical Research awarded to T.C.H and DK-17780 from the National Institutes of Health to M.G.F.
Footnotes
Abbreviations used: BHK, baby hamster kidney; CHO, Chinese hamster ovary; CT, cytoplasmic; endo H, endoglycosidase H; ER, endoplasmic reticulum; Man II, mannosidase II; RER, rough endoplasmic reticulum; RV, rubella virus; TM, transmembrane; VSV, vesicular stomatitis virus.
REFERENCES
- Allan VJ, Kreis TE. A microtubule-binding protein associated with membranes of the Golgi apparatus. J Cell Biol. 1986;103:2229–2239. doi: 10.1083/jcb.103.6.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S, Davis DL, Dahlback H, Jornvall H, Russell DW. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- Arar C, Carpentier C, le Caer J-P, Monsigny M, Legrand A, Roche A-C. ERGIC-53, a membrane protein of the endoplasmic reticulum-Golgi intermediate compartment, is identical to tMR60, an intracellular mannose-specific lectin of myelomonocytic cells. J Biol Chem. 1995;270:3551–3553. doi: 10.1074/jbc.270.8.3551. [DOI] [PubMed] [Google Scholar]
- Arendt CW, Ostergaard HL. Identification of the CD45-associated 116-kDa and 80-kDa proteins as the alpha- and beta-subunits of the alpha-glucosidase II. J Biol Chem. 1997;272:13117–13125. doi: 10.1074/jbc.272.20.13117. [DOI] [PubMed] [Google Scholar]
- Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Bannykh SI, Rowe T, Balch WE. The organization of endoplasmic reticulum export complexes. J Cell Biol. 1996;135:19–35. doi: 10.1083/jcb.135.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazolla M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bednarek S, Ravazolla M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Bergstrand A, Dallner G. Isolation of rough and smooth microsomes from rat liver by means of a commercially available centrifuge. Anal Biochem. 1969;29:351–356. doi: 10.1016/0003-2697(69)90321-2. [DOI] [PubMed] [Google Scholar]
- Farquhar M, Hauri H-P. Protein sorting and vesicular traffic in the Golgi apparatus. In: Berger E, Roth J, editors. The Golgi Apparatus. Basel, Switzerland: Birkhauser Verlag; 1997. pp. 63–128. [Google Scholar]
- Hammond C, Helenius A. Quality control in the secretory pathway: retention of a misfolded viral glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J Cell Biol. 1994;126:41–52. doi: 10.1083/jcb.126.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H-P, Schweizer A. The ER-Golgi membrane system: compartmental organization and protein traffic. In: Hoffman JF, Jamieson JD, editors. Handbook of Physiology. New York: Oxford University; 1997. pp. 605–642. [Google Scholar]
- Hidalgo J, Muniz M, Velasco A. Trimeric G proteins regulate the cytosol-induced redistribution of Golgi enzymes into the endoplasmic reticulum. J Cell Sci. 1993;108:1805–1815. doi: 10.1242/jcs.108.4.1805. [DOI] [PubMed] [Google Scholar]
- Hobman TC, Shukin R, Gillam S. Translocation of rubella virus glycoprotein E1 into the endoplasmic reticulum. J Virol. 1988;62:4259–4264. doi: 10.1128/jvi.62.11.4259-4264.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobman TC, Woodward L, Farquhar MG. The rubella virus E1 glycoprotein is arrested in a novel post-ER, pre-Golgi compartment. J Cell Biol. 1992;118:795–811. doi: 10.1083/jcb.118.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobman TC, Woodward L, Farquhar MG. Targeting of a heterodimeric membrane protein complex to the Golgi: Rubella virus E2 glycoprotein contains a transmembrane Golgi retention signal. Mol Biol Cell. 1995;6:7–20. doi: 10.1091/mbc.6.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobman TC, Woodward L, Komuro M, Farquhar MG. Identification of a novel post-ER, pre-Golgi compartment where unassembled monomers of oligomeric proteins accumulate. In: Morré DJ, Howell KE, Bergeron JJM, editors. Molecular Mechanisms of Membrane Traffic. New York, NY: Springer-Verlag; 1993. pp. 17–34. [Google Scholar]
- Howell KE, Crosby JR, Ladinsky MS, Jones SM, Schmid R, Ugelstad J. Magnetic solid supports for cell-free analysis of vesicular transport. In: Uhlen M, Hornes E, Olsvik O, editors. Advances in Biomagnetic Separation. London, United Kingdom: Eaton Publishing; 1994. pp. 195–204. [Google Scholar]
- Hurtley S, Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Kuehn M, Schekman R. COPII and secretory cargo capture into transport vesicles. Curr Opin Cell Biol. 1997;9:477–483. doi: 10.1016/s0955-0674(97)80022-1. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Hirata A, Nakano A. Inhibition of endoplasmic reticulum (ER)-to-Golgi transport induces relocation of binding protein (BiP) within the ER to form BiP bodies. Mol Biol Cell. 1994;5:1129–1143. doi: 10.1091/mbc.5.10.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Perrelet A, Ravazola M, Amherdt M, Rothman JE, Schekman R. Coatomer-rich endoplasmic reticulum. Proc Natl Acad Sci USA. 1994;91:11924–11928. doi: 10.1073/pnas.91.25.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Meda P, Holcomb C, Moore H-P, Hicke L, Schekman R. Mammalian Sec23p homologue is restricted to the endoplasmic reticulum transitional cytoplasm. Proc Natl Acad Sci USA. 1991;88:8611–8615. doi: 10.1073/pnas.88.19.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein secretion. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Behringer RR, Quaife CJ, Maxwell F, Maxwell IH, Brinster RL. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987;50:435–443. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- Raposo G, van Santen HM, Leijendekker R, Geuze HJ, Ploegh HL. Misfolded major compatibility complex class I molecules accumulate in an expanded ER-Golgi intermediate compartment. J Cell Biol. 1995;131:1403–1419. doi: 10.1083/jcb.131.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S, Brion JP, Couck AM, Flament-Durand J. Accumulation of smooth endoplasmic reticulum in Alzheimer’s disease: new morphological evidence of axoplasmic flow disturbances. J Submicrosc Cytol Pathol. 1989;21:461–467. [PubMed] [Google Scholar]
- Rose JK, Doms RW. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Rowe T, Aridor M, McCafferey J, Plutner H, Balch W. COPII vesicles derived from mammalian endoplasmic reticulum (ER) microsomes recruit COPI. J Cell Biol. 1996;135:895–911. doi: 10.1083/jcb.135.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J, Palade GE, Farquhar MG. Antibodies to rat pancreas Golgi subfractions: identification of a 58 kD cis-Golgi protein. J Cell Biol. 1987;105:2021–2029. doi: 10.1083/jcb.105.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J, Svensson K. Distribution of the intermediate elements operating in ER to Golgi transport. J Cell Sci. 1991;100:415–430. doi: 10.1242/jcs.100.3.415. [DOI] [PubMed] [Google Scholar]
- Scales S, Pepperkok R, Kreis T. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Ericsson M, Bachi T, Griffiths G, Hauri H-P. Characterization of a novel 63 kDa membrane protein: implications for the organization of the ER-to-Golgi pathway. J Cell Sci. 1993;104:671–683. doi: 10.1242/jcs.104.3.671. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Fransen JAM, Bächi T, Ginsel L, Hauri H-P. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J Cell Biol. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A, Rohrer J, Jeno P, DeMaio A, Buchman TG, Hauri H-P. A reversibly palmitoylated resident protein (p63) of an ER-Golgi intermediate compartment is related to a circulatory shock resuscitation protein. J Cell Sci. 1993;104:685–694. doi: 10.1242/jcs.104.3.685. [DOI] [PubMed] [Google Scholar]
- Shaywitz DA, Orci L, Ravazzola M, Swaroop A, Kaiser CA. Human sec13Rp functions in yeast and is located on transport vesicles budding from the endoplasmic reticulum. J Cell Biol. 1995;128:769–777. doi: 10.1083/jcb.128.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R, Meldolesi J. Endoplasmic reticulum: a dynamic patchwork of specialized subregions. Mol Biol Cell. 1992;3:1067–1072. doi: 10.1091/mbc.3.10.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B, Peter F, Krijnse Locker J, Low S, Griffiths G, Hong W. The mammalian homolog of yeast sec13p is enriched in the intermediate compartment and is essential for protein transport to the Golgi apparatus. Mol Cell Biol. 1997;17:256–266. doi: 10.1128/mcb.17.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale EJ, Plutner H, Matteson J, Balch WE. p53/58 binds COPI and is required for selective transport through the early secretory pathway. J Cell Biol. 1997;137:581–593. doi: 10.1083/jcb.137.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urade M, Mima T, Yamamoto N. Structural gene products and morphogenesis of a hybrid between rubella virus and a hamster latent virus. Res Virol. 1993;144:129–139. doi: 10.1016/s0923-2516(06)80021-x. [DOI] [PubMed] [Google Scholar]
- Urade R, Takenaka Y, Kito M. Protein degradation by ERp72 from rat and mouse liver endoplasmic reticulum. J Biol Chem. 1993;268:22004–22009. [PubMed] [Google Scholar]
- Velasco A, Hendricks L, Moremen KW, Tulsiani DRP, Touster O, Farquhar MG. Cell type-dependent variations in the subcellular distribution of α-mannosidase I and II. J Cell Biol. 1993;122:39–51. doi: 10.1083/jcb.122.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterborne DJ. Chemical assays for proteins. In: Graham JM, editor. Methods in Molecular Biology: Biomembrane Protocols. I. Isolation and Analysis. Totowa, NJ: Humana Press; 1993. pp. 197–202. [Google Scholar]
- Yoshihisa T, Barlowe C, Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science. 1993;259:1466–1468. doi: 10.1126/science.8451644. [DOI] [PubMed] [Google Scholar]